Abstract
Nonmedical use of the prescription opioid oxycodone has become a major public health problem in the United States, with special concern for adolescents. Although adults and adolescents have different sensitivities for drugs, little is known about the rewarding effects of oxycodone in adolescents compared to adults, even in rodent models. Here, we investigate sensitivity to oxycodone by the conditioned place preference assay of conditioned reward, and effect on the locomotor activity in adolescent (4 weeks old) and adult (10 weeks old) C57BL/6J mice. Mice of both ages were trained with multiple doses of oxycodone (0, 0.3, 1, and 3 mg/kg) and showed conditioned preference in a dose-dependent manner. The adult mice developed conditioned preference to the lowest dose tested (0.3 mg/kg), but adolescent mice did not. Dose-dependent oxycodone-induced increases in locomotor activity were observed across the conditioning session. Interestingly, adolescent mice developed greater sensitization to the locomotor-activating effects of oxycodone than adult mice. Thus differences in sensitivity to oxycodone, such as the lower initial sensitivity for conditioned preference but greater locomotor sensitization in adolescent mice, may indicate contributing factors in oxycodone abuse and later addiction in human adolescents.
Keywords: Adolescent, Conditioned place preference, locomotor sensitization, Oxycodone
1. Introduction
Although prescription opioid analgesics, such as oxycodone, are among the most effective medications for pain, they are also associated with the public health problem of illicit use of prescription opioids in the United States. Oxycodone is a mu-opioid agonist (MOP-r) and has been used for over half century in the United States. Compared with the prototypical MOP-r agonist morphine (also a major metabolite of heroin), potency of oxycodone is approximately twice as high, by the oral route (Benziger et al., 1997). Like morphine, oxycodone has rewarding effects (Rutten et al., 2011). However, unlike morphine, oxycodone is metabolized by Cytochrome P450 (CYP2D6 and CYP3A4) (see review Smith, 2009). Previous studies show that morphine causes MOP-r desensitization but poor internalization, compared to other MOP-r ligands (Keith et al., 1998, Whistler et al., 1999, Haberstock-Debic et al., 2003). The profile of oxycodone in this regard may differ from that of morphine, depending on in vitro conditions (Arttamangkul et al., 2008, Imai et al., 2011).
The adolescent brain is particularly susceptible to the effects of drugs of abuse. The brain circuits undergo developmental alterations during adolescence. For example, the number of dopamine receptors in the nigrostriatal and mesolimbic dopaminergic systems steadily increased after birth and peaks at 40 days of age in adolescent mice, then decreases at 60-80 days of age (Teicher et al., 1995). Mu-opioid receptor-stimulating [35S] GTPgammaS binding assessing mu-opioid receptor function showed a 20-fold increase between postnatal day five and adulthood (Talbot et al., 2005). Further, many studies have reported that adolescent rodents respond differently than adults to a number of drugs which act on the dopamine systems. For example, adolescents showed a reduced responsiveness to the locomotor effects of the psychostimulants amphetamine and cocaine (Spear and Brick, 1979, Bolanos et al., 1998). Conversely, these animals were more sensitive to the dopamine antagonist haloperidol (Spear et al., 1980) and exhibited an exaggerated locomotor response to morphine compared to that of adults (Spear et al., 1982).
We previously reported the effects of oxycodone self-administration and subsequent effects of oxycodone administration on striatal dopamine levels in adolescent and adult mice (Zhang et al., 2009b). We found that adult mice self-administered significantly more oxycodone than did adolescent mice during the acquisition of self-administration behavior. However, when examined later, in response to the lowest dose of experimenter-administered oxycodone tested, there was a significant increase in striatal dopamine levels in mice that had self-administered oxycodone during adolescence, but not in mice that had self-administered oxycodone as adults (Zhang et al., 2009b). In addition to our result, it has been reported that plasma ACTH response to CRH was enhanced in late adolescent male rats as a result of prenatal oxycodone exposure, but had no effect in females (Sithisarn et al., 2008). Taken together, these results suggest that oxycodone-induced rewarding effects differ between adolescent and adult mice. We hypothesize that adolescent and adult mice show different responses to the rewarding and locomotor stimulating effects of oxycodone, in which exposure to oxycodone is equalized across adolescents and adults. To test this hypothesis, the current study was carried out to compare oxycodone-induced conditioned rewarding effects and locomotor activity in both adolescent and adult C57BL/6J mice using a conditioned place preference paradigm. The locomotor stimulant effects may be useful markers for the addictive properties of drugs, due to a high homology between the brain regions underlying stimulant and rewarding effects of drugs (Wise and Bozarth, 1987). Sensitization of certain components of drug action has also been postulated as part of addiction mechanisms (Robinson and Berridge, 1993, 2001, 2003, Vanderschuren and Pierce, 2010).
2. Material and methods
2.1. Animals
Male adolescent and adult (4 or 10 weeks old on arrival) C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were housed in groups of four with free access to food and water in a light (12 : 12 h light/dark cycle, lights on at 0700 hours) and temperature (25 °C) controlled room. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources Commission on Life Sciences, 1996). The experimental protocols used were approved by the Institutional Animal Care and Use Committee of The Rockefeller University. See Table 1 for a description of the experimental time line and age of the mice.
Table 1. Time Line: Age in Experiment.
| On arrival | Preconditioning | Condit ioning | Postconditioning | |
|---|---|---|---|---|
| Adolescent Postnatal day | 28 | 35 | 36- 43 | 44 |
| Adult Postnatal day | 70 | 77 | 78- 85 | 86 |
2.2. Mouse place preference chambers
The mouse place preference chambers (model ENV-3013) were purchased from Med Associates (Med Associates, St.Albans, VT). Each chamber has three distinct compartments that can be separated by removable doors. Automated data collection is accomplished by individual infrared photobeams on a photobeam strip, with six beams in the white and black compartments and two beams in the smaller central gray compartment. The black compartment is 16.8×12.7×12.7 cm with a stainless-steel grid rod floor. The white compartment (also 16.8×12.7×12.7 cm) has a stainless-steel mesh floor (Zhang et al., 2002, Zhang et al., 2012).
2.3. Locomotor activity and conditioned place preference determination
Four groups of seven mice in each age groups were studied, one group at each dose: 0, 0.3, 1, and 3 mg/kg of oxycodone. Valuable strain comparisons have been generated for setting up the schedule and opioid ligand dosage for CPP (e.g., Orsini et al., 2005). However, since we focus on the effect of oxycodone in different ages in the current study, the dosage and experimental paradigm are used based on previous studies within one strain. The doses chosen in the study were based on published data in adult mice (Narita et al., 2008). The experimental paradigm for the CPP study was based on our earlier studies (Schlussman et al., 2008, Zhang et al., 2009a). Experiments were performed in a dimly lit, sound-attenuated chamber described above. The study used an unbiased, counterbalanced design in which seven mice were randomly assigned to either the oxycodone or saline compartment on the first day. Half the animals had white and half had black as the oxycodone-paired side. During the preconditioning session, each animal was placed in the center compartment with free access to the black and white compartments, and the amount of time spent in each compartment was recorded for 30 min. During the conditioning sessions, mice were placed into and restricted to the appropriate compartment for 30 min after oxycodone or saline injection. Locomotor activity was assessed as the number of “crossovers,” defined as breaking the beams at either end of the conditioning compartment. The animals were injected with oxycodone and saline on alternate days, for a total of eight conditioning sessions with four oxycodone and four saline trials for each animal. Conditioning sessions were conducted daily. The postconditioning test session (without injection; i.e., a drug-free state) was performed on the day after the last conditioning session, and was identical to the preconditioning session: Each mouse had free access to both white and black compartments. The difference between the pre- and postconditioning sessions in the amount of time spent on the drug-paired compartment was used to determine whether the mice had developed a conditioned place preference to oxycodone.
2.4. Statistical analysis
Analyses of variance (ANOVAs) of CPP, locomotor activity, and locomotor sensitization, followed by Newman–Keuls post hoc tests were used to examine the significance of differences in behavior between drug doses, ages and sessions. The results of each age group were also separately examined by ANOVA.
3. Results
A preliminary analysis was made to compare the amount of time spent during the preconditioning session in the compartment that was later paired with oxycodone to the one paired with saline. Two-way ANOVAs (Age × Side) showed no main effect of age or side, nor as there a significant interaction (means±S.E.M. are shown in Table 2). Also, in the postconditioning test session, mice of both ages showed no increased amount of time spent on the oxycodone-paired compartment, regardless of which compartment was used for pairing.
Table 2. Amount of time spent during the preconditioning session in the compartment (sec).
| Saline side | Oxycodone side | |
|---|---|---|
| Adolescent | 652±27 | 618±19 |
| Adult | 666±36 | 630±27 |
3.1. Conditioned place preference in adolescent and adult mice
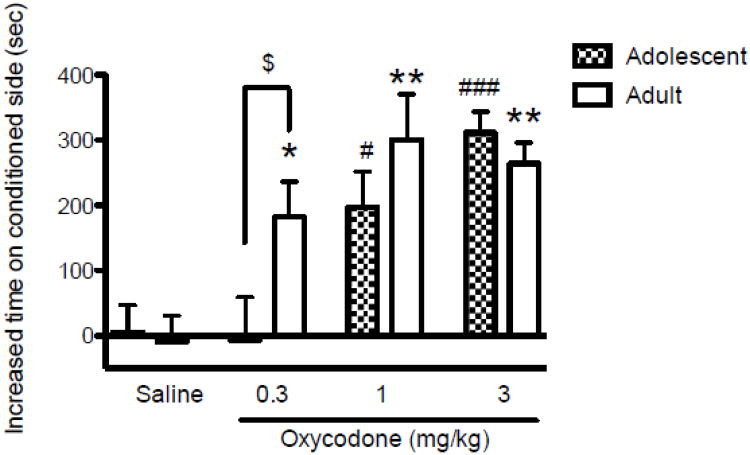
The increase in the amount of time spent on the drug-associated side, indicating a conditioned place preference, is shown in Fig. 1 in each age group of mice at each of four doses. Two-way ANOVA (Dose × Age) showed a significant main effect of Dose [F(3,48)=14.57, p<0.0001], with no significant difference between Age groups and no significant Dose × Age interaction. Of interest, Newman–Keuls post hoc tests showed that there was a significant difference in preference between the two age groups in response to the lowest dose (0.3 mg/kg) of oxycodone: adolescent mice did not show preference, whereas the adult mice did (p<0.05). When the two age groups were examined separately by one-way ANOVA, adolescent mice showed a significant main effect of Dose [F(3,24)=9.631, p<0.0002]. A significant preference for the drug-paired side was not produced by the lowest dose, 0.3 mg/kg, but there was a significant preference induced by doses of 1 and 3 mg/kg compared to the saline control (p<0.05 for 1 mg/kg, p<0.001 for 3 mg/kg). In adult mice, there was a significant main effect of Dose on increased time on the drug-associated side [F(3,24)=7.314, p<0.0012], with doses from 0.3 to 3 mg/kg inducing significant preference compared to the 0 dose (p<0.05 for 0.3 mg/kg, p<0.01 for 1 and 3 mg/kg).
Figure 1.
Conditioned place preference as reflected by the increased number of seconds spent in the oxycodone- (for oxycodone conditioned group) or saline- (for saline conditioned group) paired compartment in the postconditioning sessions (postconditioning time minus preconditioning time) in groups given oxycodone or saline (mean ±S.E.M., n=7 per group). Oxycodone produced a dose dependent preference for oxycodone-paired compartment in both age groups (*p<0.05 and *p<0.01 vs. saline in the adult group: #p<0.05 and ###p<0.001 vs. saline in the adolescent group). However, the adult mice developed conditioned preference to the lowest dose of oxycodone tested (0.3 mg/kg); the adolescent group did not ($p>0.05 between age groups).
3.2. Oxycodone-induced locomotor activity during conditioning sessions
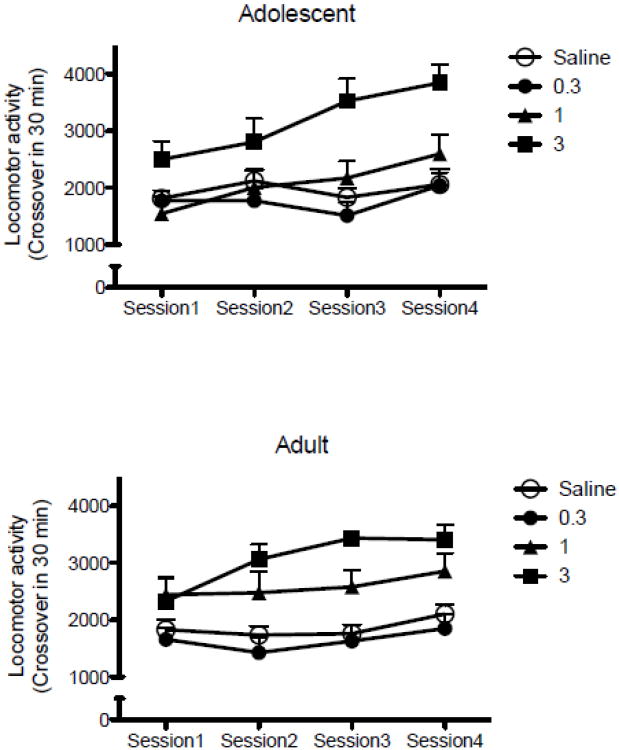
Locomotor activity in the oxycodone conditioning sessions as measured by the number of crossovers from one end of the compartment to the other for each dose from 0 (saline) to 3 mg/kg in each age group is shown in Fig. 2. Three-way ANOVA (Dose × Age × Conditioning Session) with repeated measures on the last factor revealed a dose-dependent increase in activity [F(3,192)=42.75, p<.000001], and increased locomotor activity across the sessions [F(3,192)=7.19, p<0.0002]. There was no significant difference between age groups in the number of crossovers in the 30 min period after oxycodone administration [F(1,192)<1.0]. Of interest, there was a significant Age × Dose interaction [F(3,192)=2.68, p<0.05]. Newman–Keuls post hoc tests showed that in adults both 1 and 3 mg/kg of oxycodone showed significant increases in locomotor activity compared to saline controls (both p<0.001), whereas in adolescents significance was found only at the 3 mg/kg dose of oxycodone (p<0.001).
Figure 2.
Locomotor activity in the oxycodone-paired compartment expressed as mean (±S.E.M.) number of crossovers in 30 min in each of the four oxycodone conditioning sessions (n=7 per group). Oxycodone produced a dose-dependent increase in crossovers, with no significant difference between age groups.
3.3. Sensitization of locomotor response
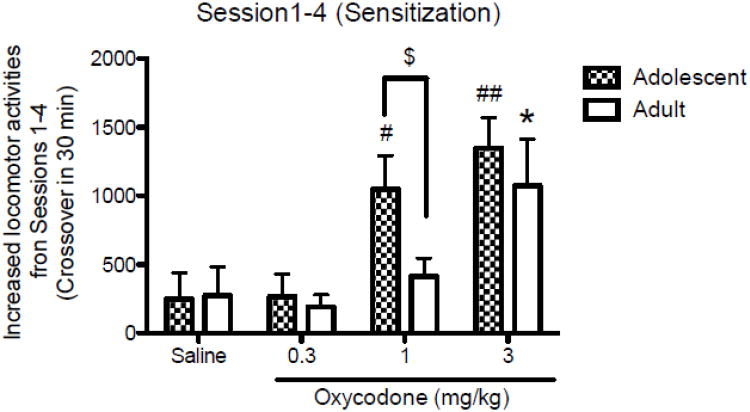
We then examined the magnitude of the locomotor response to oxycodone to see if there was sensitization to the repeated dose between the 1st and 4th day of oxycodone conditioning sessions. In Fig. 3, Two-way ANOVA (Dose × Age) of sensitization showed a significant main effect of Dose [F(3,48)=9.645, p<0.0001], with no significant difference between age groups and no significant Dose × Age interaction. Of interest, Newman–Keuls post hoc tests showed there was a significant difference in increased locomotor activity between two age groups in response to 1 mg/kg of oxycodone, with adolescent mice showing greater increased locomotor activity than the adult mice did (p<0.05). When the two age groups were examined separately by one-way ANOVA, adolescent mice showed a significant main effect of Dose [F(3,24)=7.141, p<0.0014]. Significant increased locomotor activity for oxycodone was not found at the lowest dose, 0.3 mg/kg, but there was significantly increased locomotor activity for oxycodone induced by doses of 1 and 3 mg/kg of oxycodone compared to the saline control (p<0.05 for 1 mg/kg, p<0.001 for 3 mg/kg). In adult mice, there was also a significant main effect of Dose [F(3,24)=3.543, p<0.0296]. However, there was a significant increase in locomotor activity for oxycodone only at the highest dose tested, 3 mg/kg, compared to the saline control (p<0.05).
Figure 3.
Sensitization to the locomotor stimulatory effects of oxycodone as reflected by mean (+S.E.M.) increased number of crossovers in difference between the first and fourth conditioning session of each group given oxycodone or saline (n=7 per group). Locomotor sensitization (mean crossovers from the first to the fourth sessions) was developed in both age groups (#p<0.05 and ##p<0.01 vs saline in adolescents: *p<0.05 in adults). The adolescent mice showed greater increase in mean crossovers from the first to the fourth sessions than adult mice ($p<0.05 between age groups).
4. Discussion
The rewarding effects in adolescent rodents compared with adults have been studied in several drugs of abuse. Behavioral differences between adolescent and adult rats induced by nicotine have been examined in several studies with conflicting results. Adolescents show either enhanced or reduced conditioned place preference to nicotine depending on age and dose (Vastola et al., 2002, Belluzzi et al., 2004). In cocaine, there were conflicting results. While many studies showed greater rewarding effect in adolescents than adults (Badanich et al., 2006, Brenhouse and Andersen, 2008, Zakharova et al., 2009a, Zakharova et al., 2009b), some reports showed less reward in adolescent than adults (Balda et al., 2006, Aberg et al., 2007), and some reports found no significant differences between adolescents and adults (Campbell et al., 2000, Schramm-Sapyta et al., 2004). In this study, we found that oxycodone induced a conditioned place preference in a dose-dependent manner in both age groups, but at the lowest dose, 0.3 mg/kg, there was a significant conditioned place preference in adults, but not in adolescents. The preference for the oxycodone-paired place in adolescents conditioned by 1 or 3 mg/kg oxycodone was similar to that in adults conditioned by 0.3 or 1 mg/kg oxycodone, respectively. In terms of the conditioned preference for oxydcodone, it appears that the adolescent mice were less sensitive than adult mice. Although there are no published comparisons of oxycodone-induced conditioned place preference of adolescents vs, adults, one study has reported no significant difference between adolescents and adults, in morphine-induced conditioned place preference (Campbell et al., 2000). However, the morphine study was conducted in rats, and used several methodological that differed from the present study. Thus differences in species, ligand and CPP procedures preclude a clear direct comparison of the present study and that of Campbelll et al (2000). Morphine has relatively unique properties among MOP-r agonists (and likely differs from oxycodone), in terms of propensity to cause receptor internalization and desensitization (Keith et al., 1998, Whistler et al., 1999, Haberstock-Debic et al., 2003, Arttamangkul et al., 2008, Imai et al., 2011). A further possibility involves differences in relative pharmacokinetics between oxycodone and morphine, especially in adults vs. adolescents. While morphine is metabolized by glucuronidation, oxycodone is metabolized by CYP3A4 and CYP2D6 (see review Smith, 2009). Furthermore, in a human study, CYP3A4 protein levels increased very gradually during the first 6 months of age, and levels for the 5- to 15-year age groups were lower compared with adults (Stevens et al., 2003).
In this study, we obtained a statistically significant difference for CPP data between adolescents and adults only at the smallest oxycodone doses studied, and did not detect an interaction in ANOVA between age and oxycodone dose. A power analysis for the study did reveal a relatively low power for the ANOVA interaction (0.62, vs. the more common standard of 0.8). This could potentially have militated against other significant findings that may have occurred.
The locomotor effects of drugs of abuse can be used to examine effects on drug sensitivity and drug-induced neuroplasticity. In the present study, in both age groups, locomotor activity within the conditioning chamber was significantly increased across the four sessions of oxycodone administration. Moreover, although sensitization of locomotor activity (magnitude of locomotor response to oxycodone between the 1st and 4th day of oxycodone conditioning sessions) was developed in both the adolescent and adult mice, the adults showed sensitization at 3 mg/kg, whereas the adolescents showed sensitization at both 1 and 3 mg/kg. Since there was no statistical significant difference of the acute locomotor response to 1 mg/kg oxycodone between adolescent and adult, it is unclear whether differences in sensitization in this setting were partially due to possible differences in acute locomotor effects of oxycodone in adolescents vs. adults. The present results suggest that adolescent mice are more susceptible to develop a locomotor sensitization to oxycodone at lower doses, potentially indicative of differential neuroplasticity in response to repeated MOP-r agonist exposure, and might be indicate appetitive properties considered as other substance (Quoilin et al., 2010).
In conclusion, we compared for the first time the effects of the prescription opioid oxycodone in adolescent and adult mice on conditioned place preference, locomotor activity and sensitization of locomotor activity. Adolescent mice failed to develop the conditioned preference at the lowest dose tested, whereas adult mice showed significant conditioned preference. However, locomotor sensitization in adolescent mice was found at lower dose than in adult mice. Thus, while adolescents may exhibit a decreased conditioned rewarding effect of oxycodone in this assay, adolescents may be more readily sensitized to repeated oxycodone exposure. These dual differences may together partially underlie the vulnerability of adolescent subjects to oxycodone addiction.
Highlights.
Adolescent mice did not show CPP to the lowest dose oxycodone, but adults did
Adolescent mice developed locomotor sensitization by a lower dose of oxycodone
These results may indicate to enhance motivation to take more drugs in adolescents
Acknowledgments
This research was supported by a grant from NIH-NIDA RO1-DA029147 (YZ) and NIH-NIDA P50-DA05130 (MJK). The authors have no conflicts of interest, financial or otherwise, pertaining to any aspect of the work reported in this manuscript. We thank Dr. Butelman Eduardo for his advise on writing of the manuscript, and Ms. Susan Russo for proofreading of the manuscript.
Footnotes
Authors' contributions: KN, YZ and MJK conceived the general ideas for this study and designed the experiments. KN performed the experiments, data analysis and wrote the first draft of the manuscript. AH assisted in statistical analysis. All authors provided input into the writing of the manuscript and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Institute of Laboratory Animal Resources Commission on Life Sciences National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- Aberg M, Wade D, Wall E, Izenwasser S. Effect of MDMA (ecstasy) on activity and cocaine conditioned place preference in adult and adolescent rats. Neurotoxicol Teratol. 2007;29:37–46. doi: 10.1016/j.ntt.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Balda MA, Anderson KL, Itzhak Y. Adolescent and adult responsiveness to the incentive value of cocaine reward in mice: role of neuronal nitric oxide synthase (nNOS) gene. Neuropharmacology. 2006;51:341–349. doi: 10.1016/j.neuropharm.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Benziger DP, Miotto J, Grandy RP, Thomas GB, Swanton RE, Fitzmartin RD. A pharmacokinetic/pharmacodynamic study of controlled-release oxycodone. J Pain Symptom Manage. 1997;13:75–82. doi: 10.1016/s0885-3924(96)00300-4. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H, Wein M, Barrot M, Colago EE, Rahman Z, Neve RL, Pickel VM, Nestler EJ, von Zastrow M, Svingos AL. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Sudo Y, Nakamura A, Ozeki A, Asato M, Hojo M, Devi LA, Kuzumaki N, Suzuki T, Uezono Y, Narita M. Possible involvement of beta-endorphin in a loss of the coordinated balance of mu-opioid receptors trafficking processes by fentanyl. Synapse. 2011;65:962–966. doi: 10.1002/syn.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Narita M, Nakamura A, Ozaki M, Imai S, Miyoshi K, Suzuki M, Suzuki T. Comparative pharmacological profiles of morphine and oxycodone under a neuropathic pain-like state in mice: evidence for less sensitivity to morphine. Neuropsychopharmacology. 2008;33:1097–1112. doi: 10.1038/sj.npp.1301471. [DOI] [PubMed] [Google Scholar]
- Orsini C, Bonito-Oliva A, Conversi D, Cabib S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 2005;181:327–336. doi: 10.1007/s00213-005-2259-6. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology (Berl) 2010;212:501–512. doi: 10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Rutten K, van der Kam EL, De Vry J, Tzschentke TM. Critical evaluation of the use of extinction paradigms for the assessment of opioid-induced conditioned place preference in rats. Pharmacology. 2011;87:286–296. doi: 10.1159/000327680. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhang Y, Hsu NM, Allen JM, Ho A, Kreek MJ. Heroin-induced locomotor activity and conditioned place preference in C57BL/6J and 129P3/J mice. Neurosci Lett. 2008;440:284–288. doi: 10.1016/j.neulet.2008.05.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Pratt AR, Winder DG. Effects of periadolescent versus adult cocaine exposure on cocaine conditioned place preference and motor sensitization in mice. Psychopharmacology (Berl) 2004;173:41–48. doi: 10.1007/s00213-003-1696-3. [DOI] [PubMed] [Google Scholar]
- Sithisarn T, Bada HS, Dai H, Reinhardt CR, Randall DC, Legan SJ. Effects of perinatal oxycodone exposure on the response to CRH in late adolescent rats. Neurotoxicol Teratol. 2008;30:118–124. doi: 10.1016/j.ntt.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84:613–624. doi: 10.1016/S0025-6196(11)60750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brick J. Cocaine-induced behavior in the developing rat. Behav Neural Biol. 1979;26:401–415. doi: 10.1016/s0163-1047(79)91410-9. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Spear LP, Shalaby IA, Brick J. Chronic administration of haloperidol during development: behavioral and psychopharmacological effects. Psychopharmacology (Berl) 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, Zaya MJ. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307:573–582. doi: 10.1124/jpet.103.054841. [DOI] [PubMed] [Google Scholar]
- Talbot JN, Happe HK, Murrin LC. Mu opioid receptor coupling to Gi/o proteins increases during postnatal development in rat brain. J Pharmacol Exp Ther. 2005;314:596–602. doi: 10.1124/jpet.104.082156. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JC., Jr Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res. 1995;89:167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009a;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009b;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Landthaler M, Schlussman SD, Yuferov V, Ho A, Tuschl T, Kreek MJ. Mu opioid receptor knockdown in the substantia nigra/ventral tegmental area by synthetic small interfering RNA blocks the rewarding and locomotor effects of heroin. Neuroscience. 2009a;158:474–483. doi: 10.1016/j.neuroscience.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Conditioned place preference after single doses or “binge” cocaine in C57BL/6J and 129/J mice. Pharmacol Biochem Behav. 2002;73:655–662. doi: 10.1016/s0091-3057(02)00859-6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009b;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schlussman SD, Butelman ER, Ho A, Kreek MJ. Effects of withdrawal from chronic escalating-dose binge cocaine on conditioned place preference to cocaine and striatal preproenkephalin mRNA in C57BL/6J mice. Neuropharmacology. 2012;63:322–329. doi: 10.1016/j.neuropharm.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]