Abstract
Condensed abstract
This is the first report of lenalidomide use in a large Medicare-enrolled population with MDS. The authors show that the ‘real-life’ reductions in RBC transfusions were overall consistent with clinical trials data and were greater when ≥3 lenalidomide cycles were received.
Background
Lenalidomide is approved for anemia with transfusion-dependence (TD) in lower-risk myelodysplastic syndrome (MDS) patients with 5q deletion (del5q-MDS), but its “real life” use and effect on transfusion needs are unclear. We examined its use in the Medicare population.
Methods
MDS patients enrolled in Medicare Parts A, B and D were identified using ICD-9 codes from 100% Medicare claims from 2006-8. Patients were followed until end of study or death. Claims were used to determine time to initiation of lenalidomide, daily dose, duration, and other MDS therapies. Transfusion status was defined during any 8-week period: TD required transfusions during 2 weeks, separated by ≥3 weeks; transfusion use (TU) 1 transfusion, and transfusion independence (TI), no transfusions.
Results
716 of 23,855 patients (3.2%) received lenalidomide, including 31% of 470 with del5q-MDS. At lenalidomide initiation, 33% were TD, 31% TU and 36% TI. Median time to lenalidomide initiation was shorter for del5q-MDS than for other lower-risk patients (8 vs. 20 weeks; p<0.01). The proportion of del5q-MDS patients receiving lenalidomide increased over time. Lenalidomide initiation was negatively associated with older age and baseline diabetes, stroke, and renal disease. During observation period, 44% of TU/TD patients (53% of del5q-MDS) achieved reductions in transfusion use; among TD patients receiving ≥3 cycles, 77% reduced transfusion use and 40% achieved TI.
Conclusions
This is the first report of lenalidomide use in a large Medicare-enrolled MDS population. Reductions in transfusion rates were overall consistent with clinical trials data. Response rates were higher when ≥3 lenalidomide cycles were received.
Keywords: Myelodysplastic syndromes, lenalidomide, 5q deletion
Background
Transfusion-requiring anemia represents a significant problem for patients with myelodysplastic syndromes (MDS). Historically, the primary therapeutic strategy was supportive care with red blood cell (RBC) transfusions and erythropoiesis-stimulating agents (ESAs). The addition of three new therapies, the DNA methyltransferase inhibitors (DNMTi) azacitidine and decitabine and the immunomodulatory agent lenalidomide, to the MDS therapeutic armamentarium has been a major advancement.
Lenalidomide is a potent oral derivative of thalidomide that is active in a number of hematologic malignancies. Patients with lower-risk (LR)-MDS with chromosome 5q deletions (del5q-MDS) are highly responsive to lenalidomide. In clinical trials, lenalidomide induced RBC transfusion independence (TI) in approximately two thirds of these patients, and complete cytogenetic responses in some.1,2 Lenalidomide was approved by the United States (US) Food and Drug administration (FDA) in December 2005 for use in patients with transfusion-dependent (TD) anemia due to LR del5q-MDS with or without additional cytogenetic abnormalities.3-5 Lenalidomide also induced TI in 26% of LR-MDS patients without del5q.5 Current treatment guidelines recommend consideration of lenalidomide therapy in some patients with TD LR-MDS without 5q deletion.6 A similar percentage of patients with higher-risk (HR)-del5q-MDS develop TI, but responses are typically shorter than for LR del5q-MDS.3-5
Despite accumulating evidence supporting lenalidomide therapy in some MDS subtypes, data regarding patterns of utilization and effectiveness in the “real world” setting, outside of the context of clinical trials, are scarce.7,8 To address this issue, we conducted a retrospective observational study utilizing a large 100% Medicare-enrolled claims-based MDS database to characterize lenalidomide use and effects on RBC transfusion use.
Data, Materials, and Methods
Study Population
Medicare beneficiaries with MDS were selected using 100% Medicare enrollment and claims data from 2006 through 2008. Claims for Medicare Parts A, B, and D included detailed information on dates, International Classification of Diseases 9-Clinical Modification (ICD-9-CM) diagnoses and services provided based on ICD-9-CM procedure codes, Healthcare Common Procedure Diagnosis Coding System (HCPCS) codes and/or National Drug Codes (NDC). Inclusion required one inpatient claim or two outpatient claims with MDS diagnosis within a 12-month span. Patients were observed from the date of their first MDS claim (index date) through death or study end (December 31, 2008). To ensure the observation of use of all oral therapies, the cohort was also limited to patients continuously enrolled in Medicare Part D from index date through study end [Figure 1]. All MDS risk groups were included. MDS was identified by a single ICD-9-CM diagnostic code (238.7) until October 2006, when a 5th digit was added that allowed for classification by patient MDS risk status. The ICD9 code 238.72 identified LR-MDS patients, 238.74 del5q-MDS, and 238.73 HR-MDS patients. Diagnostic codes 238.7 and 238.75 were grouped together as not otherwise specified (NOS).
Figure 1.
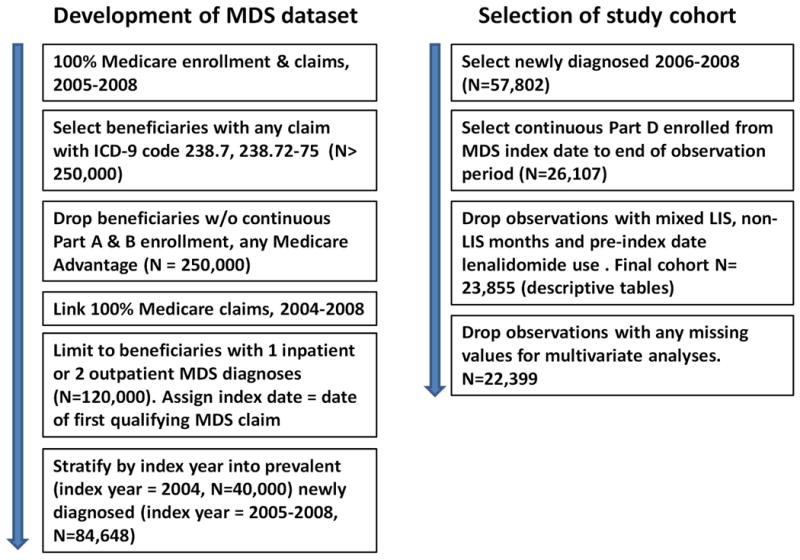
Generation of the database and the specific cohort of the study. MDS: Myelodysplastic syndromes, W/O: without, LIS: low income subsidy.
Studied Variables
Patient characteristics
Age at diagnosis, race, and sex were determined from Medicare enrollment files. Receipt of the Medicare Part D low-income subsidy (LIS) was captured based on monthly enrollment indicators. Sociodemographic factors from the 2000 US Census were linked to the enrollment files by zip code of residence. 9
Baseline health status
Baseline comorbidities were defined based on claims during the 12 months prior to MDS diagnosis. We included an indicator for poor predicted disability status (DS), a weighted claims-based measure recently described by Davidoff et al.10
MDS therapies
Prescribed treatments were identified from HCPCS or NDC in the Medicare Part B claims or Part D event files. We specified lenalidomide treatment episodes as periods of continuous claims with less than 6 months between fills. We calculated duration of lenalidomide use (first to last claim plus days supplied), daily dose, time from MDS diagnosis to initiation of lenalidomide treatment, and time until discontinuation. Small gaps in therapy were smoothed through a series of logical imputations. For example, when observing monthly fills of 14 or 21 tablets, we assumed that each fill covered a 28-day period, and we adjusted days supplied and average daily dose measures accordingly. We also measured use of DNMTi, ESAs and RBC transfusions.
Transfusion status, response
We measured transfusion status weekly based on a rolling 8-week period (current and prior 7 weeks). Patients with two or more transfusion weeks with a gap of ≥2 weeks were designated as TD. Patients with only one transfusion episode in the 8 weeks were designated as transfusion users (TU), while those who received no transfusions were designated as TI. To measure response to treatment, we selected patients who were TD or TU at lenalidomide initiation, and scanned subsequent weekly transfusion status measures during the first episode of therapy to identify changes from baseline. We categorized responses to parallel International Working Group (IWG) for MDS “major” and “minor” erythroid response criteria.11 Reductions from TD to TI status reflected “major erythroid response,” while reductions from TD to TU, or from TU to TI reflected minor response.
Statistical Considerations
Cohort analyses included univariate, bivariate and multivariate regression models. Analyses were conducted on the full cohort and on the subcohorts with del5q-MDS and other LR-MDS. All analyses were conducted using SAS v 9.2 and Stata version 12. The study was approved by the University of Maryland Institutional Review Board.
Results
Baseline characteristics and demographics
The study cohort consisted of 23,855 patients with MDS (Supplementary Table 1). MDS risk group was del5q-MDS in 480 patients (2%), other LR-MDS in 6,355 (26.6%) and HR-MDS in 1,277 (5.7%), while most (15,657 patients, 65.6%) were coded as MDS-NOS. Patients were predominantly white (87.8%) and female (58.1%), 75.3% were ≥75 years and 18.9% had poor DS. The most prevalent baseline comorbidities included ischemic cardiac disease (41.1%), diabetes (36.3%), congestive heart failure (CHF) (30.6%), renal (27.9%) and thyroid (25.1%) disease, prior solid tumor malignancy (19.4%) and prior venous thromboembolism (VTE) (4.2%).
Lenalidomide utilization
During the 3-year observation period (2006-2008), lenalidomide was prescribed to 753 (3.2%) MDS patients, of whom 19.8% had del5q-MDS and 24.2% other LR-MDS (Table 1); others had higher-risk or unclassified MDS. The proportion of del5q-MDS lenalidomide users increased over time (32.2% in first half of 2006 to 37.6% in first half of 2008; use rates for beneficiaries diagnosed in the latter half of 2008 were not reported due to inadequate follow-up to assess use). Median time from diagnosis to lenalidomide initiation was 11.5 weeks (range, 0-132 weeks) for new users; time to initiation was shorter for del5q-MDS than other LR-MDS patients (8 versus 20 weeks, p<0.01). Among patients who received lenalidomide, 72.3% had received prior ESA, and 11.7% prior DNMTi. 56.4% of lenalidomide users received ESA treatment concurrently with lenalidomide, while 29.0% received ESAs after discontinuing lenalidomide.
Table 1.
Patterns of lenalidomide initiation, use of other MDS therapies, duration and dose, overall and for del5qMDS and other lower risk MDS.
| MDS Risk Group | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Overall | del5qMDS | Other Risk | Lower- | |||||
|
|
||||||||
| N | % | N | % | N | % | |||
|
| ||||||||
| Cohort N, % of total | 23,855 | 100.0 | 480 | 2.0 | 6355 | 26.6 | ||
| Any LEN use, % of total % of cohort receiving | 753 | 100.0 | 149 | 19.8 | 182 | 24.2 | ||
| LEN† | 3.2 | 31.0 | 2.9 | |||||
| LEN use by MDS diagnosis period | ||||||||
| 2006-first half† | 143 | 3.1 | 19 | 32.2 | 21 | 3.2 | ||
| 2006-second half† | 144 | 3.6 | 26 | 35.1 | 32 | 3.2 | ||
| 2007-first half† | 165 | 3.3 | 30 | 25.9 | 46 | 3.1 | ||
| 2007-second half† | 109 | 3.2 | 32 | 36.0 | 32 | 2.8 | ||
| 2008-first half† | 123 | 3.0 | 35 | 37.6 | 33 | 2.5 | ||
| LEN use only post MDS diagnosis† | 690 | 2.9 | 141 | 29.9 | 169 | 2.7 | ||
| Time to LEN Initiation from MDS diagnosis (weeks)* | ||||||||
|
|
||||||||
| Median† | 11.5 | 8 | 20 | |||||
| Minimum | 0 | 0 | 0 | |||||
| Maximum | 132 | 89 | 125 | |||||
| Mean† | 23.7 | 14.8 | 27.7 | |||||
| S.e. | 1.0 | 1.5 | 2.0 | |||||
| Use of ESAs and DNMTi among LEN Users* | ||||||||
| N | % | N | % | N | % | |||
|
|
||||||||
| Any Use | ESA† | 545 | 79.0 | 105 | 74.5 | 148 | 87.6 | |
| DNMTi† | 149 | 21.6 | 17 | 12.1 | 50 | 29.6 | ||
| Prior to LEN initiation | ESA† | 499 | 72.3 | 91 | 64.5 | 143 | 84.6 | |
| DNMTi† | 81 | 11.7 | ** | ** | ||||
| Concurrent with LEN use | ESA† | 389 | 56.4 | 86 | 61.0 | 98 | 58.0 | |
| DNMTi† | 30 | 4.3 | ** | ** | ||||
| After LEN discontinuation | ESA | 200 | 29.0 | 36 | 25.5 | 58 | 34.3 | |
| DNMTi | 76 | 11.0 | ** | ** | ||||
| Duration, adherence of LEN use | ||||||||
| # 28 day cycles supplied | ||||||||
| Mean (s.e.) | 4.1 | 0.18 | 5.1 | 0.47 | 4.2 | 0.34 | ||
| Median | 2 | 3 | 2 | |||||
| Maximum | 29.8 | 29.8 | 26 | |||||
| Received > 3 cycles(%) | 34.3 | 44.7 | 36.7 | |||||
| Average daily dose (mg)* | ||||||||
| < 3.8 | 30 | 4.3 | ** | ** | ||||
| 3.8 -- 4.9 | 57 | 8.3 | 17 | 12.1 | 12 | 8.5 | ||
| 5.0 -- 7.5 | 286 | 41.4 | 69 | 48.9 | 66 | 46.8 | ||
| 7.6 -- 9.9 | 43 | 6.2 | 11 | 7.8 | 13 | 9.2 | ||
| 10.0 -- 15.0 | 236 | 34.2 | 34 | 24.1 | 58 | 41.1 | ||
| >15.0 | 38 | 5.5 | ** | ** | ||||
Restricted to Medicare beneficiaries with continuous Part A/B/D coverage who were first dispensed lenalidomide on or after the incident MDS diagnosis in 2006-2008,first episode of lenalidomide
Suppressed due to small sample size.
Differences between del5q and other LR MDS significant at p<0.01
Abbreviations: N: number, LEN: lenalidomide; s.e.: standard error; ESA: erythropoiesis-stimulating agents; GCSF: Granulocyte colony stimulating factors, DNMTi: DNA methyltransferase inhibitors.
Source: 100% Medicare enrollment and claims for beneficiaries with MDS, enrolled in Medicare Part D, 2006-2008)
Prior use of other MDS therapies tended to be lower for del5q-MDS compared to other LR-MDS patients, consistent with a shorter time to initiation of lenalidomide. Mean number of 4-week cycles for all patients was 4.1 (median, 2 cycles, range 0.4-29.8 cycles), with 34.3% receiving more than 3 cycles. In contrast, patients with del5q-MDS received a median of 5.1 4-week lenalidomide cycles (median, 3, range, 1-29.8), with 44.7% receiving more than 3 cycles. Median starting daily dose of lenalidomide was 7.5mg (mean 8.1mg, mode 10mg). Doses tended to be lower in del5q-MDS patients, compared to the other LR-MDS, but small sample sizes precluded statistical comparisons. There was a trend toward lower doses in the last, compared to first, treatment periods for individual patients, consistent with dose reductions with longer lenalidomide use (data not shown). Reduction in doses with longer therapy could reflect dose adjustment for side effects or reduction to lower effective doses.
Factors associated with lenalidomide prescription
Table 2 presents multivariate logistic regression estimates for factors associated with lenalidomide use. Lenalidomide initiation was negatively associated with older age (age ≥85 years, odds ratio [OR], 0.58, 95% confidence interval [95%CI], 0.46-0.74), poor DS (OR 0.56, 95%CI, 0.41-0.75), baseline diabetes (OR, 0.83, 95%CI, 0.69-0.99), renal disease (OR, 0.72, 95%CI, 0.58-0.88) and prior stroke (OR, 0.68, 95%CI, 0.51-0.91). As expected, del5q-MDS subtype was the strongest factor associated with lenalidomide prescription (OR 15.4, 95%CI, 11.8-20.0 relative to the reference category of other LR-MDS). HR-MDS was also associated with an increased use (OR 1.70, 95%CI 1.26-2.29), while MDS-NOS was associated with lower rates of use (OR 0.73, 95%CI, 0.60-0.89). Baseline pancytopenia and thrombocytopenia and high local rates of English speaking difficulty also correlated positively with prescription of lenalidomide. In contrast, LIS receipt, gender, ethnicity, area-level education, median household income, urbanicity and region of residence were not associated with lenalidomide prescription.
Table 2.
Association between patient characteristics and lenalidomide use among Medicare beneficiaries with MDS*
| Overall (n=22,399) | ||||
|---|---|---|---|---|
| Independent Variables | Odds Ratio | lower limit | upper limit | p-value |
|
| ||||
| Age at Diagnosis | ||||
| (66-74) | ||||
| 75-84 | 0.87 | 0.72 | 1.05 | 0.148 |
| 85+ | 0.58 | 0.46 | 0.74 | <.0001 |
| Sex | ||||
| (Male) | ||||
| Female | 0.90 | 0.76 | 1.07 | 0.240 |
| Race | ||||
| (White) | ||||
| Black | 0.91 | 0.62 | 1.34 | 0.638 |
| Hispanic | 1.13 | 0.65 | 1.95 | 0.669 |
| Other | 0.85 | 0.53 | 1.37 | 0.506 |
| Proportion with<high school education | 0.81 | 0.34 | 1.92 | 0.627 |
| % with English language difficulty Median household income (quartile) | 2.19 | 1.39 | 3.46 | 0.001 |
| (1st) | ||||
| 2nd | 1.27 | 1.00 | 1.61 | 0.050 |
| 3rd | 1.11 | 0.85 | 1.45 | 0.442 |
| 4th | 1.05 | 0.79 | 1.41 | 0.726 |
| Urbanicity | ||||
| (Large urban) | ||||
| Other urban/rural | 0.94 | 0.75 | 1.18 | 0.600 |
| Census Region | ||||
| (Midwest) | ||||
| Northeast | 0.90 | 0.69 | 1.16 | 0.407 |
| South | 1.09 | 0.88 | 1.34 | 0.440 |
| West | 0.90 | 0.68 | 1.19 | 0.450 |
| Drug Coverage | ||||
| (Continuous Part D, non-LIS) | ||||
| Continuous Part D, LIS | 0.99 | 0.81 | 1.23 | 0.956 |
| MDS Risk Group | ||||
| Low Risk (238.72) | ||||
| Unspecified (238.7 & 238.75) | 0.73 | 0.60 | 0.89 | 0.002 |
| Del5qMDS (238.74) | 15.36 | 11.80 | 19.98 | <.0001 |
| High Risk (238.73) | 1.70 | 1.26 | 2.29 | 0.001 |
| Disability Status | ||||
| (Good) | ||||
| Poor | 0.56 | 0.41 | 0.75 | <.0001 |
| Comorbidities (past year) | ||||
| Diabetes | 0.83 | 0.69 | 0.99 | 0.043 |
| Thyroid disorder | 1.09 | 0.90 | 1.32 | 0.371 |
| Acute myocardial infarction | 0.63 | 0.35 | 1.15 | 0.130 |
| Congestive heart failure | 1.03 | 0.83 | 1.28 | 0.771 |
| Ischemic heart disease | 1.07 | 0.89 | 1.28 | 0.491 |
| Stroke or transient ischemic attack | 0.68 | 0.51 | 0.91 | 0.009 |
| Cardiac conduction disorder | 0.96 | 0.79 | 1.17 | 0.715 |
| Renal disease | 0.72 | 0.58 | 0.88 | 0.002 |
| Liver disease | 0.69 | 0.45 | 1.07 | 0.101 |
| Venous thromboembolism | 1.42 | 0.98 | 2.06 | 0.068 |
| Gastrointestinal bleed | 1.19 | 0.93 | 1.51 | 0.173 |
| Solid tumor | 1.10 | 0.90 | 1.34 | 0.357 |
| Cytopenia | 0.83 | 0.51 | 1.35 | 0.452 |
| Neutropenia | 1.26 | 0.98 | 1.62 | 0.078 |
| Pancytopenia | 1.56 | 1.21 | 2.02 | 0.001 |
| Thrombocytopenia | 1.26 | 1.03 | 1.54 | 0.026 |
| Diagnosis period | ||||
| (2006-first half) | ||||
| 2006-2nd half | 0.99 | 0.77 | 1.28 | 0.958 |
| 2007-1st half | 0.77 | 0.60 | 1.00 | 0.047 |
| 2007-2nd half | 0.70 | 0.52 | 0.93 | 0.014 |
| 2008-1st half | 0.68 | 0.51 | 0.90 | 0.007 |
| 2008-2nd half | 0.65 | 0.46 | 0.90 | 0.009 |
Restricted to Medicare beneficiaries with continuous Part A/B/D coverage excluded beneficiaries who were first dispensed lenalidomide prior to the incident MDS diagnosis in 2006-2008
Source: 100% Medicare enrollment and claims for beneficiaries with MDS, enrolled in Medicare Part D, 2006-2008)
Lenalidomide and transfusion status
Despite the approved indication for LR-MDS with del5q with TD anemia, 36% of patients were transfusion-naïve (TN, no prior history of RBC) or TI at lenalidomide initiation, while 31% were TU and 33% were TD (Figure 2A). Point estimates indicate that 44% of patients who were either TU or TD at lenalidomide initiation (53% of del5q-MDS) experienced a transfusion response. Among those who were TD at lenalidomide initiation, 45% experienced a reduction in transfusion use during the observation period, with 15% achieving TI (Figure 2B). Moreover, 43% of patients who were TU at lenalidomide initiation (55% of del5q-MDS) achieved TI status (Figure 2A). When analysis was limited to patients who received ≥3 cycles of lenalidomide, 71% of patients who were TU or TD at lenalidomide initiation (83% of del5q-MDS) experienced a transfusion response. Among TD patients who received ≥3 lenalidomide cycles, 77% experienced a transfusion response, and 40% achieved TI (Figure 2B). Among these more extensively treated patients who were TU at time of lenalidomide initiation, 67% became TI (Figure 2B). Small sample sizes provided inadequate power to detect differences between MDS risk groups.
Figure 2.
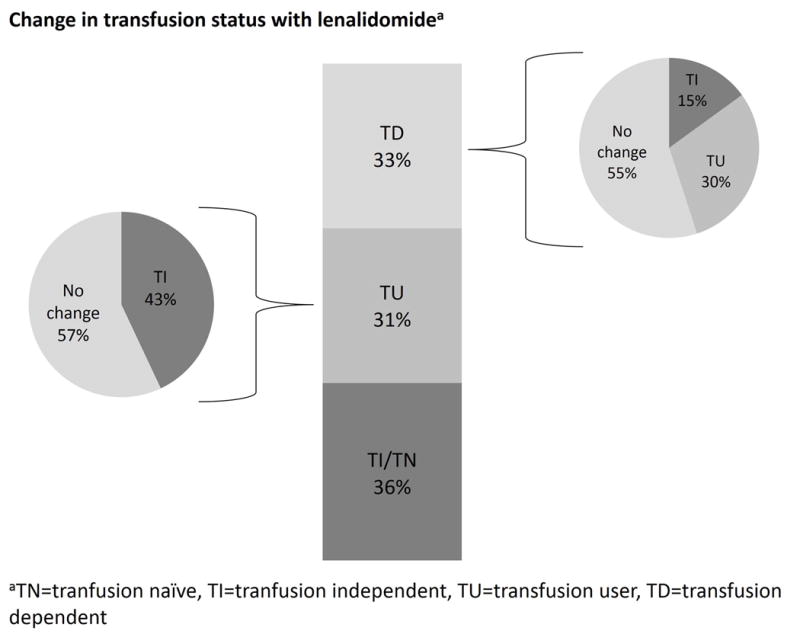
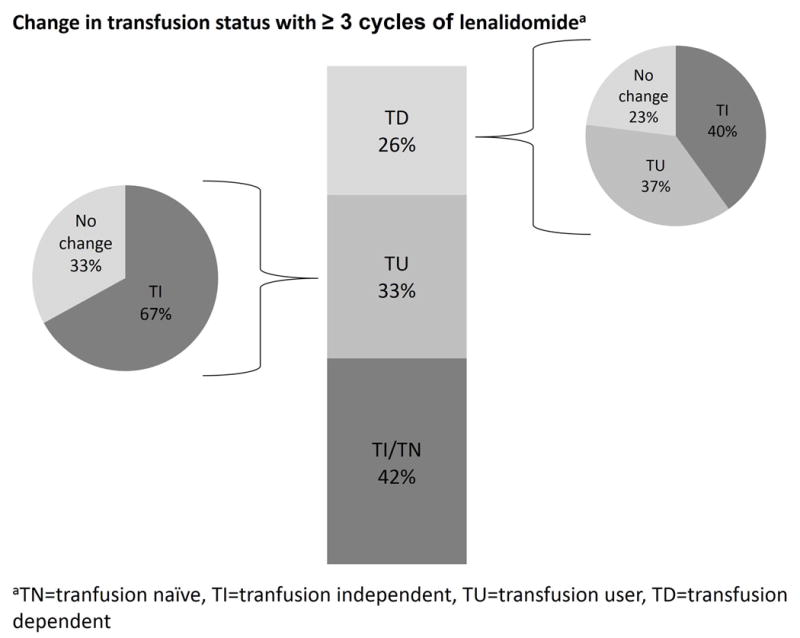
Change in transfusion status with lenalidomide therapy. The bar in the middle reports the distribution of patients by transfusion status at lenalidomide therapy initiation , while the pie charts represent the change in transfusion status during observation period. A: For all patients. B: for patients who received ≥3 cycles of lenalidomide. TI: transfusion-independent, TD: transfusion-dependent, TU: transfusion-user.
Discussion and conclusions
This study represents the first report of lenalidomide use for MDS in a large population of Part D-enrolled Medicare beneficiaries with newly diagnosed MDS. We are not aware of other large “real-life” analyses evaluating changes in transfusion status in MDS patients who received lenalidomide therapy, or, in particular, any reports of patients not enrolled in Medicare. We evaluated patterns of lenalidomide use and associated change in RBC transfusion status in a “real-world” setting during the first 3 years after MDS indication approval (2006-2008). As expected, utilization rates were highest among del5q-MDS patients and time to lenalidomide initiation was shorter for del5q-MDS versus other LR-MDS patients. These observations suggest general increased awareness of selective activity of lenalidomide for del5q-MDS on the part of prescribing physicians. Reports of hematologic response in LR-MDS patients without del5q5,12 might account partially for our finding that the majority of MDS patients (80%) who received lenalidomide were not coded as del5q-MDS. However, this finding, as well as our observation that del5q-MDS constituted an increasing proportion of lenalidomide users, may be an artifact of the change in ICD9 coding that occurred 10 months into our observation period.
Our findings with regard to lenalidomide use rates contrast with estimates from a survey of 101 hematology and medical oncology providers completed between June 2005 and January 2007.7 In that study, spanning the first year after lenalidomide approval for MDS, providers reported that 8% of recently diagnosed and 1-9% of established MDS patients received lenalidomide. However, the study cohort was not limited to Medicare beneficiaries, and did not provide information about risk status, karyotype or other patient characteristics in lenalidomide users and non-users.7
In contrast to the labeled indication for TD patients, approximately one third of MDS patients (including a similar proportion of del5q-MDS patients) were started on lenalidomide while TI. This apparent inconsistency with treatment guidelines was also found in our study examining guideline adherence in ESA use in MDS.13 It is possible that physicians were privy to clinical parameters suggesting a need for RBC transfusion, and chose to preempt transfusions by initiating lenalidomide therapy. Absent clinical measures in our claims data, we are unable to assess this scenario more definitively.
The negative correlation of advanced age, comorbidities, and poor DS with prescription of lenalidomide observed in our multivariate analysis is discouraging, since one of the goals of introducing non-intensive therapies is to allow more frail elderly MDS patients with comorbidities to receive active therapy that can be better tolerated. These findings are consistent with a recent report of patterns of use of DNMTi among elderly Medicare beneficiaries with MDS who were followed through 2007.14 In this study, only 11% of MDS patients received DNMTi therapy, and older patients and those with more comorbidities were less likely to receive DNMTi. The additional burden of physician office visits or hospitalization for administration may explain the low rates of DNMTi use, but, as an oral agent, lenalidomide should not be subject to these constraints. Instead, the lower rate of lenalidomide use in vulnerable patient populations might reflect a more cautious approach with a newly approved agent, and utilization rates may increase with improved familiarity.
Contrary to expectations, we did not find strong evidence that socioeconomic status, including LIS receipt, influenced initiation of lenalidomide. Lenalidomide is an expensive oral medication, so that beneficiaries without the LIS are subject to very large initial out-of-pocket payments and continued large payments even when reaching the catastrophic coverage phase. In contrast, those with the LIS pay minimal amounts towards their initial prescription, and pay nothing once they reach the catastrophic phase. The lack of an observed effect of LIS receipt on lenalidomide treatment may suggest that patients are not price-sensitive. Decreased price response has been demonstrated for cancer patients in other studies,15,16 and the perceived benefits of lenalidomide may be sufficiently large to justify the payment from the patient's perspective. However, patients may face income constraints that either discourage initiation of lenalidomide or encourage its early discontinuation. Our zip code-level income measure may not adequately correlate with person-specific income, and hence may not capture an association between income and treatment.
Although the transfusion response rates for the full cohort in our study reflected a mix of risk groups and relatively short duration of therapy for many patients, overall they are consistent with data in published clinical trials. In the registration study (MDS003), patients with TD anemia due to MDS with 5q deletions irrespective of karyotype complexity received lenalidomide 10 mg daily for 21 or 28 days out of 28-day cycle,1 with TI or reduction in need for transfusions in 67% and 76%, respectively.1 The randomized phase 3 MDS004 trial suggested a dose-dependent effect of lenalidomide: with the 10 mg dose (taken for 21 out of 28 days), 56.1% and 50% achieved TI for ≥26 weeks and cytogenetic response, respectively, compared with 42.6% and 25% for the 5 mg dose (taken for 28 out of 28 days).2 Lenalidomide has limited activity in LR-MDS without 5q deletions (TI, 26%, median duration 41 weeks) and HR-MDS with 5q deletions (TI, 25.5%, median duration 26 weeks), with responses typically less frequent and shorter in duration than in LR-MDS with 5q deletions.3-5
Relative to these trial data, we observed that 44% of all MDS patients and 53% of del5q-MDS patients who were either TU or TD at lenalidomide initiation experienced a reduction in transfusion use. For patients who were TD at time of lenalidomide initiation (all risk groups), 45% experienced reduction in RBC transfusions, including 15% who achieved TI [Figure 2A]. When we restricted the analysis to patients who received ≥3 cycles of lenalidomide, 71% of patients who were TU or TD at lenalidomide initiation (all risk groups, including 83% of del5q-MDS) experienced a reduction in transfusion use. The patients who were TD at time of lenalidomide initiation (all risk groups) and subsequently received ≥3 cycles of therapy had an augmented benefit; 77% had reductions in transfusions, including 40% who achieved TI [Figure 2B]. Small sample sizes provided inadequate power to detect differences between MDS risk subgroups (including del5q-MDS vs. other LR-MDS) or to compare differences based on lenalidomide dose.
The greater frequency of reduction in transfusion needs for patients who received ≥3 cycles of lenalidomide indicate that, in line with current guidelines,6 the therapeutic potential of the drug might require 3 or more months to be fully achieved in some patients. On the other hand, patients with longer treatment duration may be enriched with early responders, which could explain some of our findings. Our descriptive analysis does not account for this potential pattern; further research on the effect of therapy duration on outcomes is warranted.
The main limitations of our study relate to use of claims to select patient cohorts and to construct valid measures of treatment exposures and transfusion status. MDS risk group was assigned based on ICD9 diagnostic codes on claims, yet many in the cohort did not have a specific risk group designated. This might be due to cytogenetic evaluation not being performed in routine-care settings or, if performed, the results not being reflected in the coding. As a result, the number of patients identified as carrying del5q is relatively small, and we recognize that some portion of those designated as other LR-MDS or MDS-NOS may actually harbor the del5q abnormality. While we report lenalidomide use patterns stratified by del5q and other LR-MDS, sample size was not sufficient in either group to test for differences in treatments or outcomes.
Our measures of lenalidomide and other MDS therapies were based on claims. For oral medications, in particular, we have to presume that the medication was consumed by the patient. Our measure of erythroid response was based on changes in transfusion status, which in turn were based on observed weekly transfusion receipt. While we modeled this approach on the IWG definitions of major and minor responses, we could not fully operationalize them, absent data on hemoglobin levels.
Additionally, our analysis of changes in transfusion response should be considered descriptive and not causal. While it is possible to use observational data to examine causal relationships between lenalidomide and a variety of outcomes, including survival, progression to AML, secondary solid tumor malignancies and VTE, reporting such outcomes without adequate controls for other therapies received and without use of an analytic strategy that addresses patient selection would be inadequate and possibly misleading. This type of analysis was outside the scope of the current paper, but is clearly warranted in the future.
In summary, our data suggest that in the first 3 years following approval of lenalidomide for its MDS indication, prescribing patterns were only partly consistent with clinical guidelines and the drug label.6,17 Major deviations were the high proportion of patients who were TI when the drug was prescribed, high rates of concomitant use of lenalidomide with ESAs and other MDS therapies, and short duration of treatment episodes for a large proportion of patients. Consistency of prescribing patterns across socioeconomic strata, including in patients who may incur significant copayment, suggests that MDS-associated anemia presents a significant clinical problem for MDS patients. For patients who were TD at lenalidomide initiation, therapy appears to have had a positive impact on transfusion rates, particularly for those receiving at least 3 cycles. This experience suggests that stronger adherence to treatment guidelines would result in further improvements in outcomes.
Supplementary Material
Acknowledgments
Funding was provided by Celgene. SDG, DLM, MRB, FH, and AJD received partial funding from NIH/NCI RC1 CA145831-01(Davidoff, PI). Disclaimer: A portion of this research was undertaken while AJD was employed by the University of Maryland School of Pharmacy. The opinions expressed in this article are the author's own and do not reflect the view of the Agency for Healthcare Research and Quality, the Department of Health and Human Services or the United States government.
Footnotes
Authorship and Disclosures : AJD was the principal investigator and takes primary responsibility for the paper; AJD, AMZ, SDG, MRB, and DM developed the study concept and research design; DLM coordinated data management and performed the statistical analyses; all authors reviewed study results and interpretation; AMZ, FH, and AJD drafted the initial manuscript; all authors reviewed and signed off on the final manuscript. AJD received research funding from Celgene, GlaxoSmithKline and Novartis until September 2012. MRB and SDG receive research funding from Celgene. DM is employed by Celgene and owns Celgene stock options.
References
- 1.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 2.Fenaux P, Giagounidis A, Selleslag D, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011;118:3765–3776. doi: 10.1182/blood-2011-01-330126. [DOI] [PubMed] [Google Scholar]
- 3.Ades L, Boehrer S, Prebet T, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: Results of a phase 2 study. Blood. 2009;113:3947–3952. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 4.Ades L, Fenaux P. Immunomodulating drugs in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2011;2011:556–560. doi: 10.1182/asheducation-2011.1.556. [DOI] [PubMed] [Google Scholar]
- 5.Raza A, Reeves JA, Feldman EJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg PL, Attar E, Bennett JM, et al. NCCN clinical practice guidelines in oncology: Myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekeres MA, Schoonen WM, Kantarjian H, et al. Characteristics of US patients with myelodysplastic syndromes: Results of six cross-sectional physician surveys. J Natl Cancer Inst. 2008;100:1542–1551. doi: 10.1093/jnci/djn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekeres MA. Epidemiology, natural history, and practice patterns of patients with myelodysplastic syndromes in 2010. J Natl Compr Canc Netw. 2011;9:57–63. doi: 10.6004/jnccn.2011.0006. [DOI] [PubMed] [Google Scholar]
- 9.RTI International. RTI Spatial Impact Factor Data. https://rtispatialdata.rti.org/Home/tabid/37/Default.aspx Accessed September 5, 2011.
- 10.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. doi: 10.1016/j.jgo.2012.12.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- 12.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 13.Davidoff AJ, Smith SW, Baer MR, et al. Patterns of erythropoiesis-stimulating agent use for myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res. doi: 10.1016/j.leukres.2013.02.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R, Gross CP, Maggiore RJ, et al. Pattern of hypomethylating agents use among elderly patients with myelodysplastic syndromes. Leuk Res. 2011;35:904–908. doi: 10.1016/j.leukres.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman DP, Joyce GF, Lawless G, et al. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25:1319–1331. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldman DP, Jena AB, Lakdawalla DN, et al. The value of specialty oncology drugs. Health Serv Res. 2010;45:115–132. doi: 10.1111/j.1475-6773.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology v4. 2006 National Comprehensive Cancer Network. Myelodysplastic syndromes. 2006 doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


