Abstract
Objective:
To evaluate the relation between obstructive sleep apnea (OSA) and left ventricular mass (LVM) in morbid obesity and the influence of gender, menopausal status, anthropometry, body composition, hypertension, and other cardiovascular risk factors in this relationship.
Design:
Cross-sectional descriptive study.
Methods:
Polysomnographic and echocardiographic studies were performed in a cohort of 242 patients (86 men, 100 premenopausal (PreM) and 56 postmenopausal (PostM) women), with grade II obesity and above (BMI: 43.7 ± 0.4 kg/m2) to investigate OSA and LVM respectively. Anthropometry, body composition, glucose tolerance, and blood pressure were also recorded.
Results:
OSA to different degrees was diagnosed in 76.2% of the patients (n: 166), its prevalence being 90.9% (n: 70) for men, and 76% (n: 38) and 63.8% (n: 58) for PostM and PreM women, respectively (p < 0.01). LVM excess was greatest for PostM women (90.2%), followed by men (81.9%) and PreM females (69.6%) (p < 0.01). LVM values increased in accordance to OSA severity (absence, 193.7 ± 6.9 g; mild, 192.6 ± 7.8 g; moderate, 240.5 ± 12.5 g; severe, 273.6 ± 14.6 g; p < 0.01). LVM magnitude correlated with the menopausal state, age, central adiposity, hypertension (HT), type 2 diabetes (DM), desaturation index (DI), and apnea-hypopnea index (AHI) (r = 0.41; p < 0.01). The relationship between LVM and AHI persisted in the multivariate analysis (β = 0.25; p < 0.05) after adjusting for age, gender, menopausal state, BMI, waist circumference, neck circumference, DI, fasting plasma glucose, DM, and HT. But if tobacco habits are included, the statistical difference disappears (β = 0.22; p = 0.06).
Conclusions:
Morbid obesity is frequently associated with abnormal LVM, particularly in patients with OSA; this association is independent of HT, BMI, body composition, and other clinical factors, supporting a direct role of OSA on LVM in morbid obesity. This suggests that OSA and LVM might be taken as predictors of the cardiovascular risk in these patients.
Citation:
Pujante P; Abreu C; Moreno J; Barrero EA; Azcarate P; Campo A; Urrestarazu E; Silva C; Maria JG; Tebar J; Frühbeck G; Salvador J. Obstructive sleep apnea severity is associated with left ventricular mass independent of other cardiovascular risk factors in morbid obesity. J Clin Sleep Med 2013;9(11):1165-1171.
Keywords: Sleep apnea, left ventricular mass, morbid obesity, apnea-hypopnea index
Obstructive sleep apnea (OSA) is characterized by intermittent and repeated occlusion of the upper airways during sleep, leading to partial (hypopnea) or total (apnea) interruptions of the airflow.2 As a result, sustained variations in oxygen saturation and frequent wake up episodes take place, which may impair respiratory, cardiac, metabolic, and cognitive functions. OSA prevalence in the general population has been shown to be around 2% to 5% in women and 3% to 6% in men, although these values are probably underestimated due to difficulties in confirming the diagnosis and to the low relationship between the clinical manifestations and the number of apneas and hypopneas per hour (apnea-hypopnea index, AHI).2,3
BRIEF SUMMARY
Current Knowledge/Study Rationale: This work was designed to explore the possible relationship between obstructive sleep apnea (OSA) and alterations of the left ventricular mass (LVM) in patients with morbid obesity. The influence of gender, menopausal status, body composition, and cardiovascular risk factors were also taken into consideration. The results show a high prevalence of sleep apnea and LVM alterations in this population, especially for men and PostM women. Interestingly, the apnea-hypopnea index related to LVM independently of age, gender, menopausal status, and other cardiovascular risk factors, including hypertension. These results support a direct effect of OSA on LVM, and suggest that LVM should be assessed in all patients with morbid obesity and OSA, particularly men and postmenopausal women.
Study Impact: Morbid obesity is frequently associated to left ventricular mass alterations and obstructive sleep apnea. In this study we aimed to assess a possible direct relation between both cardiovascular risk factors and the influence played by complications such as hypertension and type 2 diabetes, often associated with obesity.
Development of OSA depends on several factors such as age, menopause, alcohol consumption, smoking, and administration of drugs that induce muscle relaxation, thus favoring respiratory airflow obstruction.2,3 However, obesity may represent the most determining factor.2–4 In the context of the present obesity epidemic, the prevalence of subjects with BMI higher than 35 kg/m2 is increasing significantly, reaching 15.5% of the population in the USA.5 Obese patients frequently display an altered breathing pattern due, at least in part, to neck fat accumulation, which provokes airway collapse during sleep. It is estimated that obesity multiplies the risk of OSA by ten, waist circumference being the anthropometric factor that best correlates with its occurrence. This high OSA prevalence in morbidly obese patients contributes to increasing their cardiometabolic morbility by different mechanisms such as the increase of the extracellular volume and sodium retention, changes in the endothoracic pressure, and increase in sympathetic nervous system tone and renin angiotensin system activity.4 Furthermore, OSA is becoming a recognized cause of resistant hyper-tension (HT),6,7 as deduced by the relation found between high AHI values and its development.8 The same may be true for the association between obesity and HT, which represent well-established factors of left ventricular mass (LVM) increase and cardiovascular risk (CVR).9 But OSA might increase the risk of developing cardiovascular disease itself.7 It is known that OSA patients display alterations in myocardial function or structure.10,11 However, it is unclear whether these changes are due to OSA, obesity, or other frequently associated comorbidities, such as type 2 diabetes mellitus (DM) or HT. Given that an integral therapeutic approach should be programmed for most patients with OSA, it is important to know whether this condition is directly associated with myocardial disturbance, independent of the presence of other potentially related factors, such as fat mass excess, HT, or DM, since in that case the interest in detecting and treating OSA for cardiovascular prevention should be reinforced.
This study was designed to explore the relationship between OSA and the changes observed in LVM in morbidly obese patients, as well as to assess whether this relation is influenced by gender, age, menopausal state, body mass index (BMI), body composition, and HT.
METHODS
Patients
We examined 242 Spanish patients with morbid obesity, BMI ≥ 35 kg/m2 (BMI: 43.7 ± 0.4 kg/m2), of whom 156 were women (56 postmenopausal [PostM] and 100 premenopausal [PreM]) and 86 men. The mean age was 43.2 ± 0.8 years (range 1,871 years). Patients with a history of coronary heart disease, heart failure, or established diagnosis of cardiac abnormalities were excluded. They were recruited in the Endocrinology Department; the cause of consultation was obesity in all cases. The response rate was 88% (n = 215).
Anthropometry and Body Composition
All the patients were subjected to routine anthropometric examinations including body weight, height, and waist and neck circumference measurements. Body composition studies were carried out by air displacement plethysmography (Bod-Pod; Life Measurements, Concord, CA, USA), which is a validated bicompartimental method to determine the percentage of fat mass and fat-free mass.12
Cardiovascular Risk Factors Studied
Smoking
Three groups were defined: non-smokers (those that never smoked), ex-smokers (who had given up smoking ≥ 6 months prior to the study), and active smokers.
Hypertension
Mean values of two measurements of blood pressure were considered. Patients undergoing antihypertensive treatment or those with systolic pressure > 140 mm Hg and/or diastolic pressure > 90 mm Hg were considered as hypertensive.
Diabetes Mellitus
An oral glucose tolerance test (OGTT) was performed in all patients with no previous history of DM. The patients were diagnosed as diabetic if the syndrome had been diagnosed previously, were being treated, or the condition was shown by OGTT according to ADA criteria.
Biochemical Measurements
Fasting plasma glucose was measured by spectrometry using an enzymatic-colorimetric method. Fasting plasma insulin was determined by immunoassay (Immulite 2500. Siemens). As an indicator of insulin resistance, the HOMA index (insulin [μU / mL] × glucose [mmol] / 22.5) was calculated in all cases.
Polysomnographic Study
All patients underwent a full night polysomnographic study. Nocturnal sleep was recorded with a Harmonie 5.2 system (Stellate, Montreal, QC, Canada) using Lamont 32-Sleep amplifiers (Lamont Medical, Madison, WI, USA). The recordings included 7 electroencephalogram channels referenced to balanced mastoids, right and left electrooculogram, oxygen saturation, airflow thoracic-abdominal bands, body position sensor and electrocardiogram. In accordance with established criteria, apnea was defined as the cessation of nasal or oral airflow > 10 seconds. Hypopnea was defined as ≥ 30% decrease in the airflow channel > 10 sec, accompanied by ≥ 4% oxygen desaturation in ≥ 90% of the events.13 The AHI and DI were automatically calculated as functions of the mean number of apneic and hypopneic or desaturation events per hour of sleep, respectively. The frequency and severity of oxygen desatu-rations were also measured. The hypnogram was visually analyzed off-line following standard criteria. The severity of OSA was classified according to the number of apnea events per hour following established criteria14: absent (< 5), mild (5-15), moderate (15-30), and severe (> 30).
Echocardiographic Study
Doppler echocardiography was carried out the day before the polysomnographic study. All determinations were performed by the same echocardiography specialist using a Sonos 7500 (Philips, Eindhoven, The Netherlands). Morphological measurements were taken in M mode with respect to a parasternal long axis view.
The left ejection fraction was measured as a systolic function parameter (EF = (TDV − TSV) / TDV), where TDV and TSV are the telediastolic and telesystolic volumes, respectively. EF alterations were classified into 4 categories15: reference, ≥ 55%; mild alteration, 45% to 54%; moderate, 30% to 44%; severe, < 30%.
The left ventricular mass (LVM) was calculated according to the formula: 0.8 {1.04 [(TDD + PPD + SD)3 – (TDD)3] + 0.6 g}16, where TDD is the telediastolic diameter, PPD the diameter of the posterior wall and SD the septal diameter. The values were adjusted according to sex into the following categories of abnormality15: women: reference, 66-150 g; mild alteration, 151-171 g; moderate, 172-182 g; severe, > 183 g; men: reference, 96-200 g; mild alteration, 201-227 g; moderate, 228-254 g; severe, > 255 g.
There is not a specific value adjusted for obesity, for this reason we adopted the most commonly used: LVM adjusted according to height (LVM/height2.7)17 and to the body surface [(BS (m2)]. In the first case a cutoff point of 51 g/m2 was taken as indicative of alteration,18,19 while in the second the formula was used to calculate the left ventricular mass index (LVMI), defined as LVM/BS. As reference values 115 g/m2 in men and 95 g/m2 in women were taken.15 The geometry of the left ventricular mass of the patients was calculated as a function of their relative wall thickness (WRT, mm) and LVMI (g/m2) and classified into 4 groups15: normal: WRT ≤ 0.42 and LVMI ≤ 95 and ≤ 115 for women and men respectively; concentric remodelling: WRT > 0.42 and LVMI ≤ 95 (women) / ≤ 115 (men); concentric hypertrophy WRT > 0.42 and LVMI > 95 (women) / > 115 (men); eccentric hypertrophy WRT ≤ 0.42 and LVMI > 95 (women) / > 115 (men).
Informed consent was obtained from each patient after full explanation of the purpose and nature of all procedures used.
Statistical Analysis
The values are expressed as mean ± standard error for quantitative variables and as percentages for categorical variables. Means were compared for groups by one-way ANOVA with Bonferroni post hoc test and contingency tables to calculate χ2 when comparing categorical variables. The correlation analyses were assessed with the Pearson correlation coefficient and with multivariate linear and logistic regression studies. All the analyses were carried out with the statistical package SPSS v.15.0 (SPSS, Inc., Chicago, IL).
RESULTS
General Characteristics of the Patients and Influence of Gender and Menopause
In Table 1 the information on the variables under study is recorded. It comprises the age of the patients, their anthropo-metric characteristics, smoking habits, prevalence of HT and DM and the echocardiographic and polysomnographic data as a function of sex and menopausal state. The mean age was roughly equivalent between men and women and, as expected, PreM women were younger than their PostM counterpart. All patients displayed an obesity degree ≥ 2, and no significant differences were observed in their respective BMIs. The body composition analysis showed a higher percentage of fat mass in women (p < 0.01) with no differences between the PreM and PostM subgroups. Men presented a more intense central fat distribution than women, as deduced from their respective waist circumference measurements (p < 0.01). Similarly, neck circumference was higher in men than in women, being similar in the PreM and PostM clusters. No differences were observed in smoking habits. Hypertension was diagnosed in 55.4% of the patients, with men and PostM subgroups displaying a higher HT prevalence than PreM women. Diabetes was found in 25.6% of the patients, with no statistical difference between men and women as a whole; however, DM was significantly more prevalent in the PostM than in the PreM consortia. HOMA index calculations showed that men were more insulin resistant, with no differences between PreM and PostM women.
Table 1.
Patient anthropometric characteristics, risk factors and echocardiographic study as a function of gender and menopausal state
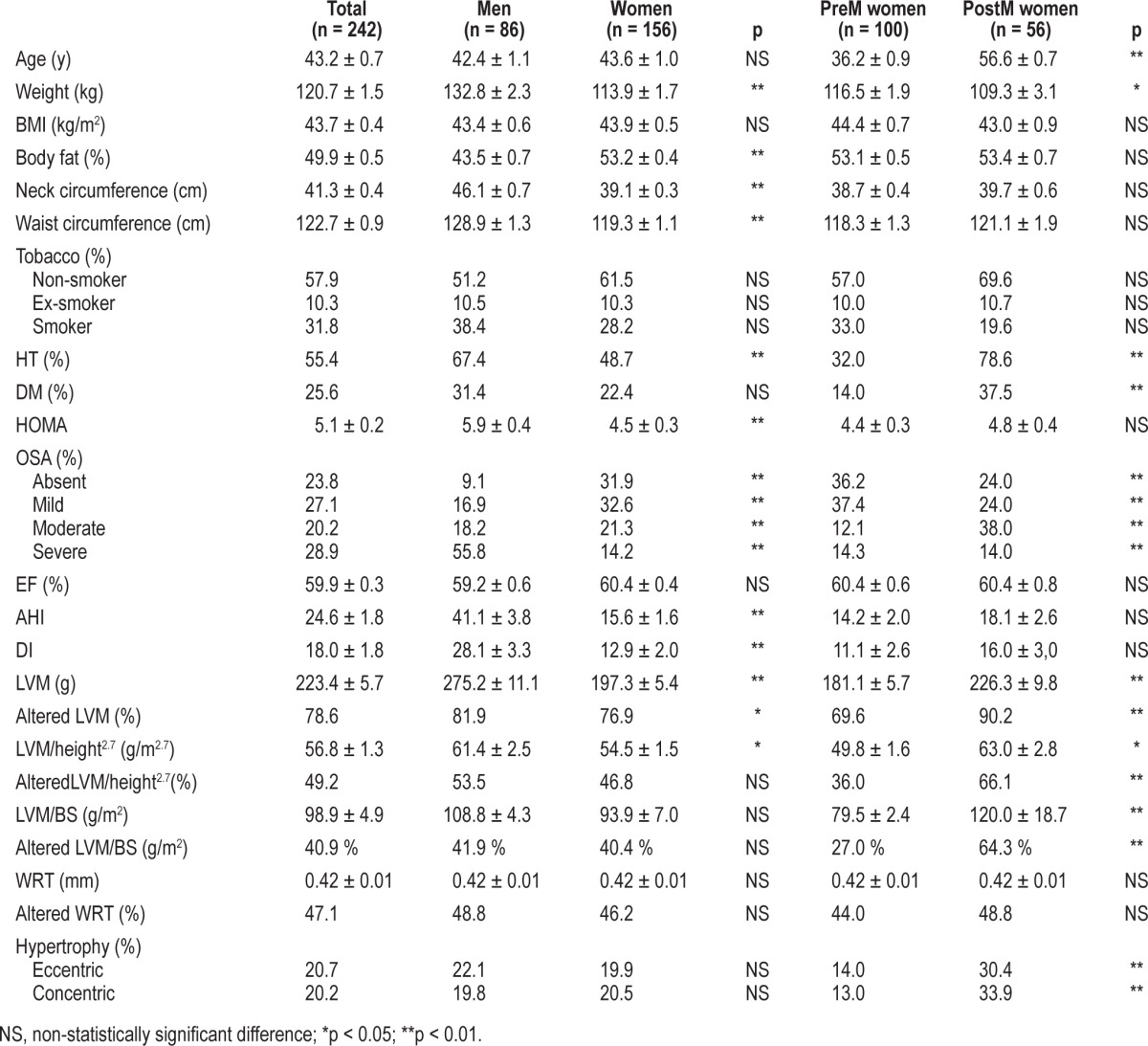
The prevalence of OSA in the whole group of patients was found to be 76.2%. However, the frequency among men rose up to 90.9%, while in women it accounted for 68.1%, the difference being statistically significant, as was that of the PreM (63.8%) and PostM (76%) clusters.
Similar echocardiographic values of the ejection fraction were found for men and PreM and PostM women, all of them being within the normal range. Significant differences (p < 0.01) were found in the LVM values of men and women and, within the latter, between the PreM and PostM subgroups. However, when LVM was adjusted for height and body surface, the differences between women and men became less (p < 0.05) and nonsignificant, respectively, although they remained as such in the case of the PreM and PostM clusters. No difference was found in the WRT of the three cohorts under study. Finally, concentric and eccentric hypertrophies were similar for both sexes taken as a whole; nevertheless, the condition affected a significantly higher proportion of PostM than PreM women (p < 0.01).
Influence of Patient Characteristics on OSA Occurrence
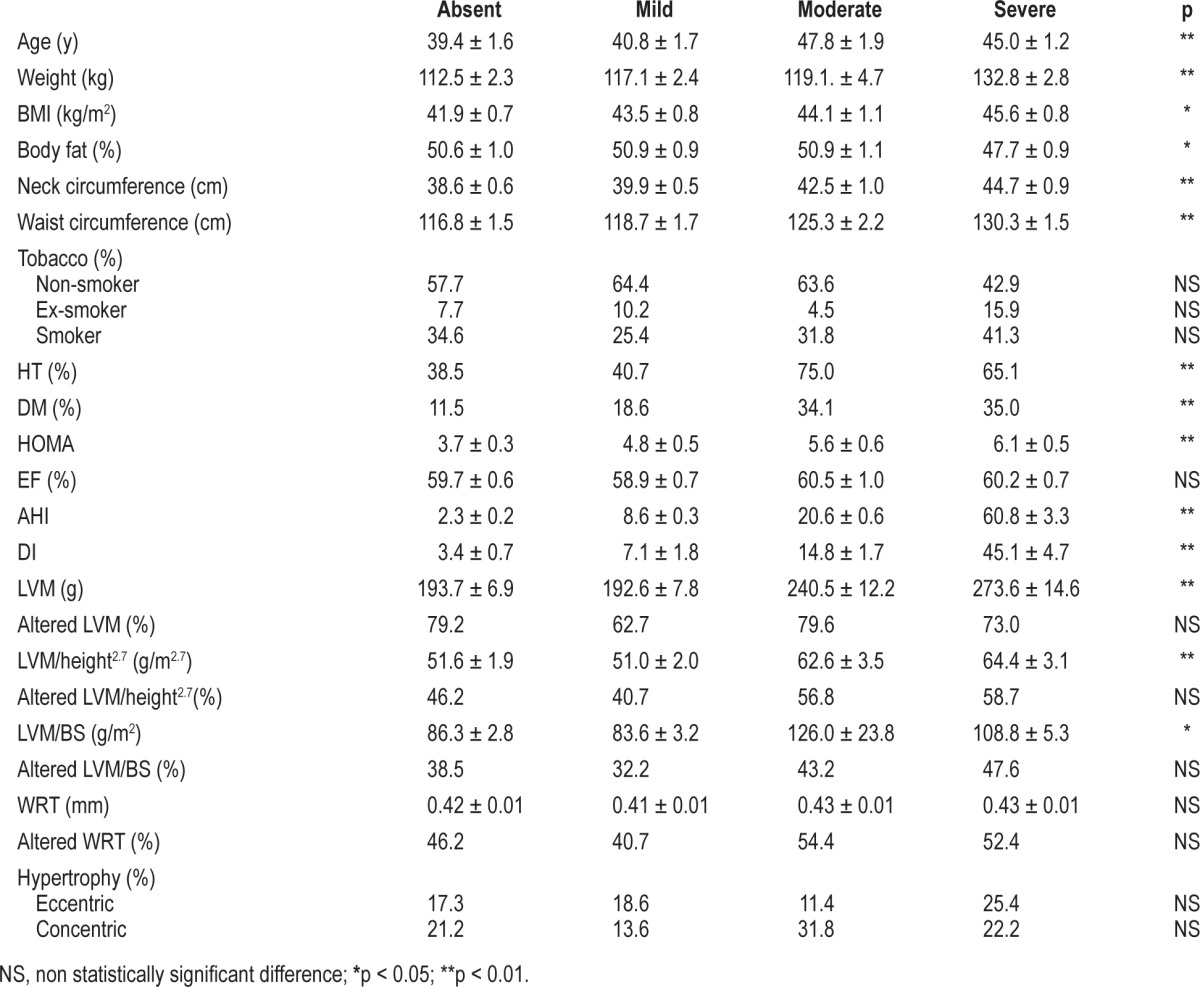
In Table 2 the characteristics of the group of patients are related to the severity of OSA. A clear tendency towards its association with increasing age, weight, BMI, body fat, waist and neck circumference, and the HOMA index was observed (p < 0.01 in all cases). Similarly, the more severe the degree of OSA, the greater the likelihood of associated comorbidities, especially HT and DM. In contrast, the systolic function, as assessed by the LV ejection fraction, was similar irrespective of the degree of OSA severity.
Table 2.
Comparison of the patient cohort anthropometric values, risk factors and echocardiographic data with their OSA severity
A gradual increase in LVM was observed concomitant with OSA severity (p < 0.01) which was clearly enhanced by HT, as can be observed in Figure 1. Similarly, a gradual increment of LVM, when adjusted with respect to height, was seen as a function of the presence and intensity of OSA (p < 0.01). However, OSA severity did not correlate to heart hypertrophy or gender.
Figure 1. LVM (g) evolution as a function of OSA severity in the absence and presence of HT.
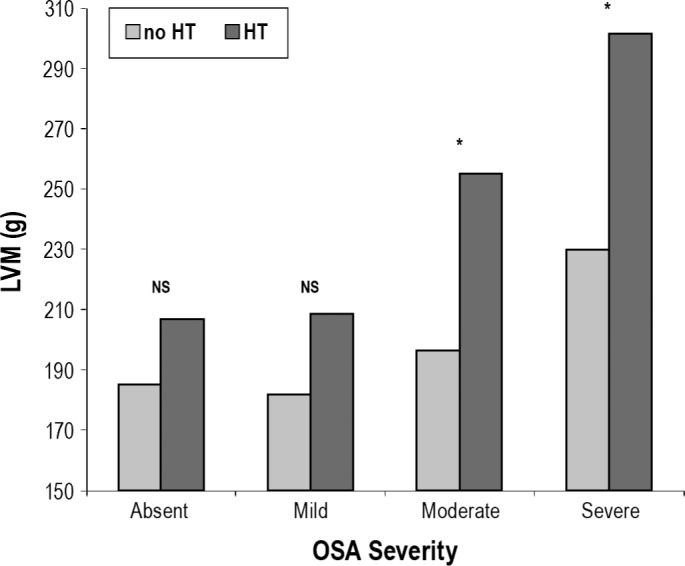
NS, non statistically significant difference; *p < 0.05.
Univariate correlation of the data showed that LVM significantly correlated with age (r = 0.24; p < 0.01), gender (r = 0.44; p < 0.01), menopausal state (r = 0.33; p < 0.01), BMI (r = 0.16; p < 0.01), percentage of fat mass (r = 0.21; p < 0.01), waist circumference (r = 0.42; p < 0.01), neck circumference (r = 0.44; p < 0.01), HT (r = 0.30; p < 0.01), DM (r = 0.35; p < 0.01), smoking habits (r = 0.15; p < 0.01), fasting plasma glucose (r = 0.19; p < 0.01), DI (r = 0.28; p < 0.01), and AHI (r = 0.41; p < 0.01). No correlations were found with the HOMA index or with fasting plasma insulin.
The correlation between LVM and AHI persisted in the multivariate regression analysis (β = 0.25; p < 0.05) after adjusting for age, gender, menopausal state, BMI, waist circumference, neck circumference, DI, fasting plasma glucose, DM, and HT. But if tobacco habits are included the statistical difference disappears (β = 0.22; p = 0.06).
Similarly, the data on LVM adjusted for height were positively associated with age (r = 0.36; p < 0.01), gender (r = 0.16; p < 0.05), menopausal state (r = 0.34; p < 0.01), BMI (r = 0.18; p < 0.05), waist circumference (r = 0.29; p < 0.01), neck circumference (r = 0.19; p < 0.05), HT (r = 0.31; p < 0.01), DM (r = 0.41; p < 0.01), fasting plasma glucose (r = 0.21; p < 0.01) DI (r = 0.22; p < 0.01), and AHI (r = 0.41, p < 0.01). However, no relationship was found with the HOMA index, fasting plasma insulin, or fat mass. The correlation between LVM/height2.7 and AHI persisted in the multivariate regression analysis (β = 0.15; p < 0.05) after adjusting for age, gender, menopausal state, BMI, waist circumference, and HT.
Influence of Anthropometrical and Metabolic Parameters on the OSA–LVH Association
The univariate analysis of the left ventricular hypertrophy data with those on the severity of OSA indicates that a clear relation between them exists (Table 3). Since OSA is dependent on several other factors, such as age, gender, menopausal state, and smoking habits, a multivariate analysis that took these variables into consideration was undertaken. The data obtained indicate that these factors significantly contributed to the outcome especially for patients that suffered moderate and severe OSA.
Table 3.
Cox proportional hazard ratios (95% CI) for the association between OSA and left ventricular hypertrophy
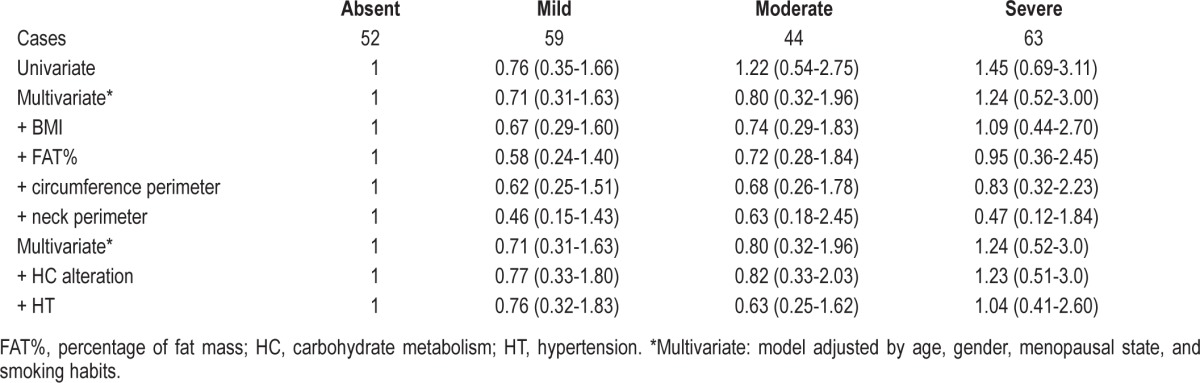
The inclusion of the BMI and fat mass data had a minor influence on the data recovered. Conversely, fat distribution played an important role, especially when neck perimeter was taken into consideration, thus confirming that blockage of the pulmonary airway is an important predisposing factor for OSA. Finally, while HT appeared to positively influence OSA severity, this relation was not noted with carbohydrate metabolism alterations, such as DM.
DISCUSSION
The correlation between OSA and alteration of myocardial function is a controversial issue. While some studies have suggested a relation between LVH, AHI and the duration of oxygen saturation periods,20–22 research carried out on obese patients with or without OSA found no differences in their LVM.23 Furthermore, the association observed between the increase in LVM and OSA was not maintained after adjusting for BMI or other associated covariates, thus suggesting that other clinical factors might influence the link between OSA and the changes in myocardial morphology. However, the results of The Sleep Heart Health Study7 showed that increases in LVM and in LVH prevalence were related to AHI, independent of age, sex, ethnicity, BMI, smoking, alcohol consumption, DM, and previous acute myocardial infarction.24
In the present study, we have addressed the relation of OSA severity with LVM increase in obese patients with BMIs over 35 kg/m2, to determine whether there was a cause/effect between both or if other cardiovascular risk factors, usually present in these patients, played a role as well. This might, in addition, promote the realization of prospective studies aiming to provide information on the possible effects of weight loss or gain on the OSA and LVM relationship.
The results obtained indicate that a high prevalence of OSA occurs in patients with morbid obesity, reaching 76.2% of the cases. A high prevalence of LVM alterations in morbidly obese patients that were affected by OSA (up to 78.6%) was observed, a result that agrees with that of a previous report where a sample of 25 obese patients (23 of which were males) was investigated.25 The frequency of LVM disturbance in women (68.1%) was significantly lower than in men, where only 9.1% of the individuals were free of the condition. The data obtained indicate that male-gender, a well-known predisposing factor for OSA in the general population, also influences its occurrence in obese patients. Nevertheless, obesity appears to increase the frequency of OSA in women as well, from about 50% found in the female population taken as a whole26 to the almost 70% found in our dataset. However, it appears that body fat distribution rather than excess total fat is responsible for the increased frequency of OSA in our patients; air displacement plethysmography indicated that men presented lower total fat figures than women but higher waist and neck circumferences, thus indicating that central fat deposition may be the real predis-posing agent for OSA pathogenesis in morbidly obese patients.
The influence of the menopausal status of the obese patients on OSA was also clear; PostM women showed a significantly higher frequency (76.0%) than PreM women (63.8%). Similar data have previously been reported,27 although in that case larger neck circumference and higher waist to hip circumference ratios were found among PostM women and, as a consequence, were taken as predisposing factors for OSA (similar to what we have just discussed for men and women). However, our female cohorts presented similar anthropometric and body composition parameters, suggesting that other factors, such as age or/and hormonal status might also be responsible for OSA prevalence in the PostM subgroup. In agreement with this assumption, the administration of hormone replacement therapy to menopausal women was associated to a lower prevalence of OSA.28
As expected, our study revealed that morbid obesity is strongly associated with cardiovascular risk factors such as hypertension and DM2, which were more prevalent in men and PostM women and showed a significant correlation with obesity and central adiposity indexes such as BMI, waist, and neck circumference, and with AHI. However, it appears that there is a direct correlation between OSA severity and LVM increase, which may be independent of the other cardiovascular risks. Furthermore, the changes in LVM were observed both in non- and hypertensive patients. However, HT induces more pronounced changes in the LVM of patients with OSA, suggesting a synergistic effect of both conditions. These data agree with those of recently published reports21,29 indicating that severe OSA impairs left ventricular function independent of age, insulin resistance or blood pressure,30 although these conditions may worsen it. LVH also represents an established cardiovascular risk factor31 particularly relevant in patients with severe OSA that exhibit an LVH prevalence of over 50% in our cohort. This relationship might be indirectly influenced by fat distribution, HT, and DM, all of which are conditions that probably influence the association between severe OSA and LVH.
It is known that insulin resistance promotes the increment of LVM and LVH through raising the extracellular volume and sodium retention32 and through hyperactivation of the reninaldosterone system.33 A direct relationship was found between HOMA and LVM alterations, which were independent of the age and other confounding factors such as HT.34 However, we could not find an association between fasting plasma insulin or HOMA index and LVM, suggesting that additional factors may be involved in the pathophysiology of LVM in patients with morbid obesity. On the other hand, the relation of fat distribution with both the degree of OSA severity and insulin resistance suggests that the latter may be considered as an important anthropometric clue when OSA is suspected and therefore, may represent an indirect marker for detecting LVM alterations in obese patients.
In contrast with a previous report,24 our study showed no differences in the ejection fraction of patients included in any of the four categories of OSA severity, suggesting that OSA was not inducing changes in their systolic function. These discrepancies may be due to the fact that patients with established cardiac disturbances were excluded from the previous report and to the age heterogeneity of our sample.
In summary, this study establishes that OSA is highly prevalent among morbidly obese patients, being especially frequent in men and PostM women. LVM alterations and LVH are also very common in these patients, and their occurrence is associated with OSA independent of other classic cardiovascular risk factors, including HT. These data suggest that OSA should be investigated and an echocardiographic study performed in all patients with morbid obesity, independent of their HT status, and especially in men and PostM women. The improvement obtained in myocardial performance following treatment with CPAP in patients with OSA,11 supports the interest of this diagnostic strategy in the prevention or treatment of myocardial dysfunction and cardiovascular risk in this group of patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed in Clinica Universidad de Navarra.
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvador J, Iriarte J, Silva C, Gomez Ambrosi J, Diez Caballero A, Fruhbeck G. [The obstructive sleep apnoea syndrome in obesity: a conspirator in the shadow] Rev Med Univ Navarra. 2004;48:55–62. [PubMed] [Google Scholar]
- 3.Akinnusi ME, Saliba R, Porhomayon J, El-Solh AA. Sleep disorders in morbid obesity. Eur J Intern Med. 2012;23:219–26. doi: 10.1016/j.ejim.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb DJ. The Sleep Heart Health Study: a progress report. Curr Opin Pulm Med. 2008;14:537–42. doi: 10.1097/MCP.0b013e328312ed61. [DOI] [PubMed] [Google Scholar]
- 8.Kurukulasuriya LR, Stas S, Lastra G, Manrique C, Sowers JR. Hypertension in obesity. Med Clin North Am. 2011;95:903–17. doi: 10.1016/j.mcna.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Hammerstingl C, Schueler R, Wiesen M, et al. Effects of untreated obstructive sleep apnea on left and right ventricular myocardial function. Int J Cardiol. 2012;155:465–9. doi: 10.1016/j.ijcard.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Colish J, Walker JR, Elmayergi N, et al. Obstructive sleep apnea: effects of continuous positive airway pressure on cardiac remodeling as assessed by cardiac biomarkers, echocardiography, and cardiac MRI. Chest. 2012;141:674–81. doi: 10.1378/chest.11-0615. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Ambrosi J, Silva C, Catalan V, et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care. 2012;35:383–8. doi: 10.2337/dc11-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caples SM, Rosen CL, Shen WK, et al. The scoring of cardiac events during sleep. J Clin Sleep Med. 2007;3:147–54. [PubMed] [Google Scholar]
- 13.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 16.Urbina EM, Gidding SS, Bao W, Pickoff AS, Berdusis K, Berenson GS. Effect of body size, ponderosity, and blood pressure on left ventricular growth in children and young adults in the Bogalusa Heart Study. Circulation. 1995;91:2400–6. doi: 10.1161/01.cir.91.9.2400. [DOI] [PubMed] [Google Scholar]
- 17.De Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–60. doi: 10.1016/0735-1097(92)90385-z. [DOI] [PubMed] [Google Scholar]
- 18.De Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62. doi: 10.1016/0735-1097(94)00540-7. [DOI] [PubMed] [Google Scholar]
- 19.Noda A, Okada T, Yasuma F, Nakashima N, Yokota M. Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest. 1995;107:1538–44. doi: 10.1378/chest.107.6.1538. [DOI] [PubMed] [Google Scholar]
- 20.Moro JA, Almenar L, Fernandez-Fabrellas E, Ponce S, Blanquer R, Salvador A. [Analysis of echocardiographic alterations observed in sleep apnea-hypopnea syndrome and how they are influenced by hypertension] Rev Esp Cardiol. 2008;61:49–57. [PubMed] [Google Scholar]
- 21.Niroumand M, Kuperstein R, Sasson Z, Hanly PJ. Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med. 2001;163:1632–6. doi: 10.1164/ajrccm.163.7.2007014. [DOI] [PubMed] [Google Scholar]
- 22.Otto ME, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298–302. doi: 10.1016/j.amjcard.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 23.Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117:2599–607. doi: 10.1161/CIRCULATIONAHA.107.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cloward TV, Walker JM, Farney RJ, Anderson JL. Left ventricular hypertrophy is a common echocardiographic abnormality in severe obstructive sleep apnea and reverses with nasal continuous positive airway pressure. Chest. 2003;124:594–601. doi: 10.1378/chest.124.2.594. [DOI] [PubMed] [Google Scholar]
- 25.Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41:610–5. doi: 10.1183/09031936.00212711. [DOI] [PubMed] [Google Scholar]
- 26.Resta O, Bonfitto P, Sabato R, De Pergola G, Barbaro MP. Prevalence of obstructive sleep apnoea in a sample of obese women: effect of menopause. Diabetes Nutr Metab. 2004;17:296–303. [PubMed] [Google Scholar]
- 27.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–92. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 28.Hedner J, Ejnell H, Caidahl K. Left ventricular hypertrophy independent of hypertension in patients with obstructive sleep apnoea. J Hypertens. 1990;8:941–6. doi: 10.1097/00004872-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Usui Y, Takata Y, Inoue Y, et al. Severe obstructive sleep apnea impairs left ventricular diastolic function in non-obese men. Sleep Med. 2013;14:155–9. doi: 10.1016/j.sleep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Gosse P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J Hypertens Suppl. 2005;23:S27–33. doi: 10.1097/01.hjh.0000165625.79933.9a. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–55. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodfriend TL, Kelley DE, Goodpaster BH, Winters SJ. Visceral obesity and insulin resistance are associated with plasma aldosterone levels in women. Obes Res. 1999;7:355–62. doi: 10.1002/j.1550-8528.1999.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 33.Sundstrom J, Arnlov J, Stolare K, Lind L. Blood pressure-independent relations of left ventricular geometry to the metabolic syndrome and insulin resistance: a population-based study. Heart. 2008;94:874–8. doi: 10.1136/hrt.2007.121020. [DOI] [PubMed] [Google Scholar]


