Abstract
Study Objectives:
The current gold-standard method of diagnosing obstructive sleep apnea (OSA) is polysomnography, which can be inefficient. We therefore sought to determine a method to triage patients at risk of OSA, without using subjective data, which are prone to mis-reporting. We hypothesized that acoustic pharyngometry in combination with age, gender, and neck circumference would predict the presence of moderate-to-severe OSA.
Methods:
Untreated subjects with suspected OSA were recruited from a local sleep clinic and underwent polysomnography. We also included a control group to verify differences. While seated in an upright position and breathing through the mouth, an acoustic pharyngometer was used to measure the minimal cross-sectional area (MCA) of the upper airway at end-exhalation.
Results:
Sixty subjects were recruited (35 males, mean age 42 years, range 21-81 years; apnea-hypopnea index (AHI) 33 ± 30 events/h (mean ± standard deviation), Epworth Sleepiness Scale score 11 ± 6, body mass index 34 ± 8 kg/m2). In univariate logistic regression, MCA was a significant predictor of mild-no OSA (AHI < 15). A multivariate logistic regression model including MCA, age, gender, and neck circumference significantly predicted AHI < 15, explaining approximately one-third of the total variance (χ2(4) = 37, p < 0.01), with only MCA being a significant independent predictor (adjusted odds ratio 54, standard error 130; p < 0.01).
Conclusions:
These data suggest that independent of age, gender, and neck size, objective anatomical assessment can significantly differentiate those with mild versus moderate-to-severe OSA in a clinical setting, and may have utility as a component in stratifying risk of OSA.
Citation:
DeYoung PN; Bakker JP; Sands SA; Batool-Anwar S; Connolly JG; Butler JP; Malhotra A. Acoustic pharyngometry measurement of minimal cross-sectional airway area is a significant independent predictor of moderate-to-severe obstructive sleep apnea. J Clin Sleep Med 2013;9(11):1161-1164.
Keywords: Acoustic pharyngometry, obstructive sleep apnea, lung, sleep, airway
Symptomatic moderate-to-severe obstructive sleep apnea (OSA) is known to affect 6-13% of the population.1 The current gold-standard method of diagnosing OSA is in-laboratory polysomnography, which can be cumbersome and expensive. Anthropometric characteristics and subjective sleepiness questionnaires have limitations in accurately predicting OSA.2,3
The current study examined the potential for acoustic pharyngometry as a method to triage efficiently patients at risk of OSA. Acoustic pharyngometry is a noninvasive method that uses sound reflection to assess quickly (< 5 min) the cross-sectional area of the upper airway as a function of distance from the oral opening.4,5 Based on the observation that anatomical compromise predisposes to OSA, prior studies have suggested that acoustic pharyngometry may be useful to predict the presence of snoring with or without OSA in both adults6–10 and children.11 Acoustic pharyngometry has also been used to assess heritability traits, such as narrow airways, in African American populations.12 Based on our experience with anatomical measurements and our work in occupational clinics where self-reports (ESS, morning headaches, SF-36) can be unreliable,13 we sought objective measurements which have good predictive value for OSA.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Due to health care reform, this study was conducted to obtain an inexpensive objective measure to predict moderate to severe obstructive sleep apnea.
Study Impact: This study shows acoustic pharyngometry is an effective measure to efficiently triage patients at risk of OSA.
In a prospective clinical study, we aimed to assess whether acoustic pharyngometry used in a daytime sleep clinic setting provides additional power to predict OSA severity beyond routine demographic and body habitus characteristics. We tested the hypothesis that acoustic pharyngometric measurement of minimal upper airway cross sectional area (MCA), in combination with objective clinical characteristics (age, gender, and neck circumference), would predict the absence of moderate-to-severe OSA, defined as an apnea-hypopnea index (AHI) ≥ 15.
METHODS
Adults (≥ 18 years) with suspected OSA were recruited from a local sleep clinic (N = 51) as well as a control group from the community (N = 9). Exclusion criteria were use of stimulants (for example amphetamines, modafinil), known head injury, dementia or retardation, alcohol or drug abuse, and pregnancy. All subjects gave informed written consent. The study was approved by Partners' Institutional Review Board.
Data Collection
The subjects recruited from the sleep clinic underwent a standard overnight laboratory polysomnography (PSG) (electroencephalogram, electrooculogram, chin and anterior tibial electromyogram, electrocardiogram, airflow using nasal pressure and oronasal thermistor, respiratory excursions using inductance plethysmography, and pulse oximetry). Those recruited from the community underwent a home sleep test level II (electroencephalogram, airflow using nasal pressure, respiratory excursions using inductance plethysmography, and pulse oximetry). All study data were manually scored by a blinded certified scorer using American Academy of Sleep Medicine alternative criteria (hypopneas defined as 50% decrease in airflow associated with 3% desaturation and/or arousal).14 We defined the presence of moderate/severe OSA as AHI ≥ 15 events/h. Clinical characteristics including body mass index (BMI) and neck circumference were measured by an experienced medical assistant naive to research study objectives during the daytime clinic visit.
Acoustic Pharyngometry
Pharyngometry data were collected as previously described15 between the hours of 08:30 and 14:30. Briefly, while seated in an upright position on a straight-back chair, subjects breathed orally through a pharyngometer (Eccovision Acoustic Pharyngometry Sleep Group Solutions, Miami FL) with the aid of a nose clip. A disposable mouthpiece was used to stabilize the tongue and provide a reproducible bite position. At normal resting lung volume (functional residual capacity), subjects were instructed to pause breathing at end-exhalation while maintaining a relaxed airway, during which acoustic measurement of upper airway cross-sectional area was made (≥ 10 pressure pulses). Measurements were repeated 3 times (see Figure 1). During a fourth measurement, subjects were asked to close their airway; subjects were coached until this occurred at the level of the glottis as observed on the pharyngo-gram.5 Data were plotted as cross-sectional area versus distance from the incisors. This pharyngogram was inspected and the minimal cross-sectional area (MCA) was measured between the oropharyngeal junction (OPJ) up to but excluding the glottis (7-18 cm from the incisors).
Figure 1. Example tracing of the recording from pharyngometry showing cross-sectional area as a function of distance from the incisors.
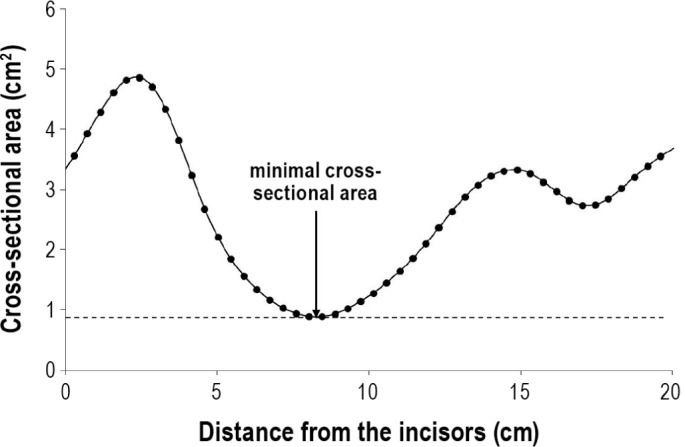
In this example, the minimal cross-sectional airway area is 0.95 cm2.
The first 3 individual MCA measurements were pooled to obtain the mean. The purpose of the fourth measurement was to determine the location of the glottis. These 4 measurements took less than 15 min per subject.
Statistical Analysis
Statistical analysis was performed using SPSS (Version 20, IBM, NY USA). Between-group differences in continuous data were assessed using t-tests or Mann-Whitney tests as appropriate for parametric and nonparametric data, respectively. Between-group differences in categorical data were assessed using χ2 tests. Univariate and multivariate logistic regression forced-entry models were used to assess the ability of hypothesized variables to predict AHI < 15. Based on our sample size, 3 predictor variables in addition to MCA were prespecified for inclusion.16 Predictors were chosen to encompass potential independent covariates or confounders of the relationship between MCA and AHI. Potential multicollinearity was investigated by assessing variance inflation factors and simple correlations. Statistical tests were considered significant when p < 0.05. A receiver operating characteristic (ROC) curve was created, and positive/negative predictive values were calculated.
RESULTS
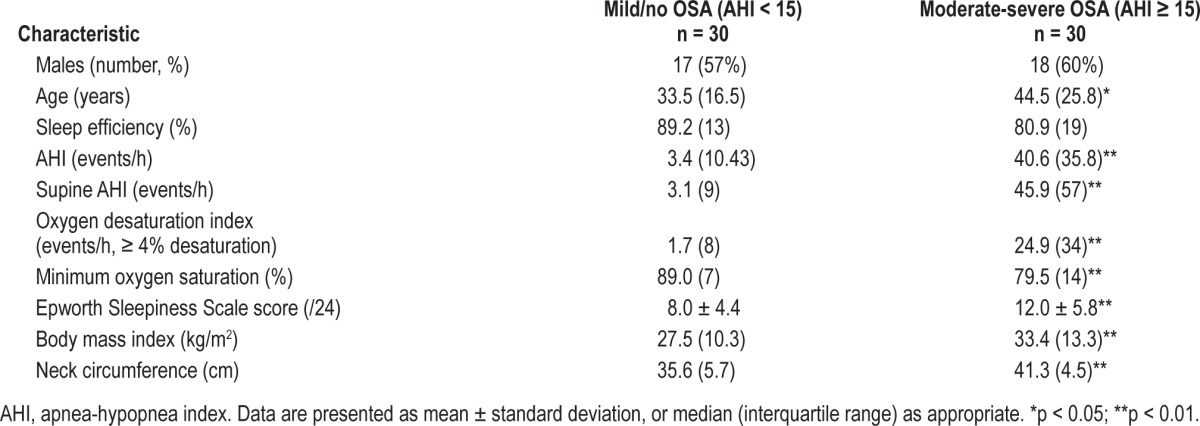
Among the 60 subjects studied, 30 patients had moderate/ severe OSA defined as AHI ≥ 15 (Table 1). The OSA (AHI ≥ 15) and mild/no-OSA (AHI < 15) groups were significantly different in terms of measures of apnea severity, BMI, age, and neck circumference. The median MCA of the OSA group was 1.66 (IQR 0.42) compared with 2.22 (IQR 0.44) in the control group (p ≤ 0.01).
Table 1.
Characteristics of patients with moderate-severe OSA and mild/no OSA
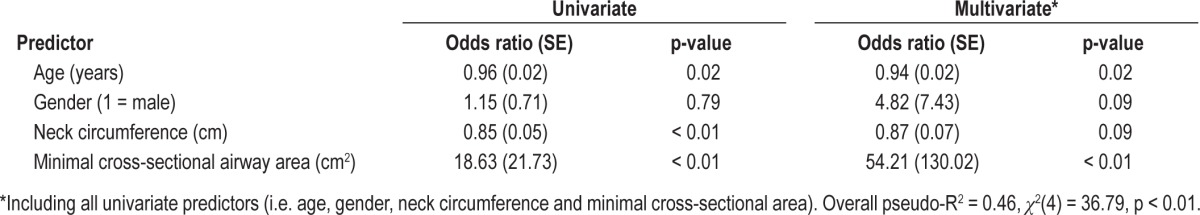
Results of the univariate and multivariate logistic regression models are shown in Table 2. Age, neck circumference, and MCA were all significant univariate predictors of AHI < 15, with age and MCA remaining as significant independent predictors of AHI < 15 after controlling for gender and neck circumference. Overall, the model had a pseudo-R2 of 0.46 (χ2(4) = 36.79, overall p < 0.01). The area under the ROC curve for MCA predicting AHI < 15 was 0.85. The optimum MCA cutoff point for detecting an AHI < 15 was 1.86 cm2 (95% CI 0.69 to 0.96); at this point, the positive predictive value was 0.87 and the negative predictive value was 0.87 (true positive: AHI < 15 and MCA < 1.86 cm2).
Table 2.
Univariate and multivariate logistic regression models with outcome AHI < 15 events/h
DISCUSSION
The current study demonstrates that MCA, determined by acoustic pharyngometry, can significantly differentiate between those with mild/no-OSA versus moderate-to-severe OSA. When analyzed alongside other variables such as gender, age, and neck circumference, acoustic pharyngometry was the only independent predictor of detecting the absence of moderate-to-severe OSA. This easily obtained measurement thus has potential utility as a component in a diagnostic algorithm for OSA. With healthcare reform, sleep testing may become less readily available, making decisions to prioritize testing in certain patients important.
Acoustic pharyngometry may also be a useful objective tool in occupational health clinics. The population in certain environments may minimize subjective symptoms in sleep questionnaires, leading to the need for simple and cost-effective tools for objective assessments. In conjunction with basic anthropometric characteristics, pharyngometry can help objectively determine those at high risk for OSA.
Other anatomical assessments have been used in the literature,17 although each has limitations. Neck circumference is easy to measure but underestimates OSA in lean individuals,18 and did not perform as well as pharyngometry in the present study. Computed axial tomography involves ionizing radiation exposure19 and is not readily available in many sleep clinics or other offsite centers; moreover, it is time consuming and costly. Similarly, cephalometrics require radiation and only have modest predictive value for OSA.8,20 Magnetic resonance imaging (MRI) avoids ionizing radiation and provides excellent definition of parapharyngeal soft tissues,21 but is also expensive, and thus unlikely to replace PSG as a method of choice for initial risk assessments of OSA. However, MRI does have theoretical advantages over pharyngometry when tissue definition and specific identification of certain tissue structures are needed, for example in preoperative assessment to determine preferred surgical procedure.
Some of the prior literature with acoustic pharyngometry has focused primarily on the OPJ, rather than the MCA per se. We chose to focus on the MCA for a number of reasons. We could reliably identify the MCA in all patients, whereas in some patients the OPJ was either difficult to identify or unclear based on review by multiple experts. We also aimed to identify a strategy which was easily implementable by clinicians in practice and thus did not rely on measurements that required expertise or experience to obtain. In addition, there are significant variabilities in anatomical factors compromising the pharyngeal airway in OSA and thus we did not want to limit our observations to the OPJ.22
Despite the study's strengths, we acknowledge a number of limitations. First, we had a limited sample size, in large part due to the move towards home sleep testing, currently very strong in Massachusetts, and few patients without complications are undergoing clinical in-laboratory polysomnography. This trend toward home testing was a major motivation underlying the present study. Second, pharyngometry does not provide insights as to the mechanisms underlying airway obstruction. For reproducibility reasons, acoustic pharyngometry is best used while awake and seated.23 We would also note that although supine sleep is clearly relevant to OSA pathogenesis, our assessments of patients during upright wakefulness still provided good predictive value in distinguishing OSA patients from controls. Thus, our data do not speak to the mechanism of decreased MCA, but do describe the phenomenon adequately. We encourage further efforts into anatomical assessment of the upper airway to determine the role of tongue anatomy, fat deposition, and mandibular structure. Third, multiple mechanisms underlie OSA, including but not limited to upper airway dilator muscle activity, endexpiratory lung volume, and ventilatory control instability. Thus, we would not expect anatomical assessments to account for all of the variance underlying OSA.24 This concept suggests the need for further efforts into apnea phenotyping,25 such that the mechanisms underlying apnea can be determined by clinicians caring for afflicted patients. Despite these limitations, we believe our findings are robust and worthy of further testing.
In conclusion, we have demonstrated that acoustic pharyngometry provides an objective and simple test with strong independent predictive value for the presence or absence of moderate-to-severe OSA. Further efforts will be required to validate these findings in an occupational setting, where self-report is often unreliable, and may contribute to the identification of mechanisms underlying apnea and promoting individualized therapy for OSA in the future.
DISCLOSURE STATEMENT
Pharyngometry equipment and support of its use was provided by Sleep Group Solutions; no other financial support was obtained for this project. Dr. Sands is supported by an American Heart Association Postdoctoral Fellowship. Dr. Malhotra was a consultant for Philips Respironics, SHC, SGS, Apnicure, Apnex, and Pfizer, but has relinquished all outside personal income from May 2012. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff at Brigham and Women's Hospital Sleep Disorders Research Program for being amazing.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- MCA
minimal cross-sectional area
- MRI
magnetic resonance imaging
- OPJ
oro-pharyngeal junction
- OSA
obstructive sleep apnea
- PSG
polysomnography
- ROC
receiver operating characteristic
- SE
standard error
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013 Apr 14; doi: 10.1093/aje/kws342. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929–38. doi: 10.1093/sleep/23.7.929. [DOI] [PubMed] [Google Scholar]
- 3.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 4.Fredberg JJ, Wohl ME, Glass GM, et al. Airway area by acoustic reflections measured at the mouth. J Appl Physiol. 1980;48:749–58. doi: 10.1152/jappl.1980.48.5.749. [DOI] [PubMed] [Google Scholar]
- 5.Kamal I. Normal standard curve for acoustic pharyngometry. Otolaryngol Head Neck Surg. 2001;124:323–30. doi: 10.1067/mhn.2001.113136. [DOI] [PubMed] [Google Scholar]
- 6.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis. 1984;130:175–8. doi: 10.1164/arrd.1984.130.2.175. [DOI] [PubMed] [Google Scholar]
- 7.Bradley TD, Brown IG, Grossman RF, et al. Pharyngeal size in snorers, nonsnorers, and patients with obstructive sleep apnea. N Engl J Med. 1986;315:1327–31. doi: 10.1056/NEJM198611203152105. [DOI] [PubMed] [Google Scholar]
- 8.Mostafiz W, Dalci O, Sutherland K, et al. Influence of oral and craniofacial dimensions on mandibular advancement splint treatment outcome in patients with obstructive sleep apnea. Chest. 2011;139:1331–9. doi: 10.1378/chest.10-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung DG, Cho HY, Grunstein RR, et al. Predictive value of Kushida index and acoustic pharyngometry for the evaluation of upper airway in subjects with or without obstructive sleep apnea. J Korean Med Sci. 2004;19:662–7. doi: 10.3346/jkms.2004.19.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamal I. Acoustic pharyngometry patterns of snoring and obstructive sleep apnea patients. Otolaryngol Head Neck Surg. 2004;130:58–66. doi: 10.1016/j.otohns.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Monahan KJ, Larkin EK, Rosen CL, et al. Utility of noninvasive pharyngometry in epidemiologic studies of childhood sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:1499–503. doi: 10.1164/rccm.200111-061OC. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Frame JM, Larkin EK, et al. Heritability of upper airway dimensions derived using acoustic pharyngometry. Eur Respir J. 2008;32:1304–8. doi: 10.1183/09031936.00029808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang C, Varvarigou V, Parks PD, et al. Psychomotor vigilance testing of professional drivers in the occupational health clinic: a potential objective screen for daytime sleepiness. J Occup Environ Med. 2012;54:296–302. doi: 10.1097/JOM.0b013e318223d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15.Kamal I. Test-retest validity of acoustic pharyngometry measurements. Otolaryngol Head Neck Surg. 2004;130:223–8. doi: 10.1016/j.otohns.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Miles J, Shevlin M. Applying regression and correlation: a guide for students and researchers. London: Sage; 2001. [Google Scholar]
- 17.Chi L, Comyn FL, Mitra N, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38:348–58. doi: 10.1183/09031936.00119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies RJ, Stradling JR. The relationship between neck circumference, radiographic pharyngeal anatomy, and the obstructive sleep apnoea syndrome. Eur Respir J. 1990;3:509–14. [PubMed] [Google Scholar]
- 19.Haponik EF, Smith PL, Bohlman ME, et al. Computerized tomography in obstructive sleep apnea. Correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–6. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Redline S, Tishler PV, Hans MG, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155:186–92. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 21.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162:740–8. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 22.Kezirian EJ, White DP, Malhotra A, et al. Interrater reliability of drug-induced sleep endoscopy. Arch Otolaryngol Head Neck Surg. 2010;136:393–7. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 23.Martin SE, Marshall I, Douglas NJ. The effect of posture on airway caliber with the sleep-apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1995;152:721–4. doi: 10.1164/ajrccm.152.2.7633733. [DOI] [PubMed] [Google Scholar]
- 24.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013 May 30; doi: 10.1164/rccm.201303-0448OC. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellman A, Eckert DJ, Jordan AS, et al. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol. 2011;110:1627–37. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


