Abstract
Objective:
In this study we examine the temporal connection between periodic leg movements (PLMs) and cortical arousals, as well as the treatment effect of pramipexole, in a clinical case with spinal cord lesion.
Methods:
A patient with complete cervical spinal cord injury and PLMs during sleep underwent two baseline sleep recordings, one recording with dopaminergic treatment, and one recording with adaptive servoventilation.
Results:
The PLMs were temporally dissociated from cortical arousals as well as from respiratory or heart rate events. PLMs were suppressed by pramipexole and persisted after treatment of apnea.
Conclusion:
The disconnection of PLMs from arousals supports a spinal generator or peripheral trigger mechanism for PLMs. The suppression of movements by a dopamine agonist suggests that its site of action is caudal to the cervical lesion and outside of the brain. Our observation provides significant new knowledge about the pathogenesis of PLMs and warrants studies in larger populations.
Citation:
Salminen AV; Manconi M; Rimpilä V; Luoto TM; Koskinen E; Ferri R; Öhman J; Polo O. Disconnection between periodic leg movements and cortical arousals in spinal cord injury. J Clin Sleep Med 2013;9(11):1207-1209.
Keywords: Periodic leg movements, spinal cord injury, dopamine agonist, cortical arousal, sleep apnea, case report
There is no common agreement on the origin of periodic leg movements (PLMs) during sleep. PLMs are suppressed by dopaminergic therapy, which has led to a hypothesis of dopaminergic dysfunction in the brain1 or in the hypothalamo-spinal inhibitory pathways.2 PLMs are accompanied by simultaneous heart rate elevations and cortical arousals.3 However, the suppression of PLMs does not affect cortical arousals, and pharmacological reduction of cortical arousals does not affect PLMs.4 This may imply that PLMs and EEG arousals are simply synchronized with each other without a necessary causal relationship.
PLMs have also been described in spinal cord injury (SCI).5 These findings do not support the hypothesis of cerebral origin of PLMs. The movements in this subgroup of patients have not been analyzed in detail. In this study, we describe a clinical case with complete SCI in whom dopaminergic-responding PLMs are not synchronized with cortical and autonomic arousals.
REPORT OF CASE
A 35-year-old male, with a cervical SCI sustained in a car accident 17 years prior to the study, underwent 4 polysomnographic studies. The initial polysomnogram was a part of a larger project focusing on neuroimaging and respiratory functions in patients with cervical SCI. Magnetic resonance imaging confirmed the complete cervical spinal lesion between vertebrae C3 and C5. The patient had no motor or sensory function below the C5 level. The patient was treated with tizanidine (12 mg daily) and baclofen (30 mg daily) for muscle spasticity. The patient did not suffer from RLS or daytime sleepiness.
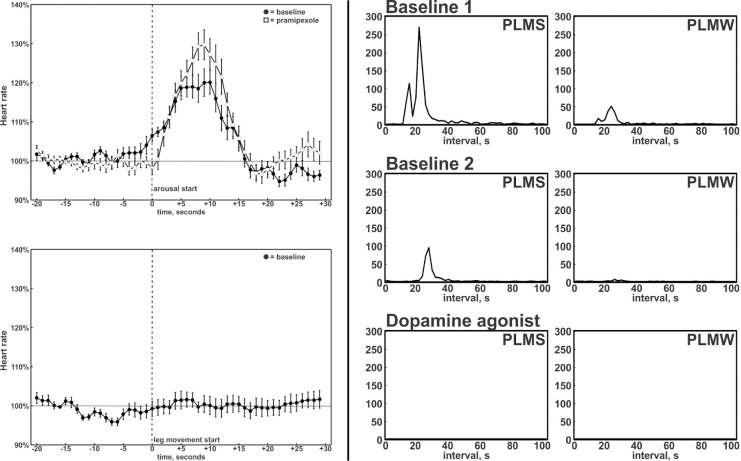
Two baseline recordings revealed excessive PLMs (PLM index 138/h and 36/h). Severe obstructive sleep apnea (apneahypopnea index 45.5/h and 74.5/h) was also discovered. The analysis of interval distributions of PLMs6 revealed a leptokurtic peak at 20-30 seconds in baseline recordings (Figure 1).
Figure 1.
Arousals resulting from events of sleep apnea were characterized by heart rate elevations (upper left panel). Heart rate elevations were not associated with PLMs (lower left panel). However, during baseline recordings, leg movements were characterized by the usual peak at 20-30 seconds in the distribution of inter-movement intervals (right panels). The peak was suppressed by pramipexole. PLMS, periodic leg movements during sleep; PLMW, periodic leg movements during wakefulness.
Cortical arousals, heart rate elevations, and respiratory events were synchronized but consistently temporally dissociated from PLMs (supplemental Figure S1). Increases in the heart rate were associated with the respiratory events, but were absent after PLMs (Figure 1). Resumption of respiration with cortical and autonomic arousals at the termination of apnea was not accompanied by PLMs.
To assess the response of the leg movements to dopamine agonists, a single test dose (0.25 mg) of pramipexole, a D3 preferential dopamine receptor agonist,7 was administered before a third recording. Pramipexole abolished PLMs completely. The sleep apnea was treated with adaptive servoventilation during a fourth night. The treatment resolved the sleep apnea, but PLMs persisted (PLM index 69.9/h).
The study was approved by the local ethics committee. Written informed consent was obtained from the patient.
DISCUSSION
Our case study indicates that PLMs may occur despite a complete lesion of the motor and sensory pathways at the level of the cervical spinal cord. However, they are not accompanied by the usual synchronous cortical events or heart rate responses. Our study also demonstrates that pramipexole is able to suppress PLMs even in the absence of a brain connection. These findings support the existence of a spinal generator or a peripheral trigger of PLMs. Suppression of PLMs by pramipexole suggests that the cerebral effects of dopamine agonists are not critical for achieving the therapeutic response.
Movements in our subject fulfill the standard criteria for PLMs. Their distribution of inter-movement intervals was typical for PLMs, and they were suppressed by pramipexole. Therefore they cannot be classified as other motor disorders frequently associated with myelopathies, such as spasticity or myoclonus.
Appearance of PLMs without synchronous cortical events in a patient with an impaired upper motor neuron suggests that the origin of the movements is outside the brain. Therefore, a spinal pacemaker or a peripheral trigger of PLMs is likely. This is supported by the absence of cardiac responses to PLMs in our subject. The findings are in line with the A11 theory of the origin of PLMs.2 Lack of supraspinal dopaminergic inhibition could result in activation of a pacemaker in the spinal cord to generate PLMs. Alternatively, a peripheral afferent stimulus triggering PLMs as a spinal reflex would also be supported by our findings.
The suppression of PLMs by pramipexole suggests that also the site of action of dopamine agonists in the suppression of PLMs is located outside of the brain. Spinal cord is one candidate for the site of action, but dopamine receptors are also present outside the nervous system.
In conclusion, PLMs may be generated independently from the brain, appear in disconnection from cortical events and from autonomic activations, and can be suppressed by a dopa-mine agonist without connection to the brain. These findings shed new light on the pathophysiology of PLMs, suggesting a spinal generator, with or without the concurrence of peripheral triggers. SCI is an interesting model for studying the mechanism of PLMs, also allowing systematic investigations in larger populations to confirm these results.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The work was performed at Tampere University Hospital and Unesta Research Centre, Tampere, Finland. The study was supported by the Tuberculosis Foundation of Tampere, Finland.
SUPPLEMENTAL MATERIAL
Temporal disconnection between leg movements and cortical arousals is demonstrated by a sample from the first baseline sleep recording
REFERENCES
- 1.Montplaisir J, Lorrain D, Godbout R. Restless legs syndrome and periodic leg movements in sleep: The primary role of dopaminergic mechanism. Eur Neurol. 1991;31:41–43. doi: 10.1159/000116643. [DOI] [PubMed] [Google Scholar]
- 2.Clemens S, Rye DB, Hochman S. Restless legs syndrome: Revisiting the dopamine hypothesis from the spinal cord perspective. Neurology. 2006;67:125–130. doi: 10.1212/01.wnl.0000223316.53428.c9. [DOI] [PubMed] [Google Scholar]
- 3.Ferri R, Zucconi M, Rundo F, Spruyt K, Manconi M, Ferini-Strambi L. Heart rate and spectral EEG changes accompanying periodic and non-periodic leg movements during sleep. Clin Neurophysiol. 2007;118:438–48. doi: 10.1016/j.clinph.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Manconi M, Ferri R, Zucconi M, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71:834–44. doi: 10.1002/ana.23565. [DOI] [PubMed] [Google Scholar]
- 5.Telles SC, Alves RS, Chadi G. Spinal cord injury as a trigger to develop periodic leg movements during sleep: An evolutionary perspective. Arq Neuropsiquiatr. 2012;70:880–4. doi: 10.1590/s0004-282x2012001100011. [DOI] [PubMed] [Google Scholar]
- 6.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 7.Manconi M, Ferri R, Zucconi M, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–7. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Temporal disconnection between leg movements and cortical arousals is demonstrated by a sample from the first baseline sleep recording