Abstract
Background
Differentiating large lipomas from atypical lipomatous tumors (ALT) is challenging and preoperative management guidelines are not well-defined. The diagnostic ambiguity leads many surgeons to refer all patients with large lipomatous masses to an oncologic specialist, perhaps unneccessarily.
Study Design
In this retrospective cohort study of patients with nonretroperitoneal lipomatous tumors, preoperative characteristics discernible without invasive diagnostic procedures were evaluated for diagnostic predictive value.
Results
319 patients (256 with lipomas, 63 with ALTs) treated between 1994 and 2012 were identified. Patients with ALTs were older (60.5 vs. 53.5 years, p<0.0001), had larger tumors (16.0 vs 8.3 cm, p<0.0001), had tumors more often located on an extremity (88.9% vs. 60.5% torso, p<0.0001), and more frequently had a history of prior operations at the same site, exclusive of excision leading to diagnosis and referral (20.6% vs. 5.9%, p=0.001). Local recurrence was observed in 2 patients with lipomas (0.8%) vs. 14 with ALTs (22.6%, p<0.0001). No patients with ALTs developed distant metastases or disease-specific mortality with a median follow-up of 27.4 months (range 0–164.6 months). On multivariate analysis age ≥55 years, tumor size ≥10 cm, extremity location, and history of prior operations were predictors for diagnosis of ALT (p<0.05).
Conclusions
Characteristics of lipomatous masses that are associated with a diagnosis of ALT include patient age ≥55 years, tumor size ≥10 cm, previous resection, and extremity location (vs torso). These easily identifiable traits may guide surgical management or referral to a specialist.
Keywords: lipoma, liposarcoma, atypical lipomatous tumor
Introduction
Lipomatous neoplasms are commonly encountered by the general surgeon as well as the surgical oncologist. Collectively, lipomatous neoplasms comprise half of all soft tissue tumors, but the individual histologic subtypes are diverse and range from the benign lipoma to the aggressive liposarcoma. While an experienced surgeon can frequently distinguish the benign lipoma from the aggressive liposarcoma, the nuances between large lipomas and similarly large atypical lipomatous tumors create a more challenging diagnostic dilemma. Given this difficulty, general surgeons often refer patients with large, ambiguous tumors to specialty centers. The focus of this analysis is to assist the general surgeon in risk-stratifying patients with large lipomatous masses.
Lipomas, the most common benign adipocytic tumor, are defined as well-circumscribed, lobulated lesions comprised of adipose often separated from surrounding adipose tissue by a thin fibrous capsule. (1) Lipomas can occur on any part of the body and, when presenting as intramuscular tumors, can be poorly circumscribed and infiltrative—similar to well-differentiated liposarcomas (WDLS). (1) Histologically, lipomas may exhibit inflammation and/or fibrosis which may be confused with the atypical features of WDLS. (2) WDLS are non-metastasizing, low-grade, lipomatous tumors with propensity for local recurrence. (3) The term atypical lipomatous tumor (ALT) is used to describe WDLS located on an extremity or torso wall, (3) and will be used accordingly throughout this manuscript. Histologically, ALTs are characterized by mature fat with a variable number of atypical, often enlarged and pleomorphic nuclei, but with low cellularity and uncommon mitotic figures. (4) The low number of mitotic figures is particularly important in distinguishing ALTs from its more aggressive counterpart, dedifferentiated liposarcoma (DDLS). (5)
After histologic classification and staging, an important prognostic factor for liposarcoma is tumor location (central/retroperitoneal versus peripheral). (1, 6) In a single-institution review of WDLS and DDLS Evans (4) showed that patients with peripherally located tumors (extremities and trunk) experienced no disease-specific mortality and all were free of disease with a minimum of 10 years follow-up. In contrast, those patients with retroperitoneal or central tumors experienced significant disease-related morbidity and mortality. In this study we excluded patients with retroperitoneal and abdominal/pelvic lipomatous tumors on the basis that they have distinct tumor biology separate from that of patients with extremity and torso lipomatous tumors. More importantly, it is appropriate for surgeons without access to a multidisciplinary team to routinely refer patients with retroperitoneal or abdominal lipomatous tumors to a specialty center.
The best opportunity for curative management of ALT is complete resection at the initial operation, yet clinical evaluation and cross-sectional imaging frequently fails to clearly establish the diagnosis. When diagnosis is challenging, patients may undergo incomplete or margin-positive resections, which subsequently can compromise local control. While this may not impact overall survival due to the low grade nature of the disease, it does contribute to recurrence, morbidity and reduced quality of life. These concerns frequently lead non-specialty surgeons to refer patients to specialists with access to multidisciplinary teams and specialized pathologists for treatment of all large, lipomatous tumors. Interestingly, with the advent of genetic analysis, many tumors thought to be ALTs have been reclassified as lipomas. (2, 7) While this is useful to surgeons in a specialty center, many surgeons do not have access to molecular testing. We sought to identify characteristics available through non-invasive methodologies that would aid the community-based general surgeon in determining who should be considered for referral to a multidisciplinary center.
Methods
Hospital clinic and billing records were queried for all patients with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code of 214.X (lipomas) or 171.X (sarcomas). To avoid identifying a large number of patients with small, clinically straightforward lipomas, the query was limited to patients treated by one of four oncologic surgeons responsible for treating the majority of patients with extremity soft tissue sarcomas at our institution. The query identified 1,796 patients, who were further screened based on age, histologic diagnosis, primary site of disease, and therapy. Adult patients (age ≥18) with a pathologic diagnosis of lipoma (including angiolipomas, fibrolipomas, and hibernomas), WDLS, or ALT on an extremity or torso wall treated with curative intent resection were included. Patients presenting with recurrent lesions were included if they underwent curative intent operation for the recurrence at our institution. Patients with retroperitoneal, intra-abdominal, and pelvic tumors were excluded on the basis that these tumors represent significantly different disease biology. (1, 3–5) Patients with a prior history of non-liposarcoma sarcoma were also excluded, on the basis that new masses in these patients warrant a higher index of suspicion. The histopathologic identification of paucicellular mature-appearing adipose tissue containing enlarged, atypical adipocyte nuclei with few to no mitotic figures was used to establish the diagnosis of ALT. (4) Retrospective chart review of clinicopathologic characteristics, operative details, and survival outcomes was performed for 319 patients (256 with lipomas, 63 with ALTs). Preoperative characteristics readily identifiable without invasive diagnostic procedures were assessed for predictive value for a diagnosis of ALT.
WDLS and ALT were considered synonymous and will be referred to herein as ALT. Regarding tumor location, a lesion was considered to be in the upper extremity if the tumor was distal to the shoulder. Similarly tumors distal to the groin were categorized as lower extremity lesions; buttock lesions were categorized as lower extremity lesions. Lesions on the neck, chest, abdomen, and back were categorized as on the torso. The anatomic depth of the tumor was evaluated relative to the superficial investing fascia of the extremity and was ascertained from the operative report. Type of resection, radical versus local excision, was obtained from the operative report as described by the operating surgeon; exact details regarding resection of investing fascia and margin of resection were not uniformly available. Direct review of imaging studies was not possible given the retrospective nature of the study and changes in how radiographic studies were stored over time.
Prior to data collection permission from Emory University’s Institutional Review Board was obtained, and compliance with Health Insurance Portability and Accountability Act of 1996 was maintained throughout the study.
Statistical Analysis
Data were analyzed using Statistical Package for the Social Sciences 19.0 for Windows (IBM, Chicago, IL). Categorical variables were compared with Pearson chi-square and Fisher exact tests, as appropriate, whereas continuous variables were compared with Student’s t-test. Two-tailed p values <0.05 were considered statistically significant. Kaplan-Meier survival analysis was used to calculate overall and recurrence-free survival. Univariate and multivariate binary logistic regression were used to identify factors predictive of a diagnosis of atypical lipomatous tumor and to assess factors predictive of recurrence.
Results
Clinicopathologic Characteristics
319 patients were identified with lipomatous masses: 80.3% (n=256) lipomas and 19.7% (n=63) ALTs. The median age was 55.0 years with a slight female predominance (53.6%); the majority of patients (51.1%, n=163) were white. The lesions most frequently involved the lower extremity (39.2%), followed by the torso (33.9%) and the upper extremity (27%). The mean duration of the presence of the mass as reported by the patient was 31.4 months (range 0–360 months). 28 patients (8.8%) reported having had similar masses excised at the same site; an additional 10 patients were referred immediately after inadequate operations for the primary tumor (3.1%). Magnetic resonance imaging (MRI) was the most frequent imaging modality utilized (77.2%); 15.7% of patients either had no imaging studies or the study was insufficiently documented within the medical record. Two patients underwent neoadjuvant radiation due to concern regarding dedifferentiated and/or myxoid areas on diagnostic biopsy; both patients had a final pathologic diagnosis consistent with ALT. No patient received neoadjuvant chemotherapy. The majority of patients (85.6%) did not have a tissue diagnosis prior to definitive surgical therapy. Radical excision was performed for the majority of tumors (62.7%) and all operations were limb-preserving. Two patients underwent adjuvant radiation, one due to concern regarding a positive margin and the other due to early focal dedifferentiation (both had ALTs). Adjuvant chemotherapy was not administered to any patient. Of the patients with ALTs only 3 (4.8%) were superficial. No distant metastases occurred. No disease-specific mortality was observed with a median follow-up of 11.5 months (3.4 months for patients with lipomas, 27.4 months for patients with ALTs). The clinicopathologic demographics of the study population are summarized in Table 1.
Table 1.
Clinicopathologic and Perioperative Characteristics of All Patients (n=319)
| Clinical characteristics | n (%) or median [range] |
|---|---|
| Age, y | 55.0 [18.7–86.5] |
| Age ≥55 y | 161 (50.5) |
| Male sex | 148 (46.4) |
| Race | |
| White | 163 (51.1) |
| Black | 90 (28.2) |
| Other | 61 (19.1) |
| Location | |
| Torso | 108 (33.9) |
| Upper Extremity | 86 (27) |
| Lower Extremity | 125 (39.2) |
| Estimated duration of mass (months) | 12 [0–360] |
| Recurrent lesion | 28 (8.8) |
| Excision immediately prior to presentation | 10 (3.1) |
| Imaging* | |
| None | 34 (10.7) |
| Computed tomography | 34 (10.7) |
| Magnetic resonance imaging | 244 (77.2) |
| Unknown | 16 (5) |
| Biopsy | |
| Core biopsy or fine needle aspiration | 29 (9.1) |
| Incisional | 7 (2.2) |
| Neoadjuvant radiation therapy | 2 (0.6) |
| Perioperative characteristics | |
| Operation | |
| Local excision | 119 (37.3) |
| Radical excision | 200 (62.7) |
| Limb preserving | 221 (100) |
| Complication (any) | 30 (9.4) |
| Wound complication requiring reoperation | 7 (2.2) |
| Postoperative radiation therapy | 2 (0.6) |
| Pathologic characteristics | |
| Pathology | |
| Lipoma | 256 (80.3) |
| Atypical lipomatous tumor | 63 (19.7) |
| Tumor size (cm) | 8.9 [1.5–35] |
| Tumor size ≥10 cm | 134 (42) |
| Margin | |
| Negative | 36 (11.3) |
| Positive | 29 (9.1) |
| Not reported | 254 (79.6) |
| Outcomes | |
| Recurrence | 16 (5) |
| Follow-up, mo | 11.5 |
| Disease-specific mortality | 0 |
Includes 9 patients who had both CT and MRI
Lipoma versus Atypical Lipomatous Tumor
The distribution of race and gender did not differ between patients with lipomas and patients with ALTs. As compared to patients with lipomas, patients with ALTs were older (60.5 vs 53.5 years, p<0.0001) and were more likely to have the tumor located on an extremity (88.9% vs 60.5%, p<0.0001). The average duration of the tumor as reported by the patient was not significantly different between patients with lipomas vs. ALTs (31.6 vs. 30.4 months, p=0.877). More patients with ALTs underwent 3-dimensional cross-sectional imaging as compared to patients with lipomas (98.3% vs 86.5%, p=0.006). There were no differences in the rate of preoperative biopsy (core, fine-needle aspiration, or incisional) between patients with ALTs vs lipomas (10.0% vs 18.3%, p=0.110). More patients with ALTs reported a history of having had similar masses excised at the same site (20.6% vs 5.9% of patients with lipomas, p=0.001), exclusive of an operation immediately prior to referral in which the referring surgeon proceeded thinking that the lesion was a lipoma. Ten patients were referred immediately after inadequate operations for the primary tumor; on final pathologic analysis, 8 patients had ALTs and 2 had lipomas (p<0.0001). Radical excision was employed more frequently amongst patients with ALTs (93.7% vs 55.1%, p <0.0001). The overall rate of complications and the wound-specific rate of complications did not differ between the two groups (p>0.05). The comparison of patients with lipomas to those with ALTs is summarized in Table 2.
Table 2.
Comparison of Clinicopathologic and Perioperative Characteristics between Patients with Lipomas and Patients with Atypical Lipomatous Tumors
| Clinicopathologic variable n (%), or mean* |
Lipomas (n=256) |
ALTs (n=63) |
p Value |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y* | 53.5 | 60.5 | <0.0001 |
| Age ≥55 y | 116 (45.3) | 45 (71.4) | <0.0001 |
| Male sex | 120 (46.9) | 28 (44.4) | 0.837 |
| Race | |||
| White | 124 (49.4) | 39 (61.9) | 0.205 |
| Black | 76 (30.3) | 14 (22.2) | |
| Other | 51 (20.3) | 10 (15.9) | |
| Location | |||
| Torso | 101 (39.5) | 7 (11.1) | <0.0001 |
| Upper Extremity | 81 (31.6) | 5 (7.9) | |
| Lower Extremity | 74 (28.9) | 51 (81) | |
| Extremity location (vs torso) | 155 (60.5) | 56 (88.9) | <0.0001 |
| Estimated duration of mass, mo* | 31.6 | 30.4 | 0.877 |
| Prior operation at same site† | 15 (5.9) | 13 (20.6) | 0.001 |
| Excision immediately prior to presentation | 2 (0.8) | 8 (12.7) | <0.0001 |
| 3-dimensional imaging (MRI or CT) | 211 (86.5) | 58 (98.3) | 0.006 |
| Preoperative tissue diagnosis | 27 (10.8) | 17 (27.9) | 0.001 |
| Neoadjuvant radiation therapy | 0 | 2 (3.3) | 0.037 |
| Perioperative characteristics | |||
| Operation | |||
| Local excision | 115 (44.9) | 4 (6.3) | <0.0001 |
| Radical excision | 141 (55.1) | 59 (93.7) | |
| Complication, any | 22 (9.8) | 8 (13.6) | 0.554 |
| Wound complication requiring reoperation | 4 (1.8) | 3 (5.1) | 0.162 |
| Postoperative radiation therapy | 0 | 2 (3.4) | 0.040 |
| Pathologic characteristics | |||
| Tumor size, cm | 8.3 | 16.0 | <0.0001 |
| Tumor size ≥10 cm | 83 (32.5) | 51 (83.6) | <0.0001 |
| Margin | |||
| Negative | 8 (3.1) | 28 (44.4) | <0.0001 |
| Positive | 2 (0.8) | 15 (23.8) | |
| Not reported | 246 (96.1) | 20 (31.7) | |
| Outcomes | |||
| Recurrence | 2 (0.8) | 14 (22.6) | <0.0001 |
| Follow-up, mo | 27.6 | 44.2 | 0.168 |
Continuous variables were compared using student’s t test; the mean is reported.
Exclusive of excision immediately prior to referral.
After excluding the 10 patients who were referred for definitive therapy immediately after insufficient operations on the grounds that these patients had an accurate tissue diagnosis a priori, the following factors were associated with increased risk of diagnosis of ALT on univariate analysis: age (both as a continuous variable and dichotomized at the median, 55 years), history of prior operations, extremity location, and tumor size (both as a continuous variable and dichotomized at 10 cm; p<0.0001 for all). Tumor size was based on the final pathologic evaluation, but given the accuracy of modern three-dimensional imaging the authors believe size can be accurately estimated preoperatively. On multivariate analysis age ≥ 55 years (HR 2.64, 95% CI 1.24–5.67, p=0.012), history of prior operations (HR 5.56, 95% CI 1.93–16.03, p=0.002), extremity location (HR 6.41, 95% CI 2.23–18.38, p=0.001), and size ≥ 10 cm (HR 16.81, 95% CI 6.49–43.53, p<0.0001) remained significantly associated with diagnosis of ALT as opposed to lipoma (Table 3).
Table 3.
Multivariate Analysis of Risk Factors for Atypical Lipomatous Tumor Versus Lipoma (n=309)*
| Risk Factor | HR | 95% CI | p Value |
|---|---|---|---|
| Age ≥ 55 y | 2.64 | 1.24–5.66 | 0.012 |
| Prior operation at same site* | 5.56 | 1.93–16.03 | 0.002 |
| Extremity location (versus torso) | 6.41 | 2.23–18.38 | 0.001 |
| Size ≥ 10 cm | 16.81 | 6.49–43.53 | <0.0001 |
Patients referred immediately after insufficient excision were excluded from analysis on the grounds that they had a pathologic diagnosis a priori; prior operation refers to operation in remote past.
Recurrence Patterns
Recurrences were observed in 16 patients (5% overall) and median time to recurrence was not reached. Of the patients experiencing recurrence, the mean time to recurrence was 16 months. All recurrences were local. Two patients (0.8%) with lipomas experienced recurrence; one was observed, the other was managed surgically and final pathology confirmed the diagnosis of lipoma. As expected, a larger proportion of patients with ALTs experienced recurrence (22.6% vs 0.8%, p<0.0001). Twelve patients were managed surgically, with final pathology confirming ALT in 11, and revealing a lipoma rather than a recurrence in 1 patient. The remaining 2 patients were followed radiographically with minimal evidence of tumor growth over 11.3 and 47.1 months respectively. Of the patients who initially presented with recurrent disease (n=28), no patients with lipomas (n=15) recurred after definitive surgical resection. Five patients with recurrent ALTs (n=13) experienced at least 1 additional recurrence. Four of the 5 patients with multiple recurrences were managed surgically; no amputations were required. In the entire study population, the risk of recurrence was significantly higher for patients who had already experienced 1 or more recurrences at presentation as compared to patients who presented with a primary lesion (HR 5.51, 95% CI 1.76–17.23, p=0.003), however this relationship was not significant when limited to patients with ALTs only (HR 1.167, 95% CI 0.21–6.54, p=0.861). Amongst patients with ALTs only (n=63) age, gender, tumor location, duration of lesion, tumor size, and tumor depth were not associated with recurrence (p>0.05). Recurrence was not associated with inadequate resection immediately prior to referral (HR 2.78, 95% CI 0.73–10.51, p=0.132) or margin status (HR 1.82, 95% CI 0.45–7.39, p=0.405).
Discussion
Differentiating ALTs from lipomas can be difficult preoperatively, but appropriate classification is important, as it may allow for observation, alter the surgical approach, or prompt referral to a specialist. In this study we identify preoperative noninvasive characteristics that favor a diagnosis of ALT over lipoma: patient age ≥ 55 years, tumor size ≥10 cm, history of prior operations at the same site (exclusive of biopsy or excision immediately prior to referral), and extremity location (vs. torso). Additionally, we characterize disease-specific morbidity (including recurrence patterns) and mortality specific to patients with ALT.
Obtaining a tissue diagnosis prior to surgical intervention is ideal, but even with fine-needle aspiration (FNA) and core-needle biopsy (CNB) the potential for complication exists (0– 10%) (8) and diagnostic ambiguity remains. (9) The nondiagnostic rate of FNA and CNB for palpable extremity soft tissue masses ranges from 8.1–12.3% and 6–23%, respectively. (8–11) When adequate tissue is obtained, lipomas comprise the most frequent subtype of benign soft tissue tumors mistaken for sarcoma. (12) Genetic analysis has enhanced the ability to distinguish between ALTs and lipomas but is frequently not available outside of a university setting and it is not uncommon for tissue obtained even by core biopsy to be inadequate for genetic analysis. Our study provides the treating surgeon with clinical risk factors not dependent on biopsy.
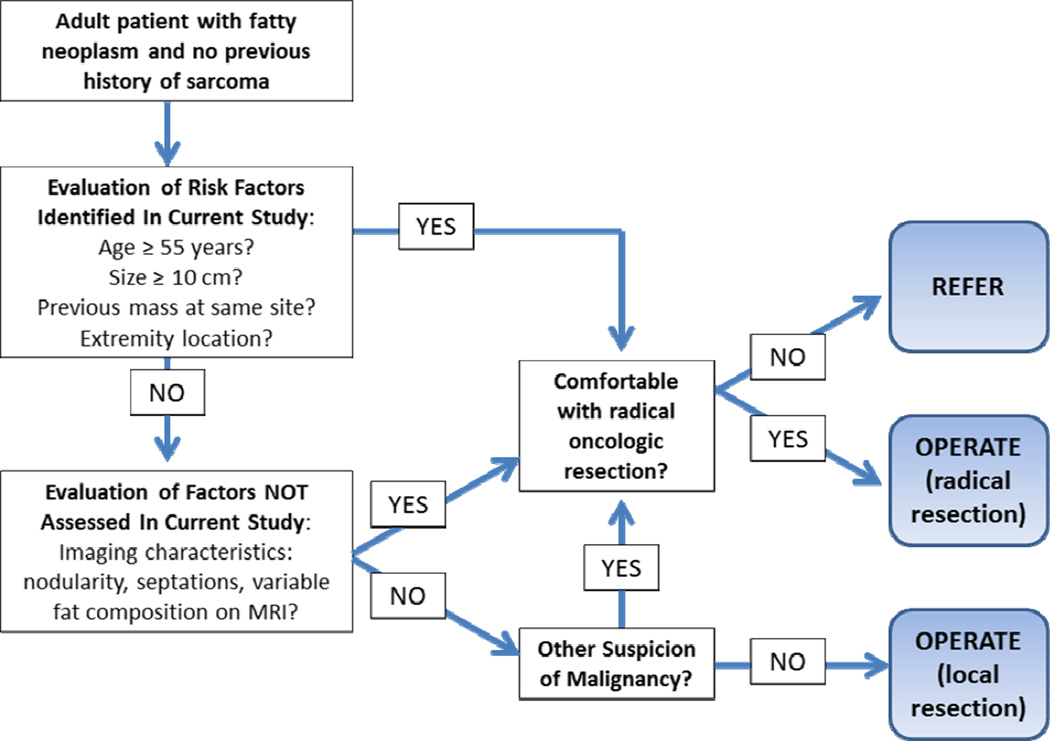
Increased patient age and tumor size have been suggested by others as markers for ALT versus lipoma. In a small study of 35 patients with lipomas and 25 with ALTs, both age and tumor size were significant on univariate but not on multivariate analysis. (13) Similarly, in a series of 54 patients with lipomas and 33 with ALTs, Brisson et al. (14) noted that patient age greater than 60 years, tumor size greater than 10 cm, and location in the lower extremity were suggestive of ALT, but only tumor size (odds ratio 1.09, 95% CI 1.02–1.18, p=0.016) remained predictive on multivariate analysis. In our series with almost twice as many patients, both increased age and tumor size remain predictive of a diagnosis of ALT on multivariate analysis. Although basing treatment decisions on retrospective data should be regarded with skepticism, a a suggested diagnostic algorithm is shown in Figure 1. If the clinician evaluating the patient is not comfortable with aggressive oncologic evaluation, referral to a specialist in any situation in which concern for malignancy exists remains appropriate.
Figure 1.
Suggested algorithm for management of a patient with a fatty neoplasm.
Given the heterogeneity of lipomatous tumors and the relative rarity of each subtype, much of the literature groups ALTs with WDLS and DDLS. Among the few ALT-specific studies, the rate of local recurrence is highly variable, ranging from 5–52%. (2, 3, 15–17) The rates of distant metastases and disease-specific mortality are more homogenous, with most studies reporting either none (2, 4, 15, 16, 18, 19) or very rare events. (3) In our study, which represents a relatively large single-institution experience comprised of only ALTs, the local recurrence rate was 22.2%, with no patient experiencing distant metastases and no disease-specific mortality.
Although others have identified patient age, tumor size, and margin status as predictors of local recurrence after resection of primary extremity soft tissue sarcoma, (20) we were unable to identify any single factor as a predictor of recurrence in the subset of patients with ALT. One potential explanation may be that other predictive models included more aggressive forms of liposarcoma and/or sarcoma in addition to ALT, and may not be applicable specifically to patients with ALT. The lack of predictors of recurrence specifically for patients with ALT has also been demonstrated by others. In a study of 51 patients with ALTs Bassett et al. (17) showed that patient age, gender, tumor size, and tumor location (extremities or torso soft tissue) were not associated with risk of recurrence. Margin status as a predictor of local recurrence has also been questioned: Sommerville et al. (18) described a low local recurrence rate (8%) in 61 patients with primary extremity ALT all treated with marginal excision and followed for a minimum of two years. A smaller study reported a similarly low rate of recurrence (11.1%) after excision of extremity ALT with positive margins in 7 of the 9 patients. (21) In our study patients with positive margins were not more likely to experience recurrence. Some have observed that patients presenting with a history of recurrences are more prone to future recurrence, (15) although others have reported no relationship. (3) Although we observed that a larger proportion of patients who initially presented with recurrence experienced re-recurrence (38.5% vs 18.4% primary), the difference was not statistically significant (p=0.243). The relatively low frequency of recurrence amongst patients with ALTs may preclude meaningful analysis. Additionally, our ability to report recurrence may be limited by our follow-up time (median 27.4 months, range 0– 164.6 months) as other studies have documented recurrence as a late event. (3, 15)
A limitation of our study involves the dependence on accurate histologic diagnosis of ALT without molecular confirmation. However, recent data suggest that there is high concordance between the initial histologic diagnosis of ALT and subsequent molecular confirmation (96%). (2) In that study, molecular cytogenetic evaluation reclassified, or “downstaged,” 21% (n=11) of ALTs to lipomas, but only 2% (n=7) of lipomas were “upstaged” to ALTs. On review of “upstaged” ALTs, tumor size is often strongly correlated. (2, 7) At our institution the majority of our soft tissue specimens are evaluated by one of three expert soft tissue pathologists who routinely review diagnostically challenging lipomatous masses in expert consultation, and we believe that the histologic diagnosis of ALT can be used with confidence for the purposes of this study. While our current practice is to obtain confirmatory MDM2 gene amplification testing when histologic or gross features of lipomatous masses are concerning for ALT (cellular atypia, retroperitoneal location, or size greater than 10.0 cm), we chose not to include molecular testing in this study since this is not readily available at all centers. Additionally, it is unclear if MDM2 testing changes management after wide excision.(2) Given the small percentage of fatty tumors upstaged to ALT upon final pathology, perhaps the role of MDM2 testing in this particular population should also be evaluated in a cost-value analysis.
Another limitation of our study is that specific imaging characteristics were not available for review. As a tertiary referral center, many of our patients underwent imaging at an outside facility, and images were not incorporated into the permanent medical record. Imaging characteristics have not been shown consistently to differentiate ALTs from lipomas, and the specificity of MRI is reported to be only 37%. (14) However, the presence of thick/irregular septations, tumor nodularity, and/or variable fatty composition is suggestive of a more aggressive biologic behavior,(13) and it is the authors practice to proceed with radical resection when any of the above imaging characteristics are present. Future studies are needed to investigate imaging features predictive of the diagnosis of ALT. Finally, we classified operative type based on the description made by the surgeon, but full details regarding resection of investing fascia and/or compartments, and measurement of margin of resection were not routinely available. It is possible that some of the defined radical resections were actually marginal excisions, and this may obscure actual differences in recurrence rates by degree of resection.
In this study we characterize a distinct population of patients with lipomatous masses (ALTs) that, while often grouped with other patients with liposarcomas, clearly have a more indolent clinical course. Our findings identify readily available, noninvasive characteristics of patients presenting with lipomatous masses that may predict the diagnosis of ALT: patient age, tumor size and location, and history of recurrence. These factors may be useful to the general surgeon in deciding surgical approach (including nonoperative management) or guiding referral to a specialist. The preoperative algorithm proposed in this retrospective study is meant as an adjunct tool for a clinically ambiguous situation. While it requires validation, it is reassuring to note that limb loss or death did not occur with a low recurrence rate.
Acknowledgments
Financial Support: Dr Fisher is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Ms Baxter has disclosed past receipt of an NIH TL1 training grant; this is not related to the current work. Dr Weiss has disclosed receipt of royalties from textbook publication; this is not related to the current work.
Abbreviations
- ALT
atypical lipomatous tumor
- WDLS
Well-differentiated liposarcoma
- DDLS
Dedifferentiated liposarcoma
- ICD-9-CM
International Classification of Diseases, ninth revision, clinical modification
- MRI
Magnetic resonance imaging
- FNA
fine-needle aspiration
- CNB
core needle biopsy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the Academic Surgical Congress, New Orleans, LA, February 2013.
References
- 1.Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008;97:298–313. doi: 10.1002/jso.20975. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Erickson-Johnson M, Wang X, et al. Molecular testing for lipomatous tumors: critical analysis and test recommendations based on the analysis of 405 extremity-based tumors. Am J Surg Pathol. 2010;34:1304–1311. doi: 10.1097/PAS.0b013e3181e92d0b. [DOI] [PubMed] [Google Scholar]
- 3.Kooby DA, Antonescu CR, Brennan MF, Singer S. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2004;11:78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- 4.Evans HL. Atypical lipomatous tumor, its variants, and its combined forms: a study of 61 cases, with a minimum follow-up of 10 years. Am J Surg Pathol. 2007;31:1–14. doi: 10.1097/01.pas.0000213406.95440.7a. [DOI] [PubMed] [Google Scholar]
- 5.Hogg ME, Wayne JD. Atypical lipomatous tumor/well-differentiated liposarcoma: what is it? Surg Oncol Clin N Am. 2012;21:333–340. doi: 10.1016/j.soc.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opinion Oncol. 2011;23:373–378. doi: 10.1097/CCO.0b013e32834796e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashima T, Halai D, Ye H, et al. Sensitivity of MDM2 amplification and unexpected multiple faint alphoid 12 (alpha 12 satellite sequences) signals in atypical lipomatous tumor. Modern Pathol. 2012 Jun 15; doi: 10.1038/modpathol.2012.90. [DOI] [PubMed] [Google Scholar]
- 8.Wu JS, Goldsmith JD, Horwich PJ, et al. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248:962–970. doi: 10.1148/radiol.2483071742. [DOI] [PubMed] [Google Scholar]
- 9.Kasraeian S, Allison DC, Ahlmann ER, et al. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthopaed Rel Res. 2010;468:2992–3002. doi: 10.1007/s11999-010-1401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams SC, Potter BK, Pitcher DJ, Temple HT. Office-based core needle biopsy of bone and soft tissue malignancies: an accurate alternative to open biopsy with infrequent complications. Clin Orthopaed Rel Res. 2010;468:2774–2780. doi: 10.1007/s11999-010-1422-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng VY, Thomas K, Crist M, et al. Fine needle aspiration for clinical triage of extremity soft tissue masses. Clin Orthopaed Rel Res. 2010;468:1120–1128. doi: 10.1007/s11999-009-1100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbiser ZK, Folpe AL, Weiss SW. Consultative (expert) second opinions in soft tissue pathology Analysis of problem-prone diagnostic situations. Am J Surg Clin Pathol. 2001;116:473–476. doi: 10.1309/425H-NW4W-XC9A-005H. [DOI] [PubMed] [Google Scholar]
- 13.Kransdorf MJ, Bancroft LW, Peterson JJ, et al. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224:99–104. doi: 10.1148/radiol.2241011113. [DOI] [PubMed] [Google Scholar]
- 14.Brisson M, Kashima T, Delaney D, et al. MRI characteristics of lipoma and atypical lipomatous tumor/well-differentiated liposarcoma: retrospective comparison with histology and MDM2 gene amplification. Skeletal Radiol. 2012 Sep 18; doi: 10.1007/s00256-012-1517-z. [DOI] [PubMed] [Google Scholar]
- 15.Mavrogenis AF, Lesensky J, Romagnoli C, et al. Atypical lipomatous tumors/welldifferentiated liposarcomas: clinical outcome of 67 patients. Orthopedics. 2011;34:e893–e898. doi: 10.3928/01477447-20111021-11. [DOI] [PubMed] [Google Scholar]
- 16.Serpell JW Chen RYCINANZJSD, Pmid. Review of large deep lipomatous tumours. ANZ J Surg. 2007;77:524–529. doi: 10.1111/j.1445-2197.2007.04042.x. [DOI] [PubMed] [Google Scholar]
- 17.Bassett MD, Schuetze SM, Disteche C, et al. Deep-seated, well differentiated lipomatous tumors of the chest wall and extremities: the role of cytogenetics in classification and prognostication. Cancer. 2005;103:409–416. doi: 10.1002/cncr.20779. [DOI] [PubMed] [Google Scholar]
- 18.Sommerville SM, Patton JT, Luscombe JC, et al. Clinical outcomes of deep atypical lipomas (well-differentiated lipoma-like liposarcomas) of the extremities. ANZ J Surg. 2005;75:803–806. doi: 10.1111/j.1445-2197.2005.03519.x. [DOI] [PubMed] [Google Scholar]
- 19.Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. A follow-up study of 92 cases with analysis of the incidence of"dedifferentiation". Am J Surg Pathol. 1992;16:1051–1058. doi: 10.1097/00000478-199211000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Cahlon O, Brennan MF, Jia X, et al. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012;255:343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubo T, Sugita T, Shimose S, et al. Conservative surgery for well-differentiated liposarcomas of the extremities adjacent to major neurovascular structures. Surg Oncol. 2006;15:167–171. doi: 10.1016/j.suronc.2006.11.004. [DOI] [PubMed] [Google Scholar]