Abstract
Spinal nerve root metastasis of renal cell carcinoma is a rare occurrence. In addition to treatment of the primary lesion, surgical resection of the nerve root metastasis, occasionally with sacrifice of the involved nerve, is the accepted standard of treatment. Resection often resolves presenting motor and pain symptoms due to relief of neural compression. We describe two patients with nerve root metastasis of renal cell carcinoma and their management. While locally advanced and metastatic renal cell carcinoma has been shown to be chemo- and radio-resistant, immunotherapy is a promising treatment. Given the high prevalence of systemic disease in patients with intradural metastases, systemic (and possibly intracranial) imaging can be used to identify other potential areas of disease.
Keywords: Cauda equina, Intradural, Metastasis, Nerve root, Renal, Spine
1. Introduction
The intradural space is a rare location of metastatic renal cell carcinoma (RCC). In a previous review of 26 patients, patients with sporadic disease (unrelated to von Hippel-Lindau [VHL] syndrome) received an initial diagnosis of primary RCC at 57 ± 13 years and diagnosis of the spinal cord metastasis at 60 ± 14.5 years.1 33% of these patients had no evidence of other metastases, similar to other series.1–7 Given the overall rarity of this lesion, each patient contributes to the overall understanding of disease presentation and management.
2. Case report
2.1 Patient 1
A 49-year-old woman with a history of left RCC status post left radical nephrectomy 8 years prior presented with a 1 year history of left buttock pain with radiation into the left lateral thigh and calf. She had also experienced some recent subjective left lower extremity weakness. On examination, she had full strength in all extremities. She had symmetric reflexes in the lower extremities without spreading or cross-activation. Straight leg raise on the left side elicited left posterior thigh pain. Her gait was normal and Romberg test was negative. The rest of her neurological exam was unremarkable. Lumbar spine MRI revealed a 2.0 cm anteroposterior × 2.7 cm transverse × 1.6 cm superior-inferior mildly gadolinium-enhancing mass underlying the pars of the left L4 vertebra without evidence of bony invasion. The left L4 neural foramen was enlarged compared to the contralateral side. Given her worsening left lower extremity pain, surgical exploration and decompression of the left L4 nerve root was undertaken. The overlying dura contained more blood vessels than was typical. The dura of the nerve root was opened medially to laterally. The lesion infiltrated the L4 nerve root and was cream-colored and hypervascular. Internal decompression was achieved despite episodes of bleeding. Resection was subtotal in an attempt to avoid injuring the motor component of the nerve, especially considering that the patient had no motor deficit preoperatively. Postoperatively, the patient had full motor capacity in the bilateral lower extremities. Two years after initial presentation, the patient remained without evidence of recurrence and with no new weakness, numbness, or parasthesias.
2.2 Patient 2
A 72-year-old man with RCC involving the right kidney was found to have a 1.5 × 1.1 cm enhancing lesion within the central spinal canal at the L2 level on a staging CT scan. He denied any back or lower extremity pain, lower extremity weakness, numbness, or paresthesias. Bowel and bladder function were normal. On examination, he had full strength in all extremities. He had symmetric 3+ patellar tendon reflexes bilaterally and 1+ bilateral ankle reflexes. The rest of his neurological exam was unremarkable. Lumbar spine MRI revealed 1.4 × 1.1 cm intensely gadolinium-enhancing intradural mass at the L2 level with mass effect on the cauda equina nerve roots. Given his hyperreflexia and mass effect demonstrated on MRI, he underwent surgical resection. Following an L2 laminectomy, the dura was opened revealing a red, highly vascularized intradural mass surrounding a single nerve rootlet. Direct stimulation of the nerve rootlet produced diffuse lower extremity stimulation, suggesting that it was a dorsal nerve rootlet. Every attempt was made to separate the tumor from the nerve rootlet; however, the tumor was highly vascularized, preventing the development of a dissection plane without significant bleeding. Thus, the tumor was resected en bloc along with the nerve rootlet. Pathology was consistent with metastatic renal cell carcinoma. Postoperatively, he recovered well, with only a slight left sided area of numbness but otherwise intact strength and normal gait. Two years after initial presentation, the patient remained without evidence of recurrence and with no new weakness, numbness, or parasthesias.
3. Discussion
3.1 Natural history of renal cell carcinoma metastasis
Intradural metastases of RCC occur throughout the spinal cord. As of 2009, there were eight reported patients with intradural RCC metastasis to the cauda equina8, some presenting as the first metastasis and others after previous metastases. RCC nerve root metastases have been described as both intramedullary and extramedullary masses.4,7–11 Some remain intramedullary, and others are similar to nerve sheath tumors in their rubbery consistency and clear demarcation.1,9 Peripheral endoneural metastases of RCC have also been diagnosed up to 4 years after surgical removal of the primary tumor.12 Although rare, the incidences of cervical, thoracic, and lumbar tumors are equal, with sacral lesions being less common.1,13
Patients with intradural metastases have a mean survival of 11 months after diagnosis of the spinal lesion, most often due to systemic disease.1 Patients with VHL have a better prognosis postoperatively, which may be attributed to being diagnosed early, with a pre-existing hemangioblastoma later being seeded by RCC.4,14–18 In fact, RCC has also been shown to seed hemangioblastomas of the medulla and cerebellum.19–23 In a single series of spinal metastases, 93% of sporadic metastasis patients were deceased at 15 months while only one-third of VHL metastasis patients were deceased at 2 years.1
3.2 Diagnostic workup
The diagnosis of patients with intradural spinal cord metastases of RCC draws attention to its systemic involvement. RCC has usually disseminated by the time it has reached the spinal cord, the most common locations being the lung (50%) and bone (49%), with the brain involved less often (8–10%).24 Despite the low incidence of concomitant brain metastasis in RCC, 41% of individuals with intradural spinal cord metastases in general (of renal and non-renal etiology) present with brain metastases and 57% develop brain metastases over time. Of course, the mode of spinal cord seeding would determine the likelihood of brain metastases, but this remains controversial. The cerebrospinal venous system is responsible for intracranial–spinal spread of the tumor.25,26 In this system, the cortical veins, dural sinuses, cavernous sinuses and ophthalmic veins of the intracranial system are connected in the suboccipital region to the vertebral venous system running along the spinal cord.26 Therefore, individuals found to have intradural RCC metastases may warrant brain imaging.1
3.3 Surgical management
In one surgical series, 94% of patients undergoing operations for sporadic intradural RCC metastases (laminectomy with tumor resection) experienced improvement in pain and/or motor function, although sensory disturbance and urinary dysfunction were less likely to improve.1 The decision to provide surgical management depends on the severity of disease. Those with advanced disease unable to undergo surgery may alternatively receive radiotherapy,4,10 even though RCC is known for its resistance to radiotherapy.27 In one review, surgery in patients with single-site metastases was shown to provide better survival than with medical therapy or radiotherapy alone, although systemic therapy is crucial given the 79% likelihood of recurrent primary disease with the discovery of a single metastasis.28 While interleukin 2 and interferon alpha have been mainstay medical therapy in addition to nephrectomy for RCC, immunotherapy such as vascular endothelial growth factor receptor antibodies, multikinase inhibitors, tyrosine kinase inhibitors, and mammalian target of rapamycin inhibitors have also shown effectiveness.29–36 Since surgery is not without its complications and operative planning is not without its contingencies, it is important to note that some nerve root metastases may not be well-demarcated lesions easily separable from the nerve root and may therefore require transection of the nerve as in Patient 2. Nerve transection due to tumor involvement, as in Patient 2 and as previously described, can sometimes alleviate pain.37
4. Conclusions
The treatment of nerve root metastases of RCC includes surgical resection of the tumor, with sacrifice of the nerve being acceptable for palliation. Identification of the need for palliation is important, as many patients with nerve root metastases suffer a large burden of systemic disease. Excellent long-term outcomes are possible with surgical treatment in some patients. Systemic imaging (including brain imaging) is warranted.
Fig. 1.
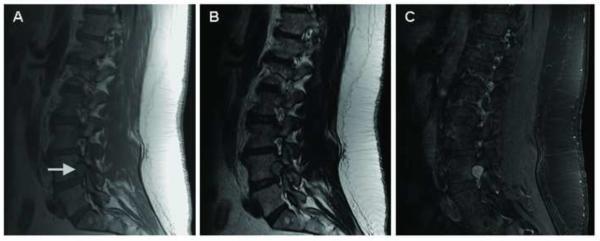
(A) T1, (B) T2, and (C) T1-weighted sagittal post-gadolinium MRI of the lumbar spine showing a 1.4 cm intradural lesion (arrow) at the level of L2 with mass effect on the nerve root of the cauda equina.
Fig. 2.
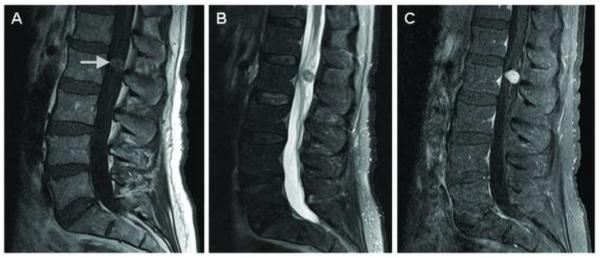
(A) T1, (B) T2, and (C) T1-weighted sagittal post-gadolinium MRI of the lumbar spine showing a T1 and T2 isointense enhancing mass (arrow) expanding the left L4–L5 neural foramen with extension into the posterior vertebral body.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Jost G, Zimmerer S, Frank S, Cordier D, Merlo A. Intradural spinal metastasis of renal cell cancer. Report of a case and review of 26 published cases. Ada Neurochir (Wien) 2009;151:815–821. doi: 10.1007/s00701-009-0358-6. discussion 821. [DOI] [PubMed] [Google Scholar]
- 2.Alfieri A, Mazzoleni G, Schwarz A, Campello M, Broger M, Vitale M, et al. Renal cell carcinoma and intradural spinal metastasis with cauda equina infiltration: case report. Spine (Phila Pa 1976) 2005;30:161–163. [PubMed] [Google Scholar]
- 3.Ateaque A, Martin JL, O'Brien C. Intramedullary spinal cord metastases from a hypernephroma 11 years following the diagnosis and treatment of the primary lesion. Br J Neurosurg. 2000;14:474–476. doi: 10.1080/02688690050175337. [DOI] [PubMed] [Google Scholar]
- 4.Fakih M, Schiff D, Erlich R, Logan TF. Intramedullary spinal cord metastasis (ISCM) in renal cell carcinoma: a series of six cases. Ann Oncol. 2001;12:1173–1177. doi: 10.1023/a:1011693212682. [DOI] [PubMed] [Google Scholar]
- 5.Kaya RA, Dalkilic T, Ozer F, Aydin Y. Intramedullary spinal cord metastasis: a rare and devastating complication of cancer--two case reports. Neurol Med Chir (Tokyo) 2003;43:612–615. doi: 10.2176/nmc.43.612. [DOI] [PubMed] [Google Scholar]
- 6.Maxwell M, Borges LF, Zervas NT. Renal cell carcinoma: a rare source of cauda equina metastasis. Case report. J Neurosurg. 1999;90:129–132. doi: 10.3171/spi.1999.90.1.0129. [DOI] [PubMed] [Google Scholar]
- 7.Schijns OE, Kurt E, Wessels P, Luijckx GJ, Beuls EA. Intramedullary spinal cord metastasis as a first manifestation of a renal cell carcinoma: report of a case and review of the literature. Clin Neurol Neurosurg. 2000;102:249–254. doi: 10.1016/s0303-8467(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 8.Kim DY, Lee JK, Moon SJ, Kim SC, Kim CS. Intradural spinal metastasis to the cauda equina in renal cell carcinoma: a case report and review of the literature. Spine (Phila Pa 1976) 2009;34:E892–895. doi: 10.1097/BRS.0b013e3181b34e6c. [DOI] [PubMed] [Google Scholar]
- 9.Kubota M, Saeki N, Yamaura A, luchi T, Ohga M, Osato K. A rare case of metastatic renal cell carcinoma resembling a nerve sheath tumor of the cauda equina. J Clin Neurosci. 2004;11:530–532. doi: 10.1016/j.jocn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Poggi MM, Patronas N, Buttman JA, Hewitt SM, Fuller B. Intramedullary spinal cord metastasis from renal cell carcinoma: detection by positron emission tomography. Clin Nucl Med. 2001;26:837–839. doi: 10.1097/00003072-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lin TK, Chen SM, Jung SM. Solitary intradural extramedullary metastasis of renal cell carcinoma to the conus medullaris. Kaohsiung J Med Sci. 2011;27:45–48. doi: 10.1016/j.kjms.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Varin S, Faure A, Bouc P, Maugars Y, Berthelot JM. Endoneural metastasis of the sciatic nerve disclosing the relapse of a renal carcinoma, four years after its surgical treatment. Joint Bone Spine. 2006;73:760–762. doi: 10.1016/j.jbspin.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 13.Shakeel M, Kumaravel M, Mackenzie JM, Knight DJ. An uncommon cause of sciatica. J Coll Physicians Surg Pak. 2009;19:127–129. [PubMed] [Google Scholar]
- 14.Jarrell ST, Vortmeyer AO, Linehan WM, Oldfield EH, Lonser RR. Metastases to hemangioblastomas in von Hippel-Lindau disease. J Neurosurg. 2006;105:256–263. doi: 10.3171/jns.2006.105.2.256. [DOI] [PubMed] [Google Scholar]
- 15.Polydorides AD, Rosenblum MK, Edgar MA. Metastatic renal cell carcinoma to hemangioblastoma in von Hippel-Lindau disease. Arch Pathol Lab Med. 2007;131:641–645. doi: 10.5858/2007-131-641-MRCCTH. [DOI] [PubMed] [Google Scholar]
- 16.Hamazaki S, Nakashima H, Matsumoto K, Taguchi K, Okada S. Metastasis of renal cell carcinoma to central nervous system hemangioblastoma in two patients with von Hippel-Lindau disease. Pathol Int. 2001;51:948–953. doi: 10.1046/j.1440-1827.2001.01298.x. [DOI] [PubMed] [Google Scholar]
- 17.Altinoz MA, Santaguida C, Guiot MC, Del Maestro RF. Spinal hemangioblastoma containing metastatic renal cell carcinoma in von Hippel-Lindau disease. Case report and review of the literature. J Neurosurg Spine. 2005;3:495–500. doi: 10.3171/spi.2005.3.6.0495. [DOI] [PubMed] [Google Scholar]
- 18.Abou-Hamden A, Koszyca B, Carney PG, Sandhu N, Blumbergs PC. Metastasis of renal cell carcinoma to haemangioblastoma of the spinal cord in von Hippel-Lindau disease: case report and review of the literature. Pathology. 2003;35:224–227. doi: 10.1080/003130203100023191. [DOI] [PubMed] [Google Scholar]
- 19.Xiong J, Chu SG, Wang Y, Zhu JJ, Li C, Mao Y. Metastasis of renal cell carcinoma to a haemangioblastoma of the medulla oblongata in von Hippel-Lindau syndrome. J Clin Neurosci. 2010;17:1213–1215. doi: 10.1016/j.jocn.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Martin SE, Al-Khatib SM, Turner MS, Douglas-Akinwande AC, Hattab EM. A 41-year-old woman with von Hippel-Lindau and a cerebellar lesion. Brain Pathol. 2010;20:511–514. doi: 10.1111/j.1750-3639.2009.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mottolese C, Stan H, Giordano F, Frappaz D, Alexei D, Streichenberger N. Metastasis of clear-cell renal carcinoma to cerebellar hemangioblastoma in von Hippel Lindau disease: rare or not investigated? Acta Neurochir (Wien) 2001;143:1059–1063. doi: 10.1007/s007010170012. [DOI] [PubMed] [Google Scholar]
- 22.Bret P, Streichenberger N, Guyotat J. Metastasis of renal carcinoma to a cerebellar hemangioblastoma in a patient with von Hippel Lindau disease: a case report. Br J Neurosurg. 1999;13:413–416. doi: 10.1080/02688699943565. [DOI] [PubMed] [Google Scholar]
- 23.Jamjoom A, Kane N, Nicoll J. Metastasis of a renal carcinoma to a cerebellar haemangioblastoma in a case of von Hippel-Lindau disease. Neurosurg Rev. 1992;15:231–234. doi: 10.1007/BF00345942. [DOI] [PubMed] [Google Scholar]
- 24.Pagano S, Franzoso F, Ruggeri P. Renal cell carcinoma metastases. Review of unusual clinical metastases, metastatic modes and patterns and comparison between clinical and autopsy metastatic series. Scand J Urol Nephrol. 1996;30:165–172. doi: 10.3109/00365599609181294. [DOI] [PubMed] [Google Scholar]
- 25.Batson OV. The Function of the Vertebral Veins and Their Role in the Spread of Metastases. Ann Surg. 1940;112:138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobinick E, Vega CP. The cerebrospinal venous system: anatomy, physiology, and clinical implications. Med Gen Med. 2006;8:53. [PubMed] [Google Scholar]
- 27.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–875. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 28.Antonelli A, Zani D, Cozzoli A, Cunico SC. Surgical treatment of metastases from renal cell carcinoma. Arch Ital Urol Androl. 2005;77:125–128. [PubMed] [Google Scholar]
- 29.Joshi DD, Banerjee T. Vascular endothelial growth factor (VEGF) receptor antibody bevacizumab (avastin) induces regression of renal cell carcinoma in an adolescent resulting in residual tumorectomy. Pediatr Blood Cancer. 2008;50:903–904. doi: 10.1002/pbc.21243. [DOI] [PubMed] [Google Scholar]
- 30.Mizutani Y. Recent advances in molecular targeted therapy for metastatic renal cell carcinoma. Int J Urol. 2009;16:444–448. doi: 10.1111/j.1442-2042.2009.02277.x. [DOI] [PubMed] [Google Scholar]
- 31.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 32.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 33.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 34.Rixe O, Bukowski RM, Michaelson MD, Wilding G, Hudes GR, Bolte O, et al. Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol. 2007;8:975–984. doi: 10.1016/S1470-2045(07)70285-1. [DOI] [PubMed] [Google Scholar]
- 35.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 36.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 37.Takada T, Doita M, Nishida K, Miura J, Yoshiya S, Kurosaka M. Unusual metastasis to the cauda equina from renal cell carcinoma. Spine (Phila Pa 1976) 2003;28:E114–117. doi: 10.1097/01.BRS.0000049910.72881.A0. [DOI] [PubMed] [Google Scholar]


