Abstract
This study was to describe symptoms, feelings, activities and medication use reported by adolescents with uncontrolled asthma on their 24-hour asthma diaries. Adolescents with uncontrolled asthma (13-17 years, N=29) completed asthma diaries and audio-recorded symptom sounds for 24 hours. A variety of symptoms were reported, and the most frequently reported symptoms were coughing followed by wheezing. Most self-reported coughing and wheezing were verified by audio-recordings. Participants reported predominantly negative feelings and low levels of activities. High discordance between self-reports and medical records in medications was noted, raising a concern of poor treatment adherence in this vulnerable group.
Keywords: uncontrolled asthma, adolescents, symptoms, emotional response, activity, medication adherence
Asthma is the most common chronic health condition in children and adolescents. According to 2009 National Health Interview Survey (Center for Disease Control and Prevention, 2011), over 7 million children (9.6%) ages 17 and younger had current asthma in the U.S. Of those, 39% (2.8 millions) were adolescents (12-17 yrs). Uncontrolled asthma takes a large toll on not only children with the disease but also the society. Over 14 million missed school days annually in the U.S. are attributable to uncontrolled asthma (American Lung Association, 2010) that is also responsible for 7.5 million outpatient office visits, over 640,000 ED visits and 157,000 hospitalizations in 2007 (Akinbami, Moorman, & Liu, 2011). Nonetheless, little is known about symptoms that adolescents commonly experience during the acute phase of uncontrolled asthma, and how they emotionally respond to the symptoms. Further, prior reports do not address the levels of activities that adolescents engage in and types of medications used by adolescents during the acute phase.
The 2007 National Asthma Education and Prevention Program (NAEPP) Asthma Guidelines (National Heart, Lung, and Blood Institute [NHLBI], 2007) classify asthma control into three categories-- “well-controlled,” “not well-controlled (NWC)” and “very poorly controlled (VPC)”-- based on the levels of impairments including daytime symptoms, night-time symptoms, interference with normal activity and use of short-acting beta agonists (SABA, “quick relief medication”). It is important to assess the levels of asthma control as it becomes the basis of determining optimum asthma management plans. For individuals ages 12 or older, uncontrolled asthma, which includes NWC and VPC, is indicated when daytime symptoms are more than 2 days per week, night-time symptoms are 1-3 times per week, “some to extreme” (NHLBI) activity limitations or use of SABA more than 2 days per week. Uncontrolled asthma has been a target of extensive research efforts that focus on effective treatments and managements. However, little is known about symptom and emotional experiences or the levels of activities in adolescents suffering from uncontrolled asthma.
Because the levels of asthma control are primarily determined by patients’ symptom reports, the NAEPP guidelines emphasize the importance of individual patients’ asthma monitoring for optimum asthma management (National Heart, Lung, and Blood Institute, 2007). An asthma diary, as recommended by the National guidelines, is one asthma self-assessment method. Clinically, asthma diaries can be used for gathering clinical information for better care of patients, guiding asthma self-management through symptom monitoring and medication adherence, and improving assessment and strengthening patient-provider communication (Cruz-Correia et al., 2007). In particular, symptom diaries are useful to those whose asthma is not under control or persistent in nature, as it can aid in the identification of asthma of higher severity (National Heart, Lung, and Blood Institute, 2007; Reznik, Sharif, & Ozuah, 2005). Asthma diaries completed on a day-to-day basis, thus eliminating recollection errors (Hensley et al., 2003), are a better means for identifying patients with persistent asthma compared to retrospective reports, as in a periodic self-assessment form completed at the time of an office visit (e.g., Asthma Control Test). Use of asthma diaries has been limited to clinical settings on an individual basis, and no attempt has been made to achieve collective understanding or descriptions of asthma experiences based on data provided by asthma diaries. Unlike a standardized questionnaire, an asthma diary is not stringent in its format or collected information, and can be modified to obtain a wide range of experiences (e.g., symptoms, activity, emotions or medication use) pertaining to the disease. Therefore, asthma diaries seem to be an appropriate tool with which to gauge experiences of adolescents with uncontrolled asthma. The range of experiences captured in this study reflects key concepts of the self-regulation theory (Johnson, 1999). The theory assumes that people use their perceptions of health events (e.g., symptoms) to regulate their functional (e.g., activity or medication adherence) and emotional responses (e.g., feelings) (Johnson).
The purpose of this descriptive study was to examine self-regulation in adolescents with uncontrolled asthma through the descriptions and in-depth analysis of information provided in the asthma diaries, including symptoms, feelings, activities, and medications, all major components of the self-regulation theory. Specific research questions of the study included: (RQ1) What are the common asthma symptoms experienced by adolescents with uncontrolled asthma? (RQ2) What are the adolescents’ emotional responses to uncontrolled asthma? (RQ3) What are the levels of activities in which the adolescents engage? and (RQ4) What are the types of medication used by the adolescents to control their symptoms, and what is the extent to which the adolescents’ self-reported medications concur with the providers’ prescriptions?
Method
This was a cross sectional, descriptive study analyzing written descriptions in 24-hour asthma diaries provided by adolescents with asthma (N=29). The asthma diaries were collected as part of a larger research study. The diary data reported in the current study capture one aspect of the self-regulation theory, perception of their experience (i.e., symptoms) to regulate their emotions (i.e., feelings) and subsequent behavior including activity and medication use.
Sample and Setting
Eligibility criteria included ages between 13-17 years, having an asthma diagnosis, meeting criteria for uncontrolled asthma by the NAEPP guidelines and currently experiencing asthma symptoms (acute asthma) as verified by healthcare providers. Only those who could speak and write English are included in the study. A total of 173 adolescents with asthma diagnosis who visited outpatient clinics or pediatric emergency department (ED) in a major academic medical center were screened. Of those, 39 were found to be eligible, with 29 enrolling and 10 declining participation. The majority were recruited from the Pediatric ED (n=19; 66%) followed by Pediatric Primary Practice (n=8; 27%), Pediatric Pulmonary Outpatient Clinic (n=2; 7%).
Data Collection Procedure
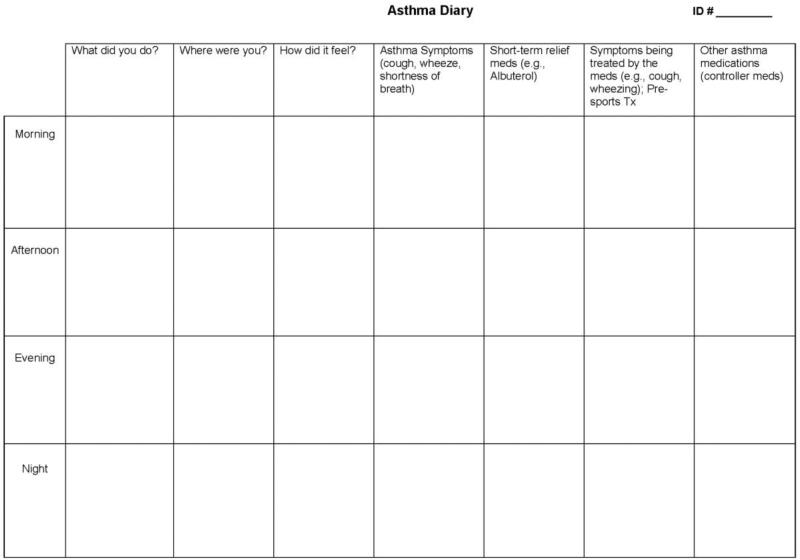
The study team devised a 24-hour asthma diary format that allowed participants to record symptoms, feelings, activities, and medication use in the morning, afternoon, evening and nighttime (Figure 1). Participants recorded this health information in the open-ended format of the diary. Simultaneously, participants continuously audio-recorded their breathing sounds to capture audible asthma symptoms (e.g., wheezing or coughing) using a commercially available compact digital recorder during the 24 hours. Electronic medical records of the participants were also reviewed to obtain information about their current asthma medications.
Figure 1.
24 Hour Asthma Diary
At enrollment, participants completed a simple sociodemographic form and were given detailed verbal and written instructions about operating the digital recorder and completing the 24-hour asthma dairy. The recorder was turned on during the enrollment meeting and the attached microphone was clipped to the free edge of the teen's shirt closest to the sternal notch. Participants recorded their breath sounds for the next 24 hours. During nighttime, participants placed the recorder and microphone on a surface close to the head of their bed for continuous recording. When privacy was desired, participants were instructed to pause and then restart the recorder later. Although participants were told not to alter their daily routine in any significant manner when recording, they were encouraged to find opportunities to record periods of time in a quieter environment with minimal talking and background noise, when feasible. Participants were required to provide a minimum of 10 hours of audio-recorded data. The asthma diary and the digital recorder were returned the following day.
The study protocol was approved by the Institutional Review Board in the academic medical center affiliated with the researchers and used for subject recruitment. Screening for prospective participants in the outpatient clinics was done by the research team via the medical center's electronic appointment /medical record system. In the ED, screening was performed by specially trained on-site ED research associates. In all recruitment sites, researchers only approached potential participants who had provided written or verbal permission to be contacted by the research team. Informed parental consent and adolescent assent were obtained prior to data collection. In the parent consent, parents specifically provided the authorization to access their teens’ medical records and use protected health information for this study. Having obtained the authorization and completed the Health Insurance Portability and Accountability Act (HIPAA) training, the research staff was given access to adolescents’ electronic medical records by the participating medical center.
Data Analysis
The recorded content of the 24-hour asthma diaries was carefully examined and summarized by two researchers. Diary data were analyzed and described for each category including symptoms, feelings, activities and medication. The digitally recorded audio data were listened to by two project nurses in order to identify and annotate each eligible asthma symptom (e.g., coughing, wheezing, shortness of breath, throat clearing) for individual participants. A randomly selected sample of annotated symptom sounds were verified by consensus among three clinicians, including a nurse practitioner, a pediatrician and a pulmonologist. Subsequently, the annotated asthma symptoms for each participant were used to confirm self-reported symptoms provided in the asthma diaries. The list of medications reported in the diary was compared with the list that the research team compiled through the review of the medical record of each participant. The percent agreements between the diaries and medical records in each type of medications were computed.
Results
Of 29 participants, females accounted for 55% (n=16), and 66% (n=19) of the sample indicated their race being non-white, primarily African-American. The mean age of adolescents was 14.6 (SD= 1.4) years. Sixty-eight percent (n=19) reported the annual household income under $30,000. Table 1 summarizes the sociodemographic characteristics of the study sample. The following are the findings that address each area of research questions.
Table 1.
Sociodemographic Characteristics of the Study Sample (N=29)
| Sex | |
| Male | 13 (44.8%) |
| Female | 16 (55.2%) |
| Race/ethnicity | |
| Black | 15 (51.7%) |
| White | 10 (34.5%) |
| Other | 4 (13.8) |
| Annual family income | |
| $9,999 or less | 8 (27.6%) |
| Between $10,000 and $29,999 | 11 (37.9%) |
| Between $30,000 and $49,000 | 4 (13.8%) |
| Between $50,000 and $69,999 | 1 (3.4%) |
| Between $70,000 and $89,000 | 1 (3.4%) |
| $100,000 or more | 3 (10.3%) |
| Missing | 1(3.4%) |
| Inner-city status | |
| Inn-city residence | 15 (52%) |
| Smokers in the house | |
| Yes | 15 (52%) |
| No | 14 (48%) |
| Number of people living in home with the participant | |
| 1-3 people | 16 (55%) |
| 4-6 people | 10 (35%) |
| 7-9 people | 3 (10%) |
| Types of health insurance | |
| Public insurance (e.g., Medicaid, CHIP*) | 16 (55%) |
| Private insurance | 6 (21%) |
| Missing | 7 (24%) |
Children's Health Insurance Program
RQ1: Symptoms
Participants reported a wide range of asthma symptoms (Table 2). Coughing was the most common asthma symptom experienced by almost all participants, followed by wheezing and shortness of breath. Chest tightness, chest pain and congestion were also common. In addition, a small number of participants reported several unusual symptoms in association with their asthma such as feeling “hot,” throat pain, headache and “itchy” lungs. Overall, symptoms, specifically coughing and wheezing, reported in the diaries were verified by listening to the corresponding breath sounds recordings. Inconsistencies between self-report and sound recordings were found only in three participants out of 29. Two participants reported symptoms, which were not verified on digital recordings. Conversely, one participant reported no asthma symptoms on the diary, yet the digital recording indicated occasional coughing.
Table 2.
Asthma symptoms reported by adolescents with uncontrolled asthma (N=29)
| Asthma Symptoms | Frequency |
|---|---|
| Cough | 96% (28) |
| Wheeze | 79% (23) |
| Shortness of breath | 72% (21) |
| Chest tightness | 24% (7) |
| Chest/back/side pain | 28% (8) |
| Hot | 14% (4) |
| Congested | 10% (3) |
| Throat pain | 10% (3) |
| Headache | 7% (2) |
| Mucus | 7% (2) |
| Can't sleep | .03% (1) |
| Cold | .03% (1) |
| Dizzy | .03% (1) |
| Itch in lungs | .03% (1) |
RQ2: Emotional Responses
Participants reported predominantly negative feelings associated with their symptoms. The most common response was “tiredness.” Other negative feelings were “afraid”, “challenging”, “difficult”, “frustrated”, “panic”, “scared”, “sad”, “upset” and “like crap.” Some responded in a neutral way downplaying symptoms by reporting “no big deal—used to it” or “feeling normal.” Positive or optimistic feelings (e.g., “relaxed” “calm” “good” or “OK”) were also reported by a small number of participants particularly when their perceived symptoms abated.
RQ3: Activity Levels
Types of activities reported in the diaries were classified into four levels based on the expected intensity. Level 1 represented sedentary activities (e.g., sitting, reading, taking a nap, watching TV), and Level 2 included mild activities involving low intensity movements such as “walking short distance”, “taking a shower” and “doing laundry.” Activities of moderate intensity were classified into Level 3 (e.g., climbing stairs, kickball, playing Wii™ boxing/hoola-hoop, walking around mall), and activities being considered intense were into Level 4 (e.g., chasing, running, wrestling, basketball, gym class fitness and exercise). Two researchers separately determined the levels of activities reported in the diary. Any discrepancies in the assigned levels of activities were resolved through discussions by the study team. A number was assigned to each reported activity based on the levels (1=Level 1; 2=Level 2; 3=Level 3; and 4=Level 4), and mean activity scores were computed for individual participants (range 1-3.6). The average activity level of the sample was 1.84 (SD=.63), indicating that the majority engaged in sedentary to mild activities during the 24 hour observation period. No significant gender differences were found in the average activity levels (t=-.73, p=.47). Likewise, activity levels in inner-city participants were not significantly different from those living in suburban areas (t=-1.73, p=.09).
RQ4: Medication Use
The most common asthma medication used by the participants was short acting beta agonist (SABA) which was reported by 93% (n=27) of the sample, followed by oral corticosteroids (48%, n=14) and combination of Inhaled corticosteroids (ICS) and Long-acting Beta Agonists (LABA) (45%, n=13). Use of Leukotriene Antagonists and ICS was reported by five participants, each. In addition, fewer reported anti-cholinergics (7%, n=2), and none reported use of LABA alone (e.g., Salmeterol). No significant gender differences were found in medication use. We compared the self-reported medications with those listed in the medical records to assess discrepancies. Medical records were unavailable for two participants whom we did not include in calculating the following percentages. Of twenty-seven participants, twelve (44%) reported no use of ICSs or an ICS/LABA combination in their diaries. However, medical records revealed that five of those actually had prescriptions for an ICS or an ICS/LABA combination, yet failed to report these in their diaries. Table 3 compares the number of participants reporting medications of each type in the diaries and medical records, and provides percent agreement. The highest agreement was found in SABA (89%) followed by the ICS/LABA combination (73%) and systemic steroids (58%). Anti-cholinergics were the most commonly omitted medications from the participants’ diaries, followed by Leukotriene Antagonists. Of twelve participants with anti-cholinergic prescriptions, only two reported taking the medication in their diaries. Of a total of 43 discrepancies in all types of medications reported in Table 3, 91% occurred due to participants’ under-reporting of medications in the diaries. Only 9% of discrepancies were due to participants reporting of medications that were not validated by the medical records.
Table 3.
Agreement between self-reported medications and medications from medical record review (N=27)
| Medications | Self-reported (n=participants) | Medical Record (n= participants) | % Agreement |
|---|---|---|---|
| Long-Term Controller Medications | |||
| Inhaled corticosteroids (ICS) | 5 | 11 | 33% |
| ICS/Long-acting Beta Agonists (LABA) Combinations | 11 | 15 | 73% |
| Systemic steroids | 11 | 19 | 58% |
| Leukotriene Antagonists | 5 | 10 | 25% |
| Methylxanthines | 1 | 1 | 100% |
| Quick-Relief Medications | |||
| Short-acting beta agonists (SABA) | 25 | 26 | 89% |
| Anti-Cholinergics | 2 | 12 | 17% |
Discussion
This study demonstrates that an asthma diary can be a useful tool in capturing and documenting experiences and treatment adherence in adolescents with uncontrolled asthma. To date, the asthma diaries have been adapted in research primarily to monitor symptoms (Hensley et al., 2003; Reznik et al., 2005). To our knowledge, this is the first study using the asthma diaries to obtain comprehensive descriptions of uncontrolled asthma involving four important areas of asthma self-regulation (symptoms, emotional responses, activities and medication use) in adolescents. Although time duration captured in the diaries was brief, 24 hours, the semi-structured asthma diaries provided a wealth of information critical to understanding how adolescents experience and manage uncontrolled asthma, which has not been previously explored in the literature.
Consistent with other studies (Davis, DiSantostefano, & Peden, 2011; Rietveld & Rijssenbeek-Nouwens, 1998; Wildhaber, Carroll, & Brand, 2012), coughing was the most common symptom experienced by those with uncontrolled asthma, followed by wheezing and shortness of breath. Participants also reported other miscellaneous symptoms that have rarely been mentioned in the literature, suggesting a wide range of asthma symptoms that adolescents perceive as a manifestation of uncontrolled asthma. Another important lesson is that adolescents’ reports of asthma symptoms, particularly coughing and wheezing, can be trustworthy given that most of those symptoms recorded in the diaries were verified by simultaneously recorded audio data of the symptoms. This finding suggests that an asthma diary can be a reliable tool for the accurate monitoring of daily symptoms in adolescents with uncontrolled asthma and can be used to learn how to cope with or self-regulate symptoms in order to minimize disruption of daily life activities. (Johnson, 1999). Asthma diaries do not rely on an individuals’ capacity to recollect symptom episodes in the past (e.g., 4 weeks before the visit), thus rendering greater accuracy.
Participants reported overwhelmingly negative feelings in association with ongoing symptoms. Of the multitude of negative emotions reported in the asthma diaries, six participants identified “tiredness” as an emotional reaction. Unlike other negative feelings such as anger, frustration, fear or sadness that are more direct or short-term response to asthma symptoms, it seems reasonable to believe that emotional exhaustion (tiredness) is a reaction to prolonged or repeated symptom episodes. In order to help the adolescents self-regulate their emotional response, it may be important to explore the frequency or duration of symptom episodes when tiredness is reported as a “feeling.” Emotional responses have an important implication for adolescents’ coping with uncontrolled asthma. Studies have shown that negative emotions could trigger or prolong asthma symptoms (Bender & Zhang, 2008; Richardson et al., 2006; Tibosch, Verhaak, & Merkus, 2011), elicit counterproductive coping mechanisms (e.g., overcompensation or denial),(Barton, Clarke, Sulaiman, & Abramson, 2003; Lahaye, Fantini-Hauwel, Van Broeck, Bodart, & Luminet, 2011) or undermine adherence to treatment (Barton et al., 2003; Drotar & Bonner, 2009). On the other hand, some participants showed a tendency to downplay or normalize asthma symptoms by expressing neutral to positive feelings. Such tendency in adolescents has been reported in other studies (Couriel, 2003; de Benedictis & Bush, 2007; Rhee, Belyea, Cirzynski, & Brasch, 2009; Rhee, Wenzel, & Steeves, 2007). This raises a concern because these individuals are more likely to underestimate the importance of symptom monitoring and adherence to treatment (Yoos, Kitzman, McMullen, Sidora-Arcoleo & Anson, 2005). Taken all together, it is critical to help adolescents define and interpret their emotional responses when assessing or predicting the course or outcomes of uncontrolled asthma in adolescents (Johnson, 1999). Accurate interpretation of symptoms can assist the adolescents in learning how to reduce discomfort with appropriate medication use and achieve the goal of well controlled asthma with low disruption in daily activities.
Given the nature of uncontrolled asthma, it is not surprising that most participants reported sedentary to mild activity levels. However, activity levels did not always correspond to symptom status as some reported low activities even during the absence of active symptoms. This finding suggests either that they may have chosen not to be involved in higher levels of activities out of precaution, or they have become accustomed to sedentary life styles in reaction to frequent asthma episodes. Literature concurs that asthma takes a substantial toll on daily activities in children and adolescents (Akinbami, LaFleur, & Schoendorf, 2002; Fuhlbrigge, Guilbert, Spahn, Peden, & Davis, 2006; Newacheck & Halfon, 2000; Wildhaber et al., 2012). Limited activity is an issue not only for those with active symptom episodes but also for those without. Many children and adolescents with well controlled asthma are inclined to avoid physical exertion or outdoor activities in order to prevent asthma episodes or keep their asthma controlled (Fuhlbrigge et al., 2006). Therefore, it is important to distinguish activity limitations that are a direct consequence of debilitating symptoms from those that are a precautionary measure taken to prevent potential symptoms. This distinction can be helpful in providing adolescents with adequate guidance concerning daily physical activity. The NAEPP guidelines recommend that adolescents be encouraged to fully participate in physical activities, and curtailing activity should be a last resort (National Heart, Lung, and Blood Institute, 2007). Given the recommendation, the overwhelmingly low activity levels in this sample are concerning and underscore an area that needs further improvement in asthma education.
Aside from SABAs that were reported by almost all participants, oral cortiocosteroids and the ICS/LABA combination were the most commonly reported medication to treat asthma in this sample. Given that the majority of participants were recruited through ED, finding the high use of oral corticosteroids in this sample is not a surprise. Despite the uncontrolled nature of asthma, it is concerning that 44% (n=12) did not report taking inhaled corticosteroids or ICS/LABA combinations, suggesting poor coping or self-regulation of symptoms by lack of preventive medication use. Of those, ICSs were actually prescribed for 5 teens according to medical records. We found substantial discordance in medications between self-reports and medical records. In their diaries, participants tended to omit the use of ICSs (not in combination with LABA), oral steroids, Leukotriene antagonists or anti-cholinergics that had been prescribed. It is unlikely that the participants forgot to record those medications that they had taken, given the short duration of observation. Instead, the failure to report those medications in the diaries may be an indication either that those prescriptions were not filled or that the medications were filled but not taken by the participants. Inaccessibility to a pharmacy database in this study prevented us from confirming the speculation. Nonetheless, the widespread discrepancies between self-reports and medical records suggests poor adherence to treatment plans despite active symptoms in participants, thus adding evidence to the literature documenting poor treatment adherence in adolescents with asthma (de Benedictis & Bush, 2007; Desai & Oppenheimer, 2011; Orrell-Valente, Jarlsberg, Hill, & Cabana, 2008). Suspected poor adherence in our sample is particularly alarming considering the uncontrolled nature of their asthma with current active symptoms. Given that most participants had a health insurance, public or private, it is less likely that the suspected poor adherence was related to the families’ financial inability to afford prescribed medication. It is also noteworthy that ICS or ICS/LABA combination was not prescribed for 1 of 4 participants despite the NAEPP guidelines recommending daily use of lCSs for uncontrolled asthma in youths 12 years or older (National Heart, Lung, and Blood Institute, 2007). Our finding is in line with earlier studies documenting that many young people do not receive warranted controller therapy (Butz et al., 2006; Fuhlbrigge et al., 2006; Halterman, Aligne, Auinger, McBride, & Szilagyi, 2000; Halterman et al., 2007; Warman, Silver, & Stein, 2001). Perhaps, the high concordance (89%) between self-reports and medical records on SABAs indicates the adolescents’ overreliance on quick relief medications for the control of their symptoms as suggested in a previous study (Butz et al., 2006). Further study is needed to address suboptimal treatment adherence by adolescent patients and their providers’ non-adherence to the guidelines.
Limitations of the Study
Several limitations of the study warrant careful interpretation of the findings. Given the small sample size primarily recruited through ED, generalization of the findings to non-ED adolescents requires cautions. Because the asthma diaries were completed only for a short period, the findings could only provide a snapshot of how adolescents deal with uncontrolled asthma, rather than reflecting changing patterns of the experiences over time. In addition, we were unable to audit the over 10 hours of sound data from each subject in its entirety due to time and resource constraints. Therefore, we may have missed legitimate symptom sounds that could have verified self-reported symptoms in the diaries. In some cases, extreme background noises in the audio data limited our ability to detect wheezing with great accuracy. Therefore, false negative symptoms from audio files might have resulted for two participants whom we were unable to confirm symptoms recorded in the diaries. Lastly, we did not have access to a pharmacy database to validate prescriptions fills, which limited us to speculate whether the suspected poor medication adherence was due to unfilled prescription.
Overall, our 24-hour diary data demonstrate congruencies among the perceptions of symptoms, emotional responses and functional regulation by limiting activities and taking rescue medications to relieve immediate symptoms. As such, this study provides support for the self-regulation theory in understanding the experiences of adolescents with uncontrolled asthma and support for future behavioral interventions that enhance coping skills of adolescents with uncontrolled asthma.
Summary
In this study, asthma diaries were used as a research tool for data collection. Nonetheless, findings from this study shed important light on clinical implications based on uncovered multidimensional experience of self-regulation in uncontrolled asthma including symptoms, emotional responses, activity levels and medication use in adolescents. Because adolescents tend to attribute a variety of symptoms to their uncontrolled asthma, it is important for nurses to be diligent and open-minded in exploring unconventional symptoms beyond coughing and wheezing. Also, the impressive accuracy of self-reported symptoms highlights the value of daily self-monitoring using asthma diaries instead of relying on potentially flawed symptom recollections from several weeks previous. Predominantly negative emotions may indicate that those adolescents’ quality of life is compromised, which in turn could have negative impact on their coping with uncontrolled asthma, such as limiting activities and poor adherence to treatment as suggested in this study. In addition, high congruency between self-reports and medical records in short-term relief medications possibly indicates adolescents’ reliance on this type of medication in managing uncontrolled asthma instead of controller therapy for which we found a considerably lesser degree of agreement. Prescribed control medications that are unreported in the diaries are unlikely to be taken, thus raising a concern of suboptimal treatment adherence. Particularly, of those who had ICS prescriptions merely 50% reported the use of the medication, which is worrisome considering the uncontrolled nature of their asthma. Nurses’ vigilant follow-ups with adolescents can be beneficial to ensure the adequate treatment adherence in this population.
This study demonstrates that asthma diaries can be a useful tool in aiding in-depth and comprehensive understanding about uncontrolled asthma and its management. Having recognized the value, nurses need to encourage adolescents to keep the asthma diaries as a means to monitor symptoms, quality of life and treatment adherence. Adolescents are more likely to keep their diaries and consider it less of a burden when they receive timely feedback from clinicians (National Heart, Lung, and Blood Institute, 2007). Asthma diaries can potentially facilitate constructive communications between adolescent patients and their nurses. Specific understanding about an individual's characteristic symptom manifestations and quality of life gleaned from the asthma diary can allow nurses or other clinicians to efficiently assess the impact of asthma and customize their intervention efforts to the individual to adequately address the identified issues. As such, the tailored communications between adolescents and clinicians can lead to mutually agreed upon asthma management plans with which the adolescents are more likely to adhere.
Acknowledgement
We gratefully acknowledge the help and support from pediatric ED staff. We also thank the adolescent participants who provided the invaluable data for this study.
Funding
This study was supported by a grant from the National Institute of Health/National Institute for Nursing Research (NINR) R01NR011169 awarded to Hyekyun Rhee.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No conflict of interest has been declared by the authors.
Contributor Information
Hyekyun Rhee, University of Rochester, School of Nursing.
Eileen Fairbanks, University of Rochester, School of Nursing.
Arlene Butz, Johns Hopkins University, School of Medicine.
References
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United states, 2005-2009. 32. U.S. Department of Health and Human Services; Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- Akinbami LJ, LaFleur BJ, Schoendorf KC. Racial and income disparities in childhood asthma in united states. Discourse Processes. 2002;2(5):382–387. doi: 10.1367/1539-4409(2002)002<0382:raidic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- American Lung Association [February 24, 2011];Trends in asthma morbidity and mortality. 2010 from http://www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf.
- Barton C, Clarke D, Sulaiman N, Abramson M. Coping as a mediator of psychosocial impediments to optimal management and control of asthma. Respiratory Medicine. 2003;97(7):747–761. doi: 10.1016/s0954-6111(03)00029-5. [DOI] [PubMed] [Google Scholar]
- Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. Journal of Allergy and Clinical Immunology. 2008;122(3):490–495. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Butz AM, Tsoukleris M, Donithan M, Hsu VD, Mudd K, Zuckerman IH, Bollinger ME. Patterns of inhaled antiinflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118(6):2504–2513. doi: 10.1542/peds.2006-1630. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention [September 8, 2011];2009 national health interview survey (NHIS) data. 2011 from http://www.cdc.gov/asthma/nhis/09/data.htm.
- Couriel J. Asthma in adolescence. Paediatric Respiratory Reviews. 2003;4(1):47–54. doi: 10.1016/s1526-0542(02)00309-3. [DOI] [PubMed] [Google Scholar]
- Cruz-Correia R, Fonseca J, Lima L, Araujo L, Delgado L, Castel-Branco MG, Costa-Pereira A. Web-based or paper-based self-management tools for asthma--patients’ opinions and quality of data in a randomized crossover study. Studies in Health Technology and Informatics. 2007;127:178–189. [PubMed] [Google Scholar]
- Davis KJ, DiSantostefano R, Peden DB. Is johnny wheezing? parent-child agreement in the childhood asthma in america survey. Pediatric Allergy and Immunology. 2011;22(1-Part-I):31–35. doi: 10.1111/j.1399-3038.2010.01016.x. [DOI] [PubMed] [Google Scholar]
- de Benedictis D, Bush A. The challenge of asthma in adolescence. Pediatric Pulmonology. 2007;42(8):683–692. doi: 10.1002/ppul.20650. [DOI] [PubMed] [Google Scholar]
- Desai M, Oppenheimer JJ. Medication adherence in the asthmatic child and adolescent. Current Allergy and Asthma Reports. 2011;11(6):454–464. doi: 10.1007/s11882-011-0227-2. doi: 10.1007/s11882-011-0227-2; 10.1007/s11882-011-0227-2. [DOI] [PubMed] [Google Scholar]
- Drotar D, Bonner MS. Influences on adherence to pediatric asthma treatment: A review of correlates and predictors. Journal of Developmental & Behavioral Pediatrics. 2009;30(6):574–582. doi: 10.1097/DBP.0b013e3181c3c3bb. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge AL, Guilbert T, Spahn J, Peden D, Davis K. The influence of variation in type and pattern of symptoms on assessment in pediatric asthma. Pediatrics. 2006;118(2):619–625. doi: 10.1542/peds.2005-2963. [DOI] [PubMed] [Google Scholar]
- Halterman JS, Aligne CA, Auinger P, McBride JT, Szilagyi PG. Inadequate therapy for asthma among children in the united states. Pediatrics. 2000;105(1 Pt 3):272–276. [PubMed] [Google Scholar]
- Halterman JS, Auinger P, Conn KM, Lynch K, Yoos HL, Szilagyi PG. Inadequate therapy and poor symptom control among children with asthma: Findings from a multistate sample. Ambulatory Pediatrics. 2007;7(2):153–159. doi: 10.1016/j.ambp.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Hensley MJ, Chalmers A, Clover K, Gibson PG, Toneguzzi R, Lewis PR. Symptoms of asthma: Comparison of a parent-completed retrospective questionnaire with a prospective daily symptom diary. Pediatric Pulmonology. 2003;36(6):509–513. doi: 10.1002/ppul.10360. [DOI] [PubMed] [Google Scholar]
- Johnson JE. Self-regulation theory and coping with physical illness. Research in Nursing & Health. 1999;22:435–448. doi: 10.1002/(sici)1098-240x(199912)22:6<435::aid-nur2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lahaye M, Fantini-Hauwel C, Van Broeck N, Bodart E, Luminet O. Emotional competence and quality of life of children with asthma: The mediating effect of coping strategies. Psychology & Health. 2011;26(12):1678–1695. doi: 10.1080/08870446.2011.562606. [DOI] [PubMed] [Google Scholar]
- National Heart, Lung, and Blood Institute . Expert panel report 3: Guidelines for the diagnosis and management of asthma. Bethesda, MD: 2007. [Google Scholar]
- Newacheck PW, Halfon N. Prevalence, impact, and trends in childhood disability due to asthma. Archives of Pediatrics & Adolescent Medicine. 2000;154(3):287–293. doi: 10.1001/archpedi.154.3.287. [DOI] [PubMed] [Google Scholar]
- Orrell-Valente JK, Jarlsberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own? Pediatrics. 2008;122(6):e1186–92. doi: 10.1542/peds.2008-0292. [DOI] [PubMed] [Google Scholar]
- Reznik M, Sharif I, Ozuah PO. Classifying asthma severity: Prospective symptom diary or retrospective symptom recall? Journal of Adolescent Health. 2005;36(6):537–538. doi: 10.1016/j.jadohealth.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Rhee H, Belyea MJ, Cirzynski S, Brasch J. Barriers to asthma self-management in adolescents: Relationships to psychosocial factors. Pediatric Pulmonology. 2009;44(2):183–191. doi: 10.1002/ppul.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: A focus group study. Journal of Pediatric Health Care. 2007;21(2):99–107. doi: 10.1016/j.pedhc.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Richardson LP, Lozano P, Russo J, McCauley E, Bush T, Katon W. Asthma symptom burden: Relationship to asthma severity and anxiety and depression symptoms. Pediatrics. 2006;118(3):1042–1051. doi: 10.1542/peds.2006-0249. [DOI] [PubMed] [Google Scholar]
- Rietveld S, Rijssenbeek-Nouwens LHM. Diagnostics of spontaneous cough in childhood asthma: Results of continuous tracheal sound recording in the homes of children. Chest. 1998;113(1):50–54. doi: 10.1378/chest.113.1.50. [DOI] [PubMed] [Google Scholar]
- Tibosch MM, Verhaak CM, Merkus PJFM. Psychological characteristics associated with the onset and course of asthma in children and adolescents: A systematic review of longitudinal effects. Patient Education and Counseling. 2011;82(1):11–19. doi: 10.1016/j.pec.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Warman KL, Silver EJ, Stein RE. Asthma symptoms, morbidity, and antiinflammatory use in inner-city children. Pediatrics. 2001;108(2):277–282. doi: 10.1542/peds.108.2.277. [DOI] [PubMed] [Google Scholar]
- Wildhaber J, Carroll WD, Brand PLP. Global impact of asthma on children and adolescents’ daily lives: The room to breathe survey. Pediatric Pulmonology. 2012;47(4):346–357. doi: 10.1002/ppul.21557. [DOI] [PubMed] [Google Scholar]
- Yoos HL, Kitzman H, McMullen A, Sidora-Arcoleo K, Anson E. The language of breathlessness: do families and health care providers speak the same language when describing asthma symptoms? Journal of Pediatric Health Care. 2005;19(4):197–205. doi: 10.1016/j.pedhc.2005.01.010. [DOI] [PubMed] [Google Scholar]