Abstract
Study Objectives:
Periodic limb movements in sleep (PLMS) are common in the elderly. A previous large polysomnographic (PSG) study examining the relationship of PLMS to sleep architecture and arousals from sleep in women found that leg movements were common in elderly women, and PLMS which were associated with EEG arousals had a strong and consistent association with markers of disturbed sleep. Since sleep differs in men and women, we now investigate the association between PLMS and PSG indices of sleep quality in a large community-based sample of older men.
Design:
Observational study, cross-sectional analyses.
Setting:
Six clinical sites participating in the Osteoporotic Fractures in Men (MrOS) Study.
Participants:
2,872 older community-dwelling men (mean age 76.4 years) who completed in-home PSG from 2003-2005.
Interventions:
N/A.
Measurements and Results:
In-home PSG was performed which included bilateral measurement of leg movements. The total number of leg movements per hour of sleep (PLMI) and the number of leg movements causing EEG-documented arousals per hour of sleep (PLMA) were computed. A PLMI ≥ 5 (70.8%) and PLMA ≥ 5 (27.4%) were both prevalent. Linear regression models were used to examine the relationship between PLMS as predictors and sleep architecture, arousal index, and sleep efficiency as outcomes. The highest quintiles of PLMI (≥ 65.1) and PLMA (≥ 6.8) showed the largest association with indices of sleep architecture; PLMA showed a larger magnitude of effect. After multivariate adjustment, participants with a higher PLMA had a small but significantly higher arousal index, lower sleep efficiency, higher percentages of stages 1 and 2 sleep, and lower percentages of stage 3-4 and REM sleep (p < 0.01). An increased PLMI was similarly associated with a higher arousal index, higher percentage of stage 2 sleep, and lower percentage of stage 3-4 (p < 0.0001), but not with an increase in stage 1, REM sleep, or sleep efficiency. Neither PLMI nor PMLA was associated with subjective sleepiness measured by the Epworth Sleepiness Scale.
Conclusions:
This study demonstrated that periodic leg movements are very common in older community-dwelling men and regardless of associated arousals, are associated with evidence of lighter and more fragmented sleep.
Citation:
Claman DM; Ewing SK; Redline S; Ancoli-Israel S; Cauley JA; Stone KL; for the Study of Osteoporotic Fractures Research Group. Periodic leg movements are associated with reduced sleep quality in older men: The MrOS Sleep Study. J Clin Sleep Med 2013;9(11):1109-1117.
Keywords: Periodic limb movements, sleep, men, geriatric, aging, sleep stage, sleep EEG arousals
Periodic limb movements in sleep (PLMS) is a disorder characterized by repetitive stereotypical movements of the legs during sleep, and has often been associated with fragmented sleep, symptoms of insomnia and excessive daytime sleepiness.1 Defining PLMS based on a PLM index (PLMI = leg movements/hour of sleep) of 5 or greater, the largest published epidemiological study reported a 3.9% prevalence of PLMS in 18,980 subjects aged 15-100 from the general population.2 In community-dwelling younger adults, the prevalence has been estimated at 5% to 6%.3 This prevalence increases in the community-dwelling elderly, with estimates between 25% and 58%.4–8 The importance of PLMS has been highlighted by recent investigations supporting a relationship between PLMS, cardiovascular disease, and autonomic activation.9–11 Because of the high prevalence of PLMS in the elderly, it has been argued that PLMS may not be a risk factor for disease, but rather a marker of aging or other health attributes.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The purpose of this study was to investigate the prevalence of periodic limb movements in a population-based sample of older men, and assess whether polysomnographic outcomes and clinical symptoms correlated with periodic limb movements. Prior work in elderly women reported detailed polysomnographic data, and showed that periodic limb movements were common and were associated with altered polysomnographic outcomes, but were not associated with changes in subjective sleepiness.
Study Impact: This study reports detailed polysomnographic data in a large population-based cohort of elderly men. Periodic limb movements were common. Periodic limb movements causing EEG arousals, and those not causing EEG arousals, were both associated with altered polysomnographic outcomes. Similar to previous reports, periodic limb movements were not associated with changes in subjective sleepiness.
PLMS and its relationship to sleep was previously reported in 455 community-dwelling elderly women in the Study of Osteoporotic Fractures (SOF).12 Results showed that in this cohort of older women, the prevalence of PLMI ≥ 5 was 66% and PLMI ≥ 15 was 52%. In SOF, when defining PLMS using a definition requiring associated arousal with each leg movement (PLMA), the prevalence for PLMA ≥ 5 was 27% and for PLMA ≥ 15 was 6%. Compared to the PLMI, an elevated PLMA was more consistently associated with poor sleep, including lower sleep efficiency, higher arousal index, more time spent in stages 1 and 2, and less time in stage 3-4 and REM sleep. Although both the PLMI and PLMA have been used clinically, it is not clear which, if not both, of these measures may be most useful to identify those with disturbed sleep architecture, which may indicate a need for treatment. The clinical significance of PLMs on daytime function remains unknown, and the results in elderly women did not show an association with Epworth score.
The present study determined the prevalence of PLMS and their association with objective measures of sleep architecture in 2,872 older men participating in the Osteoporotic Fractures in Men Study (MrOS).13,14 The present study assessed: (1) whether increasing severity of PLMS was associated with objective evidence of poorer sleep and subjective daytime sleepiness; and (2) whether stronger associations were evident when PLMS were defined by leg movements associated with cortical EEG arousals (PLMA) as compared to indices that included periodic leg movements without cortical arousals (PLMI).
METHODS
Participants
From March 2000 through April 2002, 5,994 men who were at least 65 years of age were recruited for participation in the baseline examination of MrOS.14 Men were recruited from population based listings in 6 regions of the United States.13 Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded from the study. From December 2003 through March 2005, MrOS participants were invited to participate in an ancillary study to identify outcomes of sleep disorders in older men (MrOS Sleep Study). To participate in the MrOS Sleep Study, men had to agree to a comprehensive sleep assessment that included validated sleep questionnaires, an in-clinic interview, a series of clinical measures, and a single overnight in-home PSG study. Of the 5,994 men enrolled in the overall study, 3,135 (> 100% of goal of 3,000) completed the MrOS Sleep examination. Of these, 2,872 men underwent PSG with PLMS measurement and are the subject of this analysis. The remaining 263 men were missing PSG data because the measure was not performed (n = 179), the collected data was unusable due to poor signal quality (n = 45), or the wake/sleep scoring was unreliable (n = 39).
The institutional review board (IRB) at each center approved the study protocol, and written informed consent was obtained from all subjects.
Polysomnography (PSG)
In-home sleep studies using unattended PSG (Safiro, Compumedics, Inc., Melbourne, AU) were performed. The PSG recordings were completed within 1 month of the clinic visit (mean 6.9 ± 15.8 days from visit), with 78% of recordings gathered within 1 week of the clinic visit. The recording montage consisted of C3/A2 and C4/A1 electroencephalograms, bilateral electroculograms, a bipolar submental electromyo-gram, thoracic and abdominal respiratory inductance plethysmography, airflow (using nasal-oral thermocouple and nasal pressure cannula), finger pulse oximetry; lead I electrocardiogram, body position (mercury switch sensor), and bilateral leg movements (piezoelectric sensors). Centrally trained and certified staff members performed home visits for setup of the sleep study units. After sensors were placed and calibrated, signal quality and impedance were checked, and sensors were repositioned as needed to improve signal quality using approaches similar to those in the Sleep Health Heart Study.15 Staff returned the next morning to collect the equipment and download the data to the Central Sleep Reading Center (Cleveland, OH) for centralized scoring by a trained technician using standard criteria.16,17
Polysomnography data quality were excellent, with a failure rate < 4%, and > 70% of studies graded as being of excellent or outstanding quality. Quality codes for signals and studies were graded using previously described approaches, which included coding the duration of artifact-free data per channel and overall study quality (reflecting the combination of grades for each channel).15
PLMS were scored according to standard AASM criteria at the time, in which individual movements were scored as a PLM if duration was between 0.5 and 5 sec and when there was a clear amplitude increase from baseline in leg channels.18 To be considered periodic, ≥ 4 movements needed to occur in succession no less and no more than 5 and 90 sec apart. The periodic limb movement index (PLMI) was the total number of periodic leg movements per hour of sleep. Leg movements occurring after respiratory events were excluded unless they were part of a 4 (or more) movement cluster with ≥ 2 movements occurring independently of respiratory events. The periodic limb movement arousal index (PLMA) was the total number of periodic leg movements per hour of sleep in which EEG arousal occurred within 3 sec of movement termination.12
PSG data, including sleep stage distributions, apnea and hypopneas and arousals, were scored by the centralized Case Western University Reading Center, using published criteria in effect at the time of the analysis.16,19 Arousals were scored according to AASM criteria; i.e., abrupt changes in EEG following ≥ 10-sec period of sleep, which in REM also required an increase in EMG activity.17
PSG Variables
The PLMI and PLMA were examined as quintiles. Sleep efficiency was defined as the time asleep divided by the sleep period time. Sleep stage distribution was characterized by the percent of total sleep time spent in each stage of sleep (i.e., % sleep stage 1, 2, 3-4, and REM). The Arousal Index (ArI) was calculated as the number of EEG arousals per hour of sleep. The apnea-hypopnea index (AHI) was calculated as the sum of all apneas and hypopneas that were each associated with ≥ 3% desaturation divided by total sleep time and was examined continuously and using the cutpoints of ≥ 5 and ≥ 15.
Other Measurements
Self-reported information about daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS).20 The standard cutpoint of ESS > 10 was used to define excessive daytime sleepiness.21
Participants also completed questionnaires regarding demographics, medical history, physical activity, and alcohol use. History of cardiovascular disease was defined as self-report of physician diagnosis of myocardial infarction, angina, or congestive heart failure, or self-report of coronary bypass surgery, angioplasty, or pacemaker implant. History of chronic obstructive pulmonary disease (COPD) was defined as self-reported physician diagnosis of COPD, chronic bronchitis, asthma, or emphysema.
Cognitive function was assessed with the Modified Mini-Mental State examination (MMSE) with higher scores representing better cognitive functioning.22 The Physical Activity Scale for the Elderly (PASE) measured level of physical activity, with higher scores representing greater physical activity.23 To assess function, subjects were asked whether they had difficulty performing any of 5 instrumental activities of daily living (IADL), which included walking 2-3 blocks, climbing 10 steps, preparing meals, performing heavy housework, and shopping.24,25 The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, with higher scores representing more depressive symptoms.26 Prescription and nonprescription medications were inventoried, verified by pill bottle examination, and matched to ingredient(s) using the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA).27
During the home or clinic visits, body weight was measured with a standard balance beam or digital scale and height with a wall-mounted Harpenden stadiometer (Holtain, England); these measurements were used to determine body mass index (BMI), calculated as weight (kilograms)/height (meters2).
Statistical Analysis
Participant characteristics and sleep outcomes were summarized using means and standard deviations (SD) for continuous data and counts and percentages for categorical data. Participant characteristics were examined to determine if there was a significant difference between the 2,872 men in our analysis and those 263 who did not have PLMS data gathered. Participant characteristics and sleep parameter differences across the PLMI and PLMA quintiles were also examined using ANOVA for normally distributed continuous data, Kruskal-Wallis tests for skewed continuous data, and χ2 tests for categorical data.
Linear regression models were used to examine the relationships between the PLMS predictors and the continuous outcomes of sleep architecture, the arousal index, and sleep efficiency. Though the distributions of stage 1, stage 3-4, the arousal index, and sleep efficiency were skewed, nonparametric (bootstrap) analysis on the original (untransformed) scale showed virtually the same results as the normality-based analyses on the original scale. Since those levels of skewness had virtually no impact, the outcomes were not transformed and were analyzed on the original scale. Adjusted means and 95% confidence intervals (CI) for the PLMI and PLMA quintiles were calculated using the least-squares means procedure. Covariates were included in the models if they were related to either the PLMI or PLMA predictors at p < 0.10 in univariate analyses. All models were adjusted for age, race, site, PASE score, IADL impairment, GDS score, history of cardiovascular disease, history of hyper-tension, antidepressant medication use, and AHI. To determine whether the associations between PLMS predictors and sleep outcomes were driven by men with obstructive sleep apnea, the models were re-run in the subset of men with AHI < 15.
Statistical analyses were completed using SAS version 9.1.3 (SAS Inc., Cary, NC) and Stata version 12.0 (Stata Corporation, College Station, TX).
RESULTS
Participants
The mean age of the 2,872 men included in this analysis was 76.4 years; over 90% were Caucasian; and half were hypertensive (Table 1). There were no differences between the 2,872 men with PLMS data and the 263 without PLMS data, except that a higher percentage of the men with PLMS data were Caucasian (90.7% vs. 80.6%, p < 0.0001), their mean (± SD) MMSE score was higher (92.7 ± 6.2 vs. 91.5 ± 8.3, p = 0.02), and their mean GDS score was lower (1.8 ± 2.2 vs. 2.2 ± 2.5, p = 0.008) (data not shown).
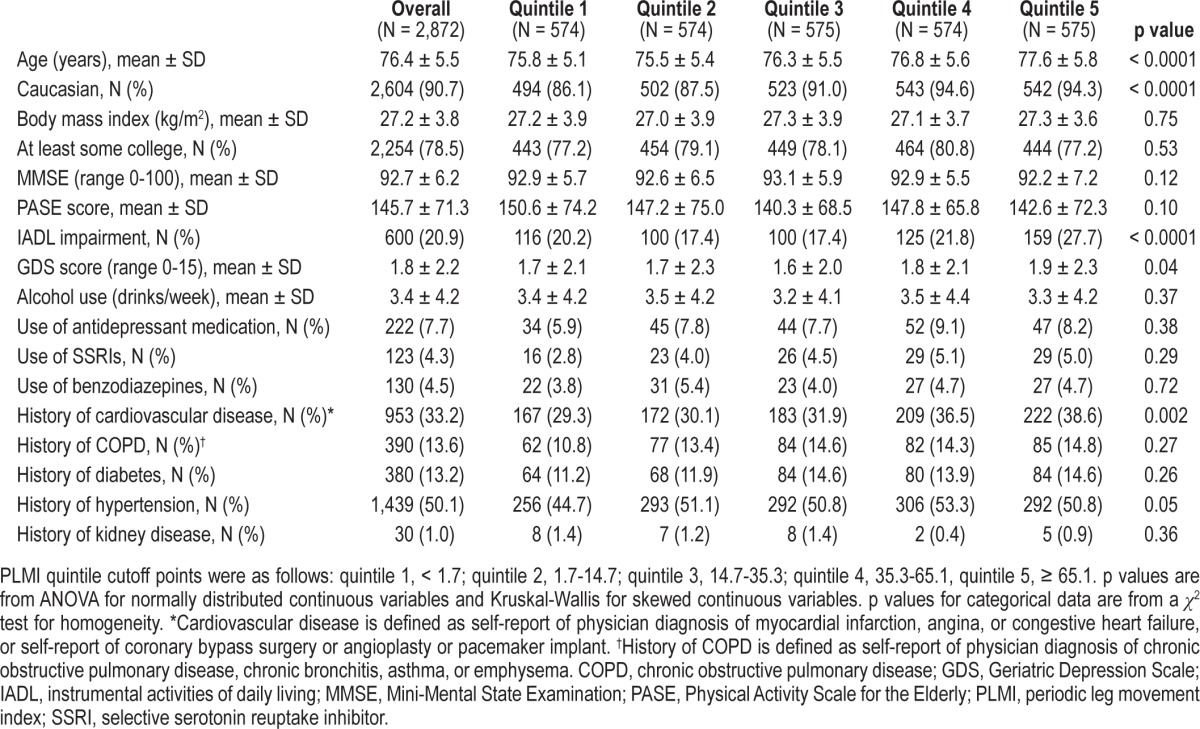
Table 1.
Characteristics by PLMI quintile
Distribution of PLMI and Association with Sleep Measures
2,034 participants (70.8%) had PLMI ≥ 5.323 (11.3%) had PLMI of 5 to < 15, and 1,711 (59.6%) had PLMI ≥ 15. Mean PLMI was 35.6 ± 37.5, with a median of 23.9 (interquartile range 3.3-56.1). Characteristics of the participants by quintiles of PLMI are presented in Table 1. Men in the higher PLMI quintiles were older on average and more likely to be Caucasian than men in the lower quintiles (p < 0.0001). These men were more likely to have an IADL impairment (p < 0.0001), and, on average, had more depressive symptoms (p = 0.04). A higher percentage of the men with elevated PLMI reported cardiovascular disease (p = 0.002), but there was no association with renal disease.
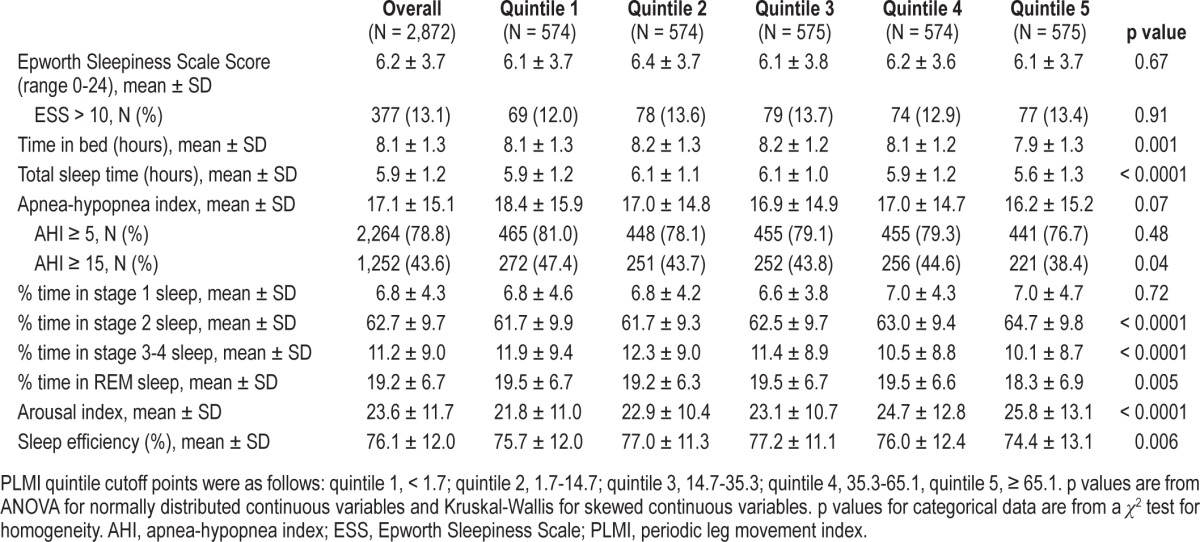
In unadjusted analyses, PLMI was significantly, although only modestly, associated with percentage time spent in stage 2, stage 3-4, REM, arousal index, and sleep efficiency (Table 2). Higher PLMI was associated with more time in stage 2 sleep (p < 0.0001), less time in stage 3-4 sleep (p < 0.0001), less time in REM sleep (p = 0.005), lower sleep efficiency (p = 0.006), and higher ArI (p < 0.0001). Differences in sleep architecture indices were largest when comparing men in the highest PLMI quintile (≥ 65.1) to men with PLMI quintiles of 1 to 4, This comparison showed that those with a PLMI in the highest quintile spent a greater percentage of time in stage 2 sleep (unadjusted mean ± SD 64.7% ± 9.8% vs. 62.2% ± 9.6%), less time in stage 3-4 sleep (10.1% ± 8.7% vs. 11.5% ± 9.1%) and REM sleep (18.3% ± 6.9% vs. 19.4% ± 6.6%), had higher ArI (25.8 ± 13.1 vs. 23.1 ± 11.3), and lower sleep efficiency (74.4% ± 13.1% vs. 76.5% ± 11.7%) (all p < 0.004). These differences were statistically significant, but small in magnitude. There was no significant association between PLMI and stage 1 or ESS score.
Table 2.
Sleep characteristics by PLMI quintile
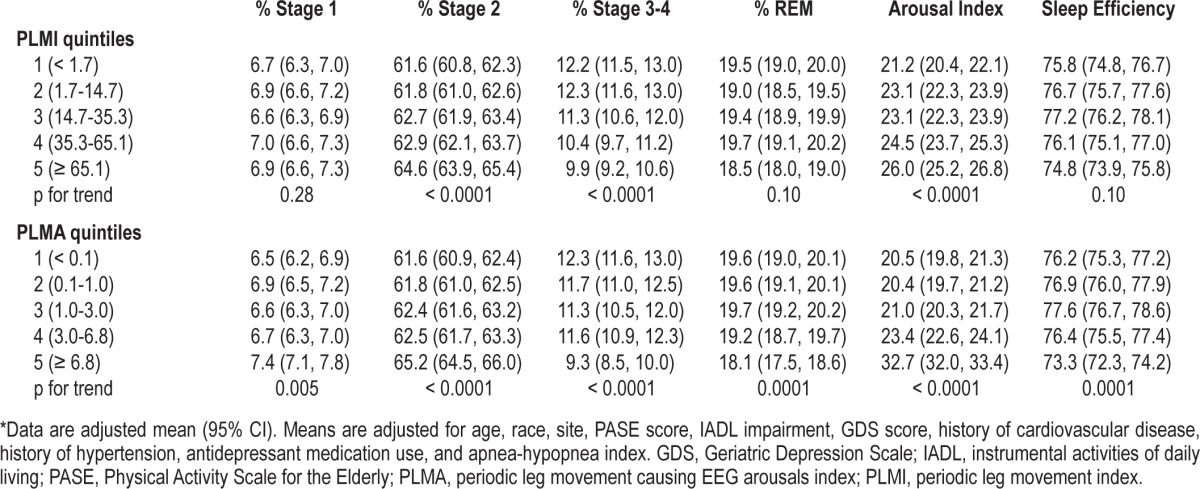
Multivariable analyses were performed to determine if the unadjusted associations remained after adjustment for age, race, site, physical activity score, IADL impairment, GDS score, history of cardiovascular disease, history of hypertension, antidepressant medication use, and AHI (Table 3). Adjusted linear regression analyses showed that higher PLMI continued to be significantly associated with many sleep outcomes, including more time spent in stage 2 sleep (p-trend < 0.0001), decreased stage 3-4 sleep (p-trend < 0.0001), and higher ArI (p-trend < 0.0001). Multivariate analyses were repeated in the subset with AHI < 15 (n = 1,620) and confirmed these results. Comparison of the highest quintile of PLMI to quintiles 1-4 by multivariate analyses showed virtually the same results as the unadjusted analyses. Quintile comparison in the subset with AHI < 15, confirmed results for stages 2, 3-4, and ArI, and were also statistically significant for REM and sleep efficiency.
Table 3.
Sleep architecture and arousal index by quintile of PLMI and PLMA*
Distribution of PLMA and Association with Sleep Measures
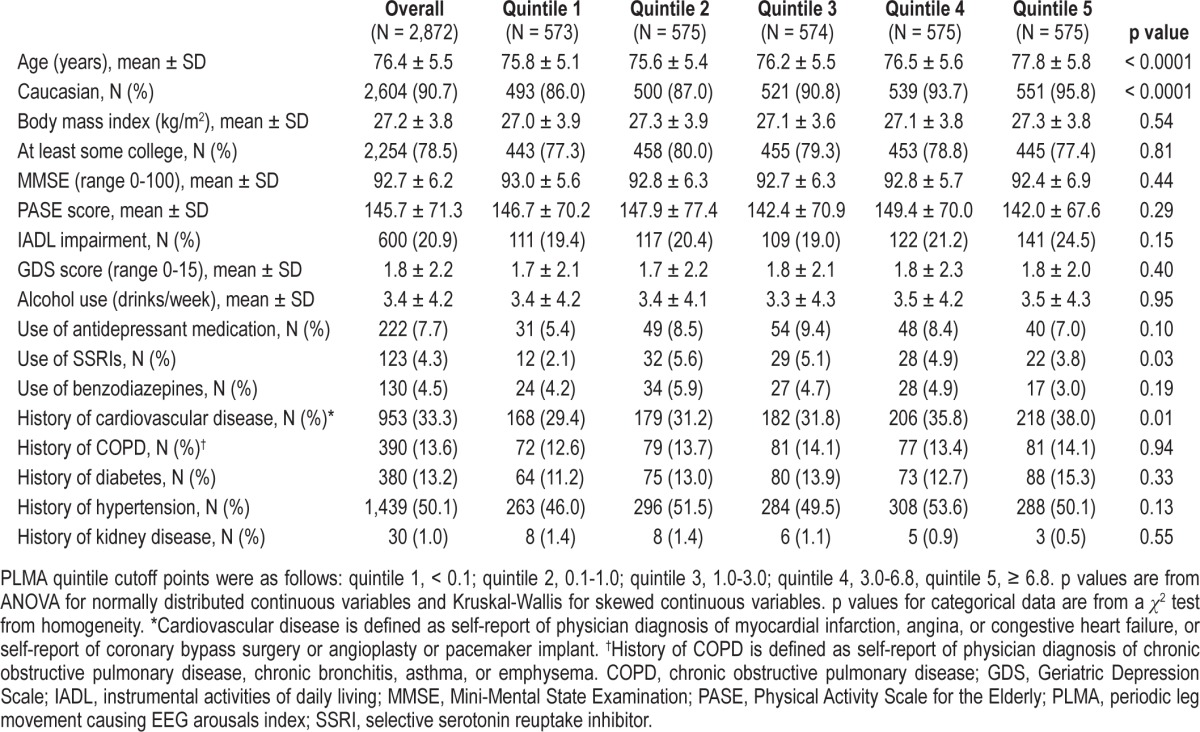
Seven hundred eighty-eight participants (27.4%) had PLMA ≥ 5; 623 (21.7%) had PLMA 5 to < 15; and 165 (5.8%) had PLMA ≥ 15. Mean PLMA was 4.1 ± 5.7, with a median of 1.8 (interquartile range 0.3-5.5). The Spearman correlation between PLMI and PLMA was 0.86 (p < 0.0001). Characteristics of the participants by quintiles of PLMA are shown in Table 4. Men in the higher PLMA quintiles were older on average and more likely to be Caucasian than men in the lower quintiles (p < 0.0001). A higher percentage of the men with elevated PLMA reported cardiovascular disease (p = 0.01) and were more likely to be taking SSRI medication (p = 0.03), but there were no differences between the PLMA quintiles for other comorbidities, including history of renal disease.
Table 4.
Characteristics by PLMA quintile
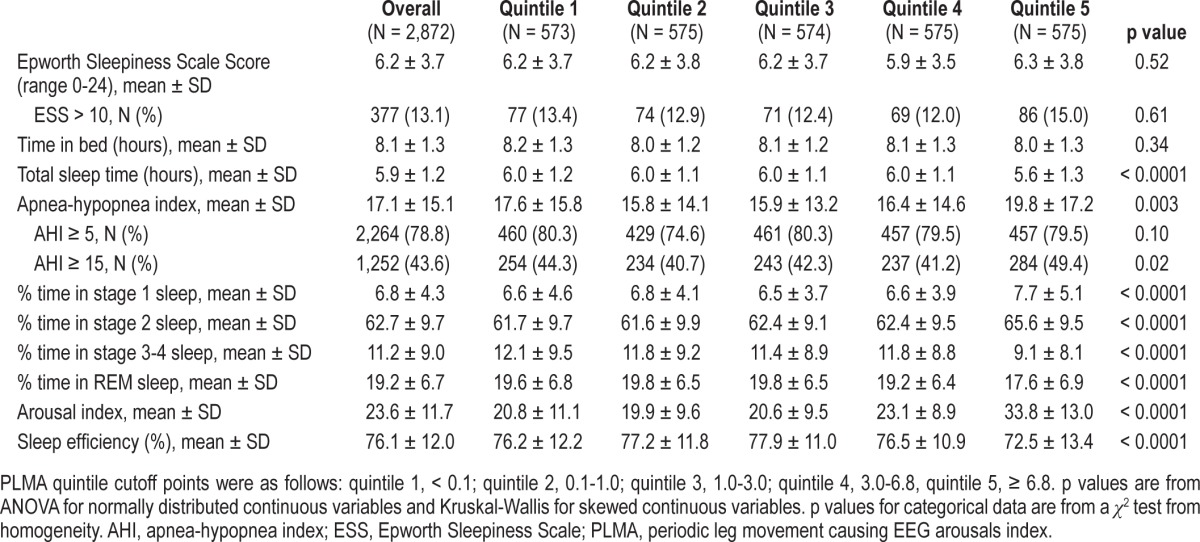
In unadjusted analyses, participants with a higher PLMA had, on average, a shorter sleep duration, spent greater percentage time in stage 1 and stage 2 sleep and less percentage time in stage 3-4 and REM sleep, had a higher ArI, and had lower sleep efficiency (all p < 0.0001) (Table 5). Similar to the analysis of PLMI, these associations were driven by men with the highest PLMA quintile (≥ 6.8). Compared to men in quin-tiles 1-4 of PLMA, men in the highest quintile spent a greater percentage of time in Stage 1 sleep (unadjusted mean ± SD 7.7% ± 5.1% vs. 6.6% ± 4.1%), more time in stage 2 sleep (65.6% ± 9.5% vs. 62.0% ± 9.6%), less time in stage 3-4 sleep (9.1% ± 8.1% vs. 11.8% ± 9.1%) and REM sleep (17.6% ± 6.9% vs. 19.6% ± 6.6%), had higher ArI (33.8 ± 13.0 vs. 21.1 ± 9.9), and lower sleep efficiency (72.5% ± 13.4% vs. 77.0% ± 11.5%) (all p < 0.0001). Participants with a higher PLMA also had a higher AHI (p = 0.003). There was no significant association between PLMA and ESS score. The relationship between PLMA and sleep stage distribution, the arousal index, and sleep efficiency remained significant after multivariate adjustment (all p ≤ 0.005) (Table 3). Comparison of the highest quintile of PLMA to quintiles 1-4 by multivariate analyses showed virtually the same results as the unadjusted analysis. Multivariate analyses and quintile comparison were repeated in the cohort subset with AHI < 15 (n = 1,620) which confirmed both analyses.
Table 5.
Sleep characteristics by PLMA quintile
DISCUSSION
The results of this study in 2,872 subjects demonstrate that periodic limb movements in sleep are common in elderly men. Overall, we found a strikingly high prevalence of PLMS when using a PLMI threshold of 5 (71%) or 15 (60%). When using a PLMA threshold of 5 (27%) or 15 (6%) as a disease-defining metric, PLMS was common but less prevalent. Importantly, both metrics were associated with poorer sleep outcomes after multivariate adjustment for various possible confounders. A higher PLMA was consistently associated with more frequent arousals, lower sleep efficiency, and a pattern of altered sleep stages suggesting lighter sleep (more stage 1 and 2 and less stage 3-4 and REM). Except for stage 1, REM and sleep efficiency, similar findings were consistently observed for PLMI. These results suggest that leg movements that do not cause arousals as well as those that do cause arousals are associated with perturbed sleep quality, although on average, these associations are modest in this sample with relatively low stage 3-4 sleep and a high arousal index. The highest quintile of PLMI (≥ 65.1) and PLMA (≥ 6.8) had the strongest association and showed the largest magnitude of effect on sleep outcomes, and PLMA had a larger magnitude effect across more sleep outcomes. Despite associations with PSG indices of sleep quality, neither PLMI nor PLMA was associated with daytime sleepiness, based on ESS score.
A significant association of sleep quality with PLMI differs from our prior report in the SOF (female) cohort. Potential explanations for why PLMI had a stronger effect on sleep outcomes in men could include the higher power of the larger MrOS study and ability to detect relatively small effects. Alternatively, sleep architecture in older women is generally better than in older men,28 and thus it is possible that the sleep of women is less sensitive to disturbances than in men.
The results of this study in older men and the SOF study in elderly women support the hypothesis that PLMS increase with age, as suggested by Coleman29 and Ancoli-Israel.5 However, studies to date including the current study have been cross-sectional, and the extent to which longitudinal changes in PLMS occur within aging subjects is unclear. The longitudinal data of Gehrman4 in a small subset of the San Diego community cohort did not show an increase at 18-year follow-up, emphasizing the need for further longitudinal assessments to better determine the extent to which PLMS represents age-dependent pathophysiology.
Subjective sleepiness, as defined by an ESS > 10, was present only in 13.1% of the cohort. The lack of an association of either the PLMI or PLMA with subjective sleepiness may be due to several factors, including the low prevalence of subjective sleepiness in the cohort, potential insensitivity of the ESS to daytime functional impairment in elderly populations,30 lack of tight correlation between subjective sleep (ESS) and objective sleep measures (PSG),30 or because of the relative modest association between PLMS and indices of sleep quality. Consistent with other studies, the clinical effect of PLMs on daytime sleepiness has not been established; the sleep stage changes in this study did not correlate with subjective sleepiness based on ESS score. Since both an elevated PLMI and elevated PLMA were previously shown in MrOS to predict cardiovascular disease, other serious health outcomes besides daytime sleepiness should be considered when evaluating PLMS.19
In considering prevalence estimates for PLMS, it is important to note that the optimal criterion for diagnosing PLMS is unknown, as are the implications of using alternative sensors for quantifying frequency of leg movements. More specifically, PLMS has been defined using cutoff values of both PLMI ≥ 531 and PLMI ≥ 15.1 Since it is evident that leg movements increase with aging, it is likely that cutoff values may need to be modified to reflect the age distribution of the study sample. Although the PLMI and PLMA were highly correlated, the frequency of leg movements associated with arousals was much lower. Our data that the highest quintiles of PLMI (≥ 65.1) and PLMA (≥ 6.8) showed the largest associations with indices of sleep quality supports the position that alternative threshold values for identifying abnormality may need to be used that account for the underlying distributions of PLMS in each respective population, reflecting age and comorbidity as well as recording techniques.
Our study had a number of strengths including a large sample size with standard scoring of PSG-derived data. Our sample focused on an older male population in whom there has been only limited prior objective sleep data. Enrollment of these community-dwelling men was not determined on the basis of PLMS, so our results are generalizable to other community samples. The importance of understanding determinants and measurement issues related to sleep disturbances in older men is underscored by the high prevalence of subjective symptoms of sleep problems and the frequency of chronic comorbidities in this population. The sleep and leg movement data were objectively scored independently of one another by a group of highly trained scorers, providing reliable information that allowed various sleep indices to be independently compared. The sleep component of MrOS was added after the original recruitment, so there was less likelihood of recruitment bias for sleep symptoms. Further, the characteristics of the large percentage of participants with PLMS data in the study were similar to those for the small percentage of the MrOS cohort without PLMS data, supporting the generalizability of the findings to other community based populations of older men.
Our cross-sectional study had a few limitations. Because our population consisted of older men, results cannot be generalized to other groups such as women, younger people, or certain ethnic groups not broadly represented in MrOS. Our scoring algorithm for leg movements explicitly excluded movement clusters that were exclusively associated with respiratory events; however, since we did not measure esophageal pressure, we could not exclude upper airway resistance events as a cause of PLMS. In addition, our questionnaire did not include validated restless leg questions or information about iron deficiency, which prevented analysis of the relationship between restless leg symptoms and objective polysomno-graphic outcomes. It is possible that the physiological impact of PLMS differs in subgroups with and without daytime symptoms of restless legs syndrome. Leg movements were measured by piezo sensors, which are commonly used clinically, rather than by EMG. The optimal method for quantifying leg movements is an area of active investigation. In preliminary unpublished work using both piezo sensors and EMG electrodes, we observed similar patterns of PLMS. Specifically, in a sample of 51 subjects studied at the Sleep Reading Center studied with both piezoelectric and leg EMG sensors, with leg movements assessed by the piezoelectrodes annotated using the rules applied to the MrOS sample and the EMG data analyzed using newly published leg movement scoring rules,32 a correlation coefficient of 0.81 was observed, indicating an excellent level of agreement. Finally, information regarding RLS symptoms and iron deficiency was not available, so whether or not these findings can be extrapolated to RLS is unclear. Although PLMS occur in approximately 80% of RLS sufferers, it occurs in several other conditions and often in the elderly without RLS symptoms.5,11,33–35 A single measurement of PLMS at baseline does not account for night-to-night variability and changes in PLMS over time.
In summary, these PSG data indicate that PLMS are very common in older men. An elevated PLMI and an elevated PLMA were both associated with a consistent, albeit a modest, pattern of sleep disruption, suggesting that both PLMI and PLMA contribute to poor sleep quality. The highest quintiles for PLMA and PLMI showed the strongest effect on sleep outcomes, particularly PLMA. Similar to data reported in elderly women in SOF, neither the PLMI nor PLMA was associated with daytime sleepiness. As the population continues to age, these results highlight the need for further research to better determine if there is a threshold level of PLMA and PLMI that identifies older participants at increased risk for comorbidity or functional limitations.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was performed at the San Francisco Coordinating Center, University of California, San Francisco, and Case Western Reserve University. The authors have indicated no financial conflicts of interest. There was no intervention. There was no off-label or investigational treatment. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839.
ACKNOWLEDGMENTS
Investigators in the Outcomes of Sleep Disorders in Older Men study (MrOS Sleep)
Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): K.L. Stone (Principal Investigator), D.C. Bauer (co-Investigator), S.R. Cummings (co-Investigator), N. Goldschlager (co-Investigator), P. Varosy (co-Investigator), K. Yaffe (co-Investigator), P.M. Cawthon (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L.Y. Lui, J. Schneider, R. Scott, D. Tanaka, J. Ziarno; Administrative Center (Oregon Health & Sciences University): E. Orwoll (Principal Investigator), K. Phipps (co-Investigator), L. Marshall (co-Investigator), J. Babich Blank (Project Director), L. Lambert, B. Chan, D. Neevel; University of Alabama, Birmingham: C.E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), C. Oden, S. House, N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, J. Smith, C. Sassaman, C. Collier, C. Atkins; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), D. King (Program Manager), N. Michaels (Asst. Program Manager), N. Nelson (Clinic Coordinator), C. Bird, D. Blanks, F. Imker-Witte, K. Moen, M. Paudel, M. Slindee; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), K. Kent, B. Malig, S. Wong; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), S. Ancoli-Israel (co-Investigator), T. Dam (co-Investigator), ML Carrion-Petersen (Project Director), P. Miller, N. Kamantigue; Central Sleep Reading Center: S. Redline (Principal Investigator), S. Surovec (Project Administrator), N. Scott (Chief Polysomnologist), N. Johnson (Programmer Analyst), J. Arnold (Polysomnologist), R. Nawabit (Polysomnologist), J. Romaniuk (Polysomnologist), S. Seicean (Polysomnologist).
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- ArI
arousal index
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- ECG
electrocardiogram
- EEG
electroenchephalogram
- EMG
electromyogram
- EOG
electrooculogram
- ESS
Epworth Sleepiness Scale
- GDS
Geriatric Depression Scale
- IADL
instrumental activities of daily living
- MMSE
Mini-Mental State Examination
- MrOS
Osteoporotic Fractures in Men Study
- PASE
Physical Activity Scale for the Elderly
- PLMA
periodic leg movement causing EEG arousals index (periodic leg movements causing EEG arousals per hour of sleep)
- PLMI
periodic leg movement index (periodic leg movements per hour of sleep)
- PLMS
periodic limb movements in sleep
- PSG
polysomnography
- REM
rapid eye movement
- SD
standard deviation
- SOF
Study of Osteoporotic Fractures
- SSRI
selective serotonin reuptake inhibitor
REFERENCES
- 1.American Academy of Sleep Medicine. International Classification of Sleep Disorders. Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Kales A, Vela-Bueno A, Jacoby JA, Scarone S, Soldatos CR. Nocturnal myoclonus and nocturnal myoclonic activity in the normal population. Res Commun Chem Pathol Pharmacol. 1982;36:129–40. [PubMed] [Google Scholar]
- 4.Gehrman P, Stepnowsky C, Cohen-Zion M, Marler M, Kripke DF, Ancoli-Israel S. Long-term follow-up of periodic limb movements in sleep in older adults. Sleep. 2002;25:340–3. doi: 10.1093/sleep/25.3.340. [DOI] [PubMed] [Google Scholar]
- 5.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 6.Bliwise D, Petta D, Seidel W, Dement W. Periodic leg movements during sleep in the elderly. Arch Gerontol Geriatr. 1985;4:273–81. doi: 10.1016/0167-4943(85)90009-3. [DOI] [PubMed] [Google Scholar]
- 7.Mosko SS, Dickel MJ, Ashurst J. Night-to-night variability in sleep apnea and sleep-related periodic leg movements in the elderly. Sleep. 1988;11:340–8. [PubMed] [Google Scholar]
- 8.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allena M, Campus C, Morrone E, et al. Periodic limb movements both in non-REM and REM sleep: relationships between cerebral and autonomic activities. Clin Neurophysiol. 2009;120:1282–90. doi: 10.1016/j.clinph.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Coelho FM, Georgsson H, Narayansingh M, Swartz RH, Murray BJ. Higher prevalence of periodic limb movements of sleep in patients with history of stroke. J Clin Sleep Med. 2010;6:428–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Claman DM, Redline S, Blackwell T, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2:438–45. [PubMed] [Google Scholar]
- 13.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Redline S, Sanders MH, Lind BK, et al. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998;21:759–67. [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A, editors. Wahington, DC: National Institutes of Health; 1968. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- 17.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 18.Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 19.Koo BB, Blackwell T, Ancoli-Israel S, Stone KL, Stefanick ML, Redline S. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 22.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 23.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 24.Fitti JE, Kovar MG. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat 1. 1987:1–115. [PubMed] [Google Scholar]
- 25.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh J YJ. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–73. [Google Scholar]
- 27.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 28.Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 29.Coleman RM, Bliwise DL, Sajben N, Boomkamp A, de Bruyn LM, Dement WC. Daytime sleepiness in patients with periodic movements in sleep. Sleep. 1982;5(Suppl 2):S191–202. doi: 10.1093/sleep/5.s2.s191. [DOI] [PubMed] [Google Scholar]
- 30.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and Validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in Older Men. J Gerontol A Biol Sci Med Sci. 2012;67:433–9. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 2005:842–3. [Google Scholar]
- 32.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.0.2. www.aasmnet.org. Darien, IL: American Academy of Sleep Medicine; 2013. [Google Scholar]
- 33.Espinar-Sierra J, Vela-Bueno A, Luque-Otero M. Periodic leg movements in sleep in essential hypertension. Psychiatry Clin Neurosci. 1997;51:103–7. doi: 10.1111/j.1440-1819.1997.tb02370.x. [DOI] [PubMed] [Google Scholar]
- 34.Hanly PJ, Zuberi-Khokhar N. Periodic limb movements during sleep in patients with congestive heart failure. Chest. 1996;109:1497–502. doi: 10.1378/chest.109.6.1497. [DOI] [PubMed] [Google Scholar]
- 35.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]


