Abstract
Background
Depression in women is a public health problem. Studies have reported positive associations between pesticides and depression, but few studies were prospective or presented results for women separately.
Objectives
We evaluated associations between pesticide exposure and incident depression among farmers’ wives in the Agricultural Health Study, a prospective cohort study in Iowa and North Carolina.
Methods
We used data on 16,893 wives who did not report physician-diagnosed depression at enrollment (1993-1997) and who completed a follow-up telephone interview (2005-2010). Among these wives, 1,054 reported physician diagnoses of depression at follow-up. We collected information on potential confounders and on ever use of any pesticide, 11 functional and chemical classes of pesticides, and 50 specific pesticides by wives and their husbands via self-administered questionnaires at enrollment. We used inverse probability weighting to adjust for potential confounders and to account for possible selection bias induced by the death or loss of 10,639 wives during follow-up. We used log-binomial regression models to estimate risk ratios and 95% confidence intervals.
Results
After weighting for age at enrollment, state of residence, education level, diabetes diagnosis, and not dropping out of the cohort, wives’ incident depression was positively associated with diagnosed pesticide poisoning, but was not associated with ever using any pesticide. Use of individual pesticides or functional or chemical classes of pesticides was generally not associated with wives’ depression. Among wives who never used pesticides, husbands’ ever use of individual pesticides or functional or chemical classes of pesticides was generally not associated with wives’ incident depression.
Conclusions
Our study adds further evidence that high level pesticide exposure, such as pesticide poisoning, is associated with increased risk of depression and sets a lower bound on the level of exposure related to depression, thereby providing reassurance that the moderate levels of pesticide exposure experienced by farmers’ wives likely do not increase risk.
Keywords: depression, female, incidence, pesticides, spouses
1. Introduction
The lifetime prevalence of doctor diagnosed depression among American women was recently reported as 20.2%, which was almost double the prevalence (11.1%) in American men (Strine et al., 2008). Although the cause of the higher prevalence of depression among women, and the cause of depression in general, remains unknown, it has been hypothesized to involve both biological susceptibilities and environmental risk factors (Kessler, 2003).
Higher rates of depression and other psychiatric conditions have been linked to exposure to pesticides, particularly organophosphate insecticides, and living on or near farms (Bazylewicz-Walczak et al., 1999; Beseler and Stallones, 2008; Beseler et al., 2006, 2008; Carruth and Logan, 2002; London et al., 2005; Mackenzie Ross et al., 2010; Meyer et al., 2010; Rehner et al., 2000; Salvi et al., 2003; Stallones and Beseler, 2002a, 2002b; Villeneuve et al., 2009; Wesseling et al., 2010). Only a few of the previous studies of pesticide exposure and depression, however, were prospective (Bazylewicz-Walczak et al., 1999; Beseler and Stallones, 2008; Salvi et al., 2003). The largest of these was a longitudinal study of about 600 farmers and their spouses in Colorado (Beseler and Stallones, 2008). In that study, depression was assessed annually for three years using the Center for Epidemiological Studies-Depression Scale (Beseler and Stallones, 2008), which assesses depression during the past week (Radloff, 1977). Farmers and their spouses who reported past pesticide poisoning at baseline were twice as likely to be classified as depressed during follow-up compared to those who did not report pesticide poisoning (Beseler and Stallones, 2008). However, associations for women were not reported separately from men in that study and associations between depression and specific pesticides, pesticide classes, or chronic, low-dose pesticide exposure were not assessed.
Four studies have evaluated pesticide exposure and depression in women (Bazylewicz-Walczak et al., 1999; Beseler et al., 2006; Carruth and Logan, 2002; Meyer et al., 2010). Bazylewicz-Walczak et al. (1999) administered the Profile of Mood States to 51 women working in the gardening industry in Poland (26 exposed to organophosphate insecticides for one season, March-June, and 25 not exposed) and found exposed women experienced greater tension, depression, and fatigue compared to unexposed women. A cross-sectional survey of 657 randomly sampled farm women in Louisiana found that women who reported pesticide use were more likely to report depressive symptoms than those who did not use pesticides (Carruth and Logan, 2002). Residents of an agricultural area of Brazil with an intensive use of pesticides had higher rates of hospitalization for mood disorders (International Classification of Diseases, 10th Revision codes F30-F39) than two reference areas (Meyer et al., 2010). In the Agricultural Health Study, wives who had ever received a physician-diagnosis of pesticide poisoning were more likely to report ever receiving a physician-diagnosis of depression than those without pesticide poisoning (Beseler et al., 2006). Relationships between specific pesticides and depression were not evaluated in any of these studies.
The Agricultural Health Study is a prospective cohort study of 57,310 licensed pesticide applicators (private and commercial) in Iowa and North Carolina and 32,345 spouses of private applicators. It was designed to assess associations between pesticides and other agricultural hazards and cancer and non-cancer endpoints (Alavanja et al., 1996). In addition to the study of wives (Beseler et al., 2006), a higher prevalence of depression was previously reported among male applicators in the Agricultural Health Study who experienced a past pesticide poisoning or who reported ever using pesticides from several different pesticide classes (Beseler et al., 2008). Neither study, however, evaluated relationships between specific pesticides and depression and both used cross-sectional designs (Beseler et al., 2006, 2008). The current analysis evaluates associations between both general and specific pesticide use and self-reported, incident depression among wives in the Agricultural Health Study.
2. Materials and Methods
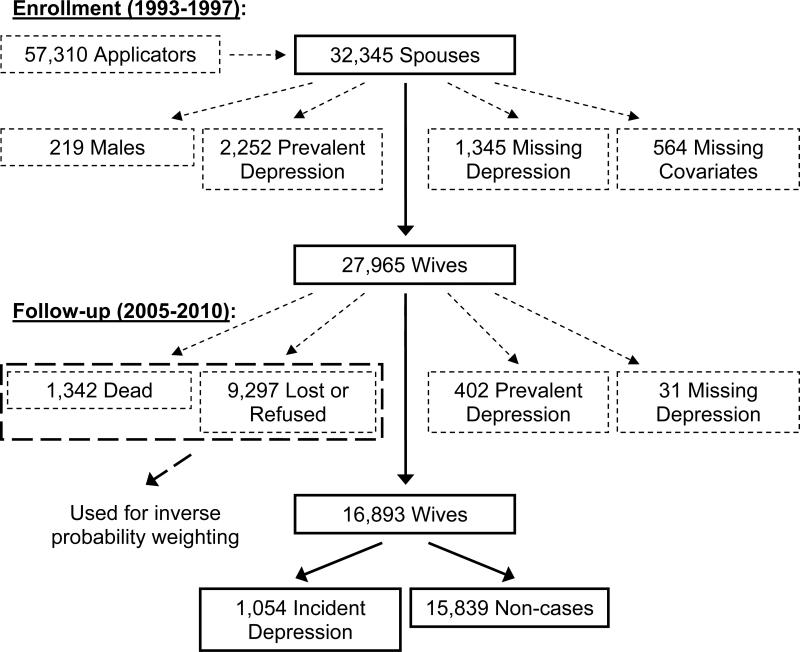
2.1. Study population and case definition (Figure 1)
Figure 1.
Flow diagram depicting the study population for an analysis of pesticide use and self-reported, incident depression in wives from Iowa and North Carolina enrolled in the Agricultural Health Study. Boxes or lines marked with solid lines represent individuals who remained in the study after each step shown, whereas boxes or lines marked with small dashes represent individuals who were excluded after each step shown (see “2.1. Study population and case definition” for more details). Boxes or lines marked with large dashes represent individuals who, although not directly included in the analysis, were incorporated into the analysis via inverse probability weighting (see “2.3. Statistical analyses” for more details).
The Agricultural Health Study cohort was assembled in 1993-1997 by enrolling pesticide applicators who were at state facilities to receive or renew their pesticide-use licenses (Alavanja et al., 1996); 84% of eligible applicators enrolled by completing a questionnaire. Additional questionnaires were sent home with married private applicators to enroll their spouses (Alavanja et al., 1996); 32,345 spouses (75% of those eligible) enrolled. We excluded 4,380 spouses from this analysis because they were male (219; < 1%), reported having been diagnosed with depression by a physician at enrollment (2,252; 7%; prevalent depression), were missing data on depression at enrollment (1,345; 4%), or were missing covariate data (564; 2%).
Incident depression was ascertained through a follow-up telephone interview completed in 2005-2010. On average, the time between enrollment in the Agricultural Health Study and the follow-up interview was 11.9 years. Of 27,965 eligible wives, 10,639 (38%) did not complete the follow-up interview (1,342 because of death). We further excluded 433 wives because they reported an age at depression diagnosis prior to their age at enrollment in the Agricultural Health Study (402; 1%; prevalent depression) or were missing data on age at depression diagnosis (31; < 1%). In total, we included 16,893 wives in this analysis: 1,054 (6%) who reported ever having been diagnosed with depression (incident depression cases) and 15,839 (94%) who did not (non-cases) (Figure 1).
Information on depression was ascertained using four different questions (Agricultural Health Study, 2012). Prevalent depression was ascertained via the enrollment questionnaire using the question “Has a DOCTOR ever told you that you had (been diagnosed with)...[d]epression requiring medication? (No, Yes)”. Incident depression was ascertained through a follow-up telephone interview via the question “Have you ever been diagnosed with depression? (No, Yes)”. Age at depression diagnosis was ascertained at follow-up via the question “How old were you when you were first diagnosed with depression? (years)”. We assigned any wife who reported an age at depression diagnosis that was less than her age at enrollment to have prevalent depression. Treatment of depression with medications was ascertained among incident cases at follow-up via the question “Are you currently taking any prescribed medicines for depression? (No, Yes)”. We used all incident depression cases for our main analyses, but conducted a sensitivity analysis in which we refit models restricting incident depression cases to wives who had taken medication for their depression.
The Agricultural Health Study was approved by the Institutional Review Boards of the National Institutes of Health and its contractors; the current analysis involving coded data was exempted from review by the Institutional Review Board of the University of North Carolina at Chapel Hill. All participants provided implied informed consent by completing and returning the enrollment questionnaires after the study was explained to them.
2.2. Exposure assessment
Information on demographics, medical conditions, lifestyle, pesticide use, and other agricultural hazards and practices was collected from wives and their applicator husbands via self-administered questionnaires at enrollment in the Agricultural Health Study (Agricultural Health Study, 2012; Alavanja et al., 1996). Exposure variables used in this analysis included wives’ and husbands’ ever use of 1) any pesticide, 2) 11 pesticide classes (four functional: fumigants, fungicides, herbicides, and insecticides; and seven chemical: carbamates, chloroacetanilide herbicides, organochlorine insecticides, organophosphate insecticides, phenoxy herbicides, pyrethroid insecticides, and triazine herbicides), and 3) 50 individual pesticides. We present results for only those pesticides for which there were at least five exposed cases. The variables for the 11 pesticide classes were created from the responses for the individual pesticides that comprised each class. We additionally analyzed data on wives’ exposure to pesticides in the non-farm job held longest and physician-diagnosed pesticide poisoning. Information on duration (years) and frequency (days per year) was collected for wives’ overall use of pesticides, but not for their use of individual pesticides or pesticide classes. We also had information on duration and frequency for husbands’ overall use of pesticides. We created variables representing wives’ and husbands’ cumulative lifetime days of overall pesticide use by multiplying the values of the duration and frequency variables and then categorizing the result into quartiles.
2.3. Statistical analyses
We evaluated associations between both general and specific pesticide use and self-reported, incident depression among wives in the Agricultural Health Study. We treated the 16,893 wives included in this analysis as a closed cohort and used log-binomial regression models to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for each association. Although using Cox proportional hazards regression models to calculate hazard ratios is often preferred for analyses of prospective cohort data because they can incorporate information on censoring and the amount of time at risk for disease (Allison, 2010), we did not have information on the exact date of depression diagnosis. Even if date of depression diagnosis was available, it may not represent the earliest occurrence because depression is an ongoing condition that may begin before first recognition or diagnosis (Farr et al., 2010). Therefore, we used log-binomial regression models to calculate RRs for our main analyses, but conducted a sensitivity analysis in which we used Cox proportional hazards regression models with time on study as the time scale to calculate hazard ratios and 95% CIs for each association. For the latter analysis, we calculated time at risk for incident depression by first assigning the date corresponding to the midpoint of the year of the age at depression diagnosis as the date of depression diagnosis. We then calculated the time at risk for incident depression as the difference (in days) between the date of enrollment in the Agricultural Health Study and the approximate date of depression diagnosis (cases), date of death (non-cases who died), or date of the follow-up telephone interview (living non-cases.
We used information from the enrollment questionnaire on potential confounders identified from the previous literature, i.e., age, state, race/ethnicity, education (as a measure of socioeconomic status), number of children in family (as a measure of social connection), farm size, frequency of alcohol use in past year, cigarette smoking, number of doctor visits in past year (as a measure of general health), diabetes or heart disease diagnoses (as measures of longstanding illness, disability, or infirmity), number of years lived or worked on a farm, working a job off a farm, and solvent (other than gasoline) exposure at the non-farm job held the longest. We obtained information on number of children in the family and farm size from participants’ husbands’ responses. Ever diagnosed with heart disease was defined as reporting myocardial infarction, angina, or arrhythmia.
We used a directed acyclic graph (Glymour and Greenland, 2008; Greenland et al., 1999) to analyze potential confounders listed above and identified two minimally sufficient adjustment sets: 1) age, alcohol use, diabetes, smoking, solvents, and state; and 2) age, diabetes, education, and state (Supplementary Data, Figure S.1). We used the second minimally sufficient adjustment set as the final model because it had less missing covariate information than the first set.
We used stabilized inverse probability weights (a type of propensity score) to adjust for the covariates in the second minimally sufficient adjustment set and to account for the loss of the 10,639 wives who did not complete the follow-up interview (Figure 1; Cole and Hernán, 2008; Hernán et al., 2004). Specifically, we calculated two types of stabilized weights for each exposure, confounding weights and selection weights, and then calculated the overall stabilized weight as the product of the two weights (Cole and Hernán, 2008; Robins et al., 2000). We then applied the overall stabilized weight to log-binomial regression models for incident depression that contained the exposure of interest as the only explanatory variable in the same way sampling weights are applied when analyzing data from complex survey sampling designs (Cole and Hernán, 2008; Robins et al., 2000). We used robust variance estimates to calculate 95% CIs because using weights for analysis induces within-subject correlation (Hernán et al., 2000). More details regarding the rationale behind, assumptions for, and references describing inverse probability weights are provided in the Supplementary Data, p. S4.
We used linear, logistic, or polytomous logistic regression models, depending on the nature of the exposure variable, to calculate the stabilized confounding weights. Specifically, we calculated the numerators of these weights as predicted probabilities of exposure from an intercept only model and the denominators of these weights as predicted probabilities of exposure from a model including the covariates in the second minimally sufficient adjustment set as explanatory variables. In the denominator model, we fit age as a restricted, quadratic spline with three equally spaced knots at ages 36, 43, and 52 years based on percentiles of the age distribution in all cases. Diabetes, education, and state were modeled as shown in Table 1.
Table 1.
Association of incident depression with characteristics of wives enrolled in the Agricultural Health Study.
| All wives | Wives who never used pesticides | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (n = 1,054) | Total (n = 16,893) | Adjusteda | Cases (n = 444) | Total (n = 6,830) | Adjusteda | |||||||
| Characteristic | No. | % | No. | % | RR | 95% CI | No. | % | No. | % | RR | 95% CI |
| Age at enrollment (years) | ||||||||||||
| ≤ 25 | 19 | 2 | 266 | 2 | 0.99 | 0.64, 1.55 | 11 | 2 | 162 | 2 | 0.86 | 0.48, 1.55 |
| 26-35 | 211 | 20 | 2,739 | 16 | 1.07 | 0.91, 1.26 | 107 | 24 | 1,338 | 20 | 1.01 | 0.80, 1.28 |
| 36-45 | 370 | 35 | 5,138 | 30 | 1.00 | Referent | 155 | 35 | 1,964 | 29 | 1.00 | Referent |
| 46-55 | 259 | 25 | 4,648 | 28 | 0.77 | 0.66, 0.90 | 89 | 20 | 1,612 | 24 | 0.70 | 0.54, 0.90 |
| 56-65 | 153 | 15 | 3,230 | 19 | 0.66 | 0.55, 0.79 | 60 | 14 | 1,304 | 19 | 0.58 | 0.44, 0.78 |
| > 65 | 42 | 4 | 872 | 5 | 0.67 | 0.49, 0.91 | 22 | 5 | 450 | 7 | 0.62 | 0.40, 0.96 |
| State of residence | ||||||||||||
| Iowa | 718 | 68 | 12,146 | 72 | 1.00 | Referent | 291 | 66 | 4,483 | 66 | 1.00 | Referent |
| North Carolina | 336 | 32 | 4,747 | 28 | 1.28 | 1.13, 1.46 | 153 | 34 | 2,347 | 34 | 1.12 | 0.93, 1.36 |
| Race/Ethnicity | ||||||||||||
| Non-Hispanic white | 1,036 | 98 | 16,508 | 98 | 1.00 | Referent | 428 | 96 | 6,586 | 96 | 1.00 | Referent |
| Other | 18 | 2 | 367 | 2 | 0.82 | 0.52, 1.29 | 16 | 4 | 240 | 4 | 1.11 | 0.68, 1.79 |
| Missing | 0 | 18 | 0 | 4 | ||||||||
| Education level | ||||||||||||
| Some high school or less or something else | 177 | 17 | 2,238 | 13 | 1.32 | 1.11, 1.57 | 70 | 16 | 821 | 12 | 1.42 | 1.08, 1.86 |
| High school graduate or General Equivalency Diploma | 341 | 32 | 5,867 | 35 | 1.00 | Referent | 149 | 34 | 2,535 | 37 | 1.00 | Referent |
| 1-3 years of vocational education beyond high school, some college, or college graduate | 473 | 45 | 7,743 | 46 | 0.95 | 0.83, 1.10 | 202 | 45 | 3,040 | 45 | 0.99 | 0.81, 1.23 |
| One or more years of graduate or professional school | 63 | 6 | 1,045 | 6 | 0.97 | 0.75, 1.26 | 23 | 5 | 434 | 6 | 0.82 | 0.54, 1.26 |
| Number of children in family | ||||||||||||
| 0 | 90 | 9 | 914 | 6 | 1.00 | Referent | 44 | 10 | 441 | 7 | 1.00 | Referent |
| 1 | 97 | 9 | 1,547 | 9 | 0.66 | 0.50, 0.88 | 53 | 12 | 771 | 12 | 0.73 | 0.50, 1.08 |
| 2 | 390 | 38 | 5,511 | 34 | 0.79 | 0.63, 0.98 | 169 | 39 | 2,280 | 35 | 0.83 | 0.60, 1.14 |
| 3-4 | 382 | 37 | 6,857 | 42 | 0.64 | 0.51, 0.80 | 138 | 32 | 2,516 | 38 | 0.64 | 0.46, 0.90 |
| > 4 | 67 | 7 | 1,489 | 9 | 0.56 | 0.41, 0.77 | 26 | 6 | 557 | 8 | 0.60 | 0.37, 0.98 |
| Missing | 28 | 575 | 14 | 265 | ||||||||
| Size of farm worked last year (acres) | ||||||||||||
| Didn't work on a farm or < 5 | 67 | 7 | 855 | 5 | 1.46 | 1.15, 1.85 | 32 | 8 | 420 | 7 | 1.36 | 0.96, 1.94 |
| 5-49 | 90 | 9 | 1,216 | 8 | 1.35 | 1.09, 1.66 | 36 | 9 | 566 | 9 | 1.10 | 0.79, 1.54 |
| > 49 | 817 | 84 | 13,609 | 87 | 1.00 | Referent | 338 | 83 | 5,250 | 84 | 1.00 | Referent |
| Missing | 80 | 1,213 | 38 | 594 | ||||||||
| Frequency of alcohol consumption during past 12 months | ||||||||||||
| Never | 432 | 41 | 7,341 | 44 | 1.00 | Referent | 182 | 41 | 3,274 | 48 | 1.00 | Referent |
| < 1 time a month | 327 | 31 | 4,706 | 28 | 1.07 | 0.93, 1.24 | 135 | 31 | 1,807 | 27 | 1.19 | 0.95, 1.49 |
| 1 time a month to 1 time a week | 232 | 22 | 3,794 | 23 | 0.93 | 0.80, 1.09 | 97 | 22 | 1,403 | 21 | 1.09 | 0.86, 1.40 |
| > 1 time a week | 58 | 6 | 929 | 6 | 1.01 | 0.78, 1.32 | 27 | 6 | 294 | 4 | 1.56 | 1.06, 2.29 |
| Missing | 5 | 123 | 3 | 52 | ||||||||
| Cigarette smoking status | ||||||||||||
| Never | 694 | 67 | 12,411 | 75 | 1.00 | Referent | 292 | 68 | 5,035 | 76 | 1.00 | Referent |
| Past | 194 | 19 | 2,685 | 16 | 1.31 | 1.12, 1.53 | 82 | 19 | 1,016 | 15 | 1.39 | 1.10, 1.75 |
| Current | 142 | 14 | 1,357 | 8 | 1.82 | 1.54, 2.17 | 55 | 13 | 593 | 9 | 1.57 | 1.19, 2.06 |
| Missing | 24 | 440 | 15 | 186 | ||||||||
| Times visited a medical doctor or medical assistant about a health concern in past 12 months | ||||||||||||
| 0 | 170 | 16 | 3,521 | 21 | 1.00 | Referent | 70 | 16 | 1,474 | 22 | 1.00 | Referent |
| 1 | 267 | 25 | 5,120 | 30 | 1.10 | 0.91, 1.32 | 117 | 27 | 2,063 | 30 | 1.21 | 0.91, 1.62 |
| > 1 | 614 | 58 | 8,216 | 49 | 1.60 | 1.36, 1.89 | 254 | 58 | 3,272 | 48 | 1.70 | 1.32, 2.20 |
| Missing | 3 | 36 | 3 | 21 | ||||||||
| Ever diagnosed with heart diseaseb | ||||||||||||
| No | 957 | 91 | 15,750 | 93 | 1.00 | Referent | 406 | 91 | 6,421 | 94 | 1.00 | Referent |
| Yes | 97 | 9 | 1,139 | 7 | 1.55 | 1.27, 1.90 | 38 | 9 | 405 | 6 | 1.68 | 1.22, 2.31 |
| Missing | 0 | 4 | 0 | 4 | ||||||||
| Ever diagnosed with diabetes (other than while pregnant) | ||||||||||||
| No | 1,023 | 97 | 16,439 | 97 | 1.00 | Referent | 426 | 96 | 6,610 | 97 | 1.00 | Referent |
| Yes | 31 | 3 | 454 | 3 | 1.28 | 0.90, 1.81 | 18 | 4 | 220 | 3 | 1.58 | 1.00, 2.50 |
| Years lived or worked on a farm over lifetime | ||||||||||||
| < 5 | 84 | 8 | 925 | 6 | 1.56 | 1.23, 1.97 | 56 | 13 | 611 | 9 | 1.55 | 1.13, 2.13 |
| 5-10 | 93 | 9 | 1,127 | 7 | 1.38 | 1.10, 1.75 | 54 | 12 | 619 | 9 | 1.44 | 1.03, 2.00 |
| 11-20 | 203 | 19 | 2,716 | 16 | 1.31 | 1.10, 1.56 | 88 | 20 | 1,198 | 18 | 1.28 | 0.97, 1.68 |
| 21-30 | 211 | 20 | 3,036 | 18 | 1.25 | 1.06, 1.48 | 85 | 19 | 1,218 | 18 | 1.25 | 0.95, 1.63 |
| > 30 | 455 | 44 | 8,924 | 53 | 1.00 | Referent | 155 | 35 | 3,094 | 46 | 1.00 | Referent |
| Missing | 8 | 165 | 6 | 90 | ||||||||
| Ever have a job off a farm | ||||||||||||
| No | 91 | 9 | 1,685 | 10 | 1.00 | Referent | 43 | 10 | 729 | 11 | 1.00 | Referent |
| Yes | 961 | 91 | 15,088 | 90 | 1.06 | 0.86, 1.31 | 399 | 90 | 6,032 | 89 | 0.95 | 0.70, 1.30 |
| Missing | 2 | 120 | 2 | 69 | ||||||||
| Exposed to solvents (other than gasoline) at non-farm job held longest | ||||||||||||
| No | 931 | 88 | 15,344 | 91 | 1.00 | Referent | 407 | 92 | 6,344 | 94 | 1.00 | Referent |
| Yes | 121 | 12 | 1,429 | 9 | 1.33 | 1.60 | 35 | 8 | 417 | 6 | 1.22 | 0.87, 1.70 |
| Missing | 2 | 120 | 2 | 69 | ||||||||
Abbreviations: CI, confidence interval; RR, risk ratio.
Adjusted for age at enrollment (modeled with a linear term).
Derived from questions regarding myocardial infarction, angina, and arrythmia.
We used logistic regression models to calculate the stabilized selection weights. Specifically, for the numerators of the weights, we calculated the predicted probabilities of not dropping out of the cohort conditional on the exposure of interest; and, for the denominators of the weights, we calculated the predicted probabilities conditional on age, diabetes, education, state, the exposure of interest, and pairwise interaction terms between each covariate and the exposure of interest. We modeled age, diabetes, education, and state the same way as for the confounding weights.
To informally assess the bias-variance (validity-precision) tradeoff (Greenland, 2008; Winer, 1978), we progressively truncated the overall stabilized weights by resetting weights less (or greater) than a certain percentile to the value of that percentile (Cole and Hernán, 2008). Regarding the RRs derived from the untruncated weights as the “true” values, we informally evaluated the bias-variance tradeoff by looking at how features of both the weights and the corresponding RRs changed with increasing truncation. We considered nearness of the mean weight to one, reduction in number of extreme weights (e.g., < 0.05 or > 20), and a balance between increased “bias” and reduced variance in the estimated RRs (Cole and Hernán, 2008). Truncating the overall stabilized weights at the first and 99th percentiles appeared to be the best balance of validity and precision in this analysis.
We conducted two main analyses to evaluate 1) associations between pesticide use and incident depression among all 16,893 wives and 2) associations between husbands’ pesticide use (i.e., indirect exposure) and wives’ incident depression among 6,830 wives who had never used any pesticides. We used within-category medians to assess linear dose-response trends in the wives’ and husbands’ cumulative lifetime days of pesticide use variables.
We performed additional analyses by adding race/ethnicity, number of children, farm size, number of doctor visits in past year, ever use of any pesticides, husbands’ age, husbands’ depression status, or husbands’ use of individual pesticides to the models for the weights one-ata-time. We also added the pesticide that was the most strongly correlated with the pesticide of interest to the models for the weights to account for correlations between use of different pesticides. We refit the models for the weights adjusting for covariates in the first minimally sufficient adjustment set instead of the second set. We separately evaluated associations with cases that occurred within five years of enrollment in the Agricultural Health Study or more than five years after enrollment. Finally, we repeated analyses without weighting (i.e., using standard regression adjustment methods) and, therefore, without adjustment for potential selection bias from drop out.
We used the P1REL20100501 release of the Phase I data set, the P3REL1000.00 release of the Phase III data set, and the AHSREL201103.00 release of the demographic data set.
3. Results
After adjusting for age at enrollment, risk of incident depression was higher among wives who lived in North Carolina, had completed some high school or less compared to high school graduate, worked on a farm less than 50 acres in size compared to 50 acres or more, were a current or past cigarette smoker compared to never having smoked, visited a medical doctor more than once in the past year compared to no visits, were ever diagnosed with diabetes or heart disease, lived or worked on a farm less than 31 years compared to 31 years or more, and were exposed to solvents (other than gasoline) at the non-farm job held the longest (Table 1). Depression was inversely associated with being older than 45 years compared to 36-45, of a race/ethnicity other than non-Hispanic white, and having at least one child compared to no children (Table 1). In general, these descriptive associations were in the same directions and of similar magnitudes when we restricted analyses to wives who had never used any pesticides (Table 1). Additionally adjusting for state gave similar results except for farm size and years lived or worked on a farm, which had RRs attenuated toward 1.00 (data not shown).
After weighting for age, diabetes diagnosis, education, state, and not dropping out of the cohort, wives’ incident depression was not associated with ever use or cumulative lifetime days of use of any pesticide, but physician-diagnosed pesticide poisoning was positively, albeit imprecisely, associated with depression (Table 2). Wives’ depression was inversely associated with ever use of chloroacetanilide, phenoxy, and triazine herbicides (Table 2) as well as use of several individual pesticides, especially herbicides (Table 3). Ever use of metalaxyl and permethrin (for crops) were significantly positively associated with depression (Table 3).
Table 2.
Pesticide use and self-reported, incident depression in wives from Iowa and North Carolina enrolled in the Agricultural Health Study.
| Cases (n = 1,054) | Total (n = 16,893) | Inverse Probability Weighteda | ||||
|---|---|---|---|---|---|---|
| Variable | No. | % | No. | % | RR | 95% CI |
| Ever personally mixed or applied pesticides | ||||||
| No | 444 | 42 | 6,830 | 40 | 1.00 | Referent |
| Yes | 610 | 58 | 10,063 | 60 | 0.96 | 0.85, 1.09 |
| Cumulative lifetime days personally mixed or applied pesticidesb | ||||||
| 0 (Median = 0.00) | 444 | 49 | 6,830 | 48 | 1.00 | Referent |
| 1-9 (8.75) | 132 | 15 | 1,952 | 14 | 1.05 | 0.87, 1.27 |
| 10-51 (24.50) | 121 | 13 | 1,972 | 14 | 0.95 | 0.78, 1.16 |
| 52-179 (108.50) | 107 | 12 | 1,935 | 13 | 0.85 | 0.69, 1.04 |
| > 179 (369.75) | 99 | 11 | 1,667 | 12 | 0.88 | 0.72, 1.09 |
| Missing | 151 | 2,537 | ||||
| Trend (IQR = 169.75)c | 0.94 | 0.85, 1.04 | ||||
| Exposed to pesticides at non-farm job held longest | ||||||
| No | 1,004 | 95 | 16,177 | 96 | 1.00 | Referent |
| Yes | 48 | 5 | 596 | 4 | 1.23 | 0.92, 1.64 |
| Missing | 2 | 120 | ||||
| Ever diagnosed with pesticide poisoning | ||||||
| No | 1,048 | 100 | 16,831 | 100 | 1.00 | Referent |
| Yes | 5 | < 1 | 49 | < 1 | 1.78 | 0.76, 4.14 |
| Missing | 1 | 13 | ||||
| Functional pesticide classesd: ever personally mixed or applied | ||||||
| Fumigants | ||||||
| No | 1,014 | 98 | 16,214 | 98 | 1.00 | Referent |
| Yes | 21 | 2 | 316 | 2 | 0.94 | 0.61, 1.47 |
| Missing | 19 | 363 | ||||
| Fungicides | ||||||
| No | 979 | 95 | 15,606 | 95 | 1.00 | Referent |
| Yes | 54 | 5 | 905 | 5 | 0.93 | 0.70, 1.23 |
| Missing | 21 | 382 | ||||
| Herbicides | ||||||
| No | 649 | 62 | 10,045 | 60 | 1.00 | Referent |
| Yes | 397 | 38 | 6,646 | 40 | 0.95 | 0.84, 1.08 |
| Missing | 8 | 202 | ||||
| Insecticides | ||||||
| No | 629 | 60 | 9,622 | 58 | 1.00 | Referent |
| Yes | 420 | 40 | 7,087 | 42 | 0.96 | 0.85, 1.08 |
| Missing | 5 | 184 | ||||
| Chemical pesticide classese: ever personally mixed or applied | ||||||
| Carbamates | ||||||
| No | 712 | 68 | 10,953 | 66 | 1.00 | Referent |
| Yes | 328 | 32 | 5,697 | 34 | 0.92 | 0.80, 1.04 |
| Missing | 14 | 243 | ||||
| Chloroacetanilide herbicides | ||||||
| No | 979 | 96 | 15,397 | 94 | 1.00 | Referent |
| Yes | 42 | 4 | 992 | 6 | 0.72 | 0.52, 0.99 |
| Missing | 33 | 504 | ||||
| Organochlorine insecticides | ||||||
| No | 946 | 93 | 14,886 | 91 | 1.00 | Referent |
| Yes | 67 | 7 | 1,395 | 9 | 0.84 | 0.65, 1.09 |
| Missing | 41 | 612 | ||||
| Organophosphate insecticides | ||||||
| No | 773 | 74 | 11,922 | 72 | 1.00 | Referent |
| Yes | 269 | 26 | 4,655 | 28 | 0.95 | 0.83, 1.09 |
| Missing | 12 | 316 | ||||
| Phenoxy herbicides | ||||||
| No | 912 | 89 | 13,779 | 84 | 1.00 | Referent |
| Yes | 118 | 11 | 2,676 | 16 | 0.71 | 0.58, 0.88 |
| Missing | 24 | 438 | ||||
| Pyrethroid insecticides | ||||||
| No | 993 | 95 | 15,876 | 95 | 1.00 | Referent |
| Yes | 55 | 5 | 864 | 5 | 1.07 | 0.81, 1.40 |
| Missing | 6 | 153 | ||||
| Triazine herbicides | ||||||
| No | 983 | 96 | 15,367 | 94 | 1.00 | Referent |
| Yes | 39 | 4 | 1,019 | 6 | 0.69 | 0.49, 0.96 |
| Missing | 32 | 507 | ||||
Abbreviations: 2,4-D, (2,4-dichlorophenoxy)acetic acid; 2,4,5-T, (2,4,5-trichlorophenoxy)acetic acid; 2,4,5-TP, (RS)-2-(2,4,5-trichlorophenoxy)propionic acid; CI, confidence interval; DDT, 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane; EPTC, S-ethyl dipropyl(thiocarbamate); IQR, interquartile range; RR, risk ratio.
Weights adjusted for age at enrollment (modeled with a restricted, quadratic spline with three equally spaced knots at ages 36, 43, and 52 years based on percentiles of the age distribution in all cases), ever diagnosed with diabetes (other than while pregnant), education level, state of residence, and not dropping out of the Agricultural Health Study cohort (conditional on the exposure of interest and on the same covariates just listed as well as pairwise interaction terms between each covariate and the exposure of interest). Robust variance estimates were used to calculate 95% CIs.
Category boundaries set at the quartiles of cumulative lifetime days of pesticide use among all wives who used any pesticides.
Used within-category medians and scaled the RR to an IQR-unit (days) increase in cumulative lifetime days of pesticide use among all wives who used any pesticides.
Fumigants included aluminum phosphide, methyl bromide, carbon tetrachloride/carbon disulfide (80/20 mix), and ethylene dibromide. Fungicides included benomyl, captan, chlorothalonil, maneb/mancozeb, metalaxyl, and ziram. Herbicides included 2,4-D; 2,4,5-T; 2,4,5-TP; alachlor; atrazine; butylate; chlorimuron-ethyl; cyanazine; dicamba; EPTC; glyphosate; imazethapyr; metolachlor; metribuzin; paraquat; pendimethalin; petroleum oil; and trifluralin. Insecticides included aldicarb, aldrin, carbaryl, carbofuran, chlordane, chlorpyrifos, coumaphos, DDT, dichlorvos, diazinon, dieldrin, fonofos, heptachlor, lindane, malathion, parathion, permethrin (for animals), permethrin (for crops), phorate, terbufos, toxaphene, and trichlorfon.
Carbamates included aldicarb, benomyl, carbaryl, and carbofuran. Chloroacetanilide herbicides included alachlor and metolachlor. Organochlorine insecticides included aldrin, chlordane, DDT, dieldrin, heptachlor, lindane, and toxaphene. Organophosphate insecticides included chlorpyrifos, coumaphos, dichlorvos, diazinon, fonofos, malathion, parathion, phorate, terbufos, and trichlorfon. Phenoxy herbicides included 2,4-D; 2,4,5-T; and 2,4,5-TP. Pyrethroid insecticides included permethrin (for animals) and permethrin (for crops). Triazine herbicides included atrazine, cyanazine, and metribuzin.
Table 3.
Ever use of specific pesticides and self-reported, incident depression in wives from Iowa and North Carolina enrolled in the Agricultural Health Study.
| Casesb (n = 1,054) | Totalb (n = 16,893) | Inverse Probability Weightedc, d | ||||
|---|---|---|---|---|---|---|
| Ever personally mixed or applieda | No. | % | No. | % | RR | 95% CI |
| Fumigants | ||||||
| Carbon tetrachloride/carbon disulfide (80/20 mix) | 6 | 1 | 108 | 1 | 0.88 | 0.40, 1.95 |
| Methyl bromide | 16 | 2 | 202 | 1 | 1.13 | 0.68, 1.87 |
| Fungicides | ||||||
| Benomyl | 9 | 1 | 157 | 1 | 0.87 | 0.45, 1.68 |
| Captan | 25 | 2 | 428 | 3 | 0.83 | 0.56, 1.24 |
| Chlorothalonil | 7 | 1 | 180 | 1 | 0.62 | 0.29, 1.31 |
| Maneb/mancozeb | 11 | 1 | 290 | 2 | 0.64 | 0.33, 1.25 |
| Metalaxyl | 25 | 2 | 268 | 2 | 1.61 | 1.03, 2.52 |
| Herbicides | ||||||
| 2,4-D | 118 | 11 | 2,662 | 16 | 0.72 | 0.58, 0.89 |
| Alachlor | 32 | 3 | 774 | 5 | 0.71 | 0.50, 1.03 |
| Atrazine | 28 | 3 | 828 | 5 | 0.61 | 0.41, 0.90 |
| Butylate | 6 | 1 | 257 | 2 | 0.48 | 0.21, 1.07 |
| Chlorimuron-ethyl | 13 | 1 | 304 | 2 | 0.71 | 0.41, 1.23 |
| Cyanazine | 16 | 2 | 517 | 3 | 0.50 | 0.30, 0.84 |
| Dicamba | 31 | 3 | 730 | 4 | 0.75 | 0.52, 1.08 |
| EPTC | 6 | 1 | 247 | 2 | 0.44 | 0.19, 1.00 |
| Glyphosate | 359 | 34 | 6,017 | 36 | 0.95 | 0.83, 1.08 |
| Imazethapyr | 17 | 2 | 517 | 3 | 0.58 | 0.35, 0.95 |
| Metolachlor | 23 | 2 | 586 | 4 | 0.68 | 0.45, 1.04 |
| Metribuzin | 8 | 1 | 324 | 2 | 0.47 | 0.23, 0.98 |
| Paraquat | 14 | 1 | 211 | 1 | 1.08 | 0.64, 1.83 |
| Pendimethalin | 15 | 1 | 408 | 2 | 0.62 | 0.37, 1.04 |
| Petroleum oil | 32 | 3 | 644 | 4 | 0.80 | 0.56, 1.14 |
| Trifluralin | 38 | 4 | 947 | 6 | 0.71 | 0.50, 0.99 |
| Insecticides | ||||||
| Aldrin | 10 | 1 | 139 | 1 | 1.37 | 0.74, 2.52 |
| Carbaryl | 325 | 31 | 5,574 | 33 | 0.94 | 0.82, 1.07 |
| Carbofuran | 7 | 1 | 347 | 2 | 0.31 | 0.14, 0.67 |
| Chlordane | 34 | 3 | 766 | 5 | 0.79 | 0.55, 1.13 |
| Chlorpyrifos | 36 | 4 | 687 | 4 | 0.95 | 0.68, 1.32 |
| Coumaphos | 10 | 1 | 228 | 1 | 0.77 | 0.41, 1.43 |
| DDT | 28 | 3 | 659 | 4 | 0.63 | 0.43, 0.94 |
| Dichlorvos | 27 | 3 | 473 | 3 | 1.05 | 0.71, 1.56 |
| Diazinon | 101 | 10 | 1,864 | 11 | 0.87 | 0.71, 1.07 |
| Fonofos | 16 | 2 | 333 | 2 | 0.87 | 0.52, 1.45 |
| Heptachlor | 6 | 1 | 129 | 1 | 0.68 | 0.31, 1.50 |
| Lindane | 14 | 1 | 276 | 2 | 0.84 | 0.50, 1.43 |
| Malathion | 203 | 20 | 3,563 | 21 | 0.96 | 0.82, 1.12 |
| Parathion | 10 | 1 | 175 | 1 | 0.89 | 0.48, 1.66 |
| Permethrin (for animals) | 34 | 3 | 633 | 4 | 0.83 | 0.58, 1.18 |
| Permethrin (for crops) | 32 | 3 | 342 | 2 | 1.44 | 1.02, 2.03 |
| Phorate | 16 | 2 | 338 | 2 | 0.77 | 0.46, 1.27 |
| Terbufos | 21 | 2 | 501 | 3 | 0.79 | 0.51, 1.23 |
| Toxaphene | 6 | 1 | 126 | 1 | 0.80 | 0.36, 1.80 |
Abbreviations: 2,4-D, (2,4-dichlorophenoxy)acetic acid; 2,4,5-T, (2,4,5-trichlorophenoxy)acetic acid; 2,4,5-TP, (RS)-2-(2,4,5-trichlorophenoxy)propionic acid; CI, confidence interval; DDT, 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane; EPTC, S-ethyl dipropyl(thiocarbamate); RR, risk ratio.
Fewer than five cases ever personally mixed or applied 2,4,5-T; 2,4,5-TP; aldicarb; aluminum phosphide; dieldrin; ethylene dibromide; trichlorfon; or ziram.
Information for specific pesticides was missing for 1–4% of wives.
The wives who did not use each specific pesticide served as the reference group.
Weights adjusted for age at enrollment (modeled with a restricted, quadratic spline with three equally spaced knots at ages 36, 43, and 52 years based on percentiles of the age distribution in all cases), ever diagnosed with diabetes (other than while pregnant), education level, state of residence, and not dropping out of the Agricultural Health Study cohort (conditional on the exposure of interest and on the same covariates just listed as well as pairwise interaction terms between each covariate and the exposure of interest). Robust variance estimates were used to calculate 95% CIs.
Among wives who reported never using any pesticides, husbands’ ever use of carbamates was the only functional or chemical pesticide class significantly positively associated with wives’ depression, although RRs for husbands’ ever use of insecticides, chloroacetanilide herbicides, organochlorine insecticides, and organophosphate insecticides were elevated (Table 4). With the exception of a few individual herbicides, wives’ depression was not associated with husbands’ use of most individual pesticides (Table 5).
Table 4.
Husbands’ pesticide use and self-reported, incident depression in wives from Iowa and North Carolina who never used pesticides, but who were enrolled in the Agricultural Health Study.
| Cases (n = 444) | Total (n = 6,830) | Inverse Probability Weightedb | ||||
|---|---|---|---|---|---|---|
| Variablea | No. | % | No. | % | RR | 95% CI |
| Cumulative lifetime days personally mixed or applied pesticidesc | ||||||
| 0 (Median = 0.00) | 8 | 2 | 82 | 1 | 1.00 | Referent |
| 1-64 (38.75) | 120 | 27 | 1,829 | 27 | 0.67 | 0.33, 1.35 |
| 65-225 (178.50) | 142 | 32 | 2,188 | 32 | 0.67 | 0.34, 1.34 |
| 226-457 (369.75) | 95 | 21 | 1,392 | 20 | 0.71 | 0.35, 1.43 |
| > 457 (767.25) | 78 | 18 | 1,297 | 19 | 0.62 | 0.31, 1.26 |
| Missing | 1 | 42 | ||||
| Trend (IQR = 393.50)d | 0.95 | 0.83, 1.09 | ||||
| Functional pesticide classese: ever personally mixed or applied | ||||||
| Fumigants | ||||||
| No | 305 | 74 | 4,833 | 77 | 1.00 | Referent |
| Yes | 105 | 26 | 1,477 | 23 | 1.11 | 0.85, 1.44 |
| Missing | 34 | 520 | ||||
| Fungicides | ||||||
| No | 266 | 65 | 4,091 | 64 | 1.00 | Referent |
| Yes | 144 | 35 | 2,260 | 36 | 0.94 | 0.75, 1.19 |
| Missing | 34 | 479 | ||||
| Herbicides | ||||||
| No | 9 | 2 | 164 | 2 | 1.00 | Referent |
| Yes | 430 | 98 | 6,536 | 98 | 1.25 | 0.64, 2.47 |
| Missing | 5 | 130 | ||||
| Insecticides | ||||||
| No | 17 | 4 | 342 | 5 | 1.00 | Referent |
| Yes | 405 | 96 | 6,199 | 95 | 1.42 | 0.88, 2.30 |
| Missing | 22 | 289 | ||||
| Chemical pesticide classesf: ever personally mixed or applied | ||||||
| Carbamates | ||||||
| No | 139 | 34 | 2,268 | 35 | 1.00 | Referent |
| Yes | 273 | 66 | 4,139 | 65 | 1.27 | 1.01, 1.60 |
| Missing | 32 | 423 | ||||
| Chloroacetanilide herbicides | ||||||
| No | 110 | 27 | 1,914 | 30 | 1.00 | Referent |
| Yes | 296 | 73 | 4,430 | 70 | 1.16 | 0.93, 1.44 |
| Missing | 38 | 486 | ||||
| Organochlorine insecticides | ||||||
| No | 201 | 50 | 3,013 | 48 | 1.00 | Referent |
| Yes | 198 | 50 | 3,254 | 52 | 1.17 | 0.94, 1.44 |
| Missing | 45 | 563 | ||||
| Organophosphate insecticides | ||||||
| No | 37 | 9 | 656 | 10 | 1.00 | Referent |
| Yes | 385 | 91 | 5,897 | 90 | 1.21 | 0.86, 1.71 |
| Missing | 22 | 277 | ||||
| Phenoxy herbicides | ||||||
| No | 91 | 22 | 1,244 | 19 | 1.00 | Referent |
| Yes | 324 | 78 | 5,262 | 81 | 0.94 | 0.74, 1.19 |
| Missing | 29 | 324 | ||||
| Pyrethroid insecticides | ||||||
| No | 302 | 74 | 4,843 | 77 | 1.00 | Referent |
| Yes | 107 | 26 | 1,471 | 23 | 1.08 | 0.87, 1.36 |
| Missing | 35 | 516 | ||||
| Triazine herbicides | ||||||
| No | 80 | 19 | 1,239 | 19 | 1.00 | Referent |
| Yes | 347 | 81 | 5,325 | 81 | 1.00 | 0.78, 1.30 |
| Missing | 17 | 266 | ||||
Abbreviations: 2,4-D, (2,4-dichlorophenoxy)acetic acid; 2,4,5-T, (2,4,5-trichlorophenoxy)acetic acid; 2,4,5-TP, (RS)-2-(2,4,5-trichlorophenoxy)propionic acid; CI, confidence interval; DDT, 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane; EPTC, S-ethyl dipropyl(thiocarbamate); IQR, interquartile range; RR, risk ratio.
Fewer than five cases’ husbands never personally mixed or applied pesticides.
Weights adjusted for age at enrollment (modeled with a restricted, quadratic spline with three equally spaced knots at ages 36, 43, and 52 years based on percentiles of the age distribution in all cases), ever diagnosed with diabetes (other than while pregnant), education level, state of residence, and not dropping out of the Agricultural Health Study cohort (conditional on the exposure of interest and on the same covariates just listed as well as pairwise interaction terms between each covariate and the exposure of interest). Robust variance estimates were used to calculate 95% CIs.
Category boundaries set at the quartiles of cumulative lifetime days of pesticide use among all husbands (of wives who never used pesticides) who used any pesticides.
Used within-category medians and scaled the RR to an IQR-unit (days) increase in cumulative lifetime days of pesticide use among all husbands (of wives who never used pesticides) who used any pesticides.
Fumigants included aluminum phosphide, methyl bromide, carbon tetrachloride/carbon disulfide (80/20 mix), and ethylene dibromide. Fungicides included benomyl, captan, chlorothalonil, maneb/mancozeb, metalaxyl, and ziram. Herbicides included 2,4-D; 2,4,5-T; 2,4,5-TP; alachlor; atrazine; butylate; chlorimuron-ethyl; cyanazine; dicamba; EPTC; glyphosate; imazethapyr; metolachlor; metribuzin; paraquat; pendimethalin; petroleum oil; and trifluralin. Insecticides included aldicarb, aldrin, carbaryl, carbofuran, chlordane, chlorpyrifos, coumaphos, DDT, dichlorvos, diazinon, dieldrin, fonofos, heptachlor, lindane, malathion, parathion, permethrin (for animals), permethrin (for crops), phorate, terbufos, toxaphene, and trichlorfon.
Carbamates included aldicarb, benomyl, carbaryl, and carbofuran. Chloroacetanilide herbicides included alachlor and metolachlor. Organochlorine insecticides included aldrin, chlordane, DDT, dieldrin, heptachlor, lindane, and toxaphene. Organophosphate insecticides included chlorpyrifos, coumaphos, dichlorvos, diazinon, fonofos, malathion, parathion, phorate, terbufos, and trichlorfon. Phenoxy herbicides included 2,4-D; 2,4,5-T; and 2,4,5-TP. Pyrethroid insecticides included permethrin (for animals) and permethrin (for crops). Triazine herbicides included atrazine, cyanazine, and metribuzin.
Table 5.
Husbands’ ever use of specific pesticides and self-reported, incident depression in wives from Iowa and North Carolina who never used pesticides, but who were enrolled in the Agricultural Health Study.
| Casesb (n = 444) | Totalb (n = 6,830) | Inverse Probability Weightedc, d | ||||
|---|---|---|---|---|---|---|
| Ever personally mixed or applieda | No. | % | No. | % | RR | 95% CI |
| Fumigants | ||||||
| Aluminum phosphide | 26 | 7 | 301 | 5 | 1.35 | 0.92, 2.00 |
| Carbon tetrachloride/carbon disulfide (80/20 mix) | 25 | 6 | 350 | 6 | 1.41 | 0.94, 2.12 |
| Ethylene dibromide | 12 | 3 | 228 | 4 | 0.86 | 0.45, 1.63 |
| Methyl bromide | 70 | 16 | 1,010 | 15 | 1.04 | 0.61, 1.77 |
| Fungicides | ||||||
| Benomyl | 46 | 12 | 657 | 11 | 1.25 | 0.80, 1.96 |
| Captan | 37 | 9 | 654 | 11 | 0.85 | 0.61, 1.19 |
| Chlorothalonil | 42 | 10 | 547 | 8 | 1.14 | 0.78, 1.66 |
| Maneb/mancozeb | 47 | 12 | 609 | 10 | 1.08 | 0.66, 1.78 |
| Metalaxyl | 91 | 22 | 1,385 | 22 | 0.93 | 0.70, 1.25 |
| Ziram | 10 | 3 | 89 | 1 | 1.76 | 0.93, 3.31 |
| Herbicides | ||||||
| 2,4-D | 316 | 72 | 5,130 | 77 | 0.83 | 0.67, 1.03 |
| 2,4,5-T | 75 | 19 | 1,392 | 23 | 1.00 | 0.76, 1.31 |
| 2,4,5-TP | 40 | 10 | 588 | 10 | 1.24 | 0.89, 1.73 |
| Alachlor | 230 | 58 | 3,477 | 56 | 1.13 | 0.93, 1.37 |
| Atrazine | 307 | 70 | 4,889 | 73 | 0.85 | 0.69, 1.05 |
| Butylate | 136 | 35 | 1,986 | 33 | 1.16 | 0.94, 1.43 |
| Chlorimuron-ethyl | 175 | 44 | 2,298 | 37 | 1.25 | 1.02, 1.52 |
| Cyanazine | 173 | 44 | 2,674 | 43 | 1.00 | 0.80, 1.24 |
| Dicamba | 208 | 52 | 3,196 | 52 | 0.96 | 0.77, 1.20 |
| EPTC | 80 | 21 | 1,263 | 21 | 0.97 | 0.73, 1.29 |
| Glyphosate | 330 | 75 | 4,935 | 74 | 1.04 | 0.84, 1.30 |
| Imazethapyr | 195 | 49 | 2,720 | 44 | 1.21 | 0.95, 1.55 |
| Metolachlor | 213 | 53 | 3,000 | 48 | 1.21 | 0.99, 1.47 |
| Metribuzin | 196 | 50 | 2,825 | 46 | 1.17 | 0.95, 1.43 |
| Paraquat | 101 | 25 | 1,443 | 23 | 1.22 | 0.95, 1.56 |
| Pendimethalin | 204 | 51 | 2,766 | 45 | 1.25 | 1.03, 1.52 |
| Petroleum oil | 186 | 48 | 2,951 | 48 | 0.95 | 0.78, 1.15 |
| Trifluralin | 231 | 57 | 3,309 | 53 | 1.19 | 0.97, 1.45 |
| Insecticides | ||||||
| Aldicarb | 41 | 10 | 699 | 11 | 1.16 | 0.73, 1.85 |
| Aldrin | 70 | 18 | 1,198 | 20 | 1.20 | 0.88, 1.64 |
| Carbaryl | 227 | 55 | 3,406 | 54 | 1.13 | 0.90, 1.40 |
| Carbofuran | 104 | 26 | 1,724 | 28 | 1.03 | 0.82, 1.29 |
| Chlordane | 88 | 23 | 1,584 | 26 | 1.04 | 0.80, 1.36 |
| Chlorpyrifos | 190 | 43 | 2,841 | 43 | 0.97 | 0.81, 1.17 |
| Coumaphos | 38 | 10 | 520 | 9 | 1.14 | 0.82, 1.58 |
| DDT | 90 | 23 | 1,682 | 27 | 1.16 | 0.85, 1.58 |
| Dichlorvos | 39 | 10 | 626 | 10 | 0.97 | 0.69, 1.38 |
| Diazinon | 124 | 32 | 1,895 | 31 | 1.07 | 0.86, 1.33 |
| Dieldrin | 18 | 5 | 433 | 7 | 0.77 | 0.45, 1.31 |
| Fonofos | 85 | 21 | 1,394 | 22 | 0.97 | 0.73, 1.27 |
| Heptachlor | 53 | 13 | 990 | 16 | 1.01 | 0.71, 1.43 |
| Lindane | 78 | 20 | 1,181 | 19 | 1.13 | 0.88, 1.45 |
| Malathion | 286 | 71 | 4,501 | 71 | 1.00 | 0.81, 1.23 |
| Parathion | 65 | 17 | 913 | 15 | 1.20 | 0.89, 1.62 |
| Permethrin (for animals) | 63 | 16 | 856 | 14 | 1.08 | 0.80, 1.45 |
| Permethrin (for crops) | 61 | 15 | 780 | 13 | 1.21 | 0.92, 1.58 |
| Phorate | 131 | 33 | 2,061 | 34 | 1.05 | 0.85, 1.31 |
| Terbufos | 159 | 39 | 2,527 | 40 | 0.95 | 0.77, 1.17 |
| Toxaphene | 64 | 16 | 915 | 15 | 1.35 | 1.00, 1.81 |
Abbreviations: 2,4-D, (2,4-dichlorophenoxy)acetic acid; 2,4,5-T, (2,4,5-trichlorophenoxy)acetic acid; 2,4,5-TP, (RS)-2-(2,4,5-trichlorophenoxy)propionic acid; CI, confidence interval; DDT, 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane; EPTC, S-ethyl dipropyl(thiocarbamate); RR, risk ratio.
Fewer than five cases’ husbands ever personally mixed or applied trichlorfon.
Information for specific pesticides was missing for 2–11% of wives’ husbands.
The wives whose husbands did not use each specific pesticide served as the reference group.
Weights adjusted for age at enrollment (modeled with a restricted, quadratic spline with three equally spaced knots at ages 36, 43, and 52 years based on percentiles of the age distribution in all cases), ever diagnosed with diabetes (other than while pregnant), education level, state of residence, and not dropping out of the Agricultural Health Study cohort (conditional on the exposure of interest and on the same covariates just listed as well as pairwise interaction terms between each covariate and the exposure of interest). Robust variance estimates were used to calculate 95% CIs.
Adding race/ethnicity, number of children, farm size, number of doctor visits in past year, ever use of any pesticides, husbands’ age, husbands’ depression status, husbands’ use of individual pesticides, or the pesticide that was the most strongly correlated with the pesticide of interest to the models for the weights one-at-a-time did not meaningfully change results (data not shown). Restricting cases to wives who had taken medication for their depression (n = 742; 70%) did not change results qualitatively (data not shown). Refitting the models for the weights adjusting for covariates in the first minimally sufficient adjustment set (age, alcohol use, diabetes, smoking, solvents, and state) gave similar results to those observed when we adjusted for the covariates in the second set (age, diabetes, education, and state) (data not shown). Inverse associations between use of individual herbicides and depression that occurred within the first five years of enrollment in the Agricultural Health Study were generally stronger in magnitude than were those with depression that occurred more than five years after enrollment (data not shown). Finally, results were similar when we used Cox proportional hazards regression models (data not shown) or log-binomial regression models with standard regression adjustment methods (Supplementary Data, Tables S.1-S.4).
4. Discussion
We found evidence for a positive association between self-reported, incident depression and a history of physician-diagnosed pesticide poisoning among wives in the Agricultural Health Study. However, depression was generally not associated, or it was inversely associated, with wives’ personal pesticide use on the farm. Among wives who never used pesticides, husbands’ ever use of pesticides was generally not associated with wives’ depression.
Our finding of a moderate, positive association between physician-diagnosed pesticide poisoning and depression, although based on only five poisoned cases, agrees with results of several other studies (Beseler and Stallones, 2008; Stallones and Beseler, 2002a, 2002b; Wesseling et al., 2010) and had previously been reported among applicators and wives of applicators in the Agricultural Health Study (Beseler et al., 2006, 2008). Pesticide poisoning may be a good measure for acute, high-level pesticide exposure because it indicates the occurrence of an exposure sufficient to cause a physical reaction. Poisoning may not, however, be a good measure for chronic exposure at lower levels.
The null and inverse associations we observed between reported pesticide use overall or use of specific pesticides and depression contrast with findings from several other studies (Bazylewicz-Walczak et al., 1999; Beseler et al., 2008; Carruth and Logan, 2002; London et al., 2005; Mackenzie Ross et al., 2010; Meyer et al., 2010; Rehner et al., 2000; Salvi et al., 2003; Villeneuve et al., 2009; Wesseling et al., 2010). These contradictory results may be explained by differences in the populations, designs, methods, sample sizes, or focus of our study relative to others. Some studies lacked data on pesticide use by individuals or use of specific pesticides (Meyer et al., 2010; Villeneuve et al., 2009). Instead, they compared hospitalization rates for mood disorder between an agricultural area with intensive pesticide use and two reference areas (Meyer et al., 2010) or used distance from individuals’ homes to a large hog farming operation as a measure of exposure (Villeneuve et al., 2009). Several studies used ecological (London et al., 2005; Meyer et al., 2010), case study (London et al., 2005), or cross-sectional designs (Beseler et al., 2008; Carruth and Logan, 2002; Mackenzie Ross et al., 2010; Rehner et al., 2000; Villeneuve et al., 2009; Wesseling et al., 2010). One small study (Rehner et al., 2000) focused on methyl parathion exposure in the home, which may be very different from occupational or agricultural pesticide use. Four studies (Bazylewicz-Walczak et al., 1999; Mackenzie Ross et al., 2010; Salvi et al., 2003; Wesseling et al., 2010) on organophosphate insecticide exposure among workers in the gardening, farming, and agricultural industries included farm workers who may have experienced substantially higher levels of pesticide exposure than the wives of farm owners in our study. Thus, our results place a lower bound on the level of pesticide exposure related to depression.
We did not expect the inverse associations we observed between wives’ incident depression and use of individual herbicides or the pesticide classes chloroacetanilide, phenoxy, and triazine herbicides, but they remained apparent across a range of analytic strategies. This is may be just a chance finding, but it is also possible that women who applied herbicides may be healthier or more physically active that those who did not. Results were similar, however, when we added number of doctor visits in past year to models for the weights. Because depression is a condition that may occur and persist without diagnosis (Farr et al., 2010), it is also possible that undiagnosed or unreported depression at the time of enrollment, when exposure was assessed, might cause wives who later reported depression not to use pesticides, thus creating the appearance of an inverse association. This may be the case because inverse associations between use of individual herbicides and depression that occurred within the first five years of enrollment in the Agricultural Health Study were generally stronger in magnitude than were those with depression that occurred more than five years after enrollment.
The prevalence of depression reported by wives at enrollment in the Agricultural Health Study (overall: 7.3%; Iowa: 6.9%; North Carolina: 8.3%) is lower than that recently reported for American women (20.2%) and residents of Iowa (men and women together; 14.7%) (Strine et al., 2008). There are a couple of possible explanations for the lower prevalence of depression in our study. First, married individuals have lower rates of depression than unmarried individuals (Centers for Disease Control and Prevention, 2010; Kessler et al., 2003; Strine et al., 2008) and all women in our study were married, by design. For comparison, the prevalence of depression at enrollment in the Agricultural Health Study among unmarried, female pesticide applicators was 14.7% (Iowa: 15.2%; North Carolina: 14.5%), which is much closer to the general population—married and unmarried women together—prevalence of depression. Additionally, residents of farming communities may be less likely to seek help for depression due to the stigma sometimes associated with mental problems (Fraser et al., 2005; Gregoire, 2002). Unfortunately, we were unable to determine if the relationships between pesticide use and incident depression among wives observed in our study were different in unmarried females due to small numbers.
Animal studies have assessed the neurological effects of some of the individual pesticides for which we observed positive associations with depression in our study. Toxaphene inhibited acetylcholinesterase in the brains of guinea pigs and affected dopamine binding to brain synaptosomes in rats (Chandra and Durairaj, 1993; Trottman and Desaiah, 1983). Metalaxyl did not affect acetylcholinesterase activities in rat brains (Naidu and Radhakrishnamurty, 1988), but permethrin affected acetylcholinesterase activities throughout the brains of rats (Abdel-Rahman et al., 2004) and increased striatal levels of dopamine transporter protein in mice (Gillette and Bloomquist, 2003). The positive associations we observed in our study, however, were not consistent across wives’ and husbands’ pesticide use, chemicals in the same pesticide class [e.g., wives’ use of permethrin (for animals) and permethrin (for crops)], or with the results of prior epidemiological studies. Similar discrepancies in associations between wives’ and husbands’ pesticide use were observed in a study of pesticide use and breast cancer in the Agricultural Health Study (Engel et al., 2005). Despite these inconsistencies, the lack of knowledge about the exact mechanisms of depression and the effects of pesticides on these mechanisms precludes firm conclusions about the biological plausibility of the positive associations observed in our study.
Confidence in the results of our study is increased by the fact that the associations between depression and participant characteristics shown in Table 1 followed patterns observed in other studies. Specifically, in previous studies, depression differed by state (Centers for Disease Control and Prevention, 2010; Kessler et al., 2003; Strine et al., 2008) and was less common among wives who were older (Beseler and Stallones, 2008; Centers for Disease Control and Prevention, 2010; Hölzel et al., 2011; Kessler et al., 2003; Strine et al., 2008), were of a race/ethnicity other than non-Hispanic white (Kessler et al., 2003; Strine et al., 2008), and had more children/greater social connection (Hölzel et al., 2011; Stallones and Beseler, 2002b). Depression was more common among wives who had less than a high school education (Centers for Disease Control and Prevention, 2010; Strine et al., 2008), were current or past smokers (Strine et al., 2008), visited a medical doctor in the past year/had poorer health (Beseler and Stallones, 2008; Carruth and Logan, 2002; Stallones and Beseler, 2002b), and had heart disease or diabetes (Clarke and Currie, 2009; Strine et al., 2008).
One limitation of our study is that data on pesticide use was self-reported, so exposure misclassification is likely. We also did not have information on wives’ duration and frequency of use of individual pesticides. Using husbands’ pesticide use as a measure of wives’ indirect exposure likely introduced exposure misclassification and could have overestimated the number of wives actually exposed. Self-reports of pesticide use are likely more accurate than those based on husband's use or the ecological or geographical indicators of exposure that have been used in some previous studies (London et al., 2005; Meyer et al., 2010; Villeneuve et al., 2009).
There is some information on reliability and validity of self-reported pesticide use in the literature. Engel et al. (2001), using data from orchardists in Washington State reported 25 years earlier as the gold standard, found sensitivities for reporting ever use of pesticides were 1.00 for any pesticides, 0.87-1.00 for pesticides classes included in our study, and 0.80-0.94 for individual pesticides included in our study. A validation study in Kansas found pesticide use was reported similarly between cancer cases and controls (Blair and Zahm, 1993). Although a reliability study in Iowa (Brown et al., 1991) found that wives could provide useful information on their husband's use of pesticides, there was considerable misclassification. A recent study in the Agricultural Health Study reported that misclassification was likely to be non-differential with respect to outcome(s) under study (Blair et al., 2011). This would typically bias associations toward the null.
Use of self-report to assess incident depression in the Agricultural Health Study might result in some misclassification, but the accuracy of self-reported depression has been evaluated and appears to be quite good. Williams et al. (1999) found using a single question to assess depression had similar sensitivity (0.85), but slightly lower specificity (0.66), compared to the Center for Epidemiological Studies-Depression Scale 20-Item instrument (sensitivity: 0.88; specificity: 0.75) when using diagnostic interviews as the gold standard. Two other studies (Kroenke et al., 2003; Whooley et al., 1997) found similar or higher sensitivities and specificities to those reported by Williams et al. (1999) when using a two question depression screening instrument. It should be noted, however, that the depression screening instruments evaluated in these studies assessed depression in the past two weeks (Kroenke et al., 2003), month (Whooley et al., 1997), or year (Williams et al., 1999), whereas we assessed ever depression in the current study. Nevertheless, misclassification of depression would typically bias risk ratios toward the null unless the amount of misclassification differed by pesticide exposure status, which is possible because pesticide exposure has been linked to decrements in memory (Ismail et al., 2012) and depression was reported after the pesticide exposures occurred. This explanation seems unlikely, however, and restricting incident depression cases in the present study to wives who had taken medication for their depression did not change results qualitatively (data not shown), which suggests that any bias resulting from misclassified incident depression was likely small.
Strengths of our study include the large sample size and prospective design, alleviating concern regarding study power and differential misclassification of exposure. Moreover, we had extensive information on wives’ exposures, including ever use of any pesticides, pesticide classes, individual pesticides, and husbands’ pesticide use. We were able to control for many confounders and demonstrated the robustness of our results to additional potential confounders not included in the original models. Finally, we used inverse probability weighting to account for any potential selection bias resulting from wives dropping out of the Agricultural Health Study. Overall, the effect of drop outs on our results appears to be small because results were similar when we used standard regression adjustment methods.
5. Conclusions
In conclusion, depression in women remains a major public health problem. Our study adds further evidence that high level pesticide exposure, such as physician-diagnosed pesticide poisoning, is associated with increased risk of depression. Our study provides little support, however, for positive associations between pesticide exposure and self-reported, incident depression among wives in the Agricultural Health Study. The few positive associations that we observed with individual pesticides were not consistent across wives’ and husbands’ pesticide use. Reverse causality or chance may be possible explanations for the inverse associations we observed between use of some individual herbicides and depression. Our results set a lower bound on the level of exposure related to depression, thereby providing reassurance that the moderate levels of pesticide exposure experienced by farmers’ wives likely do not increase risk.
Supplementary Material
Highlights.
A few small, prospective studies exist of pesticide use and depression among women.
We used data on 16,893 farmers’ wives in the Agricultural Health Study.
We evaluated associations between use of 50 pesticides and incident depression.
Depression was not associated with ever use of individual or classes of pesticides.
Depression was positively associated with physician-diagnosed pesticide poisoning.
Acknowledgements
The authors thank the field stations in Iowa (University of Iowa: Dr. Charles F. Lynch and Ellen Heywood) and North Carolina (Battelle: Charles Knott and Margaret Hayslip) and the Coordinating Center at Westat (Kate Torres, Stanley Legum, and Marsha Dunn) for their work on the Agricultural Health Study, and Dr. Stephen Cole for guidance on inverse probability weighting. We especially thank the participants in the Agricultural Health Study without which the study would not have been possible. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (NIEHS) (Z01 ES049030), the National Cancer Institute (Z01 CP044008), and NIEHS Training Grant T32ES007018.
The Agricultural Health Study was approved by the Institutional Review Boards of the National Institutes of Health and its contractors; the current analysis involving coded data was exempted from review by the Institutional Review Board of the University of North Carolina at Chapel Hill. All participants provided implied informed consent by completing and returning the enrollment questionnaires after the study was explained to them.
Abbreviations
- 2,4-D
2-(2,4-Dichlorophenoxy)acetic acid
- 2,4,5-T
2-(2,4,5-Trichlorophenoxy)acetic acid
- 2,4,5-TP
2-(2,4,5-Trichlorophenoxy)propanonic acid
- CI
confidence interval
- DDT
1,1’-(2,2,2-Trichloroethylidene)bis[4-chlorobenzene]
- EPTC
N,N-Dipropylcarbamothioic acid S-ethyl ester
- IQR
interquartile range
- NIEHS
National Institute of Environmental Health Sciences
- RR
risk ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare they have no competing financial interests.
References
- Abdel-Rahman A, Dechkovskaia AM, Goldstein LB, Bullman SH, Khan W, El-Masry EM, Abou-Donia MB. Neurological deficits induced by malathion, DEET, and permethrin, alone or in combination in adult rats. J. Toxicol. Environ. Health A. 2004;67:331–356. doi: 10.1080/15287390490273569. [DOI] [PubMed] [Google Scholar]
- Agricultural Health Study [December 18, 2012];Full Text of Questionnaires. 2012 Available at: < http://aghealth.nci.nih.gov/questionnaires.html>.
- Alavanja MCR, Sandler DP, McMaster SB, Zahm SH, McDonnell CJ, Lynch CF, Pennybacker M, Rothman N, Dosemeci M, Bond AE, Blair A. The Agricultural Health Study. Environ. Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD. Survival Analysis Using SAS: A Practical Guide. 2nd ed. SAS Institute Inc.; Cary, NC: 2010. [Google Scholar]
- Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology. 1999;20:819–826. [PubMed] [Google Scholar]
- Beseler CL, Stallones L. A cohort study of pesticide poisoning and depression in Colorado farm residents. Ann. Epidemiol. 2008;18:768–774. doi: 10.1016/j.annepidem.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Beseler C, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the agricultural health study cohort. J. Occup. Environ. Med. 2006;48:1005–1013. doi: 10.1097/01.jom.0000235938.70212.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseler CL, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study. Environ. Health Perspect. 2008;116:1713–1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Thomas K, Coble J, Sandler DP, Hines CJ, Lynch CF, Knott C, Purdue MP, Zahm SH, Alavanja MCR, Dosemeci M, Kamel F, Hoppin JA, Freeman LB, Lubin JH. Impact of pesticide exposure misclassification on estimates of relative risks in the Agricultural Health Study. Occup. Environ. Med. 2011;68:537–541. doi: 10.1136/oem.2010.059469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Zahm SH. Patterns of pesticide use among farmers: implications for epidemiologic research. Epidemiology. 1993;4:55–62. doi: 10.1097/00001648-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Brown LM, Dosemeci M, Blair A, Burmeister L. Comparability of data obtained from farmers and surrogate respondents on use of agricultural pesticides. Am. J. Epidemiol. 1991;134:348–355. doi: 10.1093/oxfordjournals.aje.a116096. [DOI] [PubMed] [Google Scholar]
- Carruth AK, Logan CA. Depressive symptoms in farm women: effects of health status and farming lifestyle characteristics, behaviors, and beliefs. J. Community Health. 2002;27:213–228. doi: 10.1023/a:1015206224421. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Current depression among adults—United States, 2006 and 2008. MMWR Morb. Mortal. Wkly. Rep. 2010;59:1229–1235. [PubMed] [Google Scholar]
- Chandra J, Durairaj G. Effect of toxaphene toxicity on enzyme activity & residue levels in vital organs of guineapig. Indian J. Med. Res. 1993;98:193–198. [PubMed] [Google Scholar]
- Clarke DM, Currie KC. Depression, anxiety and their relationship with chronic diseases: a review of the epidemiology, risk and treatment evidence. Med. J. Aust. 2009;190:S54–S60. doi: 10.5694/j.1326-5377.2009.tb02471.x. [DOI] [PubMed] [Google Scholar]
- Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am. J. Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel LS, Hill DA, Hoppin JA, Lubin JH, Lynch CF, Pierce J, Samanic C, Sandler DP, Blair A, Alavanja MC. Pesticide use and breast cancer risk among farmers’ wives in the Agricultural Health Study. Am. J. Epidemiol. 2005;161:121–135. doi: 10.1093/aje/kwi022. [DOI] [PubMed] [Google Scholar]
- Engel LS, Seixas NS, Keifer MC, Longstreth WT, Jr., Checkoway H. Validity study of self-reported pesticide exposure among orchardists. J. Expo. Anal. Environ. Epidemiol. 2001;11:359–368. doi: 10.1038/sj.jea.7500176. [DOI] [PubMed] [Google Scholar]
- Farr SL, Bitsko RH, Hayes DK, Dietz PM. Mental health and access to services among US women of reproductive age. Am. J. Obstet. Gynecol. 2010;203:542, e1–542, e9. doi: 10.1016/j.ajog.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Fraser CE, Smith KB, Judd F, Humphreys JS, Fragar LJ, Henderson A. Farming and mental health problems and mental illness. Int. J. Soc. Psychiatry. 2005;51:340–349. doi: 10.1177/0020764005060844. [DOI] [PubMed] [Google Scholar]
- Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and alpha-synuclein by the pyrethroid insecticide permethrin. Toxicol. Appl. Pharmacol. 2003;192:287–293. doi: 10.1016/s0041-008x(03)00326-0. [DOI] [PubMed] [Google Scholar]
- Glymour MM, Greenland S. Causal diagrams. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Philadelphia. 3rd ed. Williams & Wilkins; Lippincott: 2008. pp. 183–209. [Google Scholar]
- Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am. J. Epidemiol. 2008;167:523–529. doi: 10.1093/aje/kwm355. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- Gregoire A. The mental health of farmers. Occup. Med. (Lond.) 2002;52:471–476. doi: 10.1093/occmed/52.8.471. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- Hölzel L, Härter M, Reese C, Kriston L. Risk factors for chronic depression—a systematic review. J. Affect. Disord. 2011;129:1–13. doi: 10.1016/j.jad.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Ismail AA, Bodner TE, Rohlman DS. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup. Environ. Med. 2012;69:457–464. doi: 10.1136/oemed-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J. Affect. Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JBW. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med. Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am. J. Ind. Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- Mackenzie Ross SJ, Brewin CR, Curran HV, Furlong CE, Abraham-Smith KM, Harrison V. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol. Teratol. 2010;32:452–459. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Koifman S, Koifman RJ, Moreira JC, de Rezende Chrisman J, Abreu-Villaça Y. Mood disorders hospitalizations, suicide attempts, and suicide mortality among agricultural workers and residents in an area with intensive use of pesticides in Brazil. J. Toxicol. Environ. Health A. 2010;73:866–877. doi: 10.1080/15287391003744781. [DOI] [PubMed] [Google Scholar]
- Naidu KA, Radhakrishnamurty R. Metalaxyl-induced bradycardia in rats: mediated by alpha-adrenoreceptors. J. Toxicol. Environ. Health. 1988;23:495–498. doi: 10.1080/15287398809531130. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self report depression scale for research in the general population. Appl. Psychol. Measure. 1977;1:385–401. [Google Scholar]
- Rehner TA, Kolbo JR, Trump R, Smith C, Reid D. Depression among victims of south Mississippi's methyl parathion disaster. Health Soc. Work. 2000;25:33–40. doi: 10.1093/hsw/25.1.33. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol. Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Stallones L, Beseler C. Pesticide illness, farm practices, and neurological symptoms among farm residents in Colorado. Environ. Res. 2002a;90:89–97. doi: 10.1006/enrs.2002.4398. [DOI] [PubMed] [Google Scholar]
- Stallones L, Beseler C. Pesticide poisoning and depressive symptoms among farm residents. Ann. Epidemiol. 2002b;12:389–394. doi: 10.1016/s1047-2797(01)00298-8. [DOI] [PubMed] [Google Scholar]
- Strine TW, Mokdad AH, Balluz LS, Gonzalez O, Crider R, Berry JT, Kroenke K. Depression and anxiety in the United States: findings from the 2006 Behavioral Risk Factor Surveillance System. Psychiatr. Serv. 2008;59:1383–1390. doi: 10.1176/ps.2008.59.12.1383. [DOI] [PubMed] [Google Scholar]
- Trottman CH, Desaiah D. Effect of toxaphene on the binding of 3H-labeled ouabain and dopamine to rat brain synaptosomes. Toxicol. Lett. 1983;18:323–330. doi: 10.1016/0378-4274(83)90113-3. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Ali A, Challacombe L, Hebert S. Intensive hog farming operations and self-reported health among nearby rural residents in Ottawa, Canada. BMC Public Health. 2009;9:330. doi: 10.1186/1471-2458-9-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling C, van Wendel de Joode B, Keifer M, London L, Mergler D, Stallones L. Symptoms of psychological distress and suicidal ideation among banana workers with a history of poisoning by organophosphate or n-methyl carbamate pesticides. Occup. Environ. Med. 2010;67:778–784. doi: 10.1136/oem.2009.047266. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J. Gen. Intern. Med. 1997;12:439–445. doi: 10.1046/j.1525-1497.1997.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Jr., Mulrow CD, Kroenke K, Dhanda R, Badgett RG, Omori D, Lee S. Case-finding for depression in primary care: a randomized trial. Am. J. Med. 1999;106:36–43. doi: 10.1016/s0002-9343(98)00371-4. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistics and data analysis: trading bias for reduced mean squared error. Annu. Rev. Psychol. 1978;29:647–681. doi: 10.1146/annurev.ps.29.020178.003243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.