Abstract
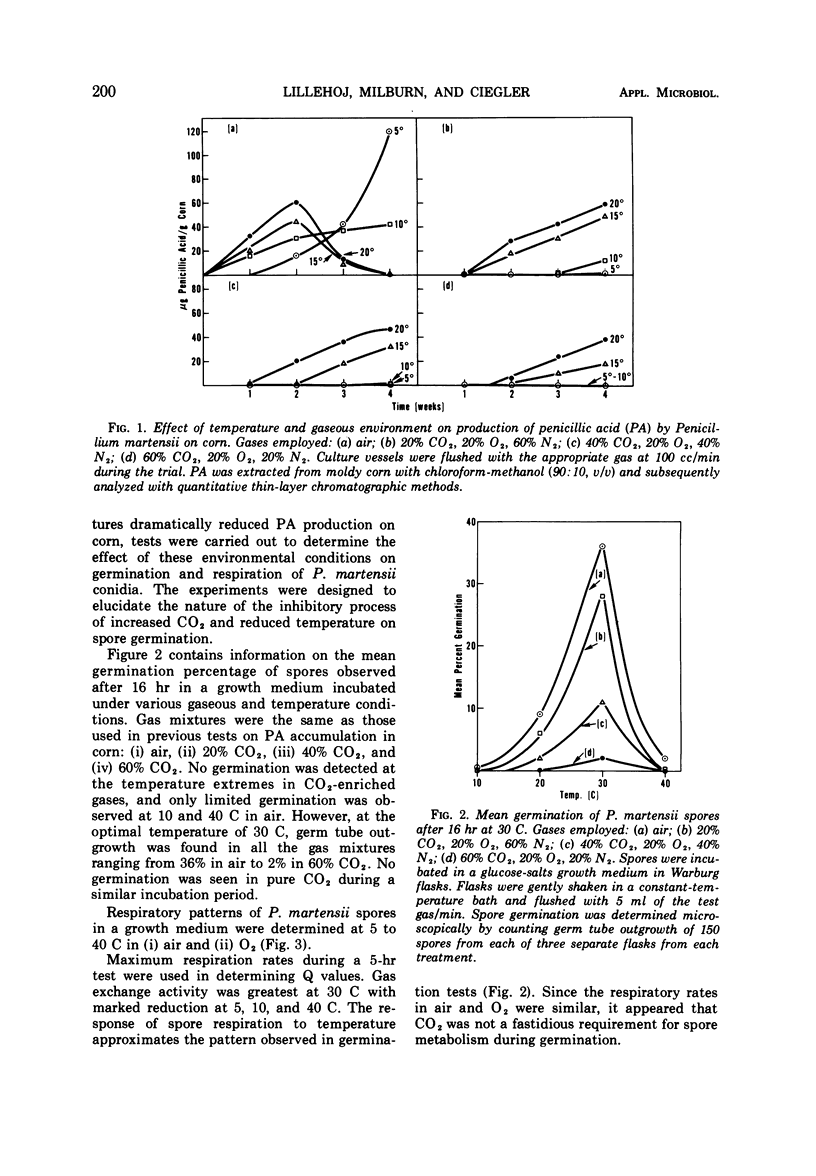
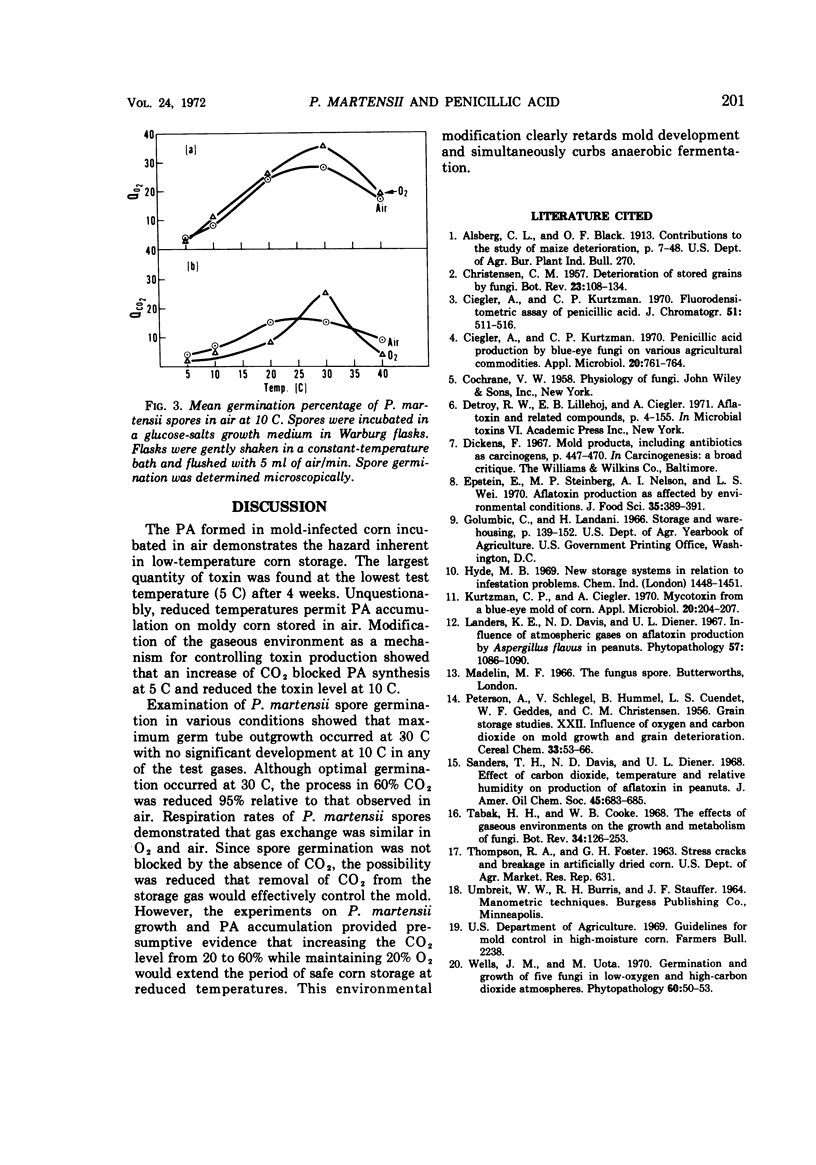
The effects of various gaseous environments and temperatures on development of Penicillium martensii NRRL 3612 and production of penicillic acid (PA) were determined. Accumulation of PA in mold-inoculated corn was measured following incubation under air; 20% CO2, 20% O2, 60% N2; 40% CO2, 20% O2, 40% N2; and 60% CO2, 20% O2, 20% N2. Although reduced temperature initially inhibited PA production, at the end of the trial the largest quantity of PA (120 μg/g of corn) was found in air-incubated corn at the lowest test temperature (5 C). Atmospheres enriched with 60% CO2 reduced PA accumulation below a detectable level at 5 and 10 C after a 4-week incubation period. Spore germination tests were carried out in a liquid growth medium incubated for 16 hr under several test conditions. Germ tube outgrowth at 30 C ranged from 36% in air to 2% in 60% CO2, whereas no germination was observed in CO2-enriched gases at 10 C. When spore respiration rates were measured in air and O2 in a liquid growth medium, complete removal of CO2 from the reaction atmosphere did not reduce O2 uptake.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciegler A., Kurtzman C. P. Fluorodensitometric assay of penicillic acid. J Chromatogr. 1970 Sep 23;51(3):511–516. doi: 10.1016/s0021-9673(01)96898-0. [DOI] [PubMed] [Google Scholar]
- Ciegler A., Kurtzman C. P. Penicillic acid production by blue-eye fungi on various agricultural commodities. Appl Microbiol. 1970 Nov;20(5):761–764. doi: 10.1128/am.20.5.761-764.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde M. B. New storage systems in relation to infestation problems. Chem Ind. 1969 Oct 11;41:1448–1451. [PubMed] [Google Scholar]
- Kurtzman C. P., Ciegler A. Mycotoxin from a blue-eye mold of corn. Appl Microbiol. 1970 Aug;20(2):204–207. doi: 10.1128/am.20.2.204-207.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers K. E., Davis N. D., Diener U. L. Influence of atmospheric gases on aflatoxin production by Aspergillus flavus in peanuts. Phytopathology. 1967 Oct;57(10):1086–1090. [PubMed] [Google Scholar]
- Sanders T. H., Davis N. D., Diener U. L. Effect of carbon dioxide, temperature, and relative humidity on production of aflatoxin in peanuts. J Am Oil Chem Soc. 1968 Oct;45(10):683–685. doi: 10.1007/BF02541257. [DOI] [PubMed] [Google Scholar]


