Abstract
Background & Aims
Dual oxidases (DUOX) are conserved NADPH oxidases that produce H2O2 at the epithelial cell surface. The DUOX enzyme comprises the DUOX and DUOXA (DUOX maturation factor) subunits. Mammalian genomes encode 2 DUOX isoenzymes (DUOX1–DUOXA1 and DUOX2–DUOXA2). Expression of these genes is upregulated during bacterial infection and chronic inflammatory diseases of the luminal gastrointestinal tract. The roles of DUOX in cellular interactions with microbes have not been determined in higher vertebrates.
Methods
Mice with disruptions of Duoxa1 and Duoxa2 genes (Duoxa−/− mice) and control mice were infected with Helicobacter felis to create a model of Helicobacter pylori infection—the most common human chronic infection.
Results
Infection with H felis induced expression of Duox2 and Duoxa2 in the stomachs of wild-type mice, and DUOX protein specifically localized to the apical surface of epithelial cells. H felis colonized the mucus layer in the stomachs of Duoxa−/− mice to a greater extent than in control mice. The increased colonization persisted into the chronic phase of infection and correlated with an increased, yet ineffective, inflammatory response. H felis colonization was also increased in Duoxa+/− mice, compared with controls. We observed reduced expression of the H2O2-inducible katA gene in H felis that colonized Duoxa−/− mice, compared with that found in controls (P=.0002), indicating that Duox causes oxidative stress in these bacteria. In vitro, induction of oxidative defense by H felis failed to prevent a direct bacteriostatic effect, at sustained levels of H2O2 as low as 30 μM.
Conclusions
Based on studies of Duoxa−/− mice, the DUOX enzyme complex prevents gastric colonization by H felis and the inflammatory response. These findings indicate the non-redundant function of epithelial production of H2O2 in restricting microbial colonization.
Keywords: dual oxidase, mouse model, mucosal immunity, gastritis
Introduction
Studies in invertebrates have been seminal in deciphering innate immune defense mechanisms that are conserved in vertebrates including humans 1. Invertebrate gut epithelial immune responses rely mainly on two types of complementary and synergistic molecular effectors that restrict proliferation of microorganisms: antimicrobial peptides and reactive oxygen species. The latter are released by dual oxidase (DUOX), a hydrogen peroxide (H2O2) generating membrane-associated NADPH oxidase 2.
Knockdown of duox in the Drosophila gut leads to a severe host defense defect against a broad spectrum of pathogens 3,4. Studies in duox deficient C. elegans confirmed that H2O2 release from mucosal surfaces is an ancient defense mechanism predating the evolution of the oxidative burst in specialized immune cells 5. More recently, knockdown of zebrafish duox was shown to increase the intracellular bacterial load of larvae infected with enteric Salmonella suggesting that an antibacterial function of DUOX is conserved in lower vertebrates 6.
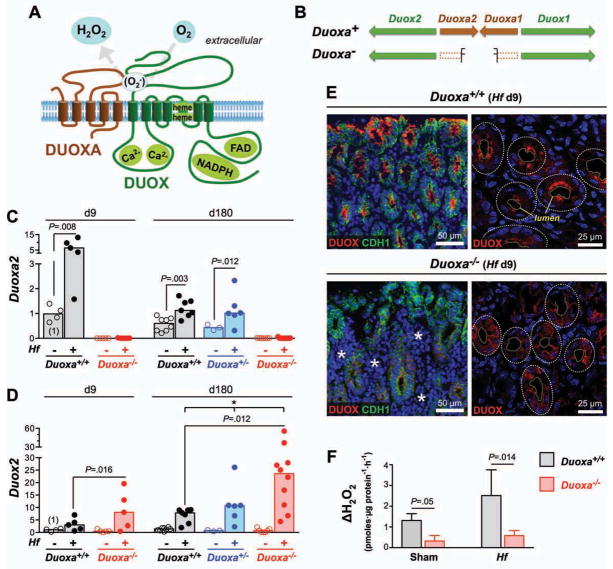
The functional DUOX enzyme complex is a heterodimer formed by association of two multipass transmembrane proteins, DUOX and DUOXA (DUOX maturation factor) (Fig. 1A). Mammalian genomes encode two DUOX isoenzymes, each representing a heterodimer of distinct DUOX and DUOXA subunits (i.e., DUOX1/DUOXA1 and DUOX2/DUOXA2) 7–9 (Fig. 1B). Both DUOX enzymes are H2O2-producing calcium-stimulated NADPH oxidases, but differ in transcriptional regulation. The conserved expression of DUOX2 throughout the luminal gastrointestinal (GI) tract of vertebrates, including humans,10 suggests it may confer a crucial evolutionary advantage. DUOX2 and DUOXA2 have been identified among the highest induced genes in Crohn’s disease 11,12, irritable bowel syndrome 13, and infectious diseases, such as Helicobacter pylori-associated gastritis 14. Despite the potential importance of this system in the pathophysiology of common human diseases, its role in mucosal defense has not been explored in any higher vertebrates in vivo.
Fig. 1. Hf infection induces DUOX2 expression in the gastric surface epithelium.
A) Topology model depicting the heterodimeric structure of a functional DUOX complex. B) Arrangement of DUOX and DUOXA subunit genes in mice (identical to other mammals). Duoxa− indicates Duoxa-deficient allele. C–D) RT-qPCR determination of Duoxa2 (C) and Duox2 (D) expression in the gastric corpus. Data points represent average expression values of individual mice. Bars indicate median expression values of N=5 to 11 mice per group. * P<.05 (Kruskal-Wallis test). E) Release of H2O2 from gastric glands isolated from Hf or mock-infected Duoxa−/− mice and wild type littermates. Extracellular H2O2 accumulation was calculated from the catalase-inhibitable change in signal in the fluorescence-based H2O2 assay, normalized for protein content in the gland preps. Data represent median and range of N=4 mice per group from two independent experiments. F) Immunostaining of DUOX proteins in the gastric epithelium of mice infected for 9 days with Hf. In Duoxa−/− mice, DUOX detection is limited to epithelial cells neighboring extensive E-cadherin (CDH1) negative infiltrates (asterisks). Confocal imaging (right panels) shows targeting of DUOX proteins to the apical surface in infected wild type but not Duoxa−/− animals. Dashed lines, lumen and circumference of individual glands.
Deletion of both Duoxa genes produces mice lacking the expression of functional DUOX enzymes (Duoxa−/− mice) 15 (Fig. 1B). We have now used this line of mice to investigate the role of DUOX-generated H2O2 in the mucosal defense against Helicobacter felis (Hf), a well-established model for H. pylori infection in humans, the most common chronic bacterial infection 16. Our results provide a paradigm for the role of DUOX in controlling microbial populations in the juxtamucosal mucus layer.
Results
Helicobacter Infection Induced DUOX2 Expression in the Gastric Epithelium
Wild type mice kept in a specific-pathogen free environment expressed both Duox2 and Duoxa2, but not Duox1 and Duoxa1 mRNA in the stomach (average threshold cycle [ct] in realtime PCR: 25.5 for Duox2; 28.5 for Duoxa2; 38.3 for Duox1; >40 for Duoxa1). The predominant expression of the DUOX2 isoenzyme in the murine stomach is consistent with the GI tract expression in other mammals 10.
In wild type mice, infection with Hf acutely induced Duox2 and Duoxa2. Nine days post infection, induction of Duoxa2 and Duox2 was 6.9 fold (P=.008) and 3.2-fold (P=.06), respectively (Fig. 1C–D). The expression of both genes remained elevated at 6 months post infection. By immunofluorescence, DUOX protein was barely detectable in control animals (Suppl. Fig. 1A–B) but showed strong multifocal expression in the gastric mucosa of Hf infected mice (Fig. 1F). Expression in infected mice was limited to epithelial cells and absent in stromal cells.
In Duoxa−/− mice, no Duoxa2 transcripts spanning the targeted deletion were detected as expected (Fig. 1C), but baseline Duox2 expression levels did not differ from wild type controls. Induction of Duox2 mRNA by Hf infection was higher than in infected wild type animals (Fig. 1D) suggesting a stronger activation of signaling pathways that induce Duox2 expression. Lack of DUOXA heterodimerization partners prevents ER-exit of DUOX proteins 15, a finding confirmed by absence of apical targeting of DUOX proteins in gastric epithelium of Duoxa−/− animals (Fig. 1E). To directly assess the functional activity of DUOX proteins, isolated gastric glands were tested for the ability to release H2O2 into the medium. Glands isolated from wild type animals generated over 4-fold higher extracellular H2O2 levels compared to those from Duoxa−/− littermates (Fig. 1F). Impaired H2O2 generation in Duoxa−/− mice was observed in Hf infected animals as wells as mock-infected controls. These data indicate that DUOX proteins are a major source of epithelial derived extracellular H2O2 in the murine stomach.
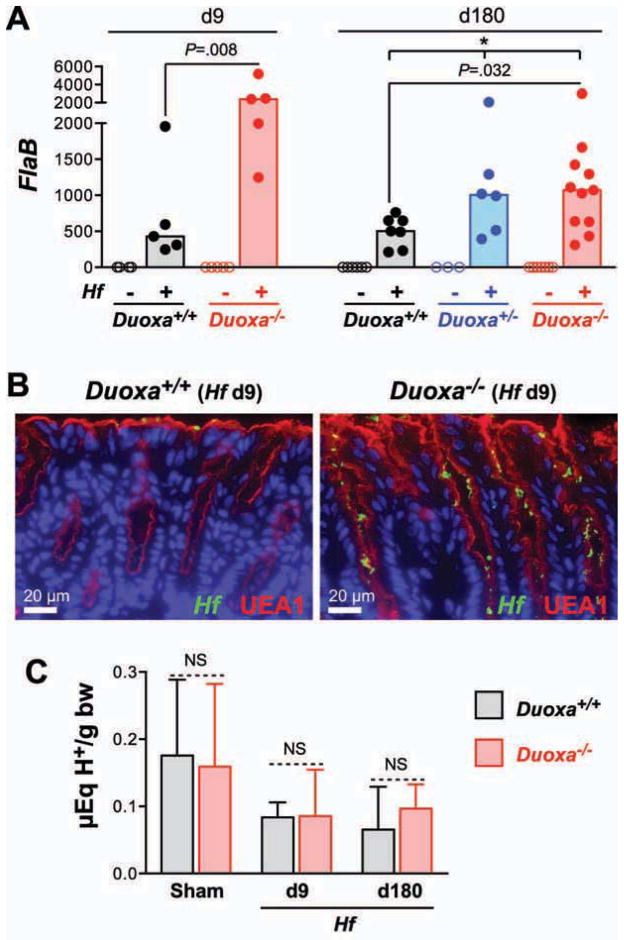
Lack of Functional DUOX Enzymes Increased Mucosal Colonization with Helicobacter
To examine whether DUOX activity affects gastric colonization and persistence of Hf, we measured colonization levels by semiquantitative realtime PCR. In Duoxa−/− animals, colonization levels were higher during both the acute and chronic phases of infection (Fig. 2A). Immunofluorescent detection of Hf revealed that colonization was limited to the mucus layer in both Duoxa−/− and wild type mice (Fig. 2B). There were no differences in gastric acidity, the number of parietal cells or the expression of H+/K+-ATPase subunit genes that could have affected Hf colonization (Fig. 2C, Suppl. Fig. S2) 17. Also, overall morphology of the gastric glands (by H&E staining) and mucus pattern (by Alcian Blue/PAS staining) were both indistinguishable between Duoxa−/− and wild type controls (data not shown).
Fig. 2. Loss of functional DUOX enzymes leads to increased mucosal colonization by Helicobacter.
A) Relative Hf colonization levels in the acute and chronic phase of infection determined by realtime qPCR. Expression of the Hf housekeeping gene flaB in gastric corpus samples was normalized for the amount of host mRNA (Hprt1). Bars indicate median values. * P<.05. B) Immunofluorescent detection of Hf in the gastric body at 9 days post infection. Mucus is visualized with Ulex Europaeus agglutinin 1 (UEA-1). C) Acidity of the gastric content expressed as μEq H+ per gram of body weight. Data represent median and range of the acidity values of N=5 to 10 mice per group. NS P>.05.
Lack of DUOX Activity Lead to Exacerbated Acute and Chronic Helicobacter-Induced Gastritis
The acute phase of Hf-induced gastritis was characterized by diffuse polymorph- and mononuclear infiltrates in the subglandular region. In infected Duoxa−/− mice, infiltrates were more widespread than in wild type controls and frequently displaced the lower portion of the glands (Fig. 3A). In accordance, mRNAs for acute inflammatory markers, such as interleukin-β, interferon-γ, the chemokine CXCL1 (a murine interleukin-8 equivalent), and TNF-α were expressed at a higher level in the stomach of infected Duoxa−/− mice compared to infected controls (Fig. 3B–C; Suppl. Fig. S3A–B).
Fig. 3. Hf-induced acute gastritis is exacerbated in Duoxa−/− mice.
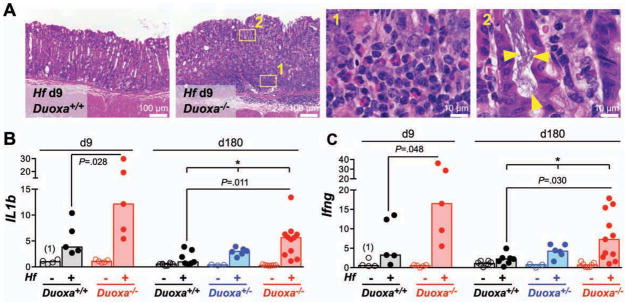
A) Representative hematoxylin and eosin stained sections of the midcorpus 9 days post infection showing marked mixed-myeloid infiltrates in the mucosa and submucosa of infected Duoxa−/− mice compared to wild type controls. Yellow triangles indicate spiral shaped bacteria within a gastric gland. B–C) mRNA expression of acute inflammation markers interleukin-1β (B) and interferon-γ (C) in the midcorpus. Bars show median expression values of N=5 to 11 mice per group. * P<.05.
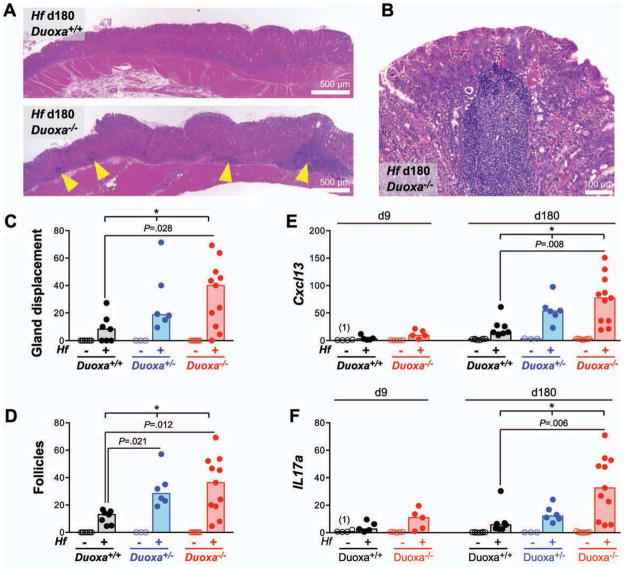
In the chronic phase of infection, the gastric mucosa of Duoxa−/− mice showed more extensive diffuse mononuclear infiltrates and higher numbers of lymphoid follicles (Fig. 4A–D). The chemokine CXCL13 (also known as B cell chemoattractant-1; BCA-1) dictates homing and motility of B-cells in lymphoid tissue, and its induction in chronic H. pylori gastritis is involved in the formation of lymphoid follicles and gastric lymphomas of the MALT type 18. In Duoxa−/− mice, the infection-induced expression of Cxcl13 and the B-cell marker Cd19 was 3.1 and 4.1-fold, respectively, higher than in wild type controls, in agreement with the histopathological finding of increased follicle development in the gastric mucosa of Duoxa−/− mice (Fig. 4E; Suppl. Fig. S3C). During Helicobacter infection, T-helper 17 (Th17) cells secrete large amounts of interleukin-17A that acts on a variety of target cells, including epithelial cells, to upregulate the production of neutrophil chemotactic factors 19. In Duoxa−/− mice, average IL17a expression at 6 months was 4.1-fold higher than in wild type controls (Fig. 4F). Overall, Helicobacter-induced Th1, Th17, and B-cell responses showed identical patterns in Duoxa−/− and control mice, but were far more robust in Duoxa−/− mice. The expression of proinflammatory markers positively correlated with the level of colonization by Hf (Suppl. Fig. S4), consistent with the concept that Hf-induced inflammation is a response to shed antigens or secreted virulence factors 20. The pivotal role of DUOX in limiting Hf infection and gastritis was underscored by the apparent haploinsufficiency of heterozygous Duoxa+/− animals: their mucosal colonization levels and inflammation scores were intermediate to wild type and homozygous Duoxa−/− animals (Fig. 3, 4; Suppl. Fig. S3, S4).
Fig. 4. Chronic follicular gastritis is more severe in Duoxa−/− mice.
A–B) Histology at 6 months post infection depicting increased number of lymphoid aggregates in the gastric corpus of chronically infected Duoxa−/− mice. C–D) Histologic scoring of gland displacement (C) and frequency of lymphoid follicles (D). E–F) mRNA expression profiles of CXCL13 (E) and interleukin-17A (F). Bars represent medians. * P<.05.
Hf Colonizing the Mucosa of Duoxa−/− Animals Expressed Lower Levels of H2O2-Inducible katA
It is well established that exposure of microbes to reactive oxygen species can modulate redox-sensitive signaling molecules and transcription factors, leading to adaptive responses, and changes in virulence or viability 21,22. While it is not feasible to directly measure the effective H2O2 concentration Hf is exposed to in its physiological niche in vivo, differential exposure of Hf to H2O2 should be reflected in differences in H2O2-responsive gene expression. Helicobacter catalase is expressed at the cell surface and its only known function is the degradation of exogenous H2O2 23. The single catalase gene of Hf (katA) 24 is therefore a prime candidate for redox-sensitive regulation by DUOX-generated H2O2. Analogous to katA of H. pylori 25, katA of Hf was indeed induced by a single H2O2 bolus (Fig. 5A). No induction was observed for flaB, a gene previously employed as a housekeeping gene in oxidative stress response studies 26. Importantly, in both acute and chronic infection, Hf colonizing control animals expressed higher katA:flaB ratio compared to Hf found in Duoxa−/− mice (Fig. 5B). These results imply that the rate of H2O2 production by the activated DUOX system is sufficient to modulate redox sensitive gene expression in Hf colonizing the overlaying mucus layer.
Fig. 5. Hf colonizing the gastric mucosa of Duoxa−/− mice express lower level of H2O2-inducible katA mRNA.
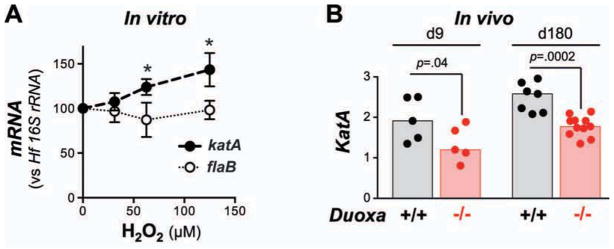
A) H2O2 induces Hf katA expression in vitro. Bacteria were cultured for 12 h following addition of H2O2 at the indicated concentrations. Data represent mean ± standard deviation of the mean of three independent experiments. * P<.05. B) In vivo katA expression of Hf colonizing the gastric mucosa of wild type and Duoxa−/− animals, respectively. Data were normalized to the Hf housekeeping gene flaB. Bars depict median values of N=5 to 11 mice per group.
Micromolar Concentrations of H2O2 have a Powerful Bacteriostatic Effect on Hf in vitro
Our results showed that inactivation of the DUOX system lead to increased bacterial colonization, which positively correlated with the inflammatory response in the Hf infection model. In addition, DUOX activity was associated with oxidative stress response in colonizing Hf in vivo. We thus hypothesized that DUOX-generated H2O2 acts as a direct suppressor of Hf colonization. To provide evidence for this concept, we assessed whether H2O2 at low concentrations would be sufficient to limit Hf proliferation. H2O2 (15 μM to 1 mM) was stable in sterile culture medium, but disappeared within one hour with the presence of Hf in the medium indicating that the bacteria efficiently scavenged H2O2 (Fig. 6C and data not shown). To test whether direct H2O2 addition could still inhibit proliferation of bacteria in the ensuing culture, bacterial DNA and intracellular ATP level were determined after culture under microaerophilic conditions. We found that a single H2O2 bolus as low as 100 μM was sufficient to suppress Hf proliferation under these conditions (Fig. 6A–B).
Fig. 6. Hf is susceptible to transient or sustained exposure to μM concentrations of H2O2.
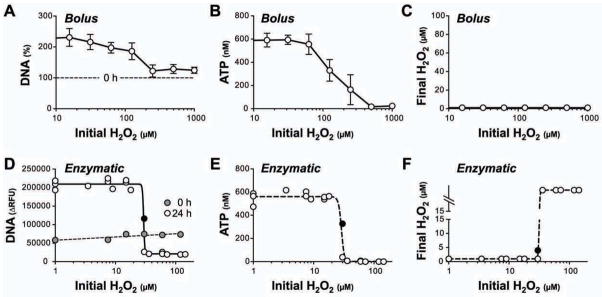
Bolus treatment: H2O2 was added to bacterial cultures (3.2 × 106 Hf/ml) at the indicated concentrations. Bacterial DNA (A) and intrabacterial ATP (B) level were determined following 18 h culture under microaerophilic conditions. Note that added H2O2 is completely scavenged by Hf resulting in transient exposure (C). Enzymatic exposure: For each experiment, Hf cultures (1.6 × 106 Hf/ml) were challenged with dilutions of glucose oxidase and cultured for 24 h. Results represent three independent experiments. Bacterial DNA (D) and ATP level (E) were measured at the end of the culture period. Values are plotted against the initial H2O2 equilibrium reached after glucose oxidase addition. H2O2 concentrations at the end of the culture period were either suppressed to background level of the medium (viable cultures; data points left to the critical threshold) or outside the measurement range (bacteria inactivated by accumulating H2O2; right to the transition). Note that the culture with 30 μM initial H2O2 level (black filled symbol) was closest to the equilibrium and had not fully outcompeted the oxidase activity at the end of the culture.
To substantiate these findings and better mimic the situation of sustained H2O2 production found in vivo, we challenged the H2O2 scavenging activity of Hf with increasing amounts of glucose oxidase as an H2O2 source. In this system, the H2O2 level is directly proportional to the ratio of glucose oxidase activity to bacterial H2O2 scavenging activity. As expected, loss of proliferation was observed when the initial H2O2 concentration exceeded a critical threshold, whereas in cultures with lower initial H2O2 equilibrium, Hf was able to outgrow the enzymatic H2O2 source. Under the culture conditions employed, the critical threshold for sustained H2O2 exposure was approximately 30 μM (Fig. 6D–F), a physiologically relevant concentration in extracellular compartments 27.
Discussion
Studies in invertebrates and zebrafish larvae have indicated a crucial role of DUOX enzymes in host defense within the luminal GI tract 3,6,28. The inducible production of hydrogen peroxide functions as a second effector arm of epithelial innate immunity, complementary and synergistic to the better characterized antibacterial peptide system 1. However, up until now, only the role of the latter has been analyzed in higher vertebrate species in vivo.
Here, using Helicobacter infection in Duoxa−/− mice as a model system, we demonstrate for the first time a nonredundant function of the DUOX system in mammalian GI immunity. Our results provide evidence that H2O2 released by DUOX at the apical surface of the gastric epithelium suppresses proliferation of Helicobacter in the overlying mucus layer. The identification of DUOX-generated H2O2 as a pivotal host factor in the homeostatic interaction with Helicobacter is consistent with prior studies showing the failure of oxidative stress resistance mutants to persist in the murine stomach 29,30. In mice lacking active DUOX enzymes, Hf was able to colonize at a higher level but did not escape from its normal niche within the mucus layer. Shedding of bacterial antigens and secretion of virulence factors trigger Hf-induced gastritis, which, as expected from the increased bacterial load, was more severe in Duoxa−/− animals. Furthermore, consistent with a primary defect in cell-autonomous epithelial defense, elevated colonization levels in Duoxa−/− persisted in the chronic phase of infection despite a stronger adaptive immune response. The pivotal role of DUOX in host defense against Hf was underscored by the severity of the Hf-induced phenotype in heterozygous mice (Duoxa+/−), which was intermediate to wild type and Duoxa−/− mice suggesting haploinsufficieny. This observation is in contrast to the normal thyroid hormone synthesis in Duoxa+/− 15, and implies that the GI epithelium expressing almost exclusively the DUOX2 isoenzyme, has a lower capacity to compensate partial DUOX defects than the thyroid epithelium expressing both DUOX isoenzymes constitutively.
The release of H2O2 from the luminal surfaces of the GI tract and its degradation within the mucus layer is expected to generate a rapidly dissipating H2O2 gradient. In fact, we were unable to detect significant H2O2 release from the luminal mucus surface ex vivo suggesting complete consumption of epithelial produced H2O2 within the mucus layer (data not shown). It remains to be shown whether H2O2 released into the mucus is primarily decomposed into H2O and O2, or whether it drives peroxidative formation of more stable oxidants, such as, HOSCN and HOCl with powerful microbicidal activity 31. Nevertheless, since the enzymatic activity of stimulated DUOX is comparable to the activated leukocyte NADPH oxidase 32, the H2O2 challenge encountered by microbes close to the epithelium should be substantial. At least in the stomachs of gerbils, the currents recorded with H2O2 sensitive microsensors indicate a greatly increased rate of H2O2 release into the juxtamucosal mucus layer following infection with H. pylori 33. Hf almost exclusively occupies a defined niche between 5–20 μm from the epithelial surface 34, a restriction dictated by the pH gradient within the mucus layer 35. These constraints on the vertical distribution of Hf may be critical for the efficient exposure to DUOX-generated H2O2. That DUOX-generated H2O2 indeed altered redox signaling in Hf in vivo was substantiated by significant higher katA expression in Hf colonizing wild type mice compared to those from Duoxa−/−. As we have shown here, growth of Hf is highly susceptible to H2O2, which exerts a bacteriostatic effect at low micromolar concentrations in vitro.
That low concentrations of exogenous H2O2 can affect bacterial redox signaling has been demonstrated previously for other catalase positive bacteria. For instance, in E. coli, 5 μM of environmental H2O2 corresponding to an intrabacterial level just above 100 nM, is sufficient to activate the redox sensor OxyR that controls the adaptive responses to H2O2 stress, including induction of catalase 36. In Campylobacter jejuni a single H2O2 bolus of 10 μM is sufficient to suppress polysaccharide capsule synthesis without direct microbicidal effects 37. In our in vitro system, sustained exposure to 30 μM H2O2 was sufficient to also interfere with bacterial growth. These results are similar to those obtained in studies of E. coli using continuous H2O2 infusion where profound growth retardation was observed between 25–50 μM, and complete bacteriostasis at 100 μM H2O2 38. It should be stressed that the antimicrobial effect of a single H2O2 bolus depends on the bacterial density, and the apparent tolerance to high millimolar H2O2 boli in other studies using concentrated cell suspensions does not seem to reflect the true bacteriostatic potential of a sustained H2O2 flux 22.
While this study illustrates how DUOX controls a noninvasive pathogen colonizing the mucus layer, we expect DUOX to be effective against a broad spectrum of pathogens including those that penetrate the inner mucus layer and make direct contact with the epithelial surface. In the latter scenario, the host defense function of DUOX could be further enhanced by recruitment of the DUOX enzyme complex to the site of bacterial contact, as shown in response to Listeria monocytogenes and Campylobacter jejuni infection in vitro 37,39. Conceivably, such targeting of DUOX should generate a high level H2O2 flux across the bacterial membrane and could explain the DUOX-mediated protection in Drosophila and zebrafish larvae from the highly invasive pathogen Salmonella Typhimurium 3,6.
Although not specifically addressed by our study, DUOX-generated H2O2 may well have additional effects on the immune response beyond its antimicrobial properties. H2O2 is now widely recognized as an important signal messenger of various intracellular signaling pathways 40. Although intracellular H2O2 signaling is spatially restricted 41, there is evidence from in vitro systems that DUOX-generated H2O2 may enter producing or neighboring cells, e.g. facilitated by specific aquaporins 42, and oxidize redox-sensitive signaling molecules in the vicinity of the plasma membrane. There is also a possibility that DUOX generated H2O2 can provide a signal for nonepithelial cells relevant in the immune response. For instance, epithelial wounding in zebrafish larvae or Drosophila embryo epidermis activates DUOX at the wound margin to release H2O2 43,44, with the resulting tissue-scale H2O2 gradient attracting immune cells to migrate to the epithelial lesion. In addition, there is evidence that DUOX activity stimulates migration of epithelial cells during wound closure 45,46. Whether DUOX plays such roles in a mammalian system in vivo will have to be tested in a model that, unlike Hf infection, induces epithelial injury.
Taken together, our study provides in vivo evidence for a nonredundant function of DUOX-generated H2O2 as epithelial immune effector in the luminal GI tract. In our chronic infection model, loss of active DUOX enzymes led to increased mucosal colonization and exacerbated, yet futile, chronic inflammatory response. The subunits of the DUOX2 isoenzyme have been identified among the highest induced genes in Crohn’s disease 11,12, irritable bowel syndrome 13, and infectious diseases 14,47. It remains to be shown whether loss of DUOX activity will also exacerbate other infectious or chronic inflammatory conditions of the luminal GI tract in which DUOX was found to be activated.
Materials and Methods
Animals
The generation of Duoxa−/− mice has been described previously 15. All animals used were females in a pure 129S6 genetic background. Within infection experiments, average body weight and age did not differ between the different genotype/treatment groups. Animals were group-housed (3–5 animals/cage; mixed genotypes) in microisolator cages under specific-pathogen-free conditions. Food and water were supplied ad libitum, with the latter including a supplemental dose of L-thyroxine to maintain euthyroidism of Duoxa−/− mice 15. Prior to necropsy, mice were fasted overnight on wire mesh with free access to water. All studies were approved by the University of Michigan Institutional Animal Care and Use Committee (PRO-00004497).
Hf culture and infection
See Supplementary Methods section.
Tissue dissection
See Supplementary Methods section.
H2O2 release of isolated mouse gastric glands
The glandular portions of the stomachs were excised and everted by cutting along the lesser curvature. The rinsed stomachs were kept in oxygenated digestion medium (Dulbecco’s Modified Eagle Medium, 20 mM Hepes pH 7.4, 10 μg/ml soybean trypsin inhibitor, 0.2% bovine serum albumin, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B). For collagenase treatment, stomachs were placed in 10 ml fresh digestion medium containing 1 mg/ml collagenase (type XI; >1200 collagenase digestion units/mg protein; Sigma, St. Louis, MO) and incubated at 37°C in a shaking incubator (130 rpm) for 40 minutes. The partially digested stomachs were held with forceps and shaken in DMEM/F12 medium supplemented with 0.5 mM dithiothreitol to liberate gastric glands. The resulting suspension was passed through a 100 μm nylon filter mesh. The filtrate was sedimentated (30 g for 4 min) and gently washed thrice each in digestion buffer and Krebs-Ringer-Hepes buffer (KRH; 124 mM NaCl, 5 mM KCl, 1.25 mM MgSO4, 1.45 mM CaCl2, 1.25 mM KH2PO4, 25 mm Hepes pH 7.4, 8 mM D-glucose, and 0.5 g/L BSA) to remove isolated cells. In the final preparations, >95% of the cells were found in multi-cellular clusters. Cell viability was confirmed by trypan blue exclusion. The glands from each stomach were incubated for one hour in KRH with or without 100 U/ml catalase (Sigma). H2O2 accumulation in the supernatant was determined using the Amplex Red/horseradish peroxidase (HRP) assay (Life Technologies). Catalase-inhibitable relative fluorescence units were converted to H2O2 concentrations using standards prepared in KRH. Gland pellets were lysed in T-PER Tissue Protein Extraction Reagent and protein content determined using the BCA assay (Thermo Fischer Scientific, Rockford, IL).
Histology and morphometric analysis
Serial 4 μm sections of formalin-fixed paraffin-embedded samples were stained with hematoxylin and eosin (H&E). For histopathological evaluation of gastric inflammation, microscopic fields at 200× magnification were scored as described by Eaton et al. 48. For each animal, scores represent the percentage of the fields displaying gastric gland displacement or lymphoid aggregates, respectively.
Immunostaining of tissue sections
For staining of frozen sections, thawed 8 μm sections were fixed again in 4% freshly prepared formaldehyde for 10 min, washed twice in PBS, and then blocked with 20% donkey serum in PBS. Primary antibodies used were a pan-DUOX antiserum 8 or normal rabbit IgG (control; Santa Cruz Biotechnology, Santa Cruz, CA), an anti-H. pylori serum (Covance, Princeton, NJ), and rat anti-E-cadherin (Invitrogen). The staining was developed using Alexa Fluor-conjugated secondary antibodies (Life Technologies) and DNA counterstained with DAPI. Mucins were visualized using biotinylated Ulex Europaeus agglutinin 1 (UEA-1; Vector Laboratories, Burlingame, CA) with Texas-Red conjugated anti-biotin antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA).
Real-time reverse transcription PCR (RT-qPCR)
See Supplementary Methods section.
Exposure of Hf to H2O2 in vitro and proliferation assays
Hf from a mid-log culture were washed and resuspended in phenol red-free RPMI-1640 medium supplemented with 2% heat inactivated FBS. Although RPMI/FBS did not meet the nutritional requirements for longterm culture of Hf 49, it supported limited proliferation within the first 24 hours (Fig. 5A). For bolus treatment, H2O2 from a 3% stock solution (880 mM) was diluted into culture medium that had been equilibrated in a microaerophilic atmosphere followed by addition of washed bacteria at 3.2·106/ml. Culture was then performed for 18 hours.
For sustained H2O2 exposure, bacterial dilutions (1.6·106/ml) were prepared in 16 ml phenol red-free DME/F12/2% FBS in vented T150 flasks that had been equilibrated in a microaerophilic atmosphere. Glucose oxidase (GOX; Sigma) was serially diluted in glucose-free phosphate buffer and mixed with the cultures (final: 2.5–40 mU/l; KM=9.8 mM; [D-glucose]=17.15 mM). In control experiments, 400 U/l catalase was added to the flasks prior to the addition of GOX. Samples of the cultures were obtained immediately after setting-up the reaction (t=0 h) and following 24 h culture (t=24 h). The initial equilibrium [H2O2] was derived from the y-intercept (t=0 h) of the kinetics of resorufin generation in the Amplex Red/HRP assay with H2O2 standards diluted in PBS (note that kHRP≫kcatalase). Repeat measurements confirmed that the initial steady state [H2O2] had been reached at less than 5 min following addition of GOX at the highest dilution. Within the linear range of the assay, [H2O2] was directly proportional to the ratio of [Hf] versus [GOX]. Values >10 μM were derived by linear extrapolation. Equilibrium [H2O2] at t=24 h had typically either shifted close to background level of the medium (growing cultures suppressing [H2O2]) or exceeded the range of the assay (bacteria inactivated by accumulating H2O2).
Intracellular ATP levels of bacteria were determined using a luciferase-based method (BacTiter-Glo Microbial cell viability assay kit; Promega, Fitchburg, WI). To determine the relative amount of bacterial DNA, cultures were diluted 1:5 in 200 mM Tris-HCl pH 8.0/50 mM EDTA containing SYBR Green I (1:2000 dilution of a 10,000× stock solution), and the fluorescence signal (ex/em, 485/535 nm) recorded. Values were corrected for the background fluorescence of sterile medium.
Statistics
The Mann-Whitney U test or unpaired Student t test were used for comparison of two groups. For multiple comparisons, data were analyzed using the Kruskal-Wallis test or one-way analysis of variance following log-transformation. Multiple comparison adjusted P values for pairwise comparisons were then calculated using Dunn’s multiple comparison test or Tukey’s post-hoc test, respectively. Data were analyzed and plotted using GraphPad Prism software, version 5 (San Diego, CA). P values <.05 were considered significant.
Supplementary Material
Acknowledgments
We thank X. De Deken and F. Miot for providing the DUOX antiserum, and M. Saqui-Salces for critical review of the manuscript. This study was supported by NIH grant DK55732 and a Pilot and Feasibility Grant of the University of Michigan Gastrointestinal Peptide Research Center (P30 DK034933).
Footnotes
Author contributions:
HG: study concept and design; data acquisition and analysis; wrote manuscript; obtained funding
MEZ: acquisition of data; review of manuscript
JLM: obtained funding; study design; review of manuscript
Disclosures:
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annual review of immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 2.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends in immunology. 2010;31:278–287. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Ha EM, Oh CT, Bae YS, et al. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–850. doi: 10.1126/science.1117311. [DOI] [PubMed] [Google Scholar]
- 4.Ha EM, Oh CT, Ryu JH, et al. An antioxidant system required for host protection against gut infection in Drosophila. Developmental cell. 2005;8:125–132. doi: 10.1016/j.devcel.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Chavez V, Mohri-Shiomi A, Garsin DA. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infection and immunity. 2009;77:4983–4989. doi: 10.1128/IAI.00627-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flores MV, Crawford KC, Pullin LM, et al. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochemical and biophysical research communications. 2010;400:164–168. doi: 10.1016/j.bbrc.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 7.Dupuy C, Ohayon R, Valent A, et al. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. The Journal of biological chemistry. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 8.De Deken X, Wang D, Many MC, et al. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. The Journal of biological chemistry. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 9.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. The Journal of biological chemistry. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 10.El Hassani RA, Benfares N, Caillou B, et al. Dual oxidase2 is expressed all along the digestive tract. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G933–942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 11.Csillag C, Nielsen OH, Vainer B, et al. Expression of the genes dual oxidase 2, lipocalin 2 and regenerating islet-derived 1 alpha in Crohn’s disease. Scandinavian journal of gastroenterology. 2007;42:454–463. doi: 10.1080/00365520600976266. [DOI] [PubMed] [Google Scholar]
- 12.Hamm CM, Reimers MA, McCullough CK, et al. NOD2 status and human ileal gene expression. Inflammatory bowel diseases. 2010;16:1649–1657. doi: 10.1002/ibd.21208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aerssens J, Camilleri M, Talloen W, et al. Alterations in mucosal immunity identified in the colon of patients with irritable bowel syndrome. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008;6:194–205. doi: 10.1016/j.cgh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornsby MJ, Huff JL, Kays RJ, et al. Helicobacter pylori induces an antimicrobial response in rhesus macaques in a cag pathogenicity island-dependent manner. Gastroenterology. 2008;134:1049–1057. doi: 10.1053/j.gastro.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasberger H, De Deken X, Mayo OB, et al. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Molecular endocrinology. 2012;26:481–492. doi: 10.1210/me.2011-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi M, Redline R, Nedrud J, et al. Role of the host in pathogenesis of Helicobacter-associated gastritis: H. felis infection of inbred and congenic mouse strains. Infection and immunity. 1996;64:238–245. doi: 10.1128/iai.64.1.238-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danon SJ, O’Rourke JL, Moss ND, et al. The importance of local acid production in the distribution of Helicobacter felis in the mouse stomach. Gastroenterology. 1995;108:1386–1395. doi: 10.1016/0016-5085(95)90686-x. [DOI] [PubMed] [Google Scholar]
- 18.Mazzucchelli L, Blaser A, Kappeler A, et al. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. The Journal of clinical investigation. 1999;104:R49–54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luzza F, Parrello T, Monteleone G, et al. Up-regulation of IL-17 is associated with bioactive IL-8 expression in Helicobacter pylori-infected human gastric mucosa. Journal of immunology. 2000;165:5332–5337. doi: 10.4049/jimmunol.165.9.5332. [DOI] [PubMed] [Google Scholar]
- 20.Every AL, Chionh YT, Skene CD, et al. Muc1 limits Helicobacter felis binding to gastric epithelial cells but does not limit colonization and gastric pathology following infection. Helicobacter. 2008;13:489–493. doi: 10.1111/j.1523-5378.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen PR, Brugarolas P, He C. Redox signaling in human pathogens. Antioxidants & redox signaling. 2011;14:1107–1118. doi: 10.1089/ars.2010.3374. [DOI] [PubMed] [Google Scholar]
- 22.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annual review of biochemistry. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stent A, Every AL, Sutton P. Helicobacter pylori defense against oxidative attack. American journal of physiology Gastrointestinal and liver physiology. 2012;302:G579–587. doi: 10.1152/ajpgi.00495.2011. [DOI] [PubMed] [Google Scholar]
- 24.Arnold IC, Zigova Z, Holden M, et al. Comparative whole genome sequence analysis of the carcinogenic bacterial model pathogen Helicobacter felis. Genome biology and evolution. 2011;3:302–308. doi: 10.1093/gbe/evr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang CH, Chiou SH. Proteomic analysis of upregulated proteins in Helicobacter pylori under oxidative stress induced by hydrogen peroxide. The Kaohsiung journal of medical sciences. 2011;27:544–553. doi: 10.1016/j.kjms.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Stewart PE, Carroll JA, Dorward DW, et al. Characterization of the Bat proteins in the oxidative stress response of Leptospira biflexa. BMC microbiology. 2012;12:290. doi: 10.1186/1471-2180-12-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B, Clement MV, Long LH. Hydrogen peroxide in the human body. FEBS letters. 2000;486:10–13. doi: 10.1016/s0014-5793(00)02197-9. [DOI] [PubMed] [Google Scholar]
- 28.Chavez V, Mohri-Shiomi A, Maadani A, et al. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetics. 2007;176:1567–1577. doi: 10.1534/genetics.107.072587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris AG, Wilson JE, Danon SJ, et al. Catalase (KatA) and KatA-associated protein (KapA) are essential to persistent colonization in the Helicobacter pylori SS1 mouse model. Microbiology. 2003;149:665–672. doi: 10.1099/mic.0.26012-0. [DOI] [PubMed] [Google Scholar]
- 30.Olczak AA, Seyler RW, Jr, Olson JW, et al. Association of Helicobacter pylori antioxidant activities with host colonization proficiency. Infection and immunity. 2003;71:580–583. doi: 10.1128/IAI.71.1.580-583.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin K, Yamauchi K, Teraguchi S, et al. Susceptibility of Helicobacter pylori and its urease activity to the peroxidase-hydrogen peroxide-thiocyanate antimicrobial system. Journal of medical microbiology. 2002;51:231–237. doi: 10.1099/0022-1317-51-3-231. [DOI] [PubMed] [Google Scholar]
- 32.Song Y, Driessens N, Costa M, et al. Roles of hydrogen peroxide in thyroid physiology and disease. The Journal of clinical endocrinology and metabolism. 2007;92:3764–3773. doi: 10.1210/jc.2007-0660. [DOI] [PubMed] [Google Scholar]
- 33.Elfvin A, Edebo A, Bolin I, et al. Quantitative measurement of nitric oxide and hydrogen peroxide in Helicobacter pylori-infected Mongolian gerbils in vivo. Scandinavian journal of gastroenterology. 2007;42:1175–1181. doi: 10.1080/00365520701288306. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber S, Stuben M, Josenhans C, et al. In vivo distribution of Helicobacter felis in the gastric mucus of the mouse: experimental method and results. Infection and immunity. 1999;67:5151–5156. doi: 10.1128/iai.67.10.5151-5156.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schreiber S, Konradt M, Groll C, et al. The spatial orientation of Helicobacter pylori in the gastric mucus. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5024–5029. doi: 10.1073/pnas.0308386101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. Journal of bacteriology. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corcionivoschi N, Alvarez LA, Sharp TH, et al. Mucosal reactive oxygen species decrease virulence by disrupting Campylobacter jejuni phosphotyrosine signaling. Cell host & microbe. 2012;12:47–59. doi: 10.1016/j.chom.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyslop PA, Hinshaw DB, Scraufstatter IU, et al. Hydrogen peroxide as a potent bacteriostatic antibiotic: implications for host defense. Free radical biology & medicine. 1995;19:31–37. doi: 10.1016/0891-5849(95)00005-i. [DOI] [PubMed] [Google Scholar]
- 39.Lipinski S, Till A, Sina C, et al. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. Journal of cell science. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 40.Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishina NM, Tyurin-Kuzmin PA, Markvicheva KN, et al. Does cellular hydrogen peroxide diffuse or act locally? Antioxidants & redox signaling. 2011;14:1–7. doi: 10.1089/ars.2010.3539. [DOI] [PubMed] [Google Scholar]
- 42.Miller EW, Dickinson BC, Chang CJ. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15681–15686. doi: 10.1073/pnas.1005776107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niethammer P, Grabher C, Look AT, et al. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razzell W, Evans IR, Martin P, et al. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Current biology: CB. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luxen S, Belinsky SA, Knaus UG. Silencing of DUOX NADPH oxidases by promoter hypermethylation in lung cancer. Cancer research. 2008;68:1037–1045. doi: 10.1158/0008-5472.CAN-07-5782. [DOI] [PubMed] [Google Scholar]
- 46.Juarez MT, Patterson RA, Sandoval-Guillen E, et al. Duox, Flotillin-2, and Src42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS genetics. 2011;7:e1002424. doi: 10.1371/journal.pgen.1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Athanasiadou S, Jones LA, Burgess ST, et al. Genome-wide transcriptomic analysis of intestinal tissue to assess the impact of nutrition and a secondary nematode challenge in lactating rats. PloS one. 2011;6:e20771. doi: 10.1371/journal.pone.0020771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eaton KA, Danon SJ, Krakowka S, et al. A reproducible scoring system for quantification of histologic lesions of inflammatory disease in mouse gastric epithelium. Comparative medicine. 2007;57:57–65. [PubMed] [Google Scholar]
- 49.Testerman TL, Conn PB, Mobley HL, et al. Nutritional requirements and antibiotic resistance patterns of Helicobacter species in chemically defined media. Journal of clinical microbiology. 2006;44:1650–1658. doi: 10.1128/JCM.44.5.1650-1658.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.