Abstract
Background
HIV-infection is characterized by chronic immune activation that persists despite effective antiretroviral therapy (ART) and is associated with elevated cardiovascular risk. Whether specific perivascular fat depots are associated with inflammation in HIV is unknown.
Methods
In a cross-sectional study, epicardial (EAT) and thoracic periaortic (TAT) adipose tissue volume were measured by computed tomography in 100 HIV-infected adults, on stable ART, with LDL-cholesterol ≤130mg/dL and evidence of heightened T-cell activation (CD8+CD38+HLA−DR+ ≥19%) or increased inflammation (high sensitivity C-reactive protein ≥2mg/L).
Results
Overall, 77% were male and 70% African American. Mean (standard deviation) age and body mass index were 47 (10) years and 28 (6.4) kg/m2, respectively. All subjects had HIV-1 RNA <1,000 copies/mL with mean (standard deviation) CD4+ T cell count of 665 (280) cells/μL; 50% were on a protease inhibitor. EAT and TAT were correlated with each other (r=0.766, p<0.0001). Both were associated with metabolic syndrome, atherogenic lipid profile, insulin resistance, total and central body fat, serum biomarkers of inflammation, and soluble CD163, but not with cellular immune activation markers. In multivariable models that adjusted for age, sex, and other measures of adiposity, both perivascular fat depots were independently associated with the presence of coronary calcium.
Conclusions
Perivascular fat is associated with soluble CD163, biomarkers of inflammation, insulin resistance, and subclinical atherosclerosis in this population of virologically suppressed HIV-infected patients on ART. The association of perivascular fat with coronary artery calcification appears to be independent of other measures of adiposity.
Keywords: Adipose tissue, Atherosclerosis, HIV, Inflammation, Macrophages
Introduction
Although the incidence of peripheral lipoatrophy appears to have decreased after the introduction of more metabolically favorable antiretroviral therapy (ART) regimens [1-3], visceral lipohypertrophy remains a common and important complication of HIV infection. This phenotype of adiposopathy (i.e. “sick fat”) is linked to chronic inflammation and immune activation[4], elevated cardiovascular risk[5], and mortality[6] in patients on ART.
Epicardial adipose tissue is a perivascular fat depot of particular interest because of dense inflammatory cell infiltration and higher production of NF-κB-dependent inflammatory cytokines such as tumor-necrosis factor-α and interleukin-6 (IL-6) compared to subcutaneous adipose tissue[7-9]. Since epicardial fat envelopes the coronary vessel adventitia without fascial separation, pathologic inflammation in the fat may promote the growth of atherosclerotic plaque in an ‘outside-in’ fashion[10]. Epicardial fat is quantitatively increased in HIV compared to un-infected controls[11] and is associated with the presence of coronary artery calcification[12] and prevalent cardiovascular disease[13].
Similarly, periaortic adipose tissue may play an important role in mediating aortic disease, although comparatively fewer studies have examined this. Periaortic fat has been associated with coronary and abdominal aortic calcification[14] as well as lower extremity peripheral arterial disease[15] in the Framingham Heart Study. HIV-infected patients appear to have increased aortic wall inflammation by 18fluorine-2-deoxy-D-glucose positron emission tomography (FDG-PET) compared to uninfected controls[16]; however, to our knowledge, no study has described periaortic fat in HIV infection.
Whether perivascular fat depots contribute to the elevated systemic inflammation seen in HIV independent of other types of abnormal fat distribution is unknown. In this study, we therefore aimed to comprehensively examine the association of epicardial and periaortic fat depots with clinical and radiologic measures of adiposity, subclinical atherosclerosis, and systemic biomarkers of inflammation and immune activation in HIV-infected patients on ART.
Methods
Study design
We performed a cross-sectional analysis of 100 subjects who underwent initial comprehensive metabolic and cardiovascular assessments at the time of enrollment into the Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV (SATURN-HIV) trial. SATURN-HIV is a randomized double-blind placebo-controlled trial designed to measure the effect of rosuvastatin 10mg daily on the progression of subclinical vascular disease. Enrollment spanned from March 2011 to January 2012. All subjects were ≥18 years of age, on stable ART with viral load <1,000 copies/mL, without known coronary disease or diabetes, and with a fasting LDL-cholesterol (LDL-C) ≤130mg/dL. Additional entry criteria included evidence of heightened T-cell activation (CD8+CD38+HLA−DR+ ≥19%) and/or increased inflammation (high sensitivity C-reactive protein (hs-CRP≥2mg/L). The study was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH) and written informed consent was obtained from each study subject. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology[17].
Study evaluations
At the initial screening visit, self-reported demographics and medical history were obtained along with a targeted physical exam including height, weight, waist, and hip measurements. Blood was drawn after at least a 12-hour fast for glucose, insulin, and lipoproteins. If enrollment criteria were met, subjects returned within 30 days for entry evaluations. HIV-1 RNA level and CD4+ cell count were obtained as part of routine clinical care.
Inflammation and Coagulation Markers
Several serum biomarkers of inflammation were measured: hs-CRP, IL-6, soluble tumor necrosis factor-α receptor I (sTNFR-I), and two adhesion molecules that are considered markers of endothelial activation—soluble vascular cell adhesion molecule-1 (sVCAM-1) and soluble intercellular adhesion molecule-1 (sICAM-1). D-dimer and fibrinogen were measured as serum markers of coagulation.
IL-6, sTNFR-I, sVCAM-1, and sICAM-1 were determined by quantitative sandwich ELISAs (R&D Systems, Minneapolis, MN). Inter-assay variability ranged from 2.02%-15.36%, 3.66%-5.77%, 4.76%-8.77%, and 3.43%-7.37%, respectively. Hs-CRP and fibrinogen were determined by particle enhanced immunonepholometric assays on a BNII nephelometer (Siemens). Inter-assay variability ranged from 3.01%-6.46% and 3.42%-7.59%, respectively. D-dimer was determined by immuno-turbidometric assay on a STA-R Coagulation Analyzer (DiagnosticaStago). Inter-assay variability ranged from 1.54%-9.03%.
Immune Activation Markers
The serum levels of two monocyte activation markers, soluble CD14 and soluble CD163, were measured by ELISA (R&D Systems, Minneapolis, MN). Inter-assay variability ranged from 0.4-8.6% for soluble CD14 and 0.7-18.3% for sCD163.
Monocytes and CD8+ T-cells were identified by size, granularity, and by expression of CD14 or CD3 and CD8, respectively. Monocyte phenotype was monitored by staining cells with the following fluorochrome-labeled antibodies: anti-Tissue Factor fluorescein isothiocyanate (FITC) (American Diagnostica, Stamford, CT), anti-CD14 Pacific Blue, anti-CD16 phycoerythrin (PE), (BD Pharmingen, San Diego, CA). In order to assure that monocyte populations were not contaminated by lymphocytes, preliminary experiments were performed that excluded CD3, CD20, and CD56 expressing cells.
T-cell activation was measured using anti-CD38 PE, anti-HLA-DR FITC, anti-CD3 Peridinin-chlorophyll-protein Complex (PerCP), anti-CD8 PerCP (BD Biosciences), and appropriate isotype control monoclonal antibodies.
Whole blood samples were incubated for 15 minutes on ice with FACS Lyse buffer (BD Biosciences) and then washed in wash buffer (phosphate buffered saline with 1% bovine serum albumin and 0.1% sodium azide). Cells were then stained for 30 minutes in the dark on ice and then washed in wash buffer, fixed in 1% formaldehyde. Monocytes were analyzed using a Miltenyi MACS Quant flow cytometer (MiltenyiBiotec, BergischGladbach, Germany) and MACS Quant software (version 2.21031.1, MiltenyiBiotec). T-lymphocytes were analyzed using an LSR II flow cytometer (Becton-Dickinson, San Jose, CA) and FACSDiva software version 6.1.1 (BD Biosciences, San Diego, CA).
Dual-Energy X-ray Absorptiometry
Evaluations included whole-body dual-energy absorptiometry (DEXA) scans. Fat distribution was measured by DEXA in the anteroposterior view using Lunar Prodigy Advance (GE Healthcare). Whole body fat volumes as well as peripheral fat depots (limb and arm fat) and central fat depots (trunk fat) were used for analysis. Technicians used the same machine on the same subject throughout the study. All DEXA scans were read at University Hospitals Case Medical Center by an experienced radiologist blinded to study information.
Coronary Calcium, Epicardial Fat, and Periaortic Fat
All subjects had a baseline computed tomography (CT) scan of the chest to quantify perivascular adipose tissue volume and coronary artery calcium score. A 64-slice multidetector CT scanner (Somatom Sensation 64, Siemens Medical Solutions USA) was used with 30 × 0.6mm collimation, 330ms rotation time, and 120kV tube voltage. Three-millimeter slices were obtained from the carina to the diaphragm with prospective ECG gating at 60% of the R-R interval.
Measurements were performed offline (Aquarius iNtuition Cloud, Terarecon, San Mateo, CA USA) by a single reader (CTL) on a single workstation using methods described previously[18]. Briefly, calcified coronary lesions were defined as areas of ≥6 pixels with density >130 Hounsfield units (HU). Total coronary calcium score was quantified using the Agatston method. Epicardial adipose tissue volumes were quantified using a semi-automatic segmentation technique in which the region of interest was defined by manually tracing the pericardial borders. Fat tissue was defined as pixels within a window of −195 to −45 HU, and epicardial fat was then selected as adipose tissue within the pericardial sac. Previous studies have used the term “pericardial” adipose tissue to describe this depot; however, because most of the fat within the pericardial sac is true epicardial fat, and to be consistent across studies in HIV, we opted to use the term “epicardial”. Similarly, thoracic periaortic adipose tissue was defined as all adipose tissue surrounding the thoracic aorta extending 69mm caudally from the level of the bifurcation of the pulmonary arteries. This method has been validated in the Framingham Heart Study and other populations[14, 18].
Carotid Artery Ultrasound
A high resolution B-mode ultrasound scan of the carotid arteries was performed at the entry visit using a Philips iU22 ultrasound system with a L9-3 MHz linear array transducer according to the consensus protocol of the American Society of Echocardiography[19]. Complete scans of the bilateral common carotid arteries (CCA), internal carotid arteries (ICA), and external carotid arteries (ECA) were used to identify plaque, defined as IMT >1.5cm or > 50% thicker than the adjacent vessel. R-wave gated still frame images of the distal 1cm of the CCA far wall were obtained at 3 separate angles bilaterally (anterior, lateral, and posterior). CCA-IMT was measured offline by a single reader (CTL) using semi-automated edge detection software (Medical Imaging Applications LLC, Coralville, IA). The mean-mean and mean-max CCA-IMT was used for analysis.
Statistical Analysis
Variables were examined for departures from normality of their distributions and log transformed when appropriate. Continuous variables were summarized using medians and inter-quartiles ranges and categorical variables with frequencies and percentages. The correlation between each of the perivascular fat depots (EAT and TAT) with the measures of interest was estimated using Spearman's correlation coefficient. In parallel analyses, multiple linear regression expressed the relationship of log EAT and separately of log TAT with selected covariates after adjusting for the contribution of traditional risk factors and clinical measures of adiposity. To further sharpen potential associations with presence of CAC, the highest and lowest quartiles of the distribution of EAT, and separately of TAT, were used as outcomes in binary logistic regressions, also adjusting for traditional risk factors. All statistical tests were two-sided with a 0.05 significance level. Nominal p-values are presented throughout. Analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC).
Results
Baseline characteristics
Baseline characteristics of study participants are displayed in Table 1. Approximately three-quarters were male and three-quarters were African American. HIV disease was well-controlled (mean CD4+ count was 665 cells/μL and 81% had HIV-1 RNA < 50 copies/mL), although participants were pre-selected to have higher levels of either CD8+ T-cell activation or hs-CRP. Half of the subjects were on a protease inhibitor-based regimen; however, only 4% were currently taking a thymidine analog nucleoside reverse transcriptase inhibitor and 12% were currently taking abacavir. One quarter of participants had metabolic syndrome as defined by Adult Treatment Panel III criteria[20]. Despite a low LDL-C (all ≤130mg/dL), over a third of patients had evidence of non-obstructive carotid plaque and a third of patients had detectable coronary artery calcium (CAC).
Table 1.
Baseline characteristics of study participants
| Demographics | n = 100 |
|---|---|
| Age (years) | 47 (10)* |
| Sex | |
| Male | 77% |
| Female | 23% |
| Race | |
| White | 29% |
| African American | 70% |
| HIV Parameters | |
| Current CD4+ count (cells/μl) | 665 (280) |
| Nadir CD4+ count (cells/μl) | 199 (99-299) |
| HIV duration (years) | 13 (6.6) |
| Antiretroviral therapy duration (years) | 6.3 (3.3-9.9) |
| Undetectable viral load (<48copies/ml) | 81% |
| Metabolic Parameters | |
| Systolic Blood Pressure (mmHg) | 121 (15) |
| HDL Cholesterol (mg/dL) | 48 (14) |
| LDL Cholesterol (mg/dL) | 95 (25) |
| Homeostatic Model Assessment of Insulin Resistance | 1.8 (1.1-3.2) |
| Metabolic Syndrome | 25% |
| Body Composition | |
| Body Mass Index (kg/m2) | 28 (6.4) |
| Total Lean Body Mass (kg) | 56 (11) |
| Total Body Fat (kg) | 26 (16) |
| Total Leg Fat (kg) | 8.2 (5.6) |
| Total Arm Fat (kg) | 2.3 (2.0) |
| Total Trunk Fat (kg) | 14 (8.2) |
| Other Cardiovascular Risk Factors | |
| Smoking | |
| Current | 62% |
| Past | 17% |
| Never | 21% |
| Family history of myocardial infarction | 32% |
| 10-year Framingham risk score (%) | 4.0 (1.0-7.3) |
| Baseline Inflammation and Immune Activation | |
| High sensitivity C-reactive protein (mg/L) | 2.3 (0.9-4.5) |
| CD8+CD38+HLA-DR+ (%) | 27 (22-39) |
| Current Medication Use | |
| Anti-hypertensive Drug | 22% |
| Fibrate | 5% |
| Fish Oil | 10% |
| Protease Inhibitor | 47% |
| AZT or D4T | 4% |
| Abacavir | 12% |
| Measures of Subclinical Vascular Disease | |
| Mean-mean common carotid artery IMT (μm) | 662 (627-803) |
| Mean-max common carotid artery IMT (μm) | 838 (773-984) |
| Carotid plaque (IMT > 1.5mm) | 38% |
| Coronary Artery Calcium | |
| 0 | 67% |
| 1-100 | 23% |
| >100 | 10% |
Data presented as median (interquartile range) for non-normally distributed continuous variables, mean (standard deviation) for normally distributed continuous variables, and percent for categorical variables
HDL = high-density lipoprotein, LDL = low-density lipoprotein, IMT = intima media thickness
Perivascular fat and traditional risk factors
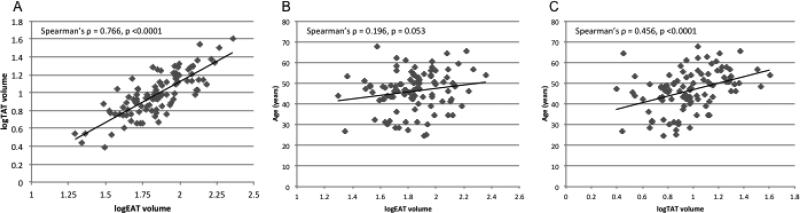
Log-transformed epicardial and thoracic periaortic fat volumes were strongly correlated with each other; although, periaortic fat was more strongly correlated with age (Figure 1). Epicardial fat did not differ by gender [median(IQR) 69(52-95) vs. 68(45-91) cm3, male vs. female, p= 0.475]; but periaortic fat volume was higher in men [9.5(7.3-15.2) vs. 7.1(5.2-9.6) cm3, p = 0.003]. Statistically significant correlations were observed between epicardial and periaortic fat and several clinical and radiologic measures of adiposity, including positive associations with both trunk fat and leg fat (Table 2). Additionally, both were significantly correlated with the metabolic syndrome and its individual components (hypertension, lower HDL, higher triglycerides, insulin resistance, and waist circumference; Figure 2). A strong and statistically significant relationship between epicardial fat and insulin resistance persisted in multivariable models (Table 3) after adjustment for age, sex, and clinical measures of adiposity (BMI and waist:hip ratio).
Figure 1.
A) Log transformed epicardial and thoracic periaortic adipose tissue volumes are linearly correlated to each other. B and C) Thoracic periaortic adipose tissue is more strongly correlated to age than epicardial adipose tissue. EAT = epicardial adipose tissue, TAT = thoracic periaortic adipose tissue.
Table 2.
Relationship of epicardial and periaortic adipose tissue to HIV parameters, metabolic parameters, body composition, biomarkers of inflammation and immune activation, and sublinical atherosclerosis
| Epicardial Adipose Tissue§ | Periaortic Adipose Tissue§ | |||
|---|---|---|---|---|
| HIV Parameters | Spearman's ρ | p value | Spearman's ρ | p value |
| Current CD4+ count | 0.207 | 0.041 | 0.092 | 0.365 |
| Nadir CD4+ count§ | −0.206 | 0.045 | −0.197 | 0.053 |
| HIV duration | 0.076 | 0.456 | 0.168 | 0.095 |
| Antiretroviral therapy duration§ | 0.232 | 0.036 | 0.304 | 0.005 |
| Duration of Protease Inhibitor Use | 0.014 | 0.910 | 0.122 | 0.311 |
| Duration of NRTI Use | 0.141 | 0.187 | 0.254 | 0.015 |
| Metabolic Parameters | ||||
| Systolic Blood Pressure | 0.230 | 0.023 | 0.227 | 0.023 |
| Use of Antihypertensive Medication | 0.215 | 0.034 | 0.258 | 0.010 |
| HDL Cholesterol | −0.340 | <0.001 | −0.345 | <0.001 |
| LDL Cholesterol | 0.044 | 0.666 | 0.051 | 0.613 |
| Triglycerides | 0.395 | <0.0001 | 0.301 | 0.002 |
| HOMA-IR§ | 0.454 | <0.0001 | 0.347 | <0.001 |
| Metabolic Syndrome | 0.347 | <0.001 | 0.321 | 0.001 |
| Body Composition | ||||
| Body Mass Index | 0.529 | <0.0001 | 0.378 | 0.0001 |
| Waist circumference | 0.650 | <0.0001 | 0.541 | <0.0001 |
| Waist:Hip Ratio | 0.576 | <0.0001 | 0.648 | <0.0001 |
| Total Lean Body Mass | 0.122 | 0.232 | 0.215 | 0.03 |
| Total Body Fat | 0.561 | <0.0001 | 0.381 | <0.0001 |
| Total Leg Fat | 0.361 | <0.001 | 0.168 | 0.094 |
| Total Arm Fat | 0.571 | <0.0001 | 0.371 | <0.001 |
| Total Trunk Fat | 0.643 | <0.0001 | 0.487 | <0.0001 |
| Biomarkers of Inflammation, Coagulation, and Immune Activation | ||||
| High sensitivity C-reactive protein | 0.307 | 0.002 | 0.186 | 0.064 |
| Interleukin-6§ | 0.245 | 0.016 | 0.195 | 0.054 |
| Soluble Tumor Necrosis Factor-α Receptor 1§ | 0.295 | 0.003 | 0.336 | <0.001 |
| Soluble Intracellular Adhesion Molecule | 0.071 | 0.490 | −0.051 | 0.615 |
| Soluble Vascular Cell Adhesion Molecule | −0.014 | 0.892 | 0.082 | 0.418 |
| Fibrinogen | 0.181 | 0.075 | 0.109 | 0.279 |
| D-dimer | −0.017 | 0.868 | −0.028 | 0.785 |
| Percent CD8+CD38+HLA-DR+ T-cells | 0.032 | 0.775 | 0.063 | 0.572 |
| Percent CD14+CD16—Monocytes | −0.004 | 0.972 | 0.021 | 0.843 |
| Percent CD14+CD16+ Monocytes | 0.030 | 0.778 | 0.017 | 0.869 |
| Soluble CD163§ | 0.282 | 0.005 | 0.274 | 0.006 |
| Soluble CD14 | 0.034 | 0.738 | −0.049 | 0.627 |
| Measures of Subclinical Atherosclerosis | ||||
| Mean-mean common carotid artery IMT§ | 0.087 | 0.401 | 0.275 | 0.006 |
| Mean-max common carotid artery IMT§ | 0.149 | 0.147 | 0.345 | <0.001 |
| Carotid plaque (IMT > 1.5mm) | 0.034 | 0.742 | 0.172 | 0.087 |
| Coronary Artery Calcium >0 | 0.228 | 0.024 | 0.244 | 0.014 |
Variable was log transformed to achieve a normal distribution
NRTI = nucleoside reverse transcriptase inhibitor, HDL = high-density lipoprotein, LDL = low-density lipoprotein, HOMA-IR = Homeostatic Model Assessment of Insulin Resistance, IMT = intima media thickness
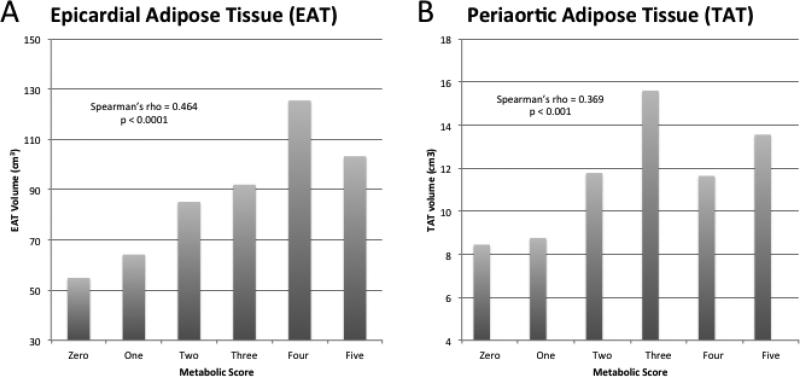
Figure 2.
Higher metabolic score is associated with larger mean volumes of epicardial (A) and thoracic periaortic (B) adipose tissue. Metabolic score is defined as the number of criteria for metabolic syndrome (out of 5) as defined by the Adult Treatment Panel III guidelines[20]. The five components are 1) waist circumference >102cm (men), >88cm (women); 2) Triglycerides ≥150mg/dL; 3) high density lipoprotein <40mg/dL (men), <50mg/dL (women); 4) blood pressure ≥130/≥85mmHg or on anti-hypertensive treatment; 5) fasting glucose ≥110mg/dL.
Table 3.
Relationship of epicardial and periaortic adipose tissue with selected biomarkers of inflammation, monocyte activation, HIV parameters, insulin resistance, and carotid intima media thickness after adjustment for other measures of adiposity and traditional risk factors.
| age, sex | age, sex, BMI | age, sex, BMI, waist:hipratio | age, sex, trunk fat | Traditional risk factors* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | Estimate | P-value | |
| Epicardial adipose tissue§ | ||||||||||
| IL-6§ | 0.371 | <0.01 | 0.293 | 0.07 | 0.33 | 0.07 | 0.298 | 0.12 | 0.317 | 0.06 |
| sTNFR-I§ | 0.234 | 0.01 | 0.234 | 0.04 | 0.123 | 0.34 | 0.134 | 0.31 | 0.263 | 0.03 |
| sCD163§ | 0.188 | 0.02 | 0.189 | 0.06 | 0.169 | 0.13 | 0.068 | 0.53 | 0.176 | 0.10 |
| ART duration§ | 0.356 | 0.06 | 0.256 | 0.26 | 0.319 | 0.22 | 0.240 | 0.37 | 0.218 | 0.37 |
| Nadir CD4+§ | −0.416 | 0.06 | −0.305 | 0.25 | −0.410 | 0.17 | −0.192 | 0.53 | −0.359 | 0.20 |
| HOMA-IR§ | 0.873 | <0.0001 | 0.666 | <0.01 | 0.637 | 0.01 | 0.641 | 0.01 | 0.580 | <0.01 |
| Mean-mean CCA-IMT§ | −0.043 | 0.20 | −0.056 | 0.18 | −0.78 | 0.10 | −0.033 | 0.50 | −0.048 | 0.24 |
| Mean-max CCA-IMT§ | −0.024 | 0.47 | −0.033 | 0.43 | −0.070 | 0.14 | −0.012 | 0.81 | −0.03 | 0.45 |
| Periaortic adipose tissue§ | ||||||||||
| IL-6§ | 0.309 | 0.01 | 0.197 | 0.20 | 0.221 | 0.20 | 0.16 | 0.37 | 0.225 | 0.15 |
| sTNFR-I§ | 0.270 | <0.01 | 0.292 | <0.01 | 0.199 | 0.11 | 0.216 | 0.09 | 0.317 | <0.01 |
| sCD163§ | 0.150 | 0.06 | 0.135 | 0.19 | 0.104 | 0.36 | 0.151 | 0.22 | 0.093 | 0.39 |
| ART duration§ | 0.231 | 0.196 | 0.092 | 0.67 | 0.099 | 0.68 | 0.016 | 0.95 | 0.033 | 0.88 |
| Nadir CD4+§ | −0.359 | 0.09 | −0.234 | 0.36 | −0.329 | 0.26 | −0.116 | 0.69 | −0.128 | 0.31 |
| HOMA-IR§ | 0.734 | <0.0001 | 0.489 | 0.02 | 0.421 | 0.08 | 0.407 | 0.10 | 0.377 | 0.07 |
| Mean-mean CCA-IMT§ | −0.008 | 0.78 | −0.004 | 0.93 | −0.011 | 0.80 | 0.037 | 0.41 | 0.02 | 0.58 |
| Mean-max CCA-IMT§ | 0.017 | 0.60 | 0.027 | 0.50 | 0.006 | 0.89 | 0.069 | 0.13 | 0.046 | 0.24 |
Adjusts for age, sex, BMI, smoking status, systolic blood pressure, HDL, non-HDL, and family history of myocardial infarction
Variable was log transformed to achieve a normal distribution
BMI = body mass index, IL-6 = interleukin 6, sTNFR-I = soluble tumor necrosis factor alpha receptor 1, sCD163, ART = antiretroviral therapy, HOMA-IR = homeostasis model of insulin resistance, CCA-IMT = common carotid artery intima-media thickness
Perivascular fat, HIV-related factors, and Inflammation
Higher epicardial fat volume was associated with higher current CD4+ count and lower nadir CD4+ count (Table 2); whereas, the association between nadir CD4+ count and periaortic fat was borderline statistically significant (p=0.053). Both fat depots were associated with total duration of antiretroviral therapy but not with total duration of protease inhibitor use. Several serum biomarkers of inflammation were correlated with epicardial and periaortic fat (Table 2); however, biomarkers of endothelial activation and coagulation were not associated. Notably, one marker of monocyte activation—soluble CD163—was significantly correlated with both fat depots, whereas sCD14 and percent circulating CD14+CD16+ monocytes were not.
Compared to those in the lowest quartile (<51.3cm3), participants in the highest quartile of epicardial fat volume (>94cm3) had significantly higher levels of inflammatory biomarkers including 3.8-fold higher mean IL-6 (p=0.022), 1.4-fold higher TNFαRI (p = 0.006), and 1.5-fold higher sCD163 (p=0.004). Similar results were seen when the highest (>13.5cm3) and lowest (<6.53cm3) quartiles of periaortic fat volumes were compared (p=0.087, p=0.002, p=0.017 for IL-6, TNFαRI, and sCD163 respectively).
In multivariable linear regression models (Table 3), adjustment for age, sex, and clinical measures of adiposity partially removed associations of epicardial and periaortic fat with markers of inflammation and soluble CD163. Adjustment for trunk fat by DEXA reduced the estimated effect even further.
Perivascular fat and subclinical atherosclerosis
Epicardial and periaortic fat were both correlated with subclinical coronary atherosclerosis as defined by the presence of detectable CAC score >0 (Table 2). An independent association between both fat depots and presence of CAC generally persisted in models that adjusted for age, sex, measures of adiposity, and traditional cardiovascular risk factors (Table 4). Participants in the highest quartile of epicardial fat had 2.2-fold higher odds of having any detectable CAC compared to those in the lowest quartile (p =0.04). Periaortic fat was significantly correlated with mean and max CCA-IMT (Table 2); however, adjustment for age and sex completely removed this association (Table 4).
Table 4.
Relationship of epicardial and periaortic adipose tissue with the presence of coronary artery calcium after adjustment for other measures of adiposity and traditional risk factors.
| age, sex | age, sex, BMI | age, sex, BMI, waist:hipratio | age, sex, trunk fat | Traditional risk factors* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | P-value | Odds Ratio | P-value | Odds Ratio | P-value | Odds Ratio | P-value | Odds Ratio | P-value | |
| Epicardial adipose tissue | 1.015 | 0.028 | 1.032 | 0.004 | 1.029 | 0.016 | 1.042 | 0.001 | 1.032 | 0.004 |
| Periaortic adipose tissue | 1.083 | 0.047 | 1.148 | 0.016 | 1.114 | 0.094 | 1.181 | 0.012 | 1.147 | 0.008 |
Odds ratio per 1cm3 increase in adipose tissue volumes
Adjusts for age, sex, body mass index, smoking status, systolic blood pressure, high density lipoprotein (HDL), non-HDL, and family history of myocardial infarction infarction
Discussion
The aim of this study was to investigate the relationship between perivascular fat depots, systemic biomarkers of inflammation, insulin resistance and subclinical atherosclerosis in HIV-infected patients on antiretroviral therapy (ART). For the first time, periaortic adipose tissue is described in this population. Our data are consistent with a model of excessive epicardial and periaortic fat as a marker of pathologic adipose tissue hypertrophy and dysfunction that contributes to chronic inflammation, insulin resistance, and subclinical atherosclerosis observed in HIV even with more metabolically favorable ART regimens.
Despite considerable variability in methods, prior studies of echocardiography-derived epicardial fat thickness and CT-derived volumes in HIV-infected patients have described similar correlations with visceral adiposity measured by MRI[21] or CT[11, 12]. Our study also confirms the associations of CT epicardial fat volume with insulin resistance[11], metabolic syndrome and its individual components[21], and coronary artery calcium[12]. Our study further demonstrates that the remarkably strong association with insulin resistance persists even after adjustment for BMI, visceral adiposity, and traditional cardiovascular disease risk factors.
HIV-infected subjects are prone to aortic aneurysms[22, 23] and have elevated levels of aortic wall inflammation[16] and atherosclerosis[24] compared to uninfected controls. Prior studies in the uninfected population have shown that periaortic fat volume increases with age and male sex, two significant risk factors for aortic aneurysms in the general population[25]. Periaortic fat has also been associated with both aortic and coronary calcification[14, 18] and peripheral arterial disease[14]. In HIV-infected patients, we demonstrate that periaortic adipose tissue volume appears to be more strongly associated with age and male sex than epicardial adipose tissue. Similarly to uninfected populations, periaortic fat was independently associated with coronary artery calcification in our study after adjustment for multiple potential confounders. Periaortic fat was also correlated with carotid IMT, a surrogate measure of abnormal arterial remodeling; although, adjustment for age and sex completely removed this association.
As in other studies of lipodystrophy and surrogate measures of cardiovascular risk in HIV[26-29], perivascular fat depots were associated with lower nadir CD4 count and longer cumulative duration of ART; although this association does not appear to be independent of age, sex, measures of adiposity, and traditional risk factors. Interestingly, neither current nor cumulative duration of protease inhibitor use was associated with perivascular fat depots in our study.
One of the principle strengths of our study was the ability to comprehensively evaluate the association of fat depots with multiple biomarkers of inflammation and immune activation. In contrast to Lo et al.[11], we found significant positive associations between perivascular adipose tissue volume and a variety of serum markers of inflammation including sCD163, a marker of monocyte activation. The pro-inflammatory role of adipose tissue is well-described[30], and the qualitative function of fat may be more important than fat quantity in mediating disease[31]. To what extent pro-inflammatory cytokines act locally in a paracrine fashion vs. systemically in an endocrine fashion is not clear; although, community based cohort studies outside of HIV have also been able to demonstrate relationships between circulating biomarkers of inflammation and quantities of visceral[32], subcutaneous[32], and perivascular fat[33].
Both macrophages[34, 35] and T-cells[36] appear to mediate inflammation in visceral and epicardial fat. In particular, sCD163, a marker of monocyte/macrophage activation, is elevated in obesity[37] and is associated with insulin resistance in the obese state[38]. While previous studies have associated sCD163 with non-calcified coronary plaque by CT angiography[39] and aortic inflammation by positron emission tomography[16], neither study adjusted for any measure of adiposity. Our findings suggest that visceral and perivascular adipose tissue volumes are associated with systemic levels of sCD163 and may partly explain the relationship between sCD163 and cardiovascular disease. On the other hand, we did not observe any relationship between perivascular fat depots and sCD14, circulating inflammatory CD14+CD16+ monocytes, or activated CD8+CD38+HLA−DR+ T-cells. Future longitudinal studies should further examine the relationship between inflamed perivascular adipose tissue and vascular disease in HIV. Future studies should also examine the relative utility of CT-derived adipose tissue volumes versus echocardiography-derived fat pad thickness in longitudinal studies of cardiovascular risk in this population.
Study Limitations
Because the study population was pre-selected to have higher levels of inflammation or CD8+ T-cell activation, our results may not be generalizeable to the entire HIV-infected population. This may also be considered a strength, however, because it allows us to characterize a subset of HIV-infected individuals that may be at high risk of cardiovascular disease despite low LDL-C. Our study also cannot prove causality or rule out the possibility of residual confounding, although this is a limitation of all cross-sectional studies. Our study was adequately powered to detect significant relationships with several serum markers of inflammation, but may have lacked power to detect more subtle relationships with other markers of immune activation. Finally, we did not measure anti-inflammatory soluble biomarkers of inflammation; although, the classic non-inflammatory monocyte population (CD14+CD16–) was not associated with either fat depot in our study.
Conclusions
Two important perivascular fat depots, epicardial and thoracic periaortic adipose tissue, are associated with biomarkers of inflammation, monocyte/macrophage activation, and insulin resistance in this population of virologically suppressed HIV-infected patients on ART, although many of these relationships appear dependent on other measures of adiposity. Furthermore, both perivascular fat depots are independently associated with subclinical atherosclerosis as defined by the presence of coronary artery calcium. The effect of statins on perivascular fat volumes and inflammation will be tested in the ongoing SATURN-HIV trial.
Acknowledgments
CTL has received a research grant from Bristol-Myers Squibb. SD currently serves on a DSMB of a Johnson and Johnson study. GAM has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Tibotec, and Gilead Sciences, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and is currently serving as the DSMB Chair for a Pfizer-sponsored study.
Grant Support: This project was supported by the National Institutes of Health [NR012642 to GM and T32 AI052067]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: YJ, CY, NS, DEL, NTF, HB, and MML have no disclosures.
References
- 1.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011 Jul 15;53(2):185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tungsiripat M, McComsey G. Pathogenesis and management of lipoatrophy. Curr HIV/AIDS Rep. 2008 May;5(2):55–63. doi: 10.1007/s11904-008-0010-8. [DOI] [PubMed] [Google Scholar]
- 3.McComsey G, Rightmire A, Wirtz V, Yang R, Mathew M, McGrath D. Changes in body composition with ritonavir-boosted and unboosted atazanavir treatment in combination with Lamivudine and Stavudine: a 96-week randomized, controlled study. Clin Infect Dis. 2009 May 1;48(9):1323–6. doi: 10.1086/597776. [DOI] [PubMed] [Google Scholar]
- 4.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009 Jan;17(1):53–9. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 5.Lake JE, Wohl D, Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011 Aug;23(8):929–38. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunfeld C, Saag M, Cofrancesco J, Jr., et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. Aids. 2010 Jul 17;24(11):1717–26. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker AR, Silva NF, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker AR, Harte AL, Howell N, et al. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab. 2009 Jan;94(1):261–7. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 9.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003 Nov 18;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 10.Campbell KA, Lipinski MJ, Doran AC, Skaflen MD, Fuster V, McNamara CA. Lymphocytes and the adventitial immune response in atherosclerosis. Circ Res. 2012 Mar 16;110(6):889–900. doi: 10.1161/CIRCRESAHA.111.263186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo J, Abbara S, Rocha-Filho JA, Shturman L, Wei J, Grinspoon SK. Increased epicardial adipose tissue volume in HIV-infected men and relationships to body composition and metabolic parameters. Aids. 2010 Aug 24;24(13):2127–30. doi: 10.1097/QAD.0b013e32833c055a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guaraldi G, Scaglioni R, Zona S, et al. Epicardial adipose tissue is an independent marker of cardiovascular risk in HIV-infected patients. Aids. 2011 Jun 1;25(9):1199–205. doi: 10.1097/QAD.0b013e3283474b9f. [DOI] [PubMed] [Google Scholar]
- 13.Gabriella O, Giovanni G, Stefano Z, et al. Ectopic fat is linked to prior cardiovascular events in men with HIV. J Acquir Immune Defic Syndr. 2012 Apr 15;59(5):494–7. doi: 10.1097/QAI.0b013e31824c8397. [DOI] [PubMed] [Google Scholar]
- 14.Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010 Jun;210(2):656–61. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox CS, Massaro JM, Schlett CL, et al. Periaortic fat deposition is associated with peripheral arterial disease: the Framingham heart study. Circ Cardiovasc Imaging. 2010 Sep;3(5):515–9. doi: 10.1161/CIRCIMAGING.110.958884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012 Jul 25;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the International Journal of Cardiology. Int J Cardiol. 2011 Dec 15;153(3):239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 18.Yun CH, Lin TY, Wu YJ, et al. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur J Radiol. 2012 Apr;81(4):749–56. doi: 10.1016/j.ejrad.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008 Feb;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. quiz 89-90. [DOI] [PubMed] [Google Scholar]
- 20.Grundy SM, Brewer HB, Jr., Cleeman JI, Smith SC, Jr., Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis G, Pellicelli AM, Grisorio B, et al. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring) 2008 Jan;16(1):179–83. doi: 10.1038/oby.2007.50. [DOI] [PubMed] [Google Scholar]
- 22.Nair R, Abdool-Carrim A, Chetty R, Robbs J. Arterial aneurysms in patients infected with human immunodeficiency virus: a distinct clinicopathology entity? J Vasc Surg. 1999 Apr;29(4):600–7. doi: 10.1016/s0741-5214(99)70304-6. [DOI] [PubMed] [Google Scholar]
- 23.Tilson MD, 3rd, Withers L. Arterial aneurysms in HIV patients: molecular mimicry versus direct infection? Ann N Y Acad Sci. 2006 Nov;1085:387–91. doi: 10.1196/annals.1383.018. [DOI] [PubMed] [Google Scholar]
- 24.Floris-Moore M, Fayad ZA, Berman JW, et al. Association of HIV viral load with monocyte chemoattractant protein-1 and atherosclerosis burden measured by magnetic resonance imaging. Aids. 2009 May 15;23(8):941–9. doi: 10.1097/QAD.0b013e328329c76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The Tromso Study. Am J Epidemiol. 2001 Aug 1;154(3):236–44. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 26.Ho JE, Scherzer R, Hecht FM, et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. Aids. 2012 Jun 1;26(9):1115–20. doi: 10.1097/QAD.0b013e328352ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010 Aug 15;51(4):435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 28.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010 Dec 15;55(5):615–9. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seminari E, Tinelli C, Minoli L, et al. Evaluation of the risk factors associated with lipodystrophy development in a cohort of HIV-positive patients. Antivir Ther. 2002 Sep;7(3):175–80. [PubMed] [Google Scholar]
- 30.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011 Jun 21;57(25):2461–73. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Farb MG, Bigornia S, Mott M, et al. Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J Am Coll Cardiol. 2011 Jul 12;58(3):232–7. doi: 10.1016/j.jacc.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007 Sep 11;116(11):1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 33.Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010 May;18(5):1039–45. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008 Sep;28(9):1654–9. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirata Y, Tabata M, Kurobe H, et al. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011 Jul 12;58(3):248–55. doi: 10.1016/j.jacc.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 36.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007 Feb 27;115(8):1029–38. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 37.Sporrer D, Weber M, Wanninger J, et al. Adiponectin downregulates CD163 whose cellular and soluble forms are elevated in obesity. Eur J Clin Invest. 2009 Aug;39(8):671–9. doi: 10.1111/j.1365-2362.2009.02170.x. [DOI] [PubMed] [Google Scholar]
- 38.Zanni MV, Burdo TH, Makimura H, Williams KC, Grinspoon SK. Relationship between Monocyte/Macrophage Activation Marker Soluble CD163 and Insulin Resistance in Obese and Normal-Weight Subjects. Clin Endocrinol (Oxf) 2011 Nov 19; doi: 10.1111/j.1365-2265.2011.04284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011 Oct 15;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]