Abstract
Study Objectives:
Sleep bruxism (SB) is reported to vary in frequency over time. The aim of this study was to assess the first night effect on SB.
Methods:
A retrospective polysomnographic (PSG) analysis was performed of data from a sample of SB patients (12 females, 4 males; age range: 17-39 years) recorded in a sleep laboratory over 2 consecutive nights. Sleep parameters and jaw muscle activity variables (i.e., rhythmic masticatory muscle activity [RMMA]) for SB were quantified and compared between the 2 nights. Subjects were classified into groups according to severity of RMMA frequency, such as low frequency (2-4 episodes/h and/or < 25 bursts/h) and moderate-high frequency (≥ 4 episodes/h and ≥ 25 bursts/h).
Results:
Overall, no first night effects were found for most sleep variables. However, total sleep time, sleep efficiency, and stage transitions showed significant time and group interactions (repeated measures ANOVAs, p ≤ 0.05). The RMMA episode index did not differ between the 2 nights, whereas the second night showed significantly higher burst index, bruxism time index, and mean burst duration (repeated measure ANOVAs, p ≤ 0.05). Five patients of 8 in the low frequency group were classified into the moderate-high frequency group on the second night, whereas only one patient in the moderate-high frequency group moved to the low frequency group.
Conclusions:
The results showed no overall first night effect on severity of RMMA frequency in young and healthy patients with SB. In clinical practice, one-night sleep recording may be sufficient for moderate-high frequency SB patients. However, low RMMA frequency in the first night could be confirmed by a second night based on the patient's medical and dental history.
Citation:
Hasegawa Y; Lavigne G; Rompré P; Kato T; Urade M; Huynh N. Is there a first night effect on sleep bruxism? A sleep laboratory study. J Clin Sleep Med 2013;9(11):1139-1145.
Keywords: Sleep bruxism, rhythmic masticatory muscle activity, first night effect, polysomnography, sleep laboratory
Sleep bruxism (SB), a sleep-related movement disorder, is characterized by repetitive jaw muscle activity associated with tooth grinding or clenching of teeth.1–4 SB can be associated with orofacial pain, masticatory muscular hypertrophy, temporomandibular joint disorders, headaches, sleep apnea, and insomnia.2,3 SB is subjectively reported by patients who are aware of jaw clenching upon awakening or who have been told by their parents or sleep partner that they grind their teeth. SB diagnosis is based on subjective reports and clinical signs and symptoms (e.g., tooth wear), and its current presence can be confirmed by electromyographic (EMG) recordings of the masseter and/or temporalis muscles.3 The SB-related EMG jaw muscle activity recorded are rhythmic masticatory muscle activity (RMMA) episodes and bursts, which are scored as sleep traces and quantified in number.2 RMMA can be observed in 60% of controls, but shows a higher frequency in bruxers (≥ 2 episodes/h).2 However, a previous study showed that SB patients may be categorized into heterogeneous frequency groups in terms of RMMA episode index, such as low frequency (2-4 episodes/h and/or < 25 bursts/h) and moderate-high frequency (≥ 4 episodes/h and ≥ 25 bursts/h) groups.5 In support of this categorization, the two groups differed in terms of certain clinical features (history of more frequent grinding reports and less jaw pain in the moderate-high than low frequency group).4
BRIEF SUMMARY
Current Knowledge/Study Rationale: The first night effects on sleep parameters and the frequency of rhythmic masticatory muscle activity (RMMA) were assessed based on two-night polysomnographic recordings in sleep bruxism (SB) patients.
Study Impact: Overall, no first night effects were found for sleep variables and RMMA frequency. However, since RMMA frequency might be underestimated in some patients for the first night, low frequency of RMMA can be confirmed by a second night based on the patient's medical and dental history in clinical practice.
To our knowledge, with the exception of a few studies using home ambulatory recordings with one to three EMG channels on masticatory muscles, the influence of the first night effect on jaw muscle activity related to SB has not been assessed.6,7 The first night effect in the sleep laboratory is reported to affect many recorded sleep variables, and thus, in clinical research settings, polysomnography (PSG) recordings obtained during the first night of a study are generally excluded from the analysis to avoid the first night effect. The main characteristics of the first night effect are short total sleep time and REM sleep, lower sleep efficiency, longer REM sleep latency, and decreased slow wave sleep.8,9
Jaw muscle activity related to SB can be recorded in a sleep laboratory or at home using ambulatory recording systems. Home sleep recordings offer a stronger likelihood of capturing environmental influences on jaw muscles activity related to SB.10,11 However, in the absence of audio-video control to distinguish RMMA from other orofacial activity (e.g., coughing, swallowing, or somniloquy), RMMA is overestimated by about 20%.12 In addition to these limitations of sleep recording, SB related EMG recording can be very challenging due to night-to-night variability. RMMA frequency has been reported to fluctuate over time for both sleep laboratory and ambulatory home sleep recordings.6,13 In a sleep laboratory setting, night-to-night variability, from second night recordings and above, was reported to be 25.3% for RMMA episodes per hour and 30.4% for burst number per hour.13 The variability of RMMA episodes over time is critical in studies assessing the benefit of management approaches, such as cognitive behavioral therapies, medication, or oral appliances or devices known to alter jaw muscle activity.14–17
The aim of this study was to assess the first night effect on categorization according to severity of RMMA frequency and in relation to sleep macro-structure, while controlling for first night to second night variability. We also assessed the first night effect on psychobehavioral variables (e.g., anxiety, stress, fatigue) in relation to consecutive nights at the sleep laboratory.
MATERIALS AND METHODS
Study Population and Screening
In this retrospective study, data were drawn from 16 SB patients (12 females; mean age ± standard error = 25.2 ± 1.5 years) who underwent ≥ 2 consecutive overnight PSG recordings in a sleep laboratory at the Hôpital du Sacré-Coeur de Montréal. Initial inclusion criteria were age from 18 to 45 years with a reported history of teeth grinding ≥ 3 times per week. Subjects were then screened for orodental and general health problems using: (1) questionnaires addressing general health, SB, SB and pain, headaches, daytime sleepiness, and sleep quality; (2) a clinical examination by a dentist to detect SB signs, including a physical assessment of the jaw, neck, and mouth; and (3) a panoramic dental X-ray to assess dental health and the temporomandibular joint. The sleep study was performed to confirm the SB diagnosis according to the Research Diagnostic Criteria for SB1,2 and to rule out additional sleep disorders (i.e., periodic limb movement syndrome, obstructive sleep apnea). All patients were nonsmokers and were not taking any medications. They were instructed to refrain from consuming caffeine and alcohol on the day before sleep recording. The sleep recording protocol was approved by the hospital's ethics committee, and all study participants read and signed a written consent form.
Data Recording
Polysomnographic recordings (≥ 7 h) were performed for each participant on 2 consecutive nights. Surface electrodes included 2 electroencephalograms (EEG: C3A2 and O2A1), bilateral electroculograms (EOGs), an electrocardiogram (ECG), and 7 EMGs on the chin/suprahyoid, bilateral masseter, temporalis, and anterior tibialis muscles. All masseter and temporalis EMG channels were used to score RMMA. A detailed description of the method is provided elsewhere.18 To assess respiratory function and exclude sleep breathing disorders, nasal airflow was measured with a thermistor sensor (Thermocouple; Protech, Woodinville, WA, USA). Respiratory efforts were assessed with thoracic and abdominal effort belts. Oximetry was continuously monitored with a finger pulse oximeter (Datex-Ohmeda; Louisville, CO). All signals were recorded using acquisition and analysis software (Harmonie Software; Stellate Systems; Montreal, QC, Canada). Simultaneous audio-video recordings were made for visual scoring of sleep motor activities, other body movements, and oropharyngeal sounds.
An independent sleep technologist scored sleep stages based on polygraphic traces according to the standard method developed by Rechtschaffen and Kales,19 using 20-sec instead of 30-sec epochs. Cortical arousals (microarousals) were scored for both nights (Night 1 and Night 2) according to the American Academy of Sleep Medicine criteria.20 PSG sleep data were first scored by a trained sleep technician working at the sleep research center. EMG muscle activity was scored by another trained research technician and calibrated by one of the investigators (GL). Masseter and temporal EMG bursts with durations > 0.25 sec were selected for sleep motor activity scoring, which was conducted according to published criteria.5,18 The presence of RMMA is recognized by contraction patterns for both the masseter and temporal muscles: phasic (≥ 3 rhythmic EMG bursts lasting from 0.25 to 2.0 sec) and tonic (sustained EMG burst lasting > 2.0 sec) burst.21–23 RMMA bursts are grouped into RMMA episodes (with ≥ 3-s interval between RMMA episodes): phasic (≥ 3 phasic bursts), tonic (≥ 1 sustained EMG tonic burst), and mixed episode (phasic and tonic bursts).21–23
Orofacial activity was defined as all types of motor activity (activity of the bilateral masseter or temporalis muscles), not including the above-mentioned RMMA characteristics.23 Audio and video recordings were carried out simultaneously to distinguish RMMA from nonspecific orofacial activities, and to document any body movements that co-occurred with orofacial movements.23 Based on the scoring, the following variables were calculated: episode index (number of RMMA episodes per hour of sleep), burst index (number of RMMA bursts per hour of sleep), orofacial index (number of orofacial episodes per hour of sleep), and bruxism time index. These indices include all types of bursts (phasic and tonic) for calculated bursts index and bruxism time index.24 The calculated episode index includes all types of episodes (phasic, tonic, and mixed), even though only 0.5% of episodes were tonic in this study.
Subjects were categorized into 2 groups according to the frequency of RMMA episodes, represented by the calculated RMMA episode index, in order to determine night-to-night variability.5,18 The low frequency group included patients who showed a low frequency of RMMA (2-4 episode index and/ or < 25 burst index) in ≥ 1 of the 2 consecutive nights. The moderate-high frequency group included patients who showed a moderate to high frequency of RMMA (≥ 4 episode index and ≥ 25 burst index) in the 2 consecutive nights.
Questionnaires
Participants answered 5-point questions concerning anxiety, stress, fatigue, and nervousness at 4 different times in relation to sleep recordings: in the evening just before PSG recording and in the morning just after PSG recording for both nights. Items of the evening and morning questionnaires were as follows: “At this moment, do you feel anxious? (Anxiety); stressed? (Stress); nervous? (Nervousness); fatigue? (Fatigue); depressed?” (Depression). The results of the 5-point questions were classified as 1 = “No” or 2-5 = “Yes.”5
Statistical Analysis
The population sample size was estimated using a preliminary analysis of the data, with a conventional α of 0.05 and a power level of 0.80 to avoid incorrect inferences in the results interpretation.
The normality of the data distribution was verified using the Shapiro–Wilk test. When data were non-normally distributed, square root or logarithmic transformations were performed. To compare data between Night 1 and Night 2 or between the low and moderate-high frequency groups, repeated measures analysis of variance (ANOVA) were performed with time (Night 1, Night 2) as a repeated measure and group (low frequency and moderate-high frequency) as the between-group factor. Paired t-tests or two-sample t-tests were performed when the interaction between time and group was significant. Correlations between Night 1 and Night 2 were assessed as either Pearson correlation coefficient (normal distributions) or Spearman correlation coefficient (non-normal distributions). Changes in self-reported psychosocial variables (anxiety, stress, fatigue) were assessed using McNemar test. Night-to-night variability was estimated with the coefficient of variation. A p value ≤ 0.05 was considered statistically significant. No p value adjustment for multiple tests was done, since the tests were in accordance with the aim of the study. All statistical analyses were performed using a commercially available software package (IBM SPSS Statistics, Version 20.0.0 for Windows; SPSS, Chicago, IL).
RESULTS
Both the low frequency and moderate-high frequency groups consisted of 8 subjects each (6 female; 2 male). Mean age was 26.1 years (17-39, min-max) for the low frequency group and 25.6 years (17-37) for the moderate-high frequency group.
Sleep Variables
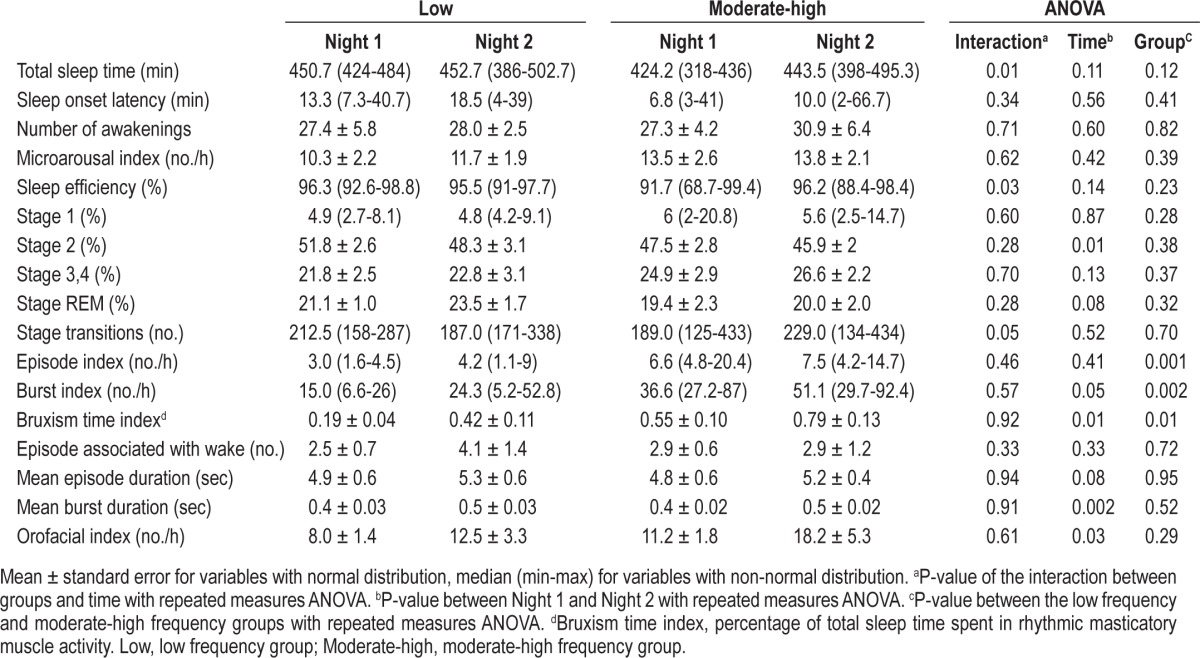
Table 1 presents the sleep characteristics and the RMMA distribution over Night 1 and Night 2. Total sleep time, sleep efficiency, and stage transitions showed significant time and group interactions (repeated measures ANOVA, p ≤ 0.05). For these variables, no significant differences were found between Night 1 and Night 2 in the low frequency group (paired t-test, p ≥ 0.32). On the other hand, the moderate-high frequency group showed higher values for these three variables in Night 2 compared to Night 1 (paired t-test, p < 0.05 for total sleep time and stage transitions, p = 0.07 for sleep efficiency). When comparing the low frequency and moderate-high frequency groups for both nights, no significant differences were found for these 3 variables (two-sample t-test), with the exception of less total sleep time in Night 1 for the moderate-high frequency group (p = 0.01). Altogether, these results suggest that the moderate-high frequency group had less total sleep time in Night 1 than in Night 2. With the exception of sleep stage 2, no other significant differences in any sleep variables were found between the Night 1 and Night or between groups (Table 1).
Table 1.
Sleep and jaw muscle activity variables
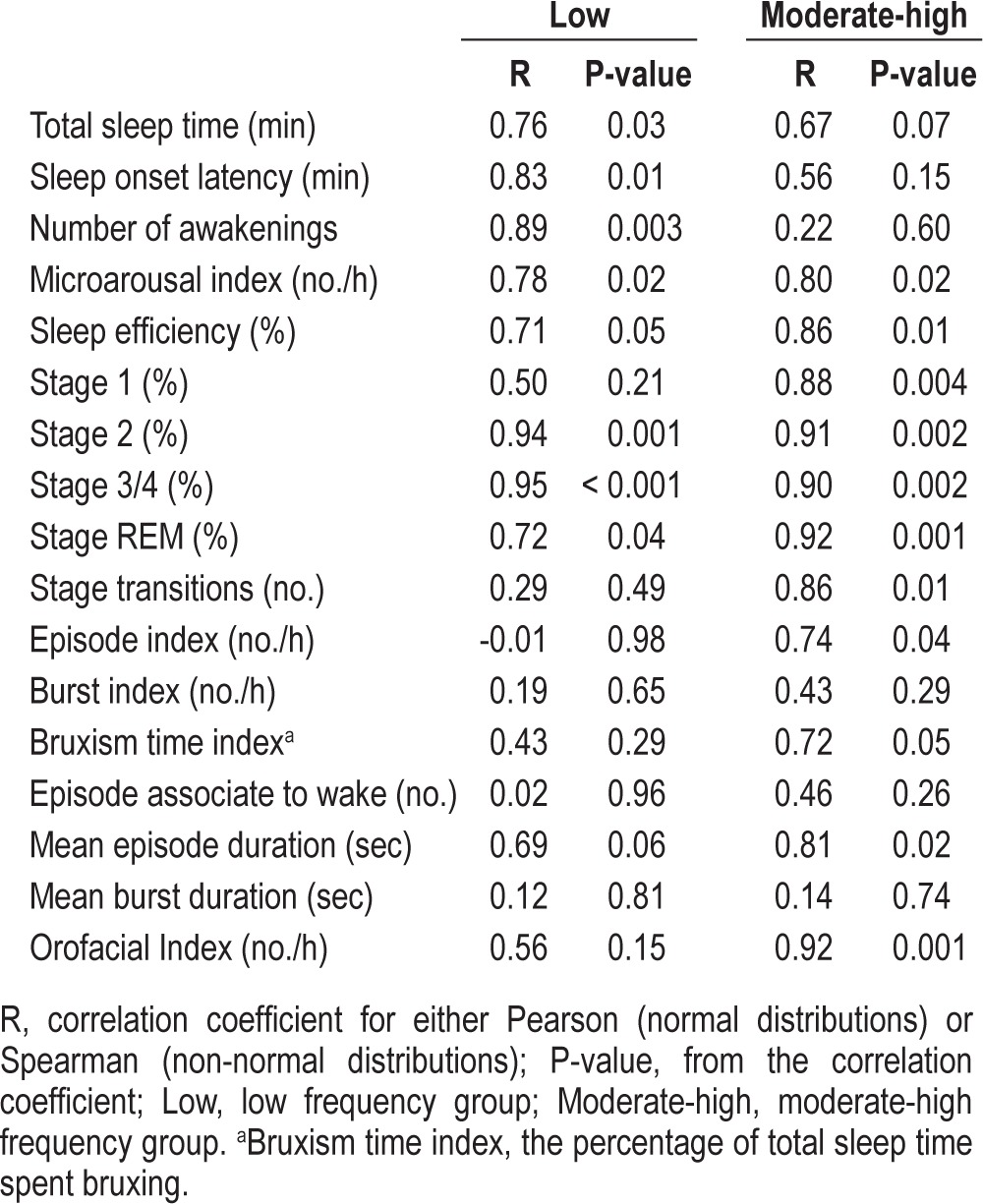
The correlations between Night 1 and Night 2 for all sleep and EMG jaw muscle activity variables are shown in Table 2. For sleep variables, the majority of correlations are medium to strong, with significant p-values.
Table 2.
Correlation coefficients for Night 1 and Night 2
Rhythmic Masticatory Muscle Activity
For all jaw muscle activity variables, no significant time and group interactions were found (repeated measure ANOVA; Table 1). For the RMMA burst index, bruxism time index, mean burst duration, and orofacial index, a significant difference was observed between Night 1 and Night 2 (repeated measure ANOVA, p ≤ 0.05). For the low frequency and moderate-high frequency groups, the burst index was 1.6 and 1.4 times lower and EMG activity was 2.2 and 1.4 times shorter (e.g., bruxism time index), respectively, in Night 1 compared to Night 2 (p ≤ 0.05, Table 1 and Figure 1). The orofacial index for Night 1 was 1.5 and 1.6 times lower than that for Night 2 for the low and moderate-high frequency groups (Table 1, p = 0.03). The other variables showed no significant differences between Night 1 and Night 2.
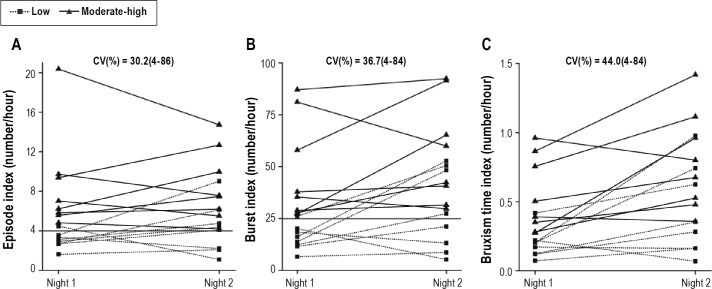
Figure 1. Individual results for RMMA variables.
(A) Episode index; (B) Burst index; (C) Bruxism time index. CV, Coefficients of variation as the mean (minimum-maximum); Low, low frequency group; Moderate-high, Moderate-high frequency group; RMMA, rhythmic masticatory muscle activity. Squares and dashed lines represent the low-frequency group. Triangles and solid lines represent the moderate-high frequency group. In panel A, the horizontal line distinguishes the low frequency group from the moderate-high frequency group. In panel B, the horizontal line distinguishes the low-frequency group from the moderate-high frequency group.
For the 2 groups, the RMMA episode index increased from Night 1 to Night 2 in 10 of 16 subjects (6 subjects in the low frequency group, 4 in the moderate-high frequency group). The RMMA burst index increased from Night 1 to Night 2 in 12 of 16 subjects (6 subjects in the low frequency group, 6 in the moderate-high frequency group). In other words, 63% of subjects showed an increase in the RMMA episode index in Night 2, and 75% of subjects showed an increase in the RMMA burst index in Night 2 (Figure 1). When the severity of RMMA frequency was assessed, 5 low frequency SB patients in Night 1 fell into the moderate-high frequency group in Night 2 based on the RMMA episode index, whereas using the RMMA burst index, 3 low frequency SB patients in Night 1 fell into the moderate-high frequency group in Night 2. However, only one patient with moderate-high frequency RMMA group in Night 1 changed to the low frequency group in Night 2 based on the RMMA episode index. Overall, 6 of 16 patients (37.5%) changed category from Night 1 to Night 2 based on the RMMA episode index.
The correlation coefficients for the RMMA episode index were -0.01 and 0.74 for the low frequency and moderate-high frequency group, respectively. The correlation coefficients for the RMMA burst index were 0.19 and 0.43 for the 2 groups, respectively. The correlation coefficients for the bruxism time index were 0.43 and 0.72 in the low frequency and moderate-high frequency groups, respectively. Thus, for these variables, the low frequency group showed weak correlation between Night 1 and Night 2, whereas the moderate-high frequency group showed strong correlation.
Questionnaires
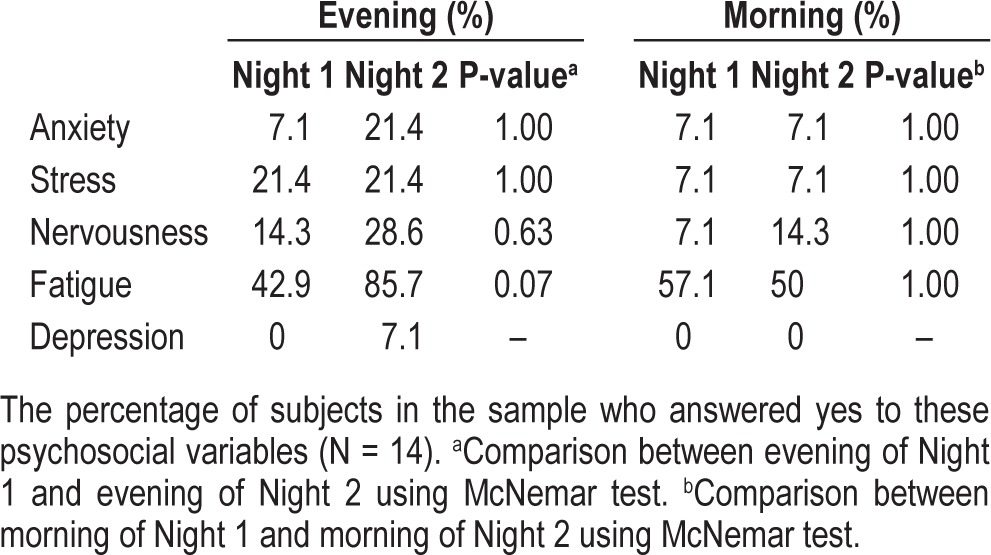
Fourteen of the 16 subjects answered the 5-point questions concerning anxiety, stress, fatigue, and nervousness (Table 3). No significant differences were observed between Night 1 and Night 2. However, the p-value for fatigue approached significance (p = 0.07), suggesting that these subjects felt slightly more fatigued in Night 1 than in Night 2.
Table 3.
Self-reports of psychosocial variables between Night 1 and Night 2
DISCUSSION
In this study, we assessed the first night effect on the frequency of RMMA and jaw muscle activity in a sleep laboratory. The results showed no first night effect on the sleep of young healthy subjects with SB. The RMMA episode index did not differ between the first and second nights, although the burst variables (e.g., burst duration) significantly changed between the two nights. However, RMMA showed first night effects in less than 40% of a low frequency group, where some patients had a low frequency of RMMA in Night 1 which increased to a moderate-high frequency in Night 2.
First Night Effect of Sleep Variables
In PSG studies, the first night effect is common: it lasts from one night up to several nights25–29 before normalizing. The main characteristics include decreased total sleep time, lower sleep efficiency, more intermittent wake time, decrease in slow wave sleep and REM, and longer REM latency.8,9 The origin of the first night effect is multifactorial and can include the following factors: (1) discomfort caused by electrodes, (2) restricted movement due to gauges and cables, (3) potential psychological consequences of being under scrutiny, and (4) changes in the environment.26 Therefore, in sleep mechanism studies and randomized controlled trials, the first night is usually used for adaptation to sleep laboratory conditions: data drawn from the first night are used mainly to exclude sleep disorders. On the other hand, several characteristics related to the variability in sleep parameters of the first night effect (e.g., sleep efficiency, total sleep time, microarousal) were not observed in some previous investigations30,31 of healthy young adults. In this study in healthy young subjects, no first night effect on sleep variables was observed, with the exception of slightly longer stage 2 sleep. The results of this study are similar to those of previous studies. Edinger et al.32 and Suetugi et al.33 demonstrated longer stage 2 sleep in Night 1 than in Night 2 in healthy young adults. Similar to our results, some studies30,31 have demonstrated no change in sleep variables from Night 1 to Night 2. It is possible that the first night effect could be diminished by better quality recording environment and improved comfort for subjects.
Interestingly, only the moderate-high frequency group showed shorter sleep and more stage transitions, in addition to a trend towards lower sleep efficiency. A bruxer has higher stress sensitivity (and panic symptoms) than a non-bruxer.34 Therefore, patients with moderate-high RMMA frequency might have greater difficulty adapting to the sleep laboratory environment.
First Night Effect of RMMA
The results showed no overall first night effect on severity of RMMA frequency in young and healthy patients with SB. Although the RMMA episode index did not change overall, a higher RMMA burst index, burst duration, and bruxism time index on the second night was observed. In other words, only the burst modality within each episode differed between Night 1 and Night 2. Thus, no effects were observed on the severity of RMMA frequency (i.e., low and moderate-high frequency). The following hypotheses might explain the low impact on the classification according to the severity of RMMA frequency. First, the number of stage transitions was significantly higher in Night 2 than in Night 1 for the moderate-high frequency group. The majority of RMMA episodes occur during NREM sleep,14,18,34 most often during the unstable sleep time immediately before transition into REM sleep.34 This explains the slight increase in the RMMA burst index during Night 2 despite the lack of difference in the RMMA episode index. Second, the results showed a substantial night-to-night fluctuation in these variables. According to previous studies, the coefficient of variation for night-to-night variation in the RMMA episode index was about 25% over 37 nights in 9 SB patients with moderate-high RMMA frequency,13 22% over 30 nights in a subject who underwent sleep laboratory PSG recordings,35 and 37% over 4 nights per subject (n = 6 with SB) using ambulatory PSG recording.6 Compared with these data, the night-to-night variation in the RMMA episode index in this study was slightly higher (at 30%) using sleep laboratory PSG recordings.
Furthermore, our results suggest that if night-to-night variability is taken into account, the first night effect on RMMA occurrence is weak. For the second night, 63% of subjects showed an increased RMMA episode index, and 75% showed an increased RMMA burst index. Under natural conditions, 50% of subjects would be expected to show an increase and 50% a decrease due to random variability. However, in our study, the percentage of subjects who showed an increase in the second night is slightly above what would be expected for random night-to-night variability.
The results of the questionnaires indicated no first night effects on anxiety, stress, fatigue, or nervousness, either before or after sleep. Additionally, no group differences were found in the results on any question items. Consequently, we could not conclude an association between psychological factors and bruxism, in accordance with previous studies.5,36 This might be explained by the absence of differences for the sleep variables and the fact that all subjects were young and healthy.
Sleep Bruxism Recordings
SB is a disorder related to sleep instability and arousal,14,22,34 and is primarily associated with RMMA. A clinical diagnosis of SB is usually based on reports of grinding sounds by sleep partners, tooth attrition, tooth mobility, tooth fracture, and damage to tooth restorations and prostheses. Because the RMMA incidence readily changes over time, it is difficult to diagnose current SB activity. Clinically useful diagnostic criteria for SB have been established by the American Academy of Sleep Medicine.1 Various assessment tools are now used for clinical and research purposes. Methods are also available for assessing SB, including the use of oral appliances and muscle activity recorders. However these methods are limited in their clinical application due to insufficient standardization of numerical criteria for jaw muscle activity and the lack of evidence-based justifications such as reliability, accuracy, and reproducibility.5,13,36 In 1996, Lavigne et al. proposed PSG cutoff criteria for SB diagnosis to be used in combination with an overall patient assessment.18 The discriminative power of these criteria was recently reconfirmed.5 However, the fluctuating nature of RMMA13 will inevitably be reflected in fluctuations in PSG recording results. According to these criteria, despite the inter-individual variability of first night effects on the RMMA occur-rence, an SB diagnosis in patients with moderate-high RMMA frequency, determined in the first night (e.g., ≥ 4 episode index and ≥ 25 burst index) remains stable, as suggested by the previous study.14 However, the severity of SB activity frequency would be underestimated on the first night when patients show low RMMA frequency.
Study Limitations
Several limitations of this study should be considered when interpreting the results. First, the sample size is small, and the subjects were young and healthy. Second, in order to focus on first night effects on SB, SB patients with no orodental or sleep problems were investigated. However, SB can occur concomitantly with other common sleep disorders such as obstructive sleep apnea and periodic leg movements in sleep.37,38 Unlike SB, these sleep disorders are more prevalent in the elderly than in young adults.37–39 Third, we did not consider sleep position, which can influence RMMA frequency as well as respiratory functions.40,41 Finally, it remains to be clarified how RMMA frequency is influenced by other proposed risk factors for SB (e.g., caffeine, alcohol).2,3 The potential influence of the above-mentioned factors on the first night effects of SB would therefore constitute a clinically relevant direction for future studies.
In conclusion, in young and healthy patients with SB, first night effects were absent for sleep macrostructure and on severity of RMMA frequency. In clinical practice, one-night sleep recording may be sufficient for SB patients with moderate-high frequency of RMMA. However, low RMMA frequency in the first night could be confirmed by a second night based on the patient's medical and dental history.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. No off-label or investigational use was made of any medical devices or pharmaceuticals. This study was supported by a grant from the Canadian Institutes of Health Research (CIHR). Dr. Hasegawa's visit to Montreal was supported by Japan's Ministry of Education, Science and Culture (Grant No. 23792263).
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Lavigne GJ, Manzini C, Huynh N. Sleep bruxism. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Saunders; 2011. pp. 1128–39. [Google Scholar]
- 3.Carra MC, Huynh N, Lavigne G. Sleep bruxism: a comprehensive overview for the dental clinician interested in sleep medicine. Dent Clin North Am. 2012;56:387–413. doi: 10.1016/j.cden.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Lobbezoo F, Ahlberg J, Glaros AG, et al. Bruxism defined and graded: an international consensus. J Oral Rehabil. 2013;40:2–4. doi: 10.1111/joor.12011. [DOI] [PubMed] [Google Scholar]
- 5.Rompré PH, Daigle-Landry D, Guitard F, Montplaisir JY, Lavigne GJ. Identification of a sleep bruxism subgroup with a higher risk of pain. J Dent Res. 2007;86:837–42. doi: 10.1177/154405910708600906. [DOI] [PubMed] [Google Scholar]
- 6.Van Der Zaag J, Lobbezoo F, Visscher CM, Hamburger HL, Naeije M. Time-variant nature of sleep bruxism outcome variables using ambulatory polysomnography: implications for recognition and therapy evaluation. J Oral Rehabil. 2008;35:577–84. doi: 10.1111/j.1365-2842.2008.01893.x. [DOI] [PubMed] [Google Scholar]
- 7.Ichiki R, Tsukiyama Y, Koyano K. Development of a portable electromyographic recording device and an application for the evaluation of the day to day variation of nocturnal masseter mascle activity. J Jpn Soc Stomatogn Func. 1999;6:67–77. [Google Scholar]
- 8.Mendels J, Hawkins DR. Sleep laboratory adaptation in normal subjects and depressed patients (“first night effect”) Electroencephalogr Clin Neurophysiol. 1967;22:556–8. doi: 10.1016/0013-4694(67)90063-6. [DOI] [PubMed] [Google Scholar]
- 9.Toussaint M, Luthringer R, Schaltenbrand N, et al. First-night effect in normal subjects and psychiatric-inpatients. Sleep. 1995;18:463–69. doi: 10.1093/sleep/18.6.463. [DOI] [PubMed] [Google Scholar]
- 10.Gallo LM, Lavigne G, Rompre P, Palla S. Reliability of scoring EMG orofacial events: polysomnography compared with ambulatory recordings. J Sleep Res. 1997;6:259–63. doi: 10.1111/j.1365-2869.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 11.Bowley J, Stockstill J, Pierce C. Reliability and validity of instrumentation used to record nocturnal clenching and/or grinding. J Orofac Pain. 1993;7:378. [PubMed] [Google Scholar]
- 12.Carra M, Huynh N, Rompré P, Lavigne GJ. Scoring sleep bruxism in absence of audio-video recording: risk of moderate overestimation. European Sleep and Research Society. 2012 [Google Scholar]
- 13.Lavigne GJ, Guitard F, Rompre PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237–44. doi: 10.1046/j.1365-2869.2001.00261.x. [DOI] [PubMed] [Google Scholar]
- 14.Huynh N, Kato T, Rompre PH, et al. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J Sleep Res. 2006;15:339–46. doi: 10.1111/j.1365-2869.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 15.Ommerborn MA, Schneider C, Giraki M, et al. Effects of an occlusal splint compared with cognitive-behavioral treatment on sleep bruxism activity. Eur J Oral Sci. 2007;115:7–14. doi: 10.1111/j.1600-0722.2007.00417.x. [DOI] [PubMed] [Google Scholar]
- 16.Landry-Schonbeck A, de Grandmont P, Rompré PH, Lavigne GJ. Effect of an adjustable mandibular advancement appliance on sleep bruxism: a crossover sleep laboratory study. Int J Prosthodont. 2009;22:251–9. [PubMed] [Google Scholar]
- 17.Jadidi F, Norregaard O, Baad-Hansen L, Arendt-Nielsen L, Svensson P. Assessment of sleep parameters during contingent electrical stimulation in subjects with jaw muscle activity during sleep: a polysomnographic study. Eur J Oral Sci. 2011;119:211–8. doi: 10.1111/j.1600-0722.2011.00822.x. [DOI] [PubMed] [Google Scholar]
- 18.Lavigne GJ, Rompre PH, Montplaisir JY. Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546–52. doi: 10.1177/00220345960750010601. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring techniques for sleep stages of human subjects. Los Angeles: Brain Res Institute; 1968. [Google Scholar]
- 20.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 21.Lavigne GJ, Rompre PH, Poirier G, Huard H, Kato T, Montplaisir JY. Rhythmic masticatory muscle activity during sleep in humans. J Dent Res. 2001;80:443–8. doi: 10.1177/00220345010800020801. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Rompre P, Montplaisir JY, Sessle BJ, Lavigne GJ. Sleep bruxism: an oromotor activity secondary to micro-arousal. J Dent Res. 2001;80:1940–4. doi: 10.1177/00220345010800101501. [DOI] [PubMed] [Google Scholar]
- 23.Dutra KMC, Pereira FJ, Rompre PH, Huynh N, Fleming N, Lavigne GJ. Oro-facial activities in sleep bruxism patients and in normal subjects: a controlled polygraphic and audio-video study. J Oral Rehabil. 2009;36:86–92. doi: 10.1111/j.1365-2842.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- 24.Van der Zaag J, Lobbezoo F, Wicks DJ, Visscher CM, Hamburger HL, Naeije M. Controlled assessment of the efficacy of occlusal stabilization splints on sleep bruxism. J Orofac Pain. 2005;19:151–8. [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Verdone P. Amount of dreaming: effect of incentive, adaptation to laboratory, and individual differences. Percept Mot Skills. 1964;19:947–58. doi: 10.2466/pms.1964.19.3.947. [DOI] [PubMed] [Google Scholar]
- 26.Le Bon O, Staner L, Hoffmann G, et al. The first-night effect may last more than one night. J Psychiatr Res. 2001;35:165–72. doi: 10.1016/s0022-3956(01)00019-x. [DOI] [PubMed] [Google Scholar]
- 27.Lorenzo JL, Barbanoj MJ. Variability of sleep parameters across multiple laboratory sessions in healthy young subjects: the “very first night effect.”. Psychophysiology. 2002;39:409–13. doi: 10.1017.S0048577202394010. [DOI] [PubMed] [Google Scholar]
- 28.Li AM, Wing YK, Cheung A, et al. Is a 2-night polysomnographic study necessary in childhood sleep-related disordered breathing? Chest. 2004;126:1467–72. doi: 10.1378/chest.126.5.1467. [DOI] [PubMed] [Google Scholar]
- 29.Agnew HW, Jr., Webb WB, Williams RL. The first night effect: an EEG study of sleep. Psychophysiology. 1966;2:263–6. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- 30.Moser D, Kloesch G, Fischmeister FP, Bauer H, Zeitlhofer J. Cyclic alternating pattern and sleep quality in healthy subjects--is there a first-night effect on different approaches of sleep quality? Biol Psychol. 2010;83:20–6. doi: 10.1016/j.biopsycho.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Sforza E, Chapotot F, Pigeau R, Buguet A. Time of night and first night effects on arousal response in healthy adults. Clin Neurophysiol. 2008;119:1590–9. doi: 10.1016/j.clinph.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Edinger JD, Fins AI, Sullivan RJ, et al. Sleep in the laboratory and sleep at home: Comparisons of older insomniacs and normal sleepers. Sleep. 1997;20:1119–26. doi: 10.1093/sleep/20.12.1119. [DOI] [PubMed] [Google Scholar]
- 33.Suetsugi M, Mizuki Y, Yamamoto K, Uchida S, Watanabe Y. The effect of placebo administration on the first-night effect in healthy young volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:839–47. doi: 10.1016/j.pnpbp.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Macaluso GM, Guerra P, Di Giovanni G, Boselli M, Parrino L, Terzano MG. Sleep bruxism is a disorder related to periodic arousals during sleep. J Dent Res. 1998;77:565–73. doi: 10.1177/00220345980770040901. [DOI] [PubMed] [Google Scholar]
- 35.Dal Fabbro C, Tufik S. A linear study of a man with sleep bruxism for 30 consecutive nights–preliminary reports. J Sleep Res. 1996;5(Suppl1):S41. [Google Scholar]
- 36.Lavigne GJ, Khoury S, Abe S, Yamaguchi T, Raphael K. Bruxism physiology and pathology: an overview for clinicians. J Oral Rehabil. 2008;35:476–94. doi: 10.1111/j.1365-2842.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 37.Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- 38.Ohayon MM, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53–61. doi: 10.1378/chest.119.1.53. [DOI] [PubMed] [Google Scholar]
- 39.Kato T, Velly AM, Nakane T, Masuda Y, Maki S. Age is associated with self-reported sleep bruxism, independently of tooth loss. Sleep Breath. 2012;16:1159–65. doi: 10.1007/s11325-011-0625-7. [DOI] [PubMed] [Google Scholar]
- 40.Bittencourt LR, Suchecki D, Tufik S, et al. The variability of the apnoeahypopnoea index. J Sleep Res. 2001;10:245–51. doi: 10.1046/j.1365-2869.2001.00255.x. [DOI] [PubMed] [Google Scholar]
- 41.Miyawaki S, Lavigne GJ, Pierre M, Guitard F, Montplaisir JY, Kato T. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003;26:461–5. [PubMed] [Google Scholar]