Abstract
The purpose of this study was to examine predictors of lymph node metastases (LN+) or extrauterine disease (ED) in low grade (FIGO grades 1 or 2) endometrioid carcinoma (LGEC) in a multi institutional setting. For LGEC with and without LNM or ED, each of the 9 participating institutions evaluated patients age, tumor size, myometrial invasion (MI), FIGO grade, % solid component, the presence or absence of papillary architecture, microcystic elongated and fragmented glands (MELF) and single cell/cell cluster invasion (SCI), lymphovascular invasion (LVI), lower uterine segment (LUS) and cervical stromal (CX) involvement and numbers of pelvic (PLN) and para-aortic (PALN) LNs sampled.302 cases were reviewed: LN+ or ED +, 96; LN-/ED-, 208. Patients' ages ranged from 23-91 yrs (median 61). Table 1 summarizes the histopathologic variables that were noted for the LN+ or ED+ group: tumor size ≥2cm, 93/96 (97%), MI >50%, 54/96 (56%), MELF, 67/96 (70%), SCI, 33/96 (34%), LVI, 79/96 (82%), >20% solid, 65/96 (68%), papillary architecture present, 68/96 (72%), LUS involved, 64/96 (67%) and CX involved, 31/96 (32%). For the LN-/ED- group, the results were as follows: tumor size ≥2cm, 152/208 (73%), MI >50%, 56/208 (27%), MELF, 79/208 (38%), single cell invasion, 19/208 (9%) , LVI, 56/208 (27%), >20% solid, 160/208 (77%), papillary architecture present, 122/208 (59%), LUS involved, 77/208 (37%), CX involved, 31/208 (15%). There was no evidence of a difference in the number of pelvic or para-aortic LNs sampled between groups (p=0.9 and 0.1, respectively). Following multivariate analysis, depth of myometrial invasion, cervical stromal involvement, lymphovascular space invasion, and the single cell pattern of invasion emerged as significant predictors of advanced stage disease. Although univariate analysis pointed to LUS involvement, MELF pattern of invasion, and papillary architecture as possible predictors of advanced stage disease, these were not shown to be significant by multivariate analysis. This study validates MI, CX involvement and LV as significant predictors of LN+ or ED. The association of SCI pattern with advanced stage LGEC is a novel finding.
Keywords: low-grade, endometrial, endometrioid, adenocarcinoma, myometrium, invasion, risk factors, lymph node, metastasis, recurrence
Introduction
Endometrial adenocarcinoma is the most common gynecologic malignancy with approximately 47,000 estimated new cases in 2012 (1). Most of these cases are low grade, low stage endometrioid adenocarcinomas (2, 3). Five to 18% of clinical stage I, low grade endometrioid adenocarcinoma may harbor a lymph node metastasis or involvement of other extrauterine sites (4-10). The low incidence of advanced stage disease in cases of low grade endometrioid adenocarcinoma has prompted a debate over the role of lymph node dissection in this setting (2-4,6,7, 11, 12) as well as studies seeking to define the subset of patients with low grade endometrioid adenocarcinoma who could most benefit from surgical staging (13-15). Factors that have been traditionally used to predict advanced stage in cases of clinical stage I endometrioid adenocarcinoma include tumor grade and depth of myometrial invasion (4, 5). In recent years, other factors including tumor size (14), lower uterine segment involvement (16), cervical involvement (17), vascular/lymphatic invasion (17,18), revisions to the 3-tiered architectural grading system (19, 20) and the pattern of myometrial invasion (21, 22) have been proposed as potential predictive indicators of extrauterine disease. In this study, we assessed the relationship of the above mentioned predictive factors to the presence of advanced stage disease in a cohort of 304 cases of low grade (FIGO grades 1 and 2) endometrial endometrioid adenocarcinoma.
Materials and Methods
This multi-institutional study encompassed cases from nine tertiary care centers from four countries, Korea, Mexico, Canada and the United States of America. Institutional Review Board (IRB) approval was obtained prior the initiation of the study. Each of the 9 participating institutions identified cases of FIGO grades 1 or 2 endometrioid, endometrial adenocarcinoma with metastases to the lymph nodes or extra uterine sites at presentation, which were treated by robotic, laparoscopic or abdominal hysterectomy over a 20 year time span from 1991 to 2011 and available follow up ranging from 1 to 239 months. All identified cases had histologic material available for review. All FIGO stages were allowed; however, cases with tumor in the ovary, fallopian tube or peritoneum associated with endometriosis and/or with a uterine tumor lacking myometrial or cervical stromal invasion were excluded as probable synchronous primaries. One to two cases of FIGO grade 1 or 2, stage I or II endometrioid adenocarcinoma with negative lymph nodes were chosen sequentially with each study case and served as the control group. The control group had no evidence of peritoneal disease or adnexal spread at the time of surgery or in follow up. Using the same criteria as for the study group, cases with synchronous primary endometrioid adenocarcinoma of the ovary, fallopian tube or peritoneum were excluded. For both the study and control groups, evidence of concomitant serous, clear cell, undifferentiated or sarcomatous components resulted in exclusion of the case from the study. Clinical and gross pathologic data were obtained from the patients’ charts and pathology reports with age, date of surgery, tumor size, presence/absence of lower uterine segment involvement, presence/absence of cervical stromal involvement, and number of lymph nodes sampled recorded in each case. Multiple representative cases from each contributing facility were reviewed with the entire group in order to achieve consensus on how the different variables would be assessed. For cases not reviewed with the entire group, slides were reviewed by at least two of the authors.
All cases and controls were reviewed using the standard FIGO grading system (23): grade 1, up to 5% solid, non-squamous component and grade 2, 6-50% solid, non-squamous component. Tumors with >50% solid non-squamous component (FIGO grade 3) were excluded. For cases with ≤5% solid architecture, the solid component was represented by tangentially cut glands. Trabecular areas or solid nests accounted for a true solid component and were distinguished from squamous differentiation by the cells’ resemblance to those lining the open glandular spaces. Areas of squamous differentiation were recognized by either bland spindle cells without obvious keratinization (morular) or polygonal to spindled cells exhibiting obvious keratinization. The percentage of the tumor’s solid component was noted, and tumors were then secondarily grouped into those with 20% or less solid architecture and those tumors with more than 20% of a solid component. Papillary architecture was defined by the presence of papillary structures with or without a fibroconnective tissue core lined by cells with grade 1 or 2 cytologic atypia. Tumors containing irregular papillae with occasional epithelial buds were included as long as the nuclear grade did not exceed 2. Depth of myometrial invasion was recorded in all cases as a percentage of the myometrial thickness. Attention was paid to the pattern of myometrial invasion, specifically whether the microcystic, elongated and fragmented (MELF) pattern (24) was present. Characteristics of the MELF pattern included the presence of small dilated glands lined by cuboidal or flattened cells with eosinophilic cytoplasm. Invasive glands often had a slit-like appearance. Lining cells could also have a squamoid appearance, and occasional intraluminal tufting of cells was seen. This invasive pattern typically had a myxoid to granulation-like reaction in the surrounding myometrium. Because some group members noted occasional cases in which single cells invaded the myometrium without gland formation or other features identified with the MELF pattern, the presence of single cell or small groups of cells (SCI) as an invasive component in the myometrium was considered separately. SCI was recognized by single cells or groups of eosinophilic cells without formation of a defined structure frequently lying in an edematous or myxoid background. Vascular/lymphatic invasion was defined by the presence of tumor fragments within endothelial-lined vascular/lymphatic spaces either within the tumor or away from it. Efforts to exclude possible retraction artifact or possible pseudovascular invasion related to use of the uterine manipulator in laparoscopic or robotically obtained specimens were made. Immunohistochemistry to demonstrate an endothelial lining in cases of suspected lymphovascular invasion was not required.
Univariate and multivariate logistic regression models were used to assess the relationship between pathologic factors and the outcomes of interest. Observations were excluded for unknown tumor size and lymph nodes (LN) not sampled. Unknown lower uterine involvement was grouped with no uterine involvement to be consistent with the coding methods for other variables. All statistical analyses were performed using SAS 9.2 for Windows.
Results
A total of 304 patients ranging from 23-91 years old (median, 61 years) were included in the study distributed as follows: lymph node metastases with or without extrauterine disease, 77 (25.3%) patients; extrauterine disease without lymph node metastases, 19 (6.3%) patients; and no lymph node metastases or extrauterine disease, 208 (68.4%) patients. For the patients with advanced stage disease (study group, n=96), tumor size ranged from 1.5 to 13 cm (mean 5.0 cm +/- 2.7 cm). For the patients with FIGO stage I/II disease (control group, n=208), tumor size ranged from 0.2 to 9 cm (mean 3.5 cm +/- 2.1 cm). Tumor size was unknown for 12 patients. For the 296 patients in which tumor size was known, 47 (15.9%) had tumors less than 2.0 cm in greatest dimension. Only two such tumors were from patients with advanced stage disease. One patient had a fallopian tube metastasis and the other patient had a positive pelvic lymph node. Sixty-four (67%) study patients had lower uterine segment involvement with unknown lower uterine segment status in 7 (7%) patients. In the control group, 77 (37%) patients had lower uterine segment involvement with unknown lower uterine segment status in 34 (16%) patients. Fifty-one study patients had involvement of the cervix: cervical stromal invasion, 41 (43%); cervical gland involvement only, 10 (10%). In the control group, 24 (12%) patients had cervical stromal involvement. Cervical glandular involvement was noted in 11 (5%) patients. All study and control cases had lymph node sampling of at least one station. There was no significant difference in the total number of pelvic or para-aortic lymph nodes sampled between patients with negative lymph nodes and those with positive lymph nodes (p=0.09, 0.10). Pelvic lymph nodes were sampled in 85 (88.5%) study cases with 1-67 pelvic lymph nodes (mean, 13.4 +/- 13.1) per patient sampled for a total of 1142 pelvic lymph nodes. Of these, 174 (15%) lymph nodes harbored a metastasis. Fifty (52%) study patients had from 1-42 (mean 8.6 +/-9.4) para-aortic lymph nodes sampled for a total of 431 lymph nodes. A metastasis was identified in sixty-six (15%) of these lymph nodes. Only two study patients had positive para-aortic lymph nodes in the setting of histologically proven negative pelvic lymph nodes. Four patients with positive para-aortic lymph nodes did not have sampling of the pelvic lymph nodes. Overall, 66 cases (16 study group, 50 control group) were classified as FIGO grade 1 endometrioid adenocarcinoma, and 238 cases (80 study group; 158 control group) were classified as FIGO grade 2 endometrioid adenocarcinoma. The percent solid component of the endometrial tumors ranged from 0-45% in both the study and control groups with 65 (68%) having >20% solid component in the study group and 160 (77%) having >20% solid component in the control group. Papillary architecture was observed at least focally in over half of the cases in the study and control groups: 68 (72%), study group and 122 (59%) control group. The depth of myometrial invasion ranged from 0-100% in the study group: 0-25%, 15 cases; 26-50%, 27 cases; 51-75%, 20 cases; >75%, 34 cases. One study patient had no myometrial invasion, but had a focus of metastatic carcinoma in the fallopian tube. For the control group, the depth of myometrial invasion ranged from 0-100%: 0-25%, 106 cases; 26-50%, 44 cases; 51-75%, 41 cases; >75%, 16 cases. MELF pattern within the myoinvasive component was observed in 146/304 (48%) patients: 67/96 (69.8%) study patients and 79/208 (38%) control patients. SCI was present in 52 of 304 patients (17%): 33/96 (34.4 %) study patients and 19/208 (9.1%) control patients. When SCI pattern of myometrial invasion was noted, MELF was also present 79% of the time (41 of 52 cases). However, the MELF pattern of myometrial invasion had no associated SCI in 105 of 146 (72%) cases. Lymphovascular invasion was observed in 135 (44%): 79/96 (82%) study patients and 56/208 (27%) control patients. A summary of histologic variables is shown in Table 1. Examples of histologic features observed in the study patients are shown in Figures 1 and 2.
Table 1. Summary of Histologic Variables in Cases with and without Lymph node Metastases or Extrauterine Disease.
| LN+ or ED+ (n=96) | LN-/ED- (n=208) | |
|---|---|---|
| Variable | Count (%) | Count (%) |
| Tumor ≥ 2.0 cm | 93 (97) | 152 (73) |
| Lower uterine segment involvement | 64 (67) | 77 (37) |
| Cervical stromal involvement | 41 (43) | 24 (12) |
| >20% Solid | 65 (68) | 160 (77) |
| Papillary architecture present | 68 (72) | 122 (59) |
| >50% Myoinvasion | 54 (56) | 56 (27) |
| MELF pattern invasion present | 67 (70) | 79 (38) |
| Single cell invasion present | 33 (34) | 19 (9) |
| Lymphovascular invasion present | 79 (82) | 56 (27) |
Figure 1.
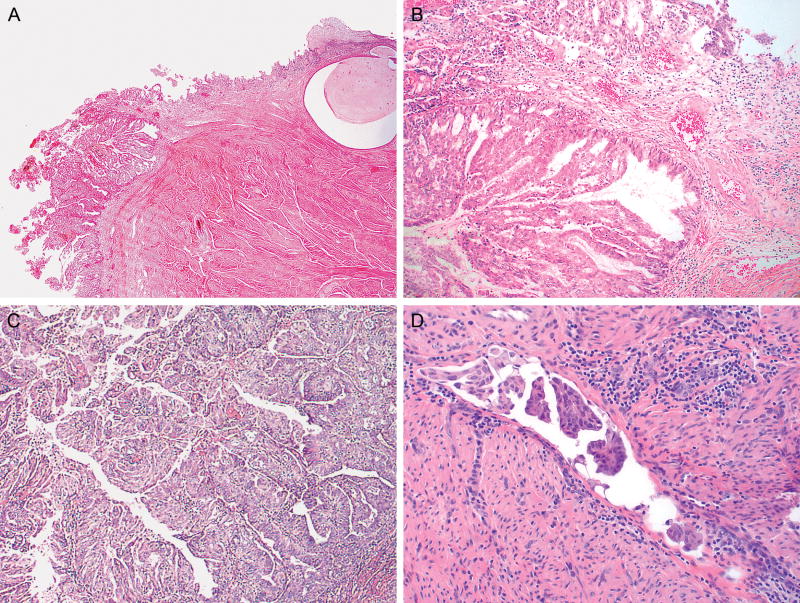
A) Endometrioid adenocarcinoma involving the endocervical glands and stroma (H&E, 2x); B) Higher power image of A depicting cervical stromal involvement (H&E, 10x); C) Example of papillary architecture observed in some cases of endometrioid adenocarcinoma (H&E, 10x); D) Lymphovascular invasion (H&E, 40x)
Figure 2.
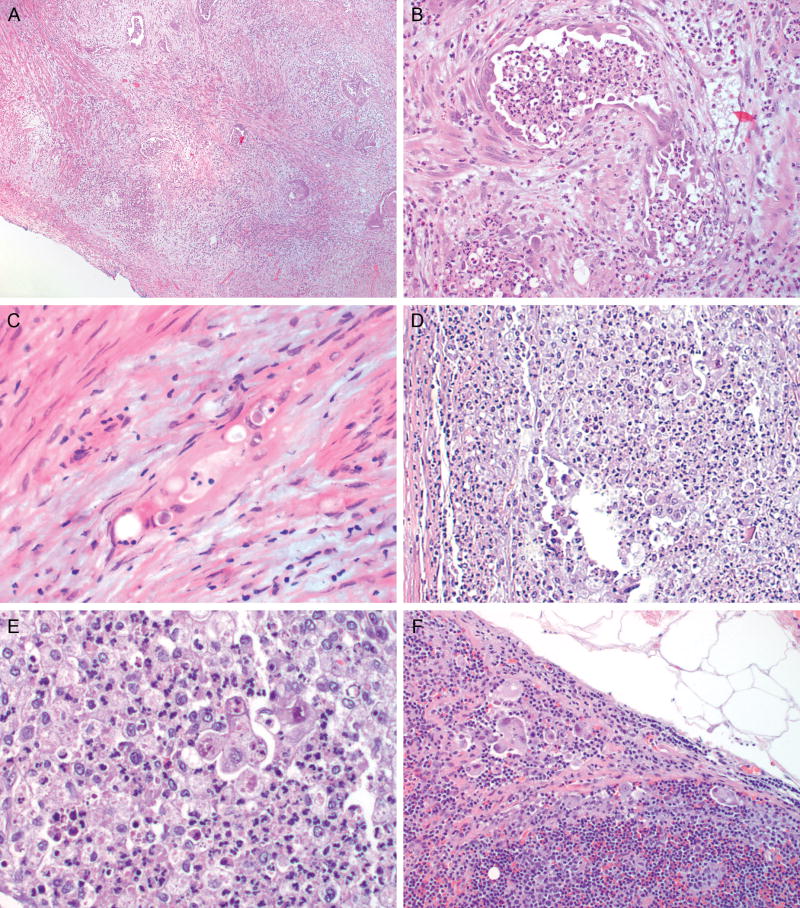
A) Low power image of deeply invasive endometrial, endometrioid adenocarcinoma, MELF pattern; note the loose, myxoid-appearing reaction surrounding the individual glands (H&E, 2x); B) Higher power image of A depicting an angulated gland with focal attenuation of the epithelial lining and an associated mixed inflammatory response (H&E, 20x); C), Compressed, microcystic gland infiltrating between myometrial fibers (H&E, 40x); D) Small cell clusters and single epithelial cells surrounded by a mixed inflammatory response (H&E, 10x); E) Higher power image of D demonstrating a small epithelial cell cluster (H&E, 40x); F) Lymph node metastasis: cells similar to those seen in D and E are present in the subcapsular cortex of a lymph node (H&E, 20x)
Preliminary work constructed univariate logistic regression analysis for lymph node metastasis alone and extra uterine disease alone. In both analyses, lymphovascular invasion and presence of cervical involvement were identified as potential predictors of the outcome modeled (lymph node metastasis only and extra uterine disease only). Additional variables identified in the analysis looking only at the patients with lymph node metastases identified additional predictive variables including tumor size ≥ 2.0 cm, depth of myometrial invasion as a continuous variable in 10% increments, presence of > 50% myometrial invasion, presence of papillary architecture, MELF pattern of invasion, SCI, lymphovascular invasion, lower uterine segment involvement, cervical stromal involvement and the number of para-aortic lymph nodes sampled. Because the sample size of the cases with extra uterine disease is small, it cannot be determined with certainty whether other variables could become important predictors were more cases to be added to this group.
When the two outcomes were combined into a single univariate logistic regression analysis modeling lymph node metastases or extrauterine disease, the analysis is very similar to that modeling the outcome of lymph node metastasis alone pointing to several possible variables predictive of advanced stage disease in FIGO I/II endometrioid adenocarcinoma including tumor size ≥ 2.0 cm, depth of myometrial invasion as a continuous variable in 10% increments, presence of > 50% myometrial invasion, presence of papillary architecture, MELF pattern of invasion, SCI, lymphovascular invasion, lower uterine segment involvement, cervical stromal involvement and the number of para-aortic lymph nodes sampled. In light of recent changes in the staging of endometrial carcinoma, it is interesting to note that endocervical glandular involvement alone was not found to be a significant predictor of lymph node metastasis in the univariate model. The results of these univariate analyses modeling lymph node metastases or extra uterine disease are summarized in Table 2.
Table 2. Univariate Logistic Regression Results Modeling Lymph Node Metastases or Extrauterine Disease.
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Age at diagnosis (continuous) | 0.99 (0.97 – 1.00) | 0.2718 |
| Tumor ≥ 2.0 cm | 13.77 (3.26 – 58.09) | 0.0004 |
| FIGO grade 2 vs 1 | 1.58 (0.85 – 2.95) | 0.1496 |
| Lower uterine segment involvement | 3.40 (2.05 – 2.66) | <0.0001 |
| Cervical stromal involvement | 5.72 (3.18 – 10.28) | <0.0001 |
| #Pelvic lymph nodes | 1.02 (1.00 – 1.05) | 0.0945 |
| #Para aortic lymph nodes | 1.09 (1.03 – 1.15) | 0.0045 |
| % Solid component (continuous) | 1.02 (1.00 – 1.04) | 0.0592 |
| >20% Solid component | 1.59 (0.93 – 2.72) | 0.0899 |
| Papillary architecture present | 1.80 (1.07 – 3.04) | 0.0276 |
| %Myometrial invasion (per 10%) | 1.36 (1.24 – 1.49) | <0.0001 |
| >50% Myometrial invasion | 3.49 (2.10 – 5.79) | <0.0001 |
| MELF pattern invasion present | 3.77 (2.25 – 6.33) | <0.0001 |
| Single cell invasion present | 5.21 (2.77 – 9.81) | <0.0001 |
| Lymphovascular invasion | 12.61 (6.87 – 23.15) | <0.0001 |
Based on the results of the univariate analysis, a multivariate regression analysis was constructed utilizing the variables predictive of lymph node metastases including tumor size (continuous variable), percent myometrial invasion (continuous variable, per 10%), percent solid component (continuous variable, per 1%), presence of MELF pattern of invasion, presence of SCI, presence of lymphovascular invasion, presence of lower uterine segment involvement, and presence of cervical stromal invasion. In constructing the multivariate model, continuous variables were chosen for statistical reasons. The number of pelvic and para-aortic lymph nodes sampled was not included as a variable in the multivariate model since too many patients had missing values for these variables and number of pelvic lymph nodes sampled was not significant in the univariate results. In addition, papillary architecture was not considered in the multivariate model since it had a very high correlation with myometrial invasion and myometrial invasion was more objectively reproducible. Of these variables, the percentage of myometrial invasion, presence of SCI, presence of lymphovascular invasion, and presence of cervical stromal involvement were independently predictive of lymph node metastases or extra uterine disease based on this model. These results are summarized in Table 3.
Table 3. Multivariate Logistic Regression Results Modeling Lymph Node Metastases or Extrauterine Disease.
| Variable | Odds Ratio (95% CI) | p-value |
| Tumor (per cm) | 1.10 (0.97 – 1.24) | 0.1505 |
| Lower uterine segment involvement | 1.92 (0.96 – 3.87) | 0.067 |
| Cervical stromal involvement | 3.15 (1.12 – 8.35) | 0.0211 |
| % Solid component (continuous, per 1%) | 0.99 (0.97 – 1.02) | 0.6704 |
| %Myometrial invasion (per 10%) | 1.15 (1.02 – 1.30) | 0.0280 |
| MELF pattern invasion present | 1.34 (0.64 – 2.82) | 0.4422 |
| Single cell invasion present | 3.46 (1.56 – 7.67) | 0.0022 |
| Lymphovascular invasion | 4.92 (2.37 – 10.19) | <.0001 |
Discussion
Based in part on earlier work suggesting that lymph node metastases correlated with other important prognostic factors in endometrial carcinoma (4), the International Federation of Gynecology and Obstetrics (FIGO) recommended surgical staging to include sampling of regional lymph nodes over clinical staging alone (23). Indeed, lymph nodes are among the most common metastatic sites in endometrial carcinoma yet the incidence of lymph node metastases in patients with FIGO grade 1 or 2 endometrial, endometrioid adenocarcinoma is low ranging from 5-18% (4-10). Although the benefit of lymphadenectomy in endometrial carcinoma continues to be debated, knowledge of lymph node status can provide important prognostic information and the opportunity to identify patients requiring adjuvant therapy. The challenge has been how to best identify patients with low grade endometrioid adenocarcinoma at greatest risk of harboring a lymph node metastasis in order to avoid over treatment of the majority of women with this disease. Most studies to date have looked at only one to a few risk factors and have been single institution studies. This multi-institutional study represents the largest contemporary series addressing the issue of predictor factors of advanced stage disease in cases of low grade endometrial endometrioid adenocarcinoma.
Although the univariate analysis confirmed previously reported findings of tumor size ≥2cm, myometrial invasion >50%, MELF pattern of invasion, lymphovascular space invasion, lower uterine segment involvement and cervical stromal involvement as predictors of lymph node metastases (4, 14-18, 21), only the percentage of myometrial invasion, cervical stromal involvement and lymphovascular space invasion were found to be independently significantly predictive in the multivariate model. The univariate model also pointed to papillary architecture, SCI pattern of myometrial invasion and the number of para-aortic lymph nodes sampled as potential predictors of lymph node metastases. Of these, only SCI was significantly associated with outcome after adjusting for the other factors in the multivariate model. Other variables investigated in the study, including patient age, FIGO grade 1 versus 2, the percentage of solid architecture, and the number of pelvic lymph nodes obtained, were not found to be significant predictors of advanced stage disease in the univariate models although the percentage of solid architecture approached significance (p=0.0527).
Little has been written about the association of age and advanced stage disease. Older series have pointed to increased age as a risk factor for poor outcome (24, 25), but these studies did not address the relationship of age to the presence of advanced stage disease. A recent study assessing nodal metastasis risk utilizing triage criteria proposed by the Mayo Clinic (14, 26) found no significant age difference between patients with and without lymph node metastases. The present study is in agreement and supports exclusion of age as a factor in the decision whether or not to surgically stage a patient.
Tumor grade in endometrial, endometrioid adenocarcinoma is typically included with staging information because grade correlates with surgical stage and ultimately prognosis (4, 5, 19) with the impact most apparent in grade 3 tumors. Later studies have grouped FIGO grade 1 and 2 tumors as a “low grade” entity in order to stratify risk with respect to lymph node metastases (14, 26), and one study showed no survival differences between grade 1 and grade 2 endometrial tumors (27). Furthermore, it has been shown that binary grading systems have improved inter and intraobserver agreement (19, 20). This study specifically excluded grade 3 endometrioid adenocarcinoma to determine whether a group with increased risk of advanced stage disease could be identified within what is conceptualized as a low risk category overall. This study found no difference in the risk of lymph node metastases or extrauterine disease between FIGO grade 1 and 2 tumors. However, FIGO grade 2 tumors represent the majority of cases in this study begging the question whether a FIGO grade 2 tumor with a lower percentage of solid non-squamous architecture would have less risk of lymph node metastases than a tumor with a percentage of solid, non squamous architecture approaching 50%. One group of investigators tested this approach using a 20% threshold and found that this threshold was exceeded in all patients who recurred (19). Although this study did not find increased odds of advanced stage disease in tumors with >20% solid architecture, the absence of difference between grade 1 and 2 in the current grading system in combination with previously published work suggests that a revision from a three-tier to a two-tier grading system should be seriously considered.
Debating the role of lymph node dissection in cases of low grade endometrial, endometrioid adenocarcinoma is beyond the scope of this study, but intuitively it seems that increased numbers of lymph nodes sampled should lead to increased detection of lymph node metastases. To that end, some have proposed a target number of lymph nodes to be sampled for optimal detection (28), and one study reported that the number of pelvic lymph nodes sampled was a factor increasing the detection of lymph node metastases (6). A later study demonstrated that the absolute lymph node count did not accurately predict the presence of lymph node metastases (8). To address whether patients with lymph node metastases were identified based on increased number of lymph nodes sampled, we compared sampling of pelvic and para aortic lymph nodes between the study and control groups. No significant differences in the number of pelvic or para aortic lymph nodes sampled between low and advanced stage groups (p=0.10 and 0.64, respectively) were identified. While the univariate model did show increased odds of the presence of a lymph node metastasis with increasing number of para-aortic lymph nodes sampled, this finding was not true for the number of pelvic lymph nodes sampled. Between the study and control patient groups there were too many missing values for numbers of lymph nodes because not every patient underwent both pelvic and para-aortic lymph node dissections. For this reason as well as the absence of significance for number of pelvic lymph nodes, these variables were excluded from the multivariate analysis. Variability with respect to individual patients and surgeons may limit the ability to ultimately assess whether an “adequate” lymph node dissection with no evidence of metastatic carcinoma is reassuring.
The univariate model pointed to both traditional and non traditional features as potential predictors of advanced stage disease, some of which could not be confirmed as independent variables on the multivariate analysis. One such variable that has been given recent attention due to its utilization as part of a staging protocol (14) is tumor size ≥2.0 cm. Although a greater percentage of patients in the study group had tumors ≥2.0 cm compared to the control group, it should be noted that for both groups, tumor size <2.0 cm was relatively uncommon. Overall only 47 patients had tumors less then 2.0 cm. Of these, 2 (2.1%) were patients with advanced stage disease and 1 of these patients had >50% myometrial invasion. Since the criterion of <50% myometrial invasion was not met, this patient would have been staged under the Mayo protocol. This suggests that tumor size did not add any information that could not be provided by another variable. While this variable may be useful in combination with others, this study does not support tumor size as the sole determinant in deciding whether or not to proceed with staging.
Another proposed predictor of lymph node metastases not supported by multivariate analysis in this study is the presence of lower uterine segment involvement. One study including all grades and histotypes of endometrial adenocarcinoma found lower uterine segment involvement to be significantly associated with lymph node metastases on multivariate analysis in patients with endometrioid histology (16). The number of patients in this study that also had endocervical involvement was not reported. Significantly, our study could not confirm the association of lower uterine segment involvement with advanced stage disease by multivariate analysis when cervical stromal involvement was included in the model. A closer look at our raw data shows that 40 of the 64 (62.5%) study patients with lower uterine segment involvement also had either endocervical gland (9/24) or stromal involvement (31/64) while 24 of the 77 (31%) control patients with documented lower uterine segment involvement had either endocervical gland or stromal involvement. This suggests that the presence of lower uterine segment involvement could be an indicator of possible endocervical involvement rather than a significant predictor of advanced stage disease.
One potential predictor of lymph node metastasis not included in the multivariate analysis was the presence of papillary architecture. The largest paper on this subject reported that the prognosis of endometrioid adenocarcinoma with papillary or villoglandular architecture was similar to endometrioid adenocarcinoma with glandular architecture (29). However, other investigators suggested that the incidence of lymphovascular invasion and lymph node metastases was higher when a villoglandular pattern was observed in the myoinvasive component of a low grade endometrial, endometrioid adenocarcinoma (30), and unpublished observations of one group member are in concordance with the latter opinion. Our study examined only the presence or absence of papillary architecture, and did not specifically address papillary architecture in the myoinvasive component. In reviewing the slides for this study, there was some variability between group members with respect to what constituted papillary architecture and whether intraglandular papillary formation should be included. Additionally, it appeared that papillary architecture correlated with the percent of myometrial invasion. Because the percent of myometrial invasion was more easily reproduced and was established in the literature as a predictor, this variable was chosen for inclusion in the multivariate model over papillary architecture. The finding that this architectural pattern could be a predictor of more aggressive behavior on the univariate analysis requires further study to determine the relationship of papillary architecture to advanced stage disease and potentially outcome.
Of the significant predictors of lymph node metastasis or extrauterine disease identified on multivariate analysis, myometrial invasion, cervical stromal invasion and lymphovascular invasion are factors used to guide clinical management evidenced by their inclusion on national and international cancer reporting checklists (31). Additionally myometrial and cervical stromal invasion establish the FIGO stage of the patient (32). Most studies of cervical stromal involvement and lymphovascular space invasion relate these features to outcome and recurrence with relatively few studies relating these features specifically to the odds of lymph node metastases or extra uterine disease. Two studies that included all grades of endometrial carcinoma found that both lymphovascular invasion and cervical stromal invasion are independent prognostic factors for lymph node metastases (17, 33). Our study confirms these findings even with the exclusion of high grade adenocarcinoma. In a real time setting such as frozen section, the utility of lymphovascular invasion or cervical stromal invasion is yet to be determined. Until that time, it would be reasonable to submit a section of cervix if there was suspicion of involvement as well as to report vascular invasion if it were observed at the time of intra operative evaluation. Post surgery, these features could be useful in determining the need for adjuvant therapy in the unstaged patient. More has been written about the relationship of deep myometrial invasion to the odds of advanced stage disease (4, 5, 14-16). Although the odds are greatest in the setting of high grade endometrioid adenocarcinoma (FIGO grade 3) and outer myometrial invasion, deeply invasive FIGO grade 2 endometrioid adenocarcinoma remains predictive of pelvic and para aortic lymph node metastases in 19% and 14% of patients respectively (5). This study specifically excluded FIGO grade 3 endometrioid and non endometrioid adenocarcinoma, which confirms and underscores the significance of myometrial invasion even within the context of so-called low grade endometrioid adenocarcinoma. This is important since myometrial invasion is a parameter that can be assessed intra operatively and guide the decision whether to perform staging. Although earlier studies question the reliability of intra operative assessment of myometrial invasion (34), recent studies have shown high frozen/permanent section concordance with respect to myometrial invasion (35, 36).
Following publication of the Gynecologic Oncology Group study demonstrating the relationship of myometrial invasion to extrauterine disease (5), two studies demonstrated a relationship between a diffuse pattern of myometrial invasion and worse patient outcome (25, 37). Although not fully characterized in either study, both noted unusual features in some infiltrating glands including a mixed inflammatory response in the myoinvasive component (37) and some glands with flattened epithelium mimicking endothelial cells (25). Both studies provide images bearing a striking resemblance to a distinct myoinvasive pattern subsequently characterized by Marshall, et al (38) and Murray, et al (39). Marshall, et al reported in a 2003 abstract the existence of a myoinvasive subtype composed of attenuated epithelium and single cells, which was frequently associated with lymphovascular invasion. Murray, et al further observed that glands with these features frequently evoke a prominent fibromyxoid stromal reaction and coined the acronym MELF (microcystic, elongated, and fragmented glands) to describe the findings in the glandular component. This study suggested that the glandular changes were possibly of a degenerative nature, but that when accompanied by an associated fibromyxoid stromal response was associated with lymphovascular invasion and a worse long term prognosis. Stewart, et al, confirmed the association of MELF pattern with lymphovascular invasion and observed that the pattern was typically associated with FIGO grade 1 or 2 endometrioid adenocarcinoma usually present along the deepest point of invasion (40). In contrast to Murray, et al, this study proposed that the changes observed in the MELF pattern represent a tumor/stromal response. Stewart and Little subsequently observed that tumors with MELF-type invasion had reduced hormone receptor and E-cadherin expression compared to areas of conventional-type invasion within the same tumor, features which are shared by epithelial-mesenchymal transition in carcinomas at other sites (41). The changes associated with epithelial-mesenchymal transition reportedly allow infiltration into the surrounding stroma and could potentiate tumor progression.
Based on these studies and others linking the pattern of myometrial invasion to patient outcome, this variable was included in the present study which focused on the presence of advanced stage disease rather than patient outcome. Modeled after one study (22), patterns of myometrial invasion such as groups of glands and single glands in addition to the MELF pattern were included in the initial case analysis. During the course of the study, consensus on the distinction of small gland groups from single glands and the percentage of each pattern could not be reached in all cases. Therefore, these categories were abandoned in favor of focusing solely on MELF and a second, single cell pattern of invasion (SCI), both of which were readily identified by all members due to the prominent stromal response typically associated with these patterns. While previous studies have included single cell invasion within the spectrum of MELF-associated changes (21, 22, 39, 40), the decision to separate the patterns was based on some in our group observing SCI outside the context of MELF. Because not all slides were available in every case and number of foci per slide was difficult to quantify, only the presence or absence of MELF or SCI was noted. The overall incidence of MELF in our study was 48%. This is higher than the reported frequency of MELF which ranges from 7-44% (21, 22, 39, 40). The higher frequency in our study could be due, in part, to a non consecutive review. The study was limited to patients who had undergone lymph node sampling potentially leading to a bias towards cases perceived to be more aggressive preoperatively. Although the amount of MELF was not quantified, it was noted to comprise ≥ 50% of the myoinvasive component in 58 of 146 cases that had MELF. However, similar to Stewart, MELF could be focal (40). As cases with focal and widespread MELF were combined into a single category, it is possible that this also contributed to our observation of this finding in a higher percentage of cases. In most cases, in agreement with Stewart, MELF was observed along the leading aspect of invasion. Overall, 52 (17%) cases in this study had SCI. There appears to be a relationship between MELF and SCI. The majority of cases with SCI also had MELF (41/52, 78.8%) although the converse was not true. The majority of cases with MELF (105/146, 71.9%) did not have SCI. It is possible that SCI could represent an evolving, more aggressive variant of MELF. However, there were 11 cases in which SCI was seen without MELF raising the possibility that this pattern could also develop outside the spectrum of MELF although a sampling issue cannot be excluded. In the univariate model, both SCI and MELF were predictive of the odds of a lymph node metastasis or extrauterine disease, but only SCI was an independent predictor of advanced stage disease on the multivariate analysis. Combining both patterns into a single category did not bring the MELF pattern to significance in keeping with the findings of Murray, et al (39). In contradistinction, only one study, which included SCI within the spectrum of MELF (21), found the MELF pattern predictive of lymph node metastases. The authors note a high rate of lymphovascular invasion in cases with MELF, but no multivariate analysis to determine whether MELF was an independent prognostic variable was undertaken. Regardless of whether SCI is part of the MELF spectrum, its presence should be regarded as possibly predictive of lymph node metastasis. The idea that the pattern of myometrial invasion could be associated with advanced stage disease is novel, and our study supports that SCI, previously included in the spectrum of MELF, increases the odds of advanced stage disease.
In summary, this multi-institutional study validates other studies’ findings that myometrial invasion, lymphovascular invasion and cervical stromal invasion are independent risk factors for lymph node metastases or extra uterine disease. Of these variables, myometrial invasion is the most readily evaluated intra operatively. The presence of single cell invasion pattern as a predictor of advanced stage disease is a novel finding. This pattern is typically associated with MELF, and MELF could be a marker for both single cell invasion and lymphovascular invasion. Single cell invasion is most likely to be identified in permanent sections. When identified, extra care should be taken when evaluating lymph node metastases as metastases associated with single cell invasion may also be composed only of single cells (21) (figure 2). Additional study is required to determine whether SCI has clinical applications and should be considered as an additional risk factor when evaluating the need for adjuvant therapy in the unstaged patient.
Acknowledgments
The authors have the following disclosures to report:
1. NIH/NCI Cancer Center Support Grant, Roland Bassett and Patricia Fox
2. Department of Pathology and Laboratory Enrichment Fund (The Ottawa Hospital, University of Ottawa) provided funds to cover administrative costs related to the study, Bojana Djordjevic
3. Department of Pathology Research Initiation Grant at Penn State provided funds to cover travel expenses related to this study, Elizabeth Frauenhoffer
Footnotes
Presented in part at the 101st Annual Meeting of the United States and Canadian Academy of Pathology
There are no conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, Cheung MK, Hugh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer. Cancer. 2006;107:1823–30. doi: 10.1002/cncr.22185. [DOI] [PubMed] [Google Scholar]
- 3.Chan JK, Huahsi Wu, Cheung MK, et al. The outcomes of 27,063 women with unstaged endometrioid uterine cancer. Gynecol Oncol. 2007;106:282–288. doi: 10.1016/j.ygyno.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Creasman WT, Boronow RC, Morrow CP, et al. Adenocarcinoma of the endometrium: its metastatic lymph node potential. Gynecol Oncol. 1976;4:239–243. doi: 10.1016/0090-8258(76)90028-7. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Morrow P, Bundy BN, et al. Surgical pathologic spread patterns of endometrial cancer. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Kilgore LC, Partridge EE, Alvarez RD, et al. Adenocarcinoma of the endometrium: survival comparisons of patients with and without pelvic node sampling. Gynecol Oncol. 1995;56:29–33. doi: 10.1006/gyno.1995.1005. [DOI] [PubMed] [Google Scholar]
- 7.Cragun JM, Havrilesky LJ, Calingaert B, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005;23:3668–3675. doi: 10.1200/JCO.2005.04.144. [DOI] [PubMed] [Google Scholar]
- 8.Huang M, Chadha M, Musa F, et al. Lymph nodes: is total number or station number a better predictor of lymph node metastasis in endometrial cancer? Gynecol Oncol. 2010;119:295–298. doi: 10.1016/j.ygyno.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Chi DS, Barakat RR, Palayekar DA, et al. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer. 2008;18:269–273. doi: 10.1111/j.1525-1438.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 10.Euscher ED, Bassett R, Malpica A. Lymph node counts in endometrial cancer: Expectations versus reality. Am J Surg Pathol. 2011;35:913–918. doi: 10.1097/PAS.0b013e31821899be. [DOI] [PubMed] [Google Scholar]
- 11.ASTEC study group. Kitchener H, Swart AM, et al. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MR ASTEC trial): a randomized study. Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panici PB, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 13.Bakkum-Gamez JN, Mariani A, Dowdy SC, et al. The impact of surgical guidelines and periodic quality assessment on the staging of endometrial cancer. Gynecol Oncol. 2011;123:58–64. doi: 10.1016/j.ygyno.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Mariani A, Dowdy SC, Cliby WA, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon JS, Mazgani M, Miller DM, et al. The significance of surgical staging in intermediate-risk endometrial cancer. Gynecol Oncol. 2011;122:50–54. doi: 10.1016/j.ygyno.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 16.Madom LM, Brown AK, Lui F, et al. Lower uterine segment involvement as a predictor for lymph node spread in endometrial carcinoma. Gynecol Oncol. 2007;107:75–78. doi: 10.1016/j.ygyno.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Akbayir O, Corbacioglu A, Goksedef B, et al. The novel criteria for predicting pelvic lymph node metastasis in endometrioid adenocarcinoma of endometrium. Gynecol Oncol. 2012;125:400–403. doi: 10.1016/j.ygyno.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Guntupalli SR, Zighelboim I, Kizer NT, et al. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol Oncol. 2012;124:31–35. doi: 10.1016/j.ygyno.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor RR, Zeller J, Lieberman RW, et al. An analysis of two versus three grades for endometrial carcinoma. Gynecol Oncol. 1999;74:3–6. doi: 10.1006/gyno.1999.5422. [DOI] [PubMed] [Google Scholar]
- 20.Lax SF, Kurman RJ, Pizer ES, et al. A binary architectural grading system for uterine endometrial endometrioid carcinoma has superior reproducibility compared with FIGO grading and identifies subsets of advance-stage tumors with favorable and unfavorable prognosis. Am J Surg Pathol. 2000;24:1201–1208. doi: 10.1097/00000478-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Pavlakis K, Messini I, Vrekoussis T, et al. MELF invasion in endometrial cancer as a risk factor for lymph node metastasis. Histopathology. 2011;58:966–973. doi: 10.1111/j.1365-2559.2011.03802.x. [DOI] [PubMed] [Google Scholar]
- 22.Quick CM, May T, Horowitz NS, et al. Low-grade, low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31:337–343. doi: 10.1097/PGP.0b013e31823ff422. [DOI] [PubMed] [Google Scholar]
- 23.FIGO Committee. Annual report on the results of treatment in gynecological cancer. Int J Gynecol, Obstet. 1989;28:189–193. [Google Scholar]
- 24.Lurain JR, Rice BL, Rademaker AW, et al. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet Gynecol. 1991;78:63–69. [PubMed] [Google Scholar]
- 25.Lee KR, Vacek PM, Belinson JL. Traditional and nontraditional histopathologic predictors of recurrence in uterine endometrioid adenocarcinoma. Gynecol Oncol. 1994;54:10–18. doi: 10.1006/gyno.1994.1158. [DOI] [PubMed] [Google Scholar]
- 26.Milam MR, Java J, Walker JL, et al. Nodal metastasis risk in endometrioid endometrial cancer. Obstet Gynecol. 2012;119:286–292. doi: 10.1097/AOG.0b013e318240de51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scholten AN, Creutzberg CL, Noordijk EM, et al. Long-term outcome in endometrial carcinoma favors a two-instead of a three-tiered grading system. Int J Radiation Oncology Biol Phys. 2002;52:1067–1074. doi: 10.1016/s0360-3016(01)02710-9. [DOI] [PubMed] [Google Scholar]
- 28.Chan JK, Urban R, Cheung MK, et al. Lymphadenectomy in endometrioid uterine cancer staging. How many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109:2454–2460. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 29.Zaino RJ, Kurman RJ, Brunetto VL, et al. Villoglandular adenocarcinoma of the endometrium: a clinicopathologic study of 61 cases: a gynecologic oncology group study. Am J Surg Pathol. 1998;22:1379–1385. doi: 10.1097/00000478-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Ambros RA, Ballouk F, Malfetano JH, et al. Significance of papillary (villoglandular) differentiation in endometrioid carcinoma of the uterus. Am J Surg Pathol. 1994;18:569–575. [PubMed] [Google Scholar]
- 31.McCluggage WG, Colgan T, Duggan M, et al. Data set for reporting of endometrial carcinomas: recommendations from the international collaboration on cancer reporting (ICCR) between United Kingdom, United States, Canada, and Australasia. Int J Gynecol Pathol. 2012;34:45–65. doi: 10.1097/PGP.0b013e31825d808b. [DOI] [PubMed] [Google Scholar]
- 32.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, Tanemura K, Ye W, et al. Clinicopathological factors predicting retroperitoneal lymph node metastasis and survival in endometrial cancer. Jpn J Clin Oncol. 1998;28:673–678. doi: 10.1093/jjco/28.11.673. [DOI] [PubMed] [Google Scholar]
- 34.Frumovitz M, Slomovitz B, Singh DK, et al. Frozen section analyses as predictors of lymphatic spread in patients with early-stage uterine cancer. J Am Coll Surg. 2004;199:388–393. doi: 10.1016/j.jamcollsurg.2004.05.258. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S, Medeiros F, Dowdy S, et al. A prospective assessment of the reliability of frozen section to direct intraoperative decision making in endometrial cancer. Gynecol Oncol. 2012;127:525–531. doi: 10.1016/j.ygyno.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Turan T, Oguz E, Unlubilgin E, et al. Accuracy of frozen-section examination for myometrial invasion and grade in endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2012 doi: 10.1016/j.ejogrb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Mittal KR, Barwick KW. Diffusely infiltrating adenocarcinoma of the endometrium. A subtype with poor prognosis. Am J Surg Pathol. 1988;12:754–758. doi: 10.1097/00000478-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Marshall DS, Hummer A, Thaler H, et al. Myometrial invasion patterns are associated with lymphovascular invasion in endometrioid adenocarcinoma of the endometrium. Mod Pathol. 2003;16:200A. [Google Scholar]
- 39.Murray SK, Young RH, Scully RE. Unusual epithelial and stromal changes in myoinvasive endometrioid adenocarcinoma: a study of their frequency, associated diagnostic problems, and prognostic significance. Int J Gynecol Pathol. 2003;22:324–333. doi: 10.1097/01.pgp.0000092161.33490.a9. [DOI] [PubMed] [Google Scholar]
- 40.Stewart CJR, Brennan BA, Leung YC, et al. MELF pattern invasion in endometrial carcinoma: association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology. 2009;41:454–459. doi: 10.1080/00313020903041135. [DOI] [PubMed] [Google Scholar]
- 41.Stewart CJR, Little L. Immunophenotypic features of MELF pattern invasion in endometrial adenocarcinoma: evidence for epithelial-mesenchymal transition. Histopathology. 2009;55:91–101. doi: 10.1111/j.1365-2559.2009.03327.x. [DOI] [PubMed] [Google Scholar]