Abstract
Background
The cholinergic system is substantially altered in individuals with major depression and is partially restored when depression remits. We quantified the availability of β2-subunit-containing nicotinic acetylcholine receptors (β2*-nAChR) in subjects with bipolar disorder.
Methods
Twenty-five subjects with bipolar disorder (15 depressed, 10 euthymic) and 25 sex- and age-matched control subjects had a [123I]5IA-85380 single photon emission computed tomography scan to quantify β2*-nAChR VT/fP (total volume of distribution, corrected for individual differences in metabolism and protein binding of the radiotracer). Average VT/fP was compared between groups and correlated with clinical characteristics. Postmortem analysis of β2*-nAChRs was conducted using equilibrium binding with [125I]5IA in subjects with bipolar disorder and matched control subjects.
Results
We showed significantly lower β2*-nAChR availability (20%–38%) in subjects with bipolar depression compared with euthymic and control subjects across all brain regions assessed (frontal, parietal, temporal, and anterior cingulate cortex, hippocampus, amygdala, thalamus, striatum). The postmortem binding study in which endogenous acetylcholine was washed out did not show a statistically significant difference in β2*-nAChR number in temporal cortex of the bipolar depressed and control groups (15% difference; p = .2).
Conclusions
We show that the alteration in the cholinergic system observed during a depressive episode appears to resolve during euthymia. We suggest that lower VT/fP observed in vivo may be due to a combination of higher endogenous acetylcholine levels during depression, which could compete with radiotracer binding to the receptor in vivo, and lower receptor number in bipolar depression. Identification of differences in cholinergic signaling in subjects with bipolar depression may improve our understanding of its etiology and reveal new treatment targets.
Keywords: Acetylcholine, β2*nAChR, bipolar disorder, depression, SPECT, tobacco smoking
Bipolar disorder is a chronic, debilitating illness with a 12-month prevalence of 2.6% and a lifetime prevalence of 3.9% (www.nimh.nih.gov/statistics). It is a leading cause of worldwide disability (1), mostly because of the depressive episodes (2), and is associated with increased risk of suicide, which is highest during depressive episodes. Although many mood stabilizers and antipsychotics are efficacious in the acute treatment and long-term prevention of manic episodes (3), fewer options are available for the treatment of the depressive phase of the illness (4,5). Antidepressant medications, which are efficacious in unipolar depression, are less effective for the acute treatment of bipolar depression (6). Therefore, there is a substantial need to develop medications that are more efficacious for the treatment of bipolar depression.
Several lines of evidence implicate dysfunction of the cholinergic system in mood disorders (7,8). Animal studies and human challenge studies suggest that decreased cholinergic activity is associated with mania, while increased acetylcholine (ACh) activity is associated with depression (9,10). ACh, a major neurotransmitter in the peripheral and central nervous systems, is released throughout the brain and binds to either muscarinic or nicotinic acetylcholine receptors (nAChRs), and brain ACh levels appear to be higher in individuals with major depressive disorder (MDD) (11). Rats bred for increased sensitivity to cholinergic agents show behaviors that resemble symptoms of depression, such as lethargy, reductions in self-stimulation, and increased behavioral despair (12). Furthermore, challenge with physostigmine, a cholinesterase inhibitor that increases ACh levels in the brain, induces significant depression-like symptoms both in human subjects with affective disorders and individuals with no history of psychiatric illness (13,14). The cholinergic system plays a major role in the sleep–wake cycle (15), learning, memory, attention (16,17), motivation, and reward (18,19); thus, dysfunction in this system may result in circadian abnormalities, cognitive deficits, and impaired reward function in individuals with bipolar depression.
Our understanding of the involvement of different neurotransmitters in bipolar depression has been hampered, in part due to lack of neuroreceptor imaging studies in this population. The substantial evidence implicating ACh and nAChRs in mood regulation and our previous findings of altered cholinergic activity in MDD that persists into remission (11) led us to determine whether there is any change in availability of β2-subunit-containing (β2*)-nAChRs in individuals with bipolar depression. We performed a [123I]-5-iodo-3-[2(S)-2-azetidinylmethoxy]pyridine ([123I]5IA) single photon emission computed tomography (SPECT) study to evaluate availability of β2*-nAChR in subjects with active bipolar depression and in subjects who are currently euthymic and comparison control subjects. [123I]5IA has high affinity for the β2*-nAChR and has been used previously to conduct studies of receptor availability (11,20,21) and occupancy (22,23). Our primary hypothesis was that subjects with bipolar disorder (currently depressed or euthymic) would have lower β2*-nAChR availability compared with control subjects based on our previous studies of individuals with unipolar depression (11). We followed with a postmortem binding study as described previously (11) to evaluate whether there are differences in β2*-nAChR number between individuals with and without bipolar disorder under conditions in which endogenous ACh is washed out. We hypothesized, based on previous findings in MDD subjects, that there would not be a difference in β2*-nAChR number between bipolar and control groups in vitro.
Methods and Materials
Study Design
Each subject participated in one magnetic resonance imaging and one [123I]5IA SPECT scan within 3 weeks of screening. In subjects with bipolar disorder, mood symptoms were reassessed at the time of the SPECT scan to confirm that each subject was still in a depressive or euthymic episode.
Regulatory Approvals
The study was approved by the Institutional Review Boards for Yale University School of Medicine and Veteran Affairs Connecticut Healthcare System. The use of [123I]5IA was allowed by the U.S. Food and Drug Administration. All subjects signed informed consent before full study participation.
Screening and Eligibility
As described previously (11), each prospective subject had an interview with an experienced psychiatrist who elicited a complete psychiatric and medical history. Subjects underwent the Structured Clinical Interview for DSM-IV Disorders (SCID-I) (24) to confirm the diagnosis of bipolar depression, current episode (depression or euthymia), or absence of any psychiatric disease. The Montgomery-Åsberg Depression Rating Scale and the Beck Depression Inventory were used to determine the severity of the current depressive episode, and the Young Mania Scale was used to rule out a current manic or mixed episode. The study participants were 25 subjects with bipolar disorder and 25 age- and gender-matched healthy comparison subjects. Fifteen of the subjects with bipolar disorder were acutely depressed (9 medicated nonsmokers, 6 unmedicated nonsmokers), and 10 were euthymic for at least 2 months (all unmedicated, 5 smokers and 5 nonsmokers). Subjects with any other significant Axis I diagnosis were excluded from the study (except nicotine dependence for smoking subjects), and only those with a primary diagnosis of bipolar disorder were included. The unmedicated subjects had been medication-free for at least 3 months, and the acutely ill subjects scored above 16 on the Hamilton Rating Scale for Depression. Comparison nonsmoking subjects were included in the study only if they had no lifetime history of any Axis I as judged with the SCID-I (except for nicotine dependence for smoking comparison controls). One control subject was recruited for each bipolar subject and matched based on age (within 3 years), smoking status, and sex. Nonsmokers defined as smoking less than 40 cigarettes in their lifetime and none in the past year; smokers were defined as smoking more than 10 cigarettes per day for at least 1 year.
Pregnancy in women was ruled out by a serum beta-human chorionic gonadotropin (hCG) test at screening and a urine beta-hCG test on the day of the SPECT scan. Nonsmoking status was confirmed by plasma cotinine levels of less than 15 ng/mL at screening and on SPECT scan day, urine cotinine levels of less than 100 ng/mL (at screening and on the day of the scan), and exhaled carbon monoxide levels of less than 8 ppm at screening and on the day of the scan. Smoking status was confirmed by plasma cotinine levels greater than 50 ng/mL, urine cotinine levels greater than 200 ng/mL, and exhaled carbon monoxide levels greater than 11 ppm at screening.
Smoking Abstinence
Nicotine has high affinity for β2*-nAChRs in the brain and blocks or displaces the radioligand from binding to these receptors (22,23). Previous studies revealed that 1 week of smoking cessation is required for all nicotine to leave the brain to accurately quantify β2*-nAChR availability (25). All smoking subjects were asked to abstain from tobacco smoking and any nicotine products for about 1 week before SPECT scan. Smoking abstinence was achieved through daily contingency management meetings and twice-daily urine cotinine and carbon monoxide collections. Subjects were paid for these appointments as described previously (26). On SPECT scan day, smoking abstinence was confirmed by plasma cotinine levels of less than 15 ng/mL, urine cotinine levels of less than 100 ng/mL, and exhaled carbon monoxide levels of less than 8 ppm.
Magnetic Resonance Imaging
Magnetic resonance imaging for coregistration was performed on a 3-Tesla Siemens Scanner (Erlangen, Germany; Magnetom Trio A Tim System; Software: Numaris/4; Version: syngo MR B17) to guide placement of regions of interest for SPECT scans (Series 1: 3 plane localizer; Series 2: Sag 3d Turbo Flash; 250 field of view; 1-mm slice thickness; 176 slices total; echo time 3.53; repetition time 2500; inversion time 1100; flip angle 7; 256 × 256 2 averages).
SPECT Imaging
SPECT emission scans were obtained on the Phillips PRISM 3000 XP (Cleveland, Ohio), a three-headed SPECT camera equipped with a low-energy, ultra-high-resolution fan beam collimator (photopeak window, 159 keV ± 10%; matrix 128 × 128) with a uniform sensitivity across the field of view. A 57Co-distributed source was measured with each experiment to control for day-to-day variation in camera sensitivity. The axial resolution (full width at half maximum) is 12.2 mm, measured with a 123I line source in water in a cylindrical phantom. [123I]5IA was synthesized as previously described (27) and administered through a venous catheter in the upper extremity, using a bolus-plus-constant-infusion paradigm with a ratio of 7.00 ± .03 hours for the bipolar depressed group, 7.00 ± .04 hours for the bipolar euthymic group, and 7.00 ± .04 hours for the control group, and a total injected dose (accounting for decay) of 341 ± 43.8 MBq for the bipolar depressed group, 347 ± 27.9 MBq for the bipolar euthymic group, and 337 ± 48.2 MBq for the control group. Blood samples for metabolite analyses were collected from another venous catheter. Approximately 6 hours after injection of [123I]5IA, a simultaneous transmission emission protocol scan and three 30-minute equilibrium emission scans were obtained. Blood samples were collected at the midpoint of the scans to quantify total parent and free fraction (fP), to correct for individual differences in metabolism and protein binding of [123I]5IA.
SPECT Image Analysis
As previously described (27), SPECT emission images were reconstructed using a filtered back projection algorithm with a ramp filter on a 128 × 128 matrix to obtain 50 slices with a pixel size of 2.06 × 2.06 × 3.56 mm in the x, y, and z axes. A three-dimensional (3D) Butterworth filter (order 10, cutoff frequency .24 cycle/pixel) was applied post hoc. A coregistered magnetic resonance image was used to guide the placement of standard two-dimensional region of interest templates using MEDx software (Medical Numerics, Germantown Maryland) as previously described (22,28). A 3D volume of interest was generated for each region and transferred to the coregistered SPECT image to determine regional radioactive densities. The chosen regions were known to contain β2*-nAChRs and included the frontal, anterior cingulate, and temporal cortices, thalamus, striatum (an average of caudate and putamen), hippocampus, and amygdala (29,30). We previously showed significant sex differences in metabolism and protein binding of [123I]5IA; thus, regional [123I] 5IA uptake was determined by VT/fP (total volume of distribution corrected for metabolism and protein binding of radiotracer), which corrects for this variability.
In Vitro Binding Assays
Samples of temporal cortex from eight depressed individuals with bipolar disorder (three nonsmoking, aged 55.7 ± 18.2 years; five smoking, aged 31.7 ± 19.2 years) who had committed suicide, and from eight sex-, smoking-, and age-matched control subjects (three nonsmoking, aged 56.7 ± 18.2 years; five smoking, aged 36.6 ± 18.6 years) were obtained from the Canadian Brain Bank. The tissue prisms were split on an ice-cold surface in an orientation that provided approximately equal amounts of gray and white matter. Tissue was homogenized in a chilled glass tissue grinder in 1 mL .1 × physiological buffer (PB) (14.4 mmol/L NaCl, .22 mmol/L KCl, .2 mmol/L CaCl2, .1 mmol/L MgSO4, 25 mmol/L (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) Hemi-Na) plus protease inhibitors (10 µg/mL aprotinin, leupeptin, pepstatin A). The resulting homogenate was centrifuged at 20,000 g for 5 minutes, and the supernatant was collected and saved. The pellet was resuspended in 1 m: of .1 × PB. For membrane binding with [125I]5IA (Perkin Elmer, Waltham, Massachusetts), a 100-µL aliquot of each homogenate was diluted 1:10 in .1 × PB, centrifuged at 20,000 relative centrifugal force for 5 minutes, supernatant discarded, and the pellet was resuspended in 1 mL .1 × PB (procedure was repeated three times). Protein content was determined using the Lowry method, aliquots of 10 µL were used for 5IA binding, taken in triplicate (duplicate total binding, one for nonspecific), and 10 µL of 3 × PB was added to each well. [125I]5IA (2200 Ci/mmol) was diluted in water, and 10 µL was added to each well at room temp for 4 hours. Nonspecific binding was measured in the presence of 100 µmol/L cytisine. Samples were collected by vacuum filtration (Packard Cell Harvester, Waltham, Massachusetts) on Pall (Washington, New York) glass fiber filters (type A/C and type A/D, sandwiched), soaked in .5% polyethylenimine, rinsed three times with approximately 1 mL per well of ice-cold PB. Radioactivity was quantified by liquid scintillation (Beckmann LS6000LL; Brea, California). Saturation of [125I]5IA was calculated using a two-parameter hyperbolic equation of the form B = Bmax[L]/Kd + [L] where B is measured binding at concentration of ligand [L] with apparent maximal binding (Bmax) and equilibrium constant (Kd). To ensure the calculated parameters conformed to simple Michaelis-Menton kinetics, a double-reciprocal plot of each saturation curve was taken, and goodness of fit was assessed to a single polynomial equation of the form y = mx + b, where y = 1/measured binding, b = 1/Bmax, and m = 1/Kd.
Statistical Analysis
Statistical analysis was performed using IBM SPSS v19.0 (Armonk, New York). Statistical significance was set at p < .05, two-tailed. Multivariate analysis of variance (MANOVA), which controls for Type II error, was used to measure differences in VT/fP between control, depressed and euthymic bipolar groups. Post hoc analyses (Bonferroni) were used to specify between group differences. Secondary analyses using MANOVA were conducted to examine the effect of medication on β2*-nAChRs in depressed bipolar subjects and the effect of smoking in euthymic bipolar subjects. The Spearman correlation coefficient was used to measure associations between receptor availability and clinical variables. Two-way analysis of variance was used to analyze the in vitro [125I]5IA binding data.
Results
Demographic and Clinical Characteristics
Clinical and demographic characteristics are shown in Table 1 for bipolar depressed group and matched control subjects; Table 2 for euthymic bipolar group and matched control subjects; and Table 3 for all euthymic bipolar, depressed bipolar, and controls. The medication list for bipolar depressed subjects was as follows: Subject 1—aripiprazole, escitalopram, and bupropion; Subject 2—lamotrigine and oxcarbazepine; Subject 3—lithium and phenelzine; Subject 4—quetiapine, aripiprazole, and hydroxyzine; Subject 5—valproic acid; Subject 6—lithium, fluoxetine, and topiramate; Subject 7—oxcarbazepine, dextroamphetamine, and citalopram; Subject 8—lamotrigine; and Subject 9—lithium, risperidone, alprazolam, and methylphenidate.
Table 1.
Demographics and Clinical Characteristics and VT/fP Data in Depressed Subjects with Bipolar Disorder and Control Subjects
| Bipolar Unmedicated (n = 6) | Bipolar Medicated (n = 9) | Control Subjects (n =15) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yrs), Mean (SD) | 42.3 ± 12.6 | 40.7 ± 14.5 | 40.8 ± 13.2 | ||||||||||
| Sex | 4 men, 2 women | 6 men, 3 women | 10 men, 5 women | ||||||||||
| Years Diagnosed with BD | 10.2 ± 8.8 | 11.7 ± 13.3 | NA | ||||||||||
| Duration of Current Episode (wks) | 27.2 ± 15.7 | 106.0 ± 176.3 | NA | ||||||||||
| Lifetime No. of Depressive Episodes | 30.2 ± 40.4 | 30 ± 40 | NA | ||||||||||
| Screening |
Scan Day |
Screening |
Scan Day |
Screening |
Scan Day |
||||||||
| Assessments | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| CES-D | 36.8 | 8.3 | 38.3 | 11.7 | 32 | 6.9 | 29.0 | 10.7 | 4.6 | 6.8 | NA | ||
| BDI | 27.5 | 10.6 | 26.7 | 6.4 | 21 | 5.0 | 17.3 | 6.6 | .8 | 1.6 | NA | ||
| STAI-S | 48 | 10 | 45.2 | 11.3 | 46 | 8.4 | 44.3 | 12.9 | NA | ||||
| STAI-T | 50 | 8.3 | 54.0 | 11.2 | 54 | 5.9 | 51 | 5.9 | NA | ||||
| HAM-D | 21 | 8.3 | 20 | 6.8 | 18 | 4.6 | 15 | 6.9 | NA | ||||
| Young | 2.8 | 2.7 | 4.0 | 4.5 | 2.9 | 1.6 | 1.6 | 1.8 | NA | ||||
| MADRS | 31 | 8.0 | 30 | 4.1 | 26 | 7.1 | 19 | 9.9 | NA | ||||
|
VT/fP | |||||||||||||
| Bipolar Med (n =9) |
Bipolar Unmed (n =6) |
All Bipolar (n =15) |
Control (n =15) |
||||||||||
| ROI | Mean | SD | Mean | SD | Mean | SD | Mean | SD | % Diff | F1,27 | pa | ||
| Frontal | 32 | 7.2 | 36 | 12.5 | 33 .8 | 9.4 | 54.4 | 12.6 | −37.70 | 24.7 | <.0001 | ||
| Anterior Cingulate | 35 | 7.7 | 42.2 | 10.8 | 37 .8 | 9.4 | 53.5 | 9.8 | −29.40 | 21.1 | <.0001 | ||
| Temporal | 38 | 7.7 | 45.2 | 11.4 | 40 .8 | 9.7 | 57.2 | 10.9 | −28.70 | 19.7 | <.0001 | ||
| Parietal | 33 | 6.4 | 55.6 | 36.5 | 34 .4 | 8.3 | 48.2 | 11.6 | −28.60 | 14.1 | .001 | ||
| Hippocampus | 58.3 | 10.8 | 52.3 | 28.8 | 56 .2 | 18.3 | 76.6 | 15.5 | −26.70 | 10.6 | .003 | ||
| Amygdala | 45 | 8.0 | 43.7 | 16.4 | 44 .8 | 11.1 | 55.7 | 11.7 | −19.50 | 6.6 | .016 | ||
| Striatum | 46.7 | 12.1 | 56.6 | 16.9 | 50 .6 | 14.5 | 72.4 | 13.7 | −33.70 | 18.1 | <.0001 | ||
| Thalamus | 80.7 | 15.8 | 97.7 | 32.9 | 87 .5 | 24.6 | 129.8 | 30.9 | −32.60 | 17.5 | <.0001 | ||
BD, bipolar disorder; BDI, Beck Depression Inventory; CES-D, Center for Epidemiologic Studies Depression scale; Diff., difference; HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery-Åsberg Depression Rating Scale; Med, medicated; NA, not applicable; ROI, region of interest; STAI-S and STAI-T, State-Trait Anxiety Inventory State and Trait subscales; Unmed, unmedicated; Young, Young Mania Scale; VT/fP, receptor availability.
p< .05 statistically significant.
Table 2.
Demographic Characteristics, Scan-Day Assessments, and VT/fP Data in Euthymic Bipolar Disorder Subjects and Matched Control Subjects
| CNS (n = 5) |
CS (n = 5) |
BDNS (n = 5) |
BDS (n = 5) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||
| Age (yrs) | 33.6 | 10.6 | 36.2 | 12.3 | 35.0 | 13.3 | 37.6 | 13.9 | ||||
| Years Diagnosed | NA | NA | 9.4 | 10.1 | 14 | 9.6 | ||||||
| Months Euthymic | NA | NA | 8.1 | 9.0 | 9.8 | 11 | ||||||
| Cigarettes/Day | NA | 18.0 | 6.7 | NA | 15.6 | 7.2 | ||||||
| Years Smoking | NA | 16.4 | 11.7 | NA | 13.0 | 13.2 | ||||||
| Scan-Day Mood Assessments | ||||||||||||
| BDI | 2.0 | 4.5 | 6.5 | 6.7 | 5.6 | 2.7 | 7.8 | 6.0 | ||||
| CES-D | 5.2 | 6.8 | 10 | 9.2 | 15 | 6.9 | 14.4 | 11.3 | ||||
| HAM-D | NA | NA | 3.6 | 2.7 | 3.8 | 1.1 | ||||||
| MADRS | NA | NA | 2.4 | 2.1 | 4.2 | 4.1 | ||||||
| STAI-S | 28 | 7.8 | 35.6 | 14.4 | 36 | 7.5 | 43 | 7.2 | ||||
| Young | NA | NA | 1.4 | 2.6 | 3.2 | 2.8 | ||||||
| Scan-Day Smoking Assessments | ||||||||||||
| Desire | NA | 7.8 | 4.5 | NA | 6.5 | 5.7 | ||||||
| Relief | NA | 8.8 | 6.3 | NA | 7.3 | 4.7 | ||||||
| Withdrawal | NA | 7.4 | 8.4 | NA | 9.4 | 8.0 | ||||||
| VT/fP | ||||||||||||
| CNS |
CS |
BDNS |
BDS |
|||||||||
| ROI | Mean | SD | Mean | SD | Mean | SD | Mean | SD | % Diff.a | % Diff.b | % Diff.c | % Diff.d |
| Frontal | 39 | 2.8 | 54.9 | 11.3 | 50.6 | 17.9 | 49.1 | 9.8 | 4.8 | 3.2 | −10.6 | 29.7 |
| Anterior Cingulate | 47 | 7.3 | 63 | 14 | 50.4 | 14.2 | 56.5 | 10.6 | −3.2 | −11 | −11 | 8.0 |
| Temporal | 52 | 7.5 | 65.6 | 13.6 | 55.4 | 16.7 | 59.6 | 12.6 | −2.6 | −7.1 | −9.1 | 6.1 |
| Parietal | 44 | 5.3 | 63.8 | 12.4 | 53.6 | 17.7 | 57.9 | 13.4 | 2.5 | −7.4 | −9.3 | 21.5 |
| Hippocampus | 56.6 | 19.3 | 71.4 | 17.8 | 67.2 | 32.8 | 75.8 | 13.2 | −2.3 | 4.2 | −8.6 | 3.3 |
| Amygdala | 54.9 | 14.7 | 68 | 16 | 53 | 22 | 60.7 | 17.9 | −2.9 | −6.8 | −8.8 | 6.5 |
| Striatum | 61 | 9.4 | 76 | 19 | 64.6 | 18.4 | 69.3 | 15.3 | 12 | −11 | 6.1 | 19 |
| Thalamus | 114 | 18.5 | 123 | 36.9 | 117 | 40.4 | 112 | 16.2 | −11 | −14 | −10 | −4.3 |
% Diff., percent difference; BDI, Beck Depression Inventory; BDS, smokers with bipolar disorder, BDNS, nonsmokers with bipolar disorder; CES-D, Center for Epidemiologic Studies Depression scale; CS, control smokers, CNS, control nonsmokers; HAM-D, Hamilton Rating Scale for Depression; NA, not applicable; MADRS, Montgomery-Åsberg Depression Rating Scale; STAI-S, State–Trait Anxiety Inventory State subscale; Young, Young Mania Scale; VT/fP, receptor availability.
All bipolar disorder vs. all control subjects.
BDNS vs. BDS.
BDS vs. CS.
BDNS vs. CNS.
Table 3.
Demographics and Clinical Characteristics and VT/fP Data in All Bipolar Disorder and Control Subjects
| Euthymic Bipolar (n =0) |
Depressed Bipolar (n =5) |
Control Subjects (n =25) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yrs) Mean (SD) | 36.3 ± 12.9 | 41.6 ± 13.1 | 38.4 ± 12.4 | ||||||||||||
| Sex | 5 men, 5 women | 10 men, 5 women | 15 men, 10 women | ||||||||||||
| Duration Current Episode (wks) |
NA | 77.9 ± 144 | NA | ||||||||||||
| Screening |
Scan Day |
Screening |
Scan Day |
Screening |
Scan Day |
||||||||||
| Assessments | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| CES-D | 16 | 9.0 | 14 | 9.4 | 33 | 6.6 | 29.3 | 10.4 | 7.6 | 8.0 | NA | ||||
| BDI | 8.8 | 7.4 | 6.7 | 4.8 | 21 | 5.0 | 19 | 6.2 | 3.5 | 5.5 | NA | ||||
| STAI-S | 39 | 9.7 | 35 | 8.8 | 47 | 8.4 | 43.6 | 11.7 | NA | ||||||
| STAI-T | 50 | 8.3 | 42 | 8.6 | 53 | 6.8 | 50 | 6.4 | |||||||
| HAM-D | 5.7 | 5.0 | 3.9 | 2.0 | 18 | 4.6 | 15 | 6.1 | |||||||
| Young | 2.4 | 2.6 | 2.4 | 3.1 | 2.6 | 2.2 | 2.6 | 2.7 | |||||||
| MADRS | 4.7 | 4.6 | 3.1 | 3.4 | 25 | 6.1 | 19.3 | 9.8 | |||||||
|
VT/fP | |||||||||||||||
| Eu BD (n =10) |
CS for Eu (n =10) |
Dep BP (n =15 |
CS for Dep BD (n =15 |
||||||||||||
| ROI | Mean | SD | Mean | SD | Diff. Eu vs. CS |
Mean | SD | Mean | SD | Diff. Dep vs. CS |
Diff. Dep vs. EU BD |
F | pa | ||
| Frontal | 49.9 | 11.9 | 47.6 | 11.9 | 4.8% | 34 | 9.4 | 54.4 | 12.6 | −37.9% | −32.3% | 10.8 | <.0001 | ||
| Anterior Cingulate | 53.4 | 13.9 | 55.2 | 13.9 | −3.3% | 38 | 9.4 | 54 | 9.8 | −29.3% | −29.2% | 11.7 | <.0001 | ||
| Temporal | 57.5 | 12.6 | 59.1 | 12.6 | −2.7% | 41 | 9.7 | 57.2 | 10.9 | −28.7% | −29.0% | 11.8 | <.0001 | ||
| Parietal | 49.7 | 14.1 | 47.3 | 10.7 | 5.1% | 34 | 8.3 | 48.2 | 11.6 | −28.6% | −30.8% | 8.6 | .001 | ||
| Hippocampus | 71.0 | 19.0 | 63.7 | 19.0 | 11.5% | 56.2 | 18.3 | 76.6 | 15.5 | −26.6% | −20.8% | 2.3 | .12 | ||
| Amygdala | 56.2 | 16.8 | 62.9 | 16.8 | −10.7% | 44.8 | 11.1 | 55.7 | 11.7 | −19.6% | −20.3% | 3.4 | .04 | ||
| Striatum | 67.0 | 16.7 | 69.0 | 16.7 | –2.9% | 50.6 | 14.5 | 72.4 | 13.7 | −30.1% | −24.5% | 9.1 | <.0001 | ||
| Thalamus | 115 | 27.8 | 118 | 27.8 | −2.3% | 87.5 | 24.6 | 130 | 30.9 | −32.6% | −23.8% | 8.5 | .001 | ||
BDI, Beck Depression Inventory; BD, bipolar disorder; CES-D, Center for Epidemiologic Studies Depression scale; CS, control subject; Dep, depressed; Diff., difference; Eu, euthymic; HAM-D, Hamilton Rating Scale for Depression; MADRS, Montgomery-Åsberg Depression Rating Scale; NA, not applicable; ROI, region of interest; STAI-S and -T, State–Trait Anxiety Inventory State and Trait subscales; Young, Young Mania Scale; VT/fP, receptor availability.
p value is for bipolar depressed subjects compared with bipolar euthymic and CS (multivariate analysis of variance).
VT/fP in the Overall Sample
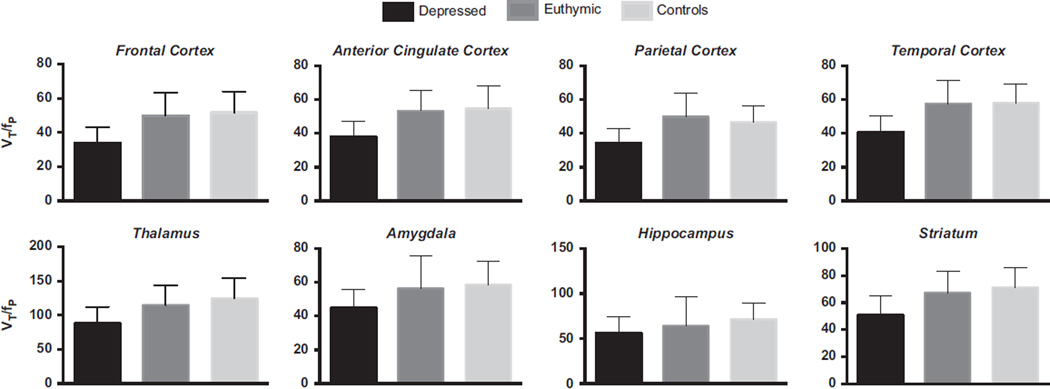
Results of the omnibus model including three groups revealed a significant effect of group (Hotelling’s trace F = 2.5, p = .004). Post hoc tests showed that this difference was due to statistically significantly lower VT/fP in bipolar depressed compared with bipolar euthymic and control subjects (Table 1 and Table 3; Figure 1). Because the euthymic bipolar group and associated controls included smoking subjects, we removed the smoking subgroups and repeated the analyses. Omnibus model including three groups (bipolar depressed, bipolar euthymic nonsmokers, and control non-smokers) revealed a trend toward a significant effect of group (Hotelling’s trace F = 1.5, p = .1). Post hoc tests showed that this difference was due to statistically significantly lower VT/fP in bipolar depressed compared with bipolar euthymic and control subjects.
Figure 1.
Bars illustrating in vivo VT/fP in bipolar depressed (n = 15), bipolar euthymic (n = 10), and control (n = 25) subjects. Lower VT/fP was observed across all brain regions in bipolar depressed compared with bipolar euthymic and control subjects (Hotelling’s trace F = 2.5, p = .004). VT/fP, receptor availability.
Differences in VT/fP and Clinical Variables Due to Medication Status in Currently Depressed Subjects
We did not detect significant differences in clinical variables between medicated and unmedicated bipolar depression groups (all ps > .1). Results of the omnibus MANOVA modeling did not detect significant differences in VT/fP between medicated and unmedicated subjects with bipolar depression (Hotelling’s trace p = .85). However, when we examined groups on the basis of medication status, in the unmedicated sample, we observed a significant negative correlation between self-reported depression score on the Beck Depression Inventory and VT/fP in the frontal cortex (r5 = −.83, p = .04).
VT/fP in Euthymic Subjects as a Function of Smoking Status
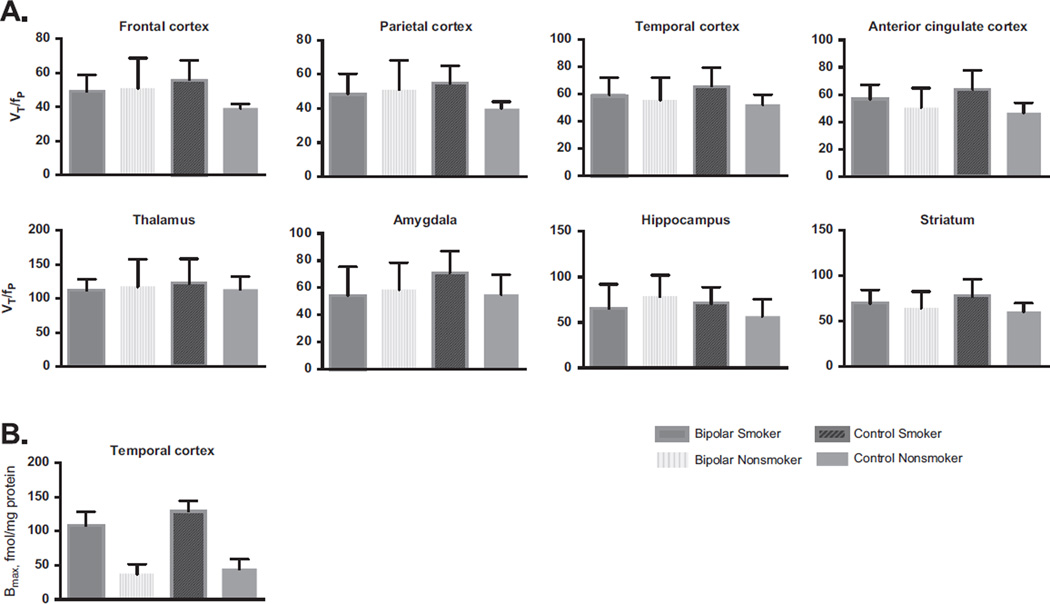
To determine whether there is upregulation of β2*-nAChRs bipolar disorder, we performed a MANOVA within the euthymic bipolar group and detected that in vivo, smokers with bipolar disorder do not have higher VT/fP compared with age- and sex-matched nonsmokers with bipolar disorder (Hotelling’s trace F = 5.6, p = .16; Figure 2). We confirmed previous findings in control smokers: significantly higher VT/fP compared with nonsmokers (Hotelling’s trace F = 10.0, p = .04), and this was significant in the anterior cingulate, frontal, and parietal cortices (F55 = 6.0, p = .04; F5,5 = 10.7, p = .01; and F5,5 = 8.8, p = .02, respectively, with a trend in the temporal cortex F5,5 = 4.0, p = .08). No significant associations between VT/fP and clinical variables were observed.
Figure 2.
(A) Illustration of in vivo VT/fP in euthymic smokers with (n = 5) and without (n = 5) bipolar disorder and nonsmokers with (n = 5) and without (n = 5) bipolar disorder. No significant upregulation was observed in VT/fP in bipolar euthymic sample (Hotelling’s trace F = 5.6, p = .16). As we have shown previously, there was higher VT/fP in control smokers compared to nonsmokers (Hotelling’s trace F = 10.0, p = .04), and this was significant in the anterior cingulate, frontal, and parietal cortices (F5,5 = 6.0,p = .04; F5,5 = 10.7,p = .01; and F5,5 = 8.8, p = .02, respectively, with a trend in the temporal cortex F55 = 4.0, p = .08). (B) Calculated Bmax (fmoles/mg protein) for [125I]5IA binding in homogenates of temporal cortex samples in control nonsmokers (n = 3), control smokers (n = 5), nonsmokers with bipolar disorder (n = 3) and smokers with bipolar disorder (n = 5). No significant differences were detected as an effect of diagnosis (F1,14 = 2.13, p = .17), but nicotinic acetylcholine receptors upregulation was greater in control smokers than in smokers with bipolar disorder (F1,14 = 104.4, p < .0009). VT/fP, receptor availability.
In Vitro [125I]5IA Binding
There were no significant differences in calculated [125I]5IA Kd values for 125I-A85380 binding in control versus bipolar smokers (p > .9) or control versus bipolar nonsmokers (p > .8; Student t test). The Kd values by group are as follows (mean ± SEM): control nonsmoker, 2.25E-8 ± 2.1E-9 mmol/L; bipolar nonsmoker, 2.6E-8 ± 4.5E-9 mmol/L; control smoker: 3.94E-8 ± 3.7E-9 mmol/L; bipolar smoker: 3.45E-9 ± 5.6E-9 mmol/L. Calculated Bmax values are as follows: control nonsmoker, 47.5 ± 13.5 fmol/mg; bipolar nonsmoker, 37 ± 8.2 fmol/mg, control smoker, 127 ± 8.6 fmol/mg; bipolar smoker, 110 ± 11.4 fmol/ mg. Two-way analysis of variance showed a main effect of smoking status on Kd (F1,15 = 7, p = .02), and post hoc two-sided t tests showed p = .017 for the comparison of control smokers versus control nonsmokers (the others were all ps > .05). A small shift in affinity in the control smoker group may reflect differential posttranslational modification that could be worthy of further consideration. Measurement of high-affinity (200 pM) [125I]5IA binding in tissue samples from human temporal cortex revealed a highly significant effect of smoking status (F1,14 = 104.4, p < .0009) and a trend toward a bipolar diagnosis effect (F1,14 = 2.13, p = .17). The effect of smoking on 200 pM [125I]5IA binding was further examined with saturation analysis of radioligand binding and detected a significant increase in the calculated Bmax of [125I]5IA (F1,15 = 24.74, p < .0009).
Discussion
We showed that compromise in the cholinergic system associated with depression in subjects with bipolar disorder appears to resolve during euthymia. Consistent with our previous study in unmedicated subjects with MDD (11), we found that currently depressed subjects with bipolar depression had significantly lower VT/fP of cortical (25%–38%) and subcortical (20%– 24%) β2*-nAChRs compared with age- and sex-matched bipolar euthymic and control subjects. Contrary to the findings of the MDD study, we did not detect significant differences in VT/fP between bipolar euthymic and control subjects. In vitro β2*-nAChR quantification in the temporal cortex did not detect significant differences in receptor number in bipolar depressed subjects compared with control subjects, although there was a trend toward significance likely due to the 15% lower Bmax/Kd in the bipolar depressed sample.
There are several possible explanations for the lower VT/fP in the bipolar depressed sample. Lower β2*-nAChR VT/fP in vivo might be due to fewer receptor complexes available on the cell surface or intracellularly in bipolar depression, lower nondisplace-able binding in the bipolar depressed group, or could result from more ACh competing with the radiotracer. In studies using intact cells, nicotinic ligands bind with equal efficiency to intracellular and extracellular nAChRs (31). Given the 29% difference in temporal cortex VT/fP and 15% difference in Bmax/Kd, it is plausible that the observed lower binding in vivo is in part due to lower intracellular or extracellular pool in bipolar depression. Use of VT/ fP as an outcome measure (sum of specific and nondisplaceable bindings) assumes uniform nondisplaceable binding across experimental groups. Although we previously calculated nondisplaceable binding in control smokers, this has not been done in individuals with mental illness. It is possible that individuals with bipolar disorder have lower nondisplaceable binding, which would reflect lower VT/fP and is a limitation to using VT/fP as outcome measure. Competition for radioligand binding by higher endogenous levels of ACh would be consistent with Janowsky’s adrenergic-cholinergic balance hypothesis of depression, which posits that a depressive state may be due to overactivity of brain ACh (7,14). Preclinical evidence (32) and current clinical evidence (33) show that increasing endogenous ACh levels by blocking acetylcholinesterase (AChE) effectively competes with the radioligand for the binding to β2*-nAChRs. Thus, we suggest that the observed lower VT/fP in the current study may be due to higher extracellular ACh levels that could be associated with a depressed state, in combination with lower intra- and extracellular complexes.
The lack of observed differences in β2*-nAChR VT/fP between control and bipolar euthymic subjects in the in vivo sample was surprising. We had previously reported that the apparent lower VT/fP in vivo (i.e., higher endogenous ACh) during depression in unmedicated subjects with unipolar depression is persistent even during euthymia (11). Our finding of no difference, which may be interpreted as a resolution in the cholinergic compromise during euthymia, may be critical in understanding the role of cholinergic system in the mood states in individuals with bipolar disorder and may also contribute to the growing literature regarding neurochemical differences between unipolar and bipolar depression.
The reduced VT/fP in bipolar depression contributes to a growing literature showing that ACh can play a role in regulation of mood and motivation. The role of ACh in mood regulation may derive from the fact that ACh output to the cortex is driven in large part by the limbic system (34), which is largely responsible for mood regulation. In turn, alterations in cholinergic tone affect signaling through nAChRs, and there are several lines of evidence suggesting that nAChR signaling may contribute to depression (7). Furthermore, some studies suggest that medications that act at nAChRs may have antidepressant properties (35,36). For example, up to 60% of individuals with bipolar depression smoke, likely because of the antidepressant effects of nicotine, which has high affinity for β2*-nAChRs and desensitizes these receptors (for review see Mineur and Picciotto) (7). In addition, partial agonists (37) and antagonists (38) of β2*-nAChR can enhance the anti-depressant effects of serotonin or norepinephrine reuptake inhibition, and a large number of studies suggest that decreasing the activity of ACh at nAChRs using antagonists or partial agonists results in antidepressant effects (39). Such data suggest that nAChRs, specifically those containing the β2 subunit, are involved in mood regulation in humans.
Medication status did not appear to affect β2*-nAChR VT/fP during a depressive episode in bipolar depressed subjects, although affirmation is required in a larger sample of medicated and unmedicated subjects. However, this finding is consistent with a previous study showing that treatment with selective serotonin reuptake inhibitors (SSRIs) did not have an effect on [123I]5IA binding (40). Although SSRIs have been shown to block nAChRs, these effects appear to be non-competitive through actions on the channel pore (41). Interestingly, although there were no significant differences in self-reported symptoms of depression between the medicated and unmedicated groups in the current sample, in the unmedicated group, lower β2*-nAChR VT/fP was significantly associated with greater numbers of reported depressive symptoms. This is an important observation that requires further investigation in a larger sample of medicated and unmedicated subjects with bipolar depression.
Our in vivo data show an apparent lack of upregulation in β2*-nAChR VT/fP in smokers versus nonsmokers with bipolar disorder, whereas postmortem findings show a significant upregulation in the temporal cortex, although the upregulation appears to be blunted in tissue from bipolar subjects. It is likely that smokers with bipolar disorder do upregulate, and, because of the small sample size, we did not observe a detectable upregulation in vivo. Similar findings exist in subjects with schizophrenia (42), where postmortem quantification shows blunted upregulation of β2*-nAChRs in smokers with versus without schizophrenia. Further examination is required to confirm whether β2*-nAChRs are regulated in a different manner in individuals with mood disorders compared with control smokers.
There are several limitations to this study. First, we included both medicated and unmedicated bipolar depressed subjects The effect of medications on β2*-nAChR VT/fP cannot be ruled out. For example, bupropion is a noncompetitive high-affinity nAChR antagonist (43) and may alter β2*-nAChR VT/fP. Some reports also suggest SSRIs may weakly bind to β2*-nAChR; however, a [123I]5IA SPECT study showed that SSRI treatment did not affect radio-ligand binding (i.e., receptor availability) (40). Because we did not include a homogenous sample of medications, evaluations and conclusions of the effect of medications on β2*-nAChR are limited. Also, subject compliance to medication routine was not evaluated (i.e., we did not ensure they adhered to their daily medication routine, and we did not check blood for therapeutic dose levels). Second, the postmortem sample is small (only three nonsmokers and five smokers) and limited to the temporal cortex; however, it is in line with previous findings in MDD sample. Furthermore, the quantitative evaluation of the static receptor number requires fewer subjects to obtain statistically relevant findings, and the greater effect of smoking on nAChR number in the postmortem study is in line with the published effects of smoking on nAChR upregulation in postmortem brain (30,42). Third, scanning smokers after smoking abstinence limits understanding of brain ACh levels during active smoking. However, given that nicotine directly competes with radioligand for binding to the β2*-nAChRs, it is required that smokers abstain from smoking.
In conclusion, this is a unique examination of the cholinergic system across mood states in individuals with bipolar depression. We showed that during depression, there is lower β2*-nAChR VT/fP in vivo regardless of medication status, which is likely due to a combined effect of lower actual VT/fP and in vivo competition between radioligand and ACh. We also showed that there appears to be a restoration of cholinergic functioning during euthymia. Additional studies are needed to clarify the molecular underpinnings of the observed lower VT/fP during depressive state and to determine whether the cholinergic system may be a novel target for drugs aimed at treating bipolar depression or to help smokers with bipolar disorder during smoking abstinence to achieve smoking cessation without relapsing into a depressive episode.
Acknowledgments
This research was funded by National Institute of Mental Health Grant No. R21MH085198-02 (to IE) and NIDA Grant No. R01DA015577 (KPC). Salary support was provided by Grant Nos. K12DA00167 (JH), MH077681 (MRP), and K01MH092681 (IE), and K02DA031750 (KPC). We thank the technologists at the Institute for Neurodegenerative Disorders for conducting single photon emission computed tomography scans and Louis Amici for metabolite analyses. We thank Gustavo Turecki, M.D., Ph.D., of McGill University for providing the brain samples.
Dr. Hannestad became a full-time employee of UCB Pharma S.A. after the study was completed; he had no competing interests while the study was ongoing. Dr. Bhagwagar is a full-time employee of Bristol-Meyers Squibb. Dr. Seibyl is a consultant for Bayer Healthcare and GE Healthcare and holds equity in Molecular Neuroimaging. Dr. Picciotto has received grant funding from Targacept, Inc.
Footnotes
Ms. DellaGioia, Ms. Perkins, and Drs. Bois, Cosgrove, McClure-Begley, and Esterlis report no biomedical financial interests or potential conflicts of interest.
References
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 3.Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: A multiple-treatments meta-analysis. Lancet. 2011;378:1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- 4.Frye MA, Ha K, Kanba S, Kato T, Ozerdem A, Vazquez G, et al. international consensus group on depression prevention in bipolar disorder. J Clin Psychiatry. 2011;72:1295–1310. doi: 10.4088/JCP.10123co1c. [DOI] [PubMed] [Google Scholar]
- 5.Vieta E, Locklear J, Gunther O, Ekman M, Miltenburger C, Chatterton ML, et al. Treatment options for bipolar depression: A systematic review of randomized, controlled trials. J Clin Psychopharmacol. 2010;30:579–590. doi: 10.1097/JCP.0b013e3181f15849. [DOI] [PubMed] [Google Scholar]
- 6.Sidor MM, Macqueen GM. Antidepressants for the acute treatment of bipolar depression: A systematic review and metaanalysis. J Clin Psychiatry. 2011;72:156–167. doi: 10.4088/JCP.09r05385gre. [DOI] [PubMed] [Google Scholar]
- 7.Mineur YS, Picciotto MR. Nicotine receptors and depression: Revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip NS, Carpenter LL, Tyrka AR, Price LH. Nicotinic acetylcholine receptors and depression: A review of the preclinical and clinical literature. Psychopharmacology (Berl) 2010;212:1–12. doi: 10.1007/s00213-010-1932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilsaver S. Pathophysiology of “cholinoceptor supersensitivity” in affective disorders. Biol Psychiatry. 1986;21:813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 10.Owens M, Overstreet D, Knight D, Rezvani A, Ritchie J, Bissette G, et al. Alterations in the hypothalamic-pituitary-adrenal axis in a proposed animal model of depression with genetic muscarinic supersensitivity. Neuropsychopharmacology. 1991;4:87–93. [PubMed] [Google Scholar]
- 11.Saricicek A, Esterlis I, Maloney KH, Mineur YS, Ruf BM, Muralidharan A, et al. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overstreet D. The Flinders sensitive line rats: A genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- 13.Janowsky D, el-Yousef M, Davis J, Sekerke H. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 14.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Szymusiak R. Magnocellular nuclei of the basal forebrain: Substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- 16.Murray C, Fibiger H. Learning and memory deficits after lesions of the nucleus basalis magnocellularis: Reversal by physostigmine. Neuroscience. 1985;14:1025–1032. doi: 10.1016/0306-4522(85)90273-8. [DOI] [PubMed] [Google Scholar]
- 17.Wesnes K, Warburton D. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- 18.Picciotto M, Zoli M, Rimondin R, Lena C, Marubio L, Pich E, et al. Acetycholine receptors containing the b2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- 19.Picciotto M, MZ M, Zachariou V, Changeux J. Contribution of nicotinic acetylcholine receptors containing the beta 2-subunit to the behavioural effects of nicotine. Biochem Soc Trans. 1997;25:824–829. doi: 10.1042/bst0250824. [DOI] [PubMed] [Google Scholar]
- 20.D’Souza D, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, et al. Lower β2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. Am J Psychiatry. 2012;169:326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esterlis I, Cosgrove K, Petrakis I, McKee S, Bois F, Krantzler E, et al. SPECT imaging of nicotinic acetylcholine receptors in nonsmoking heavy alcohol drinking individuals. Drug Alcohol Depend. 2010;108:146–150. doi: 10.1016/j.drugalcdep.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esterlis I, Mitsis E, Batis J, Bois F, Picciotto M, Stiklus S, et al. Brain β2*-nicotinic acetylcholine receptor occupancy after use of a nicotine inhaler. Int J Neuropsychopharmacology. 2011;14:389–398. doi: 10.1017/S1461145710001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esterlis I, Cosgrove K, Batis J, Bois F, Stiklus S, Perkins E, et al. Quantification of smoking induced occupancy of β2-nicotinic acetylcholine receptors: Estimation of nondisplaceable binding. J Nucl Med. 2010;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 25.Staley J, Krishnan-Sarin S, Cosgrove K, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2-nicotinic acetylcholine receptors than nonsmokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosgrove K, Esterlis I, McKee S, Bois F, Seibyl J, Krishnan-Sarin CMS, et al. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch Gen Psychiatry. 2012;69:418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staley J, Cv Dyck, Weinzimmer D, Brenner E, Baldwin R, Tamagnan G, et al. Iodine-123-5-IA-85380 SPECT measurement of nicotinic acetylcholine receptors in human brain by the constant infusion paradigm: Feasibility and reproducibility. J Nucl Med. 2005;46:1466–1472. [PubMed] [Google Scholar]
- 28.Saricicek A, Esterlis I, Maloney K, Mineur Y, Ruf B, Muralidharan A, et al. Persistent β2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry. 2012;169:851–859. doi: 10.1176/appi.ajp.2012.11101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, et al. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than non-smokers. J Neurosci. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breese C, Marks M, Logel J, Adams C, Sullivan B, Collins A, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Therap. 1997;282:7–13. [PubMed] [Google Scholar]
- 31.Whiteaker P, Sharples C, Wonnacott S. Agonist-induced upregulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: Pharmacological and spatial definition. Mol Pharm. 1998;53:950–962. [PubMed] [Google Scholar]
- 32.Fujita M, Al-Tikriti M, Tamagnan G, Zoghbi S, Bozkurt A, Baldwin R, et al. Influence of acetylcholine levels on the binding of a SPECT nicotinic acetylcholine receptor ligand [123I]5-I-A-85380. Synapse. 2003;48:116–122. doi: 10.1002/syn.10194. [DOI] [PubMed] [Google Scholar]
- 33.Esterlis I, Hannestad J, Bois F, Sewell R, Tyndale R, Seibyl J, et al. Imaging changes in synaptic acetylcholine availability in living human subjects. J Nucl Med. 2013;54:78–82. doi: 10.2967/jnumed.112.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesulam M. Cholinergic aspects of aging and Alzheimer’s disease. Biol Psychiatry. 2012;71:760–761. doi: 10.1016/j.biopsych.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mineur Y, Einstein E, Seymour P, Coe J, O’Neill B, Rollema H, et al. α4β2 nicotinic acetylcholine receptor partial agonists with low intrinsic efficacy have antidepressant-like properties. Behav Pharmacol. 2011;22:291–299. doi: 10.1097/FBP.0b013e328347546d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George T, Sacco K, Vessicchio J, Weinberger A, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: A preliminary study. J Clin Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- 37.Rollema H, Shrikhande A, Ward K, Tingley FD3rd, Coe JW, O’Neill BT, et al. Pre-clinical properties of the alpha4beta2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br J Pharmacol. 2010;160:334–345. doi: 10.1111/j.1476-5381.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caldarone B, Harrist A, Cleary M, Beech R, King S, Picciotto M. High-affinity nicotinic acetylcholine receptors are required for anti-depressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Philip NS, Carpenter LL, Tyrka AR, Price LH. The nicotinic acetylcholine receptor as a target for antidepressant drug development [published online April 24] Sci World J. 2012 doi: 10.1100/2012/104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavanagh J, Patterson J, Pimlott S, Wyper D, Dewar D. SSRI antidepressants do not confound single photon emission computed tomography (SPECT) imaging studies using the alpha4beta2 nicotinic acetylcholine receptor [123I]5-I-A85380 ligand: In vivo and in vitro evidence. Synapse. 2010;64:111–116. doi: 10.1002/syn.20705. [DOI] [PubMed] [Google Scholar]
- 41.Shytle R, Silver A, Lukas R, Newman M, Sheehan D, Sanberg P. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 42.Breese C, Lee M, Adams C, Sullivan B, Logel J, Gillen K, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 43.Slemmer J, Martin B, Damaj M. Bupropion is a nicotinic agonist. J Pharmacol Exp Therap. 2000;295:321–327. [PubMed] [Google Scholar]