Abstract
As the population ages, more individuals with autoimmune diseases are experiencing reproductive senescence. Understanding the impact of menopause and age-related androgen decline on disease onset and course, as well as the potential for hormonal interventions, is critically important. In men, lupus erythematosis (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS) are associated with lower androgen levels. However, the impact of age-related declines in testosterone, as well as of testosterone replacement, on disease course remains underexplored. In women, the course of all three diseases with onset after the age of menopause differs from that with onset before menopause. Early age at menopause is associated with increased disease risk, and after menopause, disease course changes in SLE and RA. Less is known about MS. This article summarizes what is known about the relationship between reproductive aging and autoimmune diseases in men and women, and highlights areas for further investigation.
Keywords: Multiple sclerosis, Systemic lupus erythematosus, Rheumatoid arthritis, Menopause, Andropause, Hormone replacement therapy
1. Introduction
Studies of autoimmune disease onset and course during reproductive transitions such as puberty and pregnancy have highlighted the modulatory role of gonadal hormones. Less is known about the impact of reproductive senescence (menopause and age-related androgen decline) on autoimmune diseases. This article reviews what is known about the effect of reproductive aging, first in men and then in women, on systemic lupus erythematosis (SLE), rheumatoid arthritis (RA), and multiple sclerosis (MS). Data are obtained from a Pubmed search, until November 30 2012, without language restrictions, for all papers combining one of the terms “menopause”, “andropause”, “hormone” “estrogen” or “testosterone” with one of the terms “lupus”, “rheumatoid arthritis”, “multiple sclerosis” or “autoimmune”. Where comprehensive reviews are available, these are included rather than individual studies. The aim is to highlight relatively unexplored questions, and central avenues for further research.
2. Age-related androgen decline in men and autoimmunity
2.1. Protective effects of testosterone
Testosterone’s impact on the immune system is, on aggregate, anti-inflammatory. Specific mechanisms include: (1) lower secretion of IL-1β, IL-6, TNF, and other pro-inflammatory mediators by monocytes and macrophages; (2) increased production of anti-inflammatory IL-10 by T cells and (3) inhibition of the NF κ-mediated activation of the IL-6 gene promoter in human fibroblasts, and of T cell proliferation in animal models.
MS is a chronic demyelinating disease that in addition to an inflammatory component as is the case for SLE and RA, is also characterized by neurodegeneration. Testosterone has been shown to be not only inflammatory but also neuroprotective in animal models of MS, through mechanisms including regulation of gliosis, protection of spinal cord neurons in culture from glutamate-mediated toxicity and of cultured neurons against beta amyloid toxicityinduced cell death, induction of neuronal differentiation and neurite outgrowth, and increased cell survival in the dentate gyrus promoting hippocampal neurogenesis (Reviewed in [5]).
2.2. Hypoandrogenism and inflammatory diseases
In animal models of SLE, RA and MS, androgen deficiency following castration has resulted in a more inflammatory milieu and increased disease activity [6–9]. Treatment of castrated animals with testosterone, particularly DHT (which cannot be aromatized to estrogens, thus demonstrating androgen-specific effects) has resulted in alleviation of symptoms and inflammation [7,10–12].
In men, a higher prevalence of autoimmune diseases has been suggested in patients with hypogonadism [13]. In fact, relative androgen deficiency has been noted in some, but not all, studies of men with SL [14–23], RA [24–34] and MS [35–38] (Table 1). Limitations of some of these studies include low numbers of subjects, variable definitions of androgen deficiency, and lack of control for marked diurnal cyclicity of testosterone levels. Additionally, some failed to account for the effects of corticosteroid use, which even in low doses may suppress the hypothalamic–pituitary–testicular axis [27], as well as of disease duration and disease status; a negative correlation has been noted between androgen levels and disease activity in RA [24]. In SLE and RA, these findings are not attributable to increased renal clearance [39]. Nonetheless, altogether existing studies do point to both hypogonadotropic as well as testicular patterns of hypogonadism in men with SLE, RA and MS. It is mostly not possible to establish causality, i.e. to determine whether low testosterone levels are risk factors for autoimmunity, or rather, sequelae of chronic illness. Notably, one study of subjects with RA noted low testosterone levels very early in the course of RA, with increases observed over a 2-year period in patients who responded to disease modifying treatments [33].
Table 1.
Summary of investigations of androgen levels in male patients with SLE, RA and MS.
Only studies including 5 or more male patients are presented.
Unless noted, HC’s refers to male age-matched healthy controls.
Unless noted, specimens were drawn in the am.
| Ref | Study design | N | Age (years) |
Androgen levels | Limitations/notes |
|---|---|---|---|---|---|
| SLE | |||||
| [14] | Cross-sectional descriptive |
8 SLE cases, | 4 had elevated plasma estradiol and estrone; 1 with low T and high LH; 1 with high LH and FSH |
No mention of time of day No patients on corticosteroids |
|
| [15] | Cross-sectional descriptive |
49 SLE cases | Mean 45(20–74) |
25/49 cases with T below assay range (of these, 24 on corticosteroids, which suppress LH and only 1 of these 24 had elevated LH) |
35 cases on corticosteroids |
| 49 HCs (for E assesment only) |
36/49 with androstenedione and/or DHEAs levels below normal (of these, 31 on corticosteroids). Cases with higher mean E2 (p < 0.05); Elevated E1 in 10/49 cases vs. 0% HCS (p < 0.01); elevated E2 in 18/49 cases vs 3/49 HCs (p < 0.001). |
No mention of time of day | |||
| [16] | Case control | 10 SLE cases | Mean 39.6 (25–60) |
No differences in T or E2 between cases and HCs (and no effect of steroid use on T or E2 in cases) |
5 cases on corticosteroids |
| 10 HCs | Cases with lower T/E2 ratio (p < 0.05). Lower mean T increase after HCG stimulation in cases (p < 0.05) |
||||
| [17] | Case control | 6 SLE cases | Cases with decreased androgen and increased estrogen. |
Cases without prior steroid exposure |
|
| 4 HCs | Tendency toward an increase in aromatase activity in cases (skin and SC tissue); aromatase activity varied inversely with disease activity, and significant direct correlation with estrogen levels. |
? Time of day | |||
| [18] | Case control | 14 SLE cases 17 HCs |
At least one abnormal level (FSH, LH or T) in 43% cases, 0 controls (p < 0.01); 35% cases with functional hypoandrogenism (high LH and/or low T). Cases with higher E2/T (lower T/E2) ratio (p < 0.03). |
Excluded an additional 3 SLE cases with prior cyclophosphamide therapy or pre-puberty |
|
| [19] | Case control | 7 SLE cases |
No significant difference in basal T, FreeT. |
Did not account for steroid use |
|
| 10 HCs | Significantly lower DHEAS androstenedione and E2; higher FSH levels; as well as E2 Impaired testicular (FreeT) response after HCG stimulation |
||||
| [20] | Case control | 16 SLE cases 20 HCs |
Mean 26 (19–46) |
No significant differences in FSH, LH, testosterone, oestradiol, and beta-HCG levels in cases vs. HCs Higher prolactin in cases (p = 0.012) |
All cases newly diagnosed and untreated |
| [21] | Case control | 35 SLE cases 33 HCs |
Mean40.1 (17–71) |
No difference in T, E2, PRL and E2/T ratio; Significantly higher FSH (p = 0.004) and LH (p = 0.01); 14% cases and 0% HCs with low T and high LH. Higher PRL/T ratio in cases (p = 0.04); and ratio correlated with SLEDAI |
Excluded prior cyclophosphamide, and renal failure, from study |
| [22] | Case control | 23 SLE cases | Mean 46, median45 (24–69) |
Lower mean DHEAS (1.9 nmol/L) and total T (8.9 nmol/L) levels than normal reference range. 2.5% with T levels below reference range. |
Could not disentangle effects of steroid use; However, DHEAs but not T significantly negatively associated with daily and cumu- lative corticosteroid doses Did not control for prior steroid or chemotherapy use |
| [23] | Case control | 25 SLE cases | Mean 27 (15–45) |
No difference in total T; low T levels in 24% cases vs. 0% HCs (p = 0.022); |
|
| 25 HCs | Higher mean FSH and LH levels (p <0.01) |
Also found lower penile and testicular measurements incases than HCs |
|||
| RA | |||||
| [24] | Longitudinal observational | 10 RA cases | During hospital admission for flares, significantly lower total and free T levels than in post-hospitalization follow-up (p < 0.01) |
Excluded 1 case with progressive testicular failure ? Steroids ? Time of day |
|
| [25] | Case control | 14 RA cases OA controls |
Lower T in cases than OA controls (p < 0.01) |
?Steroids ?Time of day |
|
| [25] | Case control | 8 RA cases 8 HCs |
Failure of serum T to achieve nomral levels in response to hCG stimulation, vs HC’s (p < 0.05) |
?Steroids ?Time of day |
|
| [26] | Case control | 87 RA cases 141 HC |
Cases mean 58 (SD 11) HCs mean 35 (SD 9.4) |
Significantly lower total and free T in cases vs. HCs (p < 0.001). Difference persisted after controlling for age differences. |
Samples taken from 9 am-5 pm Only excluded current cortticosteroid users |
| [27] | Case control | 12 RA cases off prednisone |
62 (38–75) |
Non-steroid users: Normal T, elevated LH and FSH (p < 0.01) vs. HCs |
No prior steroid treatment |
| 24 RA cases on 5–10 mg/d prednisone 70 HCs |
Steroid users: low T (p < 0.05), only slightly elevated LH and FSH vs. HCs; lower T, FSH and LH vs. non steroid cases |
||||
| [28,29] | Case control | 14 RA cases 8 HCs |
Lower mean T in cases (p < 0.001) | ?Steroids ?Time of day |
|
| [30] | Case control | 13 cases with new onset synovitis (<1 yr), fitting criteria for RA 32 HCs |
Lower mean free T in cases vs. HCs aged 45+ (p < 0.03) |
No prior steroid treatment | |
| Similar levels of ACTH, cortisol, DHEA, DHEA-s, and total T. |
|||||
| A steeper age-associated decline in DHEA in cases than HCs |
Morning samples | ||||
| [31] | Case control | 36 RA and eRA Cases 40s 55 HCs |
Mean Early (15–80) |
No difference in DHEAs levels | No prior steroids |
| [32] | Longitudinal observation |
18 RA cases treated with TNF antago- nists for 2Y |
Median 59 Decrease in DHEAs after 1 and (44–74) 2Y of TNF antagonists, but no changes in LH, E2 or T. |
||
| [33,34] | Cross-sectional case control; |
41 RA cases with joint symptoms b1Y |
Mean 53 | At baseline, lower mean T than HCs for cases significant for cases <50Y (p < 0.001, p = 0.004) and not for cases <50 (p = 0.06, p = 0.07); lower LH in cases <50 years (p b 0.001) |
Did not control for corticosteroid use |
| Longitudinal observation |
131 HCs | At 2 years, in clinical responders to disease modifying therapies (improved DAS28 score), significant increase in T and SHBG. LH levels were low and stable Decrease in DAS28 score during 2Y correlated with increased T (r = −0.46, p = 0.006) |
|||
|
MS [35] |
Case control | 25 MS cases (mean EDSS 5.3) |
Mean 40.8 (25–57) |
Serum T below lower limit of normal in 24% cases, 0% HCs; LH and FSH were not appropriately elevated. |
Patients were inpatients, within 4 weeks of a clinical relapse |
| 6 HCs | HC mean 34.7 |
Only 2 cases responded inappropriately to the GnRH test: 1 with delayed response, another with exaggerated response |
No discussion of steroid use | ||
| [37] | Case control | 25 MS cases, mean disease duration 6.1 years (1–26) 18 HCs |
Mean 32.3 (but mean includes F cases) |
Non-significant lower FSH in cases (p = 0.06) |
No mention of time of day No steroids in prior 2 months Positive correlation between E2 levels and MRI features: T2 lesion load (r = 0.47, p = 0.02) and T1 leion load (r = 0.43, p = 0.04). |
| [38] | Case control | 66 MS cases, mean disease duration 9.5 years |
Mean 40.6 (28–55) |
Significantly lower mean serum T, LH, FSH (p = 0.01), FAI (androgen index) (p = 0.003) and higher SHBG (p = 0.02) in cases. PRL non-significantly elevated in cases (p = 0.06). |
Morning samples |
| 48 HCs | GnRHa response inadequate in 67% cases, and lower LH and FSH levels relative to HCs (p = 0.001) |
All cases medication free for 6 months Decreased total sperm count, sperm motility and % normal sperm morphology lower in cases than HCs Pituitary- and testicular-level impairments Progressive disease with more HPT abnormalities than relapsing remitting disease |
DHEA-s = dihydroepiandrosterone sulphate E1 = estrone E2 = estradiol FSH = follicle stimulating hormone HC = healthy control (age-matched) LH = luteinizing hormone PRL = prolactin T = testosterone
2.3. Age-related androgen decline and autoimmune diseases
In humans, a gradual decline in function of the male hypothalamic–pituitary–gonadal axis occurs with age. This is marked by decreased central regulation by the hypothalamus and pituitary, as well as by decreased testicular function, including decreased number and function of testosteroneproducing Leydig cells, and decreased number and secretory function of sperm-producing testicular Sertoli cells [40]. Thus, average total testosterone levels in healthy men decrease from 617 ng/dL in men aged 25–34 years to 606 ng/dL in men aged 45–54 years, and to 471 ng/dL in men aged 75–84 years, without any abrupt cutoff [41]. Given the gradual changes that occur in men, there is likely not a clear period of symptomatic change, or “andropause”, that would be analogous to the menopausal transition in women [42,43]. Thus, while men may over time experience decreases in androgen levels, these levels may not necessarily correlate with specific symptoms such as muscle mass or libido, or with response to androgen therapies.
Epidemiologically, it has been hypothesized that the later age of onset of MS in men relative to women, and the peak presentation of SLE and RA in older men [44], may be secondary to an age-related decline in protective testosterone levels in men. Most studies summarized in Table 1 linking low androgen levels and inflammatory diseases focused on younger male populations, and hence did not explicitly assess for any effect of age-related declines in testosterone levels. To our knowledge, no studies have specifically assessed the impact of ARAD on autoimmune diseases. Furthermore, there is limited literature regarding the interaction between androgen deficiency and clinical course, in terms of either outcomes or comorbidities [45].
2.4. Limited clinical trials of androgen therapy in men with autoimmune diseases
2.4.1. SLE
2.4.1.1. Testosterone
No trials of testosterone have been reported in men with SLE.
In 34 women with mild to moderate SLE, a 150 µg testosterone patch administered for 12 weeks in a randomized, double-blind, single-centre placebo-controlled trial did not result in increased adverse events or laboratory safety parameters (e.g. full blood count, erythrocyte sedimentation rate, creatinine, liver function tests, cholesterol) but nor was it effective in affecting disease [46].
2.4.1.2. DHEA
The oral adrenal androgen DHEA has been administered in women with SLE, with three prospective studies demonstrating increases in testosterone levels after treatment, as well as decreasing disease activity [47,48] and flares [49]. Main side effects were mild acne and hirsutism. However, a Cochrane review of seven randomized controlled trials found that DHEA offered little clinical benefit in patients with mild or moderate SLE, but did yield some modest increases in health-related quality of life measures [50].
2.4.2. RA
2.4.2.1. Testosterone
Two trials of testosterone therapy have been reported in men. In an open-label study, oral testosterone undecanoate administered daily for 6 months to 7 male RA patients of mean age 57.9 years (+/−9) led to a significant increase in testosterone levels and in the number of CD8+ T cells, and to a decrease in the CD4 + (helper): CD8+ T-cell ratio and in the IgM rheumatoid factor. There was also a decrease in the number of affected joints and daily intake of NSAIDS [51]. However, in a placebo-controlled trial of 30 men aged 34−79 years with RA, randomized to receive monthly injections of testosterone enanthate 250 mg or placebo as an adjunct therapy for 9 months, there were significant rises in serum testosterone, dihydrotestosterone and estradiol in the treatment group but no effect of treatment on disease activity [52].
In women, early studies suggested that testosterone propionate treatment led to significant improvement or remission in RA, but treatment was limited by masculinizing side effects [53]. More recently, a double-blind placebo-controlled study of testosterone administration in 57 postmenopausal women revealed clinically relevant improvement in 21% patients [54]. Furthermore, testosterone therapy has been shown to increase DHEA-s levels in RA patients.
Synergistic interactions in RA between androgens and both immunosuppressive agents (cyclosporin A (CSA) [1–3], methotrexate [4]) and anti-IL-6 receptor antibody [55], including androgenizing effects, have also been explored. These associations should be confirmed, as they may provide a rationale for androgen therapy as an adjuvant to disease modifying treatments.
2.4.3. MS
2.4.3.1. Testosterone
In a pilot clinical trial of 10men with MS (age < 65 years), treatment with testosterone gel for 12 months was associated with significant improvement in cognitive performance, slowing of the rate of brain atrophy [56], reduced delayed type hypersensitivity (DTH) skin recall responses, and immunologic shift in peripheral lymphocyte composition, with fewer CD4+ T cells and more NK cells [57]).
3. Menopause and changes in endocrine and immune function
3.1. Menopause
The menopausal transition involves a much more defined and time-limited series of physiological changes associated with reproductive senescence, divided into a series of stages [58]. Operationally, menopause is defined as the final menstrual period (FMP), after which no further menses occur during a 12-month interval. The mean age at menopause in Western societies is 49–52 [59,60].
3.2. Endocrine changes at menopause
The endocrine changes associated with menopause have historically been thought to arise from ovarian follicular depletion and degradation, with compensatory hypothalam-ic and pituitary changes. As follicles become exhausted, the ability of the ovary to produce estradiol is compromised, and hence the ability of estradiol to stimulate ovulation and endometrial buildup [61]. As ovarian production of estradiol and estrone declines premenopausally, FSH production by the pituitary is stimulated and can rise or fluctuate in an attempt to drive increased estrogen production [62].
Decreases in estradiol may occur only very late perimenopausally, in the last six months before menopause ([58], Fig. 1). However, starting in the fourth decade, or early perimenopause, there is gradual decline in progesterone, leading to luteal phase defects. As estradiol and progesterone levels become more erratic, cycle length may fluctuate and even accelerate premenopausally, before the eventual spacing and cessation of cycles [61,63–66]. After menopause, the primary endogenous source of estrogen is estrone, which is synthesized in adipocytes from androstenedione (progesterone derivative), via aromatase. In contrast to the sudden fall in estradiol during menopause, the levels of total and free testosterone, as well as dehydroepiandrosterone sulfate (DHEA-s) and androstenedione appear to decline more or less steadily with age. An effect of natural menopause on circulating androgen levels has not been observed [67].
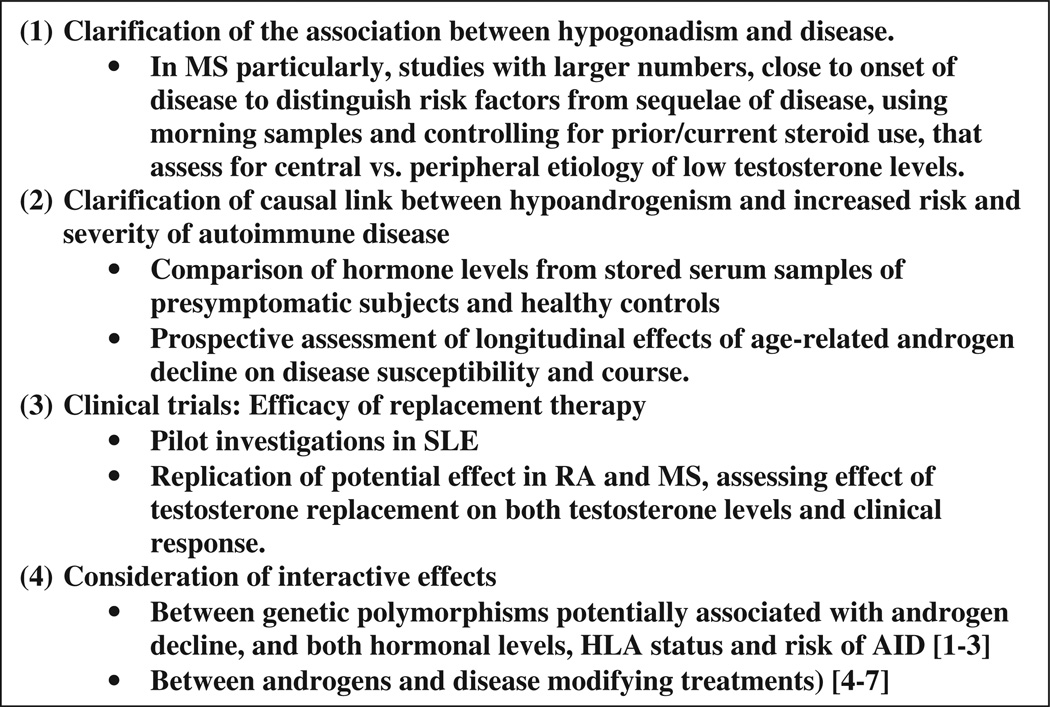
Figure 1.
Key areas required to clarify association between autoimmune diseases and male reproductive aging.
However, more recently, a more central role for hypothalamic–pituitary–gonadal axis (HPG axis) dysfunction in triggering menopause, independently of its compensatory role in response to follicular depletion, has been implicated in rodent, primate and human studies (first rodent studies [68], reviewed in [69]). There is impaired hypothalamic response in middle-aged females to the positive feedback that E2 typically exertson GnRH neurons, and on the balance between excitatory and inhibitory signals leading to the LH surge; this impaired response can lead to an LH surge induction ranging from normal to failed. In humans these changes accelerate in the years closer to menopause [70]. This aberrant HPG responsiveness likely leads to ovarian dysregulation, including luteal phase abnormalities due to aberrant E2 secretion, variable cycle length, and increasing ovarian folliculogenesis in turn accelerating follicular depletion [71,72].
Altogether then, menopause is characterized by a complex interaction between central endocrine regulation and peripheral follicular responsiveness, leading to fluctuating cycle length and E2 levels, and ultimately to lower E2 and P levels, peaking of FSH levels, follicular depletion, and irreversible cessation of menses.
3.3. Immune changes at menopause
During periods of endocrine change, such as menses and pregnancy and with exogenous use (HRT, OCP), immune changes have been observed [73–75]. The endocrine changes occurring at menopause induce changes in immune function,
in addition to those associated with immunosenescence [76]. Thus, declining levels of estrogen and DHEA sulfate may be associated with increased production of proinflammatory cytokines (IL1, IL6, TNF-alpha), and increased physiologic response to these cytokines, decreased secretion of antiinflammatory cytokines (INF-gamma), decreased lymphocyte levels (CD4+ T cells, B cells), and decreased cytotoxic activity of NK cells. Conversely, immune changes may also lead to endocrine changes associated with menopause. For example, changes in lymphocyte composition and continuous rather than cyclical secretion of cytokines may accelerate ovarian follicular atresia (See [77,78] for review). Postmenopausal hormone therapy may mitigate these changes in the cellular immune response [79–81].
3.4. Associations between menopause and autoimmune diseases
Investigations into the association between menopause and autoimmune disease onset and course have centered around four main areas of inquiry: (1) effect of age at menopause on disease onset and course; (2) subject and disease characteristics in individuals with “late onset”, typically after 50; (3) impact of menopause on disease course and (4) modulatory effects of HRTs (Table 2).
Table 2.
Menopause and autoimmunity.
| SLE | RA | MS | |
|---|---|---|---|
| Age at menopause | Nurses Health Study: Increased risk of SLE with earlier menopause, esp surgical [82] |
Increased risk of RA with earlier menopause, <45Y vs. <51Y [100]. Menopause <45Y vs. <45Y: increased risk of seronegative RA, and trend towards increased risk of seropositive RA [101]. Earlier menopause associated with milder disease course [102]. |
MS onset typically in 3rd-5th decades |
| Late onset disease | 16% patients with onset <50Y F:M ratio: lower (3.2:1 vs. 13.3:1) |
Late Onset RA typically <60Y F:M ratio: lower (1:1 vs. 3.7:1 in individuals younger than 30 [99]) |
3–12% individuals with onset <50Y F:M ratio: lower (1.9:1 vs. 2.8:1) |
| More insidious presentation | More acute onset | Disease type at onset: less frequently relapsing remitting (80% vs. 95% for females) and more frequently primary progressive |
|
| Symptoms: lower incidence nephritis, malar rash and photosensitivity Serology: lower incidence anti ds DNA and anti-Ro antibodies [83–85]. |
Symptoms: Greater disease activity and functional decline, more systemic manifestations, proximal large joint involvement, similarity with polymyalgia rheumatica [103–105]. |
Symptoms: more motor and coordination symptoms, fewer visual symptoms. Shorter time to progression to EDSS 6. |
|
| Menopausal | Decreased frequency of flares after menopause | A cohort study following individuals early in disease | Unknown |
| Transition | Decrease SLEDAI Greater damage accrual in affected organs from individual flares |
course over 6 years found higher Radiographic Joint Damage scores and higher physical disability scores as reported in a Health Assessment Questionnaire in postmenopausal women than in premenopausal women or in male subjects [106]. |
|
| Hormone | Disease risk: increased risk of SLE in NHS [82,92,93] as well as others [82,92,93], but this may be biased by misattribution of SLE symptoms to menopause, prompting HRT use. |
Disease risk: WHI: no significant reduction in RA risk [107]. |
Unknown |
| Replacement Therapies |
Disease course: SELENA trial: no increase in severe, and a modest increase in mild-moderate flares in women taking HRT [94]. |
Disease course: WHI: non-significant improvement in joint pain scores [107], additional studies have yielded no significant association (e.g.[109]). |
|
| Disease sequelae: Increased risk of venous thrombosis or thromboembolism [94,97]. Protective effects on bone density in one small study [98]. |
Disease sequelae: Protective effects on bone density [110–113]. |
3.4.1. SLE
3.4.1.1. Age at menopause
In the Nurses Health Study I and II, two landmark longitudinal epidemiological studies following 121,700 female registered nurses since 1976 (NHS I) and 116,000 female nurses since 1989 (NHS II) to assess risk factors for major chronic diseases in women, an early age at menopause, especially surgical, was associated with an increased risk of developing SLE [82].
3.4.1.2. Age of disease onset
Approximately 16% patients experience disease onset after the age of 50 [83]. In this group with later onset, the F:M ratio is usually lower than in individuals with earlier onset [84] (3:1 vs. 13:1 [85]). The median time to diagnosis is also longer than in subjects in addition to those associated with earlier presentation (5 years vs. 3 years), and there is a lower incidence of high titer anti-ds DNA and anti-Ro antibodies [83–85]. Finally, in subjects with later onset there may be a different distribution of specific symptoms; for example, a lower incidence of nephritis, malar rash, and photosensitivity has been observed. Women undergoing hysterectomy before disease onset were noted to have similar characteristics as individuals with onset of disease after age 50, suggesting a role for gonadal hormones in these observed differences [86].
3.4.1.3. Menopausal transition
Several longitudinal studies following female subjects through the menopausal transition have noted decreased frequency of flares after menopause, modestly decreased SLE Disease Activity Index (SLEDAI), but greater damage accrual in affected organs from individual flares in the postmenopausal period [87–90]. Cohort studies comparing pre- and post-menopausal subjects yielded similar findings [89]. It should be noted that the disease trajectories in these subjects were not compared with those of age-matched males to control for the effect of advancing age. Fewer flares were also observed among younger women experiencing cyclophosphamide-induced ovarian failure than age-matched controls, suggesting a causative role for estrogen decline associated with menopause and not just age per se [91].
3.4.1.4. HRT
3.4.1.4.1. Disease risk
An increased risk of developing SLE has been associated with HRT use for 2 or more years in the Nurses Health Studies [82,92,93] as well as others [82,92,93], but this may be biased by misattribution of SLE symptoms to menopause, hence prompting HRT use.
3.4.1.4.2. Disease course
While there have been case reports of worsening flares after initiation of HRT, the randomized prospective placebo-controlled multicenter SELENA (Safety of Estrogens in Lupus Erythematosus-National Assessment) trial of HRT revealed no increase in severe, and a modest increase in mild-moderate flares in women taking combined estrogen-progestin replacement [94]. In contrast to the SELENA, other studies did not sub-stratify flares by disease severity, and found no increased rate of flares with HRT relative to placebo [95,96]. These results cannot be generalized to women with certain severe co-morbidities, such as severe disease activity at baseline, ischemic heart disease, prior history of thrombosis, or positive antiphospholipid antibody and lupus anticoagulant.
3.4.1.4.3. Disease sequelae
Increased risk of venous thrombosis or thromboembolism was noted in one large randomized controlled trial [97], but risk did not reach significance in SELENA (which excluded women with prior thrombosis and/or history of anticardiolipin antibody) [94]. Bone mineral density was better preserved in women with SLE taking 50 mg transdermal 17beta-estradiol than in women taking placebo, in one small study (N = 32, p < 0.005) [98].
3.4.2. RA
3.4.2.1. Age at menopause
It has been estimated that an average woman develops RA at the time of her menopause [99]. Later age at menopause has been associated with a decreased risk of RA (adjusted risk ratio of 0.64 with menopause after age 51 years vs. prior to age 45 [100]). This is true both for seronegative, and perhaps seropositive, RA [101]. These findings suggest that exposure to estrogen may be protective against the onset of disease. Interestingly, earlier age at menopause has also been associated with a greater proportion of patients presenting with a milder disease course [102], pointing to more complex interactions between hormone levels and disease subtypes.
3.4.2.2. Age of disease onset
Because median age of first symptoms of RA occurs later than in other inflammatory diseases (45 in women and 50 in men), late onset disease is usually considered after age 60. Late Onset RA is associated with a F:M ratio of 1 (vs. 3.7:1 in individuals younger than 30 [99]), as well as with higher frequency of acute onset, greater disease activity and functional decline, more systemic manifestations, proximal large joint involvement, similarity with polymyalgia rheumatica [103–105].
3.4.2.3. Menopausal transition
A cohort study following individuals early in disease course over 6 years found higher Radiographic Joint Damage s vcores and higher physical disability scores as reported in a Health Assessment Questionnaire in postmenopausal women than in premenopausal women or in male subjects [106].
3.4.2.4. HRT
3.4.2.4.1. Disease risk
The Women's Health Initiative (WHI), a landmark longitudinal study that included randomized controlled trials evaluating the effects of (1) unopposed estrogen and (2) estrogen plus progestin compared with placebo on a diverse set of health outcomes, noted no significant reduction in the risk of RA [107].
3.4.2.4.2. Disease course
While previous studies suggested decreased both risk of RA and disease activity with HRT [108], The Women's Health Initiative (WHI) noted only a non-significant improvement in joint pain scores among HRT users [107], and additional studies have yielded no significant association (e.g.[109]).
3.4.2.4.3. Disease sequelae
HRT has protective effects on bone density in women with RA [110–113].
3.4.3. MS
Less is known about the association between menopause and MS.
3.4.3.1. Age at menopause
The peak age of MS onset in women is 24 [114], i.e. before the menopausal transition.
3.4.3.2. Age of disease onset
In subjects with disease onset after age 50, F:M ratios are lower (1.9:1 vs. 2.8:1), disease type at onset is less frequently relapsing remitting (80% vs. 95% for females) and more frequently primary progressive, course is more rapidly progressive with fewer relapses or new gadolinium enhancing lesions, and symptoms more often involve motor and coordination symptoms and less often visual symptoms, than in individuals with disease onset between ages 18–49 [115–120]
Further, time to progression to an Expanded Disability Severity Scale (EDSS) of 6 (Intermittent or unilateral constant assistance (cane, crutch, brace) required to walk about 100 meters with or without resting) in women with late onset MS is more rapid than in women with earlier onset MS, and in contrast to the latter group, is as fast as that of men.
3.4.3.3. Menopausal
transition. In MS, advanced age is associated with worsening disability and accelerated conversion to progressive forms [116,117,121]. There are no objective data regarding the effect of menopause on MS disease outcomes. Menopause has been associated with patient-reported worsening of symptoms in 40–54% of women in two small studies [122,123] but not in a third study [124].
3.4.3.4. HRT
No objective data exist on the impact of HRTs on disease course. Patient reports differ vastly in the reported effectiveness of HRT, from limited utility to improvement in 75% patients [122–124].
3.5. Potential mechanisms
While epidemiologic data summarized in the previous sections point to an association between estrogen decline and disease onset or activity, the pathophysiological mechanisms are likely to be complex. Typically, high levels of estrogens (such as seen during pregnancy) are felt to shift T helper cell ratios from 1 to 2, leading to improved disease activity in RA but worsened in SLE. It would follow that the low levels of estrogens postmenopausally would lead to a decrease in the progression and severity of disease activity in SLE and worsened activity in RA, which has been observed.
However as summarized above, early age at menopause seems to increase risk of both SLE and RA. Furthermore, F:M ratios in later onset disease are decreased for SLE, RA and MS, relative to sex ratios in earlier-onset disease.
This points to complex interactions between estrogen levels and inflammation, and to immune changes regulated not only by changing estrogen levels but also by changes in androgens, progesterone, and estrogen/androgen ratios. Furthermore, some effects are likely mediated by hormonal changes on end organs affected by AIDs. Finally, in MS, decreasing estrogen levels postmenopausally are likely to affect not only inflammation but also neurodegeneration, yielding varying aggregate effects on disease course.
3.6. Non-immunological associations between menopause and autoimmune diseases
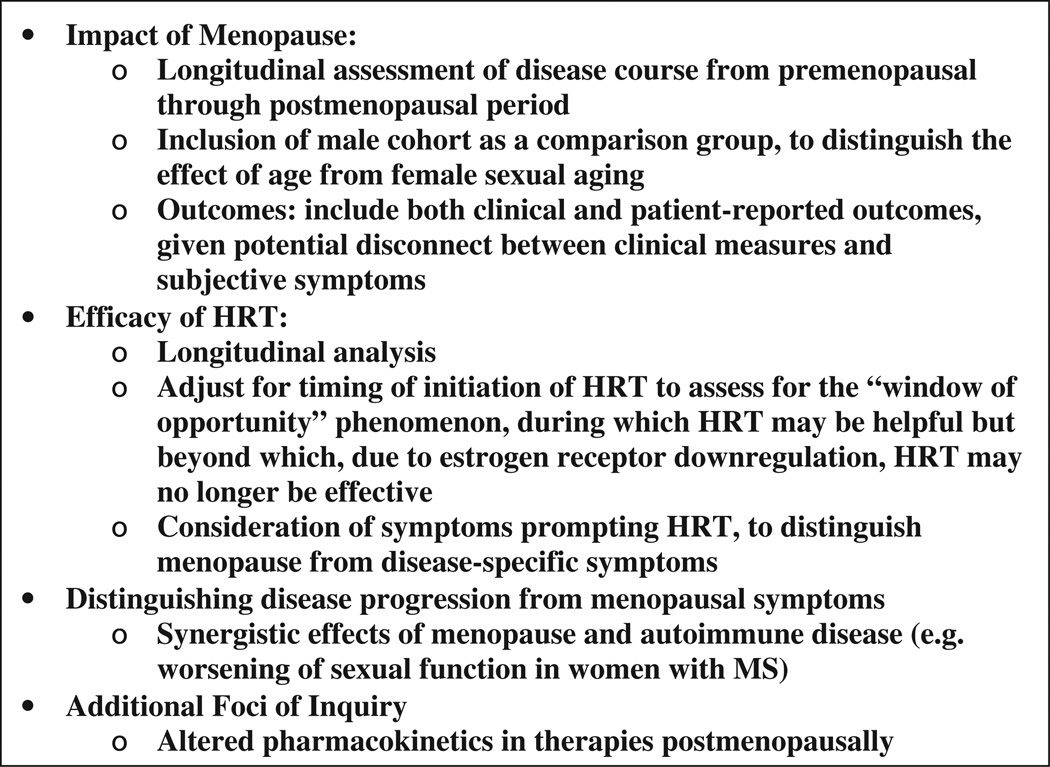
Other important aspects of menopause in autoimmune disease include but are not limited to: impact of inflammatory disease and its treatments (e.g. cyclophosphamide) on premature ovarian failure and age of menopause; additive effects of estrogen decline and inflammatory disease on other systems, e.g. cardiovascular; and exacerbation of postmenopausal bone loss in patients subjected to long-term glucocorticoid use (reviewed in [125]) (Fig. 2).
Figure 2.
Key areas required to clarify association between autoimmune diseases and female reproductive aging.
4. Conclusion
In summary, the endocrine changes occurring during the menopausal transition are associated with changes in disease risk, onset and course in women with SLE and RA. The effects of HRT on these outcomes, if any, are likely modest. In women with MS, the potential impact of menopause on disease course is unknown, and may be more complex given that both the inflammatory and neurodegenerative aspects of MS are under estrogenic regulation. Longitudinal assessment of disease course and of HRT effects through the menopausal transition and into the later postmenopausal years, is required. Comparison should be performed with men, to control for changes attributable to age itself. For MS, SLE and RA, exploring the synergistic effects of menopause and diseasespecific pathophysiology on function is important in evaluating the potential impact of HRT. In men, more longitudinal investigations are needed to determine a potential causal link between age-related hypoandrogenism and disease risk and progression, as well as the potential for intervention with androgen therapies.
Understanding this complex interplay between reproductive senescence and the course of autoimmune diseases is of critical importance to address the needs of, and minimize the progression of disability in, affected patients in our aging population.
Footnotes
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
References
- 1.Cutolo M, Sulli A, Giusti M, Barone A, Seriolo B, Accardo S. Increase in serum 5 alpha-androstane-3 alpha,17 beta-diol glucuronide as a possible marker of the androgen-mediated immunosuppressive activity exerted by cyclosporin A: preliminary results. Clin. Exp. Rheumatol. 1994;12:350–351. [PubMed] [Google Scholar]
- 2.Sulli A, Pizzorni C, Scotto-Busato R, Accardo S, Cutolo M. Androgenizing effects of cyclosporin A in rheumatoid arthritis. Ann. N. Y. Acad. Sci. 1999;876:391–396. doi: 10.1111/j.1749-6632.1999.tb07663.x. [DOI] [PubMed] [Google Scholar]
- 3.Giltay EJ, van den Borne BE, van Schaardenburg D, Gooren LJ, Popp-Snijders C, Blankenstein MA, Dijkmans BA. Androgenizing effects of low-dose cyclosporin in male patients with early RA. Br. J. Rheumatol. 1998;37:470–472. doi: 10.1093/rheumatology/37.4.470. [DOI] [PubMed] [Google Scholar]
- 4.Cutolo M, Sulli A, Craviotto C, Felli L, Pizzorni C, Seriolo B, Villaggio B. Antiproliferative-antiinflammatory effects of meth-otrexate and sex hormones on cultured differentiating myeloid monocytic cells (THP-1) Ann. N. Y. Acad. Sci. 2002;966:232–237. doi: 10.1111/j.1749-6632.2002.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 5.Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog. Brain Res. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganesan K, Balachandran C, Manohar BM, Puvanakrishnan R. Comparative studies on the interplay of testosterone estrogen and progesterone in collagen induced arthritis in rats. Bone. 2008;43:758–765. doi: 10.1016/j.bone.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Panchanathan R, Shen H, Bupp MG, Gould KA, Choubey D. Female male sex hormones differentially regulate expression of Ifi202, an interferon-inducible lupus susceptibility gene within the Nba2 interval. J. Immunol. 2009;183:7031–7038. doi: 10.4049/jimmunol.0802665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bebo BF, Jr, Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. Gonadal hormones influence the immune response to PLP 139–151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1998;84:122–130. doi: 10.1016/s0165-5728(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 9.Fillmore PD, Blankenhorn EP, Zachary JF, Teuscher C. Adult gonadal hormones selectively regulate sexually dimorphic quantitative traits observed in experimental allergic encephalomyelitis. Am. J. Pathol. 2004;164:167–175. doi: 10.1016/S0002-9440(10)63107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganesan K, Selvam R, Abhirami R, Raju KV, Manohar BM, Puvanakrishnan R. Gender differences and protective effects of testosterone in collagen induced arthritis in rats. Rheumatol. Int. 2008;28:345–353. doi: 10.1007/s00296-007-0446-y. [DOI] [PubMed] [Google Scholar]
- 11.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Maccio DR, Calfa G, Roth GA. Oral testosterone in male rats and the development of experimental autoimmune encephalo-myelitis. Neuroimmunomodulation. 2005;12:246–254. doi: 10.1159/000085656. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Balderas FJ, Tapia-Serrano R, Fonseca ME, Arellano J, Beltran A, Yanez P, Camargo-Coronel A, Fraga A. High frequency of association of rheumatic/autoimmune diseases and untreated male hypogonadism with severe testicular dysfunction. Arthritis Res. 2001;3:362–367. doi: 10.1186/ar328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inman RD, Jovanovic L, Markenson JA, Longcope C, Dawood MY, Lockshin MD. Systemic lupus erythematosus in men. Genetic and endocrine features. Arch. Intern. Med. 1982;142:1813–1815. [PubMed] [Google Scholar]
- 15.Miller MH, Urowitz MB, Gladman DD, Killinger DW. Systemic lupus erythematosus in males. Medicine (Baltimore) 1983;62:327–334. doi: 10.1097/00005792-198309000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Carrabba M, Giovine C, Chevallard M, Angelini M, Ambrosi B, Travaglini P. Abnormalities of sex hormones in men with systemic lupus erythematosus. Clin. Rheumatol. 1985;4:420–425. doi: 10.1007/BF02031894. [DOI] [PubMed] [Google Scholar]
- 17.Folomeev M, Dougados M, Beaune J, Kouyoumdjian JC, Nahoul K, Amor B, Alekberova Z. Plasma sex hormones and aromatase activity in tissues of patients with systemic lupus erythematosus. Lupus. 1992;1:191–195. doi: 10.1177/096120339200100312. [DOI] [PubMed] [Google Scholar]
- 18.Sequeira JF, Keser G, Greenstein B, Wheeler MJ, Duarte PC, Khamashta MA, Hughes GR. Systemic lupus erythematosus: sex hormones in male patients. Lupus. 1993;2:315–317. doi: 10.1177/096120339300200507. [DOI] [PubMed] [Google Scholar]
- 19.Vilarinho ST, Costallat LT. Evaluation of the hypothalamic- pituitary-gonadal axis in males with systemic lupus erythematosus. J. Rheumatol. 1998;25:1097–1103. [PubMed] [Google Scholar]
- 20.Chang DM, Chang CC, Kuo SY, Chu SJ, Chang ML. Hormonal profiles and immunological studies of male lupus in Taiwan. Clin. Rheumatol. 1999;18:158–162. doi: 10.1007/s100670050075. [DOI] [PubMed] [Google Scholar]
- 21.Mok CC, Lau CS. Profile of sex hormones in male patients with systemic lupus erythematosus. Lupus. 2000;9:252–257. doi: 10.1191/096120300680198926. [DOI] [PubMed] [Google Scholar]
- 22.Bhattoa HP, Kiss E, Bettembuk P, Balogh A. Bone mineral density, biochemical markers of bone turnover, and hormonal status in men with systemic lupus erythematosus. Rheumatol. Int. 2001;21:97–102. doi: 10.1007/s00296-001-0149-8. [DOI] [PubMed] [Google Scholar]
- 23.Vecchi AP, Borba EF, Bonfa E, Cocuzza M, Pieri P, Kim CA, Silva CA. Penile anthropometry in systemic lupus erythematosus patients. Lupus. 2011;20:512–518. doi: 10.1177/0961203310384121. [DOI] [PubMed] [Google Scholar]
- 24.Gordon D, Beastall GH, Thomson JA, Sturrock RD. Prolonged hypogonadism in male patients with rheumatoid arthritis during flares in disease activity. Br. J. Rheumatol. 1988;27:440–444. doi: 10.1093/rheumatology/27.6.440. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo M, Balleari E, Giusti M, Monachesi M, Accardo S. Sex hormone status of male patients with rheumatoid arthritis: evidence of low serum concentrations of testosterone at baseline and after human chorionic gonadotropin stimulation. Arthritis Rheum. 1988;31:1314–1317. doi: 10.1002/art.1780311015. [DOI] [PubMed] [Google Scholar]
- 26.Spector TD, Ollier W, Perry LA, Silman AJ, Thompson PW, Edwards A. Free serum testosterone levels in 276 males: a comparative study of rheumatoid arthritis, ankylosing spondy-litis and healthy controls. Clin. Rheumatol. 1989;8:37–41. doi: 10.1007/BF02031066. [DOI] [PubMed] [Google Scholar]
- 27.Martens HF, Sheets PK, Tenover JS, Dugowson CE, Bremner WJ, Starkebaum G. Decreased testosterone levels in men with rheumatoid arthritis: effect of low dose prednisone therapy. J. Rheumatol. 1994;21:1427–1431. [PubMed] [Google Scholar]
- 28.Masi AT. Sex hormones and rheumatoid arthritis: cause or effect relationships in a complex pathophysiology? Clin. Exp. Rheumatol. 1995;13:227–240. [PubMed] [Google Scholar]
- 29.Masi AT, Feigenbaum SL, Chatterton RT. Hormonal and pregnancy relationships to rheumatoid arthritis: convergent effects with immunologic and microvascular systems. Semin. Arthritis Rheum. 1995;25:1–27. doi: 10.1016/s0049-0172(95)80014-x. [DOI] [PubMed] [Google Scholar]
- 30.Kanik KS, Chrousos GP, Schumacher HR, Crane ML, Yarboro CH, Wilder RL. Adrenocorticotropin glucocorti-coid and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J. Clin. Endocrinol. Metab. 2000;85:1461–1466. doi: 10.1210/jcem.85.4.6534. [DOI] [PubMed] [Google Scholar]
- 31.Straub RH, Paimela L, Peltomaa R, Scholmerich J, Leirisalo-Repo M. Inadequately low serum levels of steroid hormones in relation to interleukin-6 and tumor necrosis factor in untreated patients with early rheumatoid arthritis and reactive arthritis. Arthritis Rheum. 2002;46:654–662. doi: 10.1002/art.10177. [DOI] [PubMed] [Google Scholar]
- 32.Ernestam S, Hafstrom I, Werner S, Carlstrom K, Tengstrand B. Increased DHEAS levels in patients with rheumatoid arthritis after treatment with tumor necrosis factor antagonists: evidence for improved adrenal function. J. Rheumatol. 2007;34:1451–1458. [PubMed] [Google Scholar]
- 33.Tengstrand B, Carlstrom K, Hafstrom I. Gonadal hormones in men with rheumatoid arthritis- from onset through 2 years. J. Rheumatol. 2009;36:887–892. doi: 10.3899/jrheum.080558. [DOI] [PubMed] [Google Scholar]
- 34.Tengstrand B, Carlstrom K, Hafstrom I. Bioavailable testosterone in men with rheumatoid arthritis-high frequency of hypogonadism. Rheumatology. 2002;41:285–289. doi: 10.1093/rheumatology/41.3.285. [DOI] [PubMed] [Google Scholar]
- 35.Wei T, Lightman SL. The neuroendocrine axis in patients with multiple sclerosis. Brain. 1997;120(Pt 6):1067–1076. doi: 10.1093/brain/120.6.1067. [DOI] [PubMed] [Google Scholar]
- 36.Foster SC, Daniels C, Bourdette DN, Bebo BF., Jr Dysregulation of the hypothalamic-pituitary-gonadal axis in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Neuroimmunol. 2003;140:78–87. doi: 10.1016/s0165-5728(03)00177-2. [DOI] [PubMed] [Google Scholar]
- 37.Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, Nicoletti F, Pozzilli C. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J. Neurol. Neurosurg. Psychiatry. 2005;76:272–275. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Safarinejad MR. Evaluation of endocrine profile hypothalamic- pituitary-testis axis and semen quality in multiple sclerosis. J. Neuroendocrinol. 2008;20:1368–1375. doi: 10.1111/j.1365-2826.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 39.Straub RH, Weidler C, Demmel B, Herrmann M, Kees F, Schmidt M, Scholmerich J, Schedel J. Renal clearance and daily excretion of cortisol and adrenal androgens in patients with rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 2004;63:961–968. doi: 10.1136/ard.2003.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pines A. Male menopause: is it a real clinical syndrome? Climacteric. 14(2011):15–17. doi: 10.3109/13697137.2010.507442. [DOI] [PubMed] [Google Scholar]
- 41.Vermeulen A. Declining androgens with age: an overview. In: Vermeulen A, Oddens BJ, editors. New York: Androgens and the aging male, Parthenon Publishing; 1996. pp. 3–14. [Google Scholar]
- 42.Gould DC, Petty R, Jacobs HS. For and against: the male menopause—does it exist. BMJ. 2000;320:858–861. doi: 10.1136/bmj.320.7238.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins ES. The medicalisation of male menopausein America. Soc. Hist. Med. 2007;20:369–388. doi: 10.1093/shm/hkm039. [DOI] [PubMed] [Google Scholar]
- 44.Tengstrand B, Ahlmen M, Hafstrom I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J. Rheumatol. 2004;31:214–222. [PubMed] [Google Scholar]
- 45.Tengstrand B, Hafstrom I. Bone mineral density in men with rheumatoid arthritis is associated with erosive disease and sulfasalazine treatment but not with sex hormones. J. Rheumatol. 2002;29:2299–2305. [PubMed] [Google Scholar]
- 46.Gordon C, Wallace DJ, Shinada S, Kalunian KC, Forbess L, Braunstein GD, Weisman MH. Testosterone patches in the management of patients with mild/moderate systemic lupus erythematosus. Rheumatology. 2008;47:334–338. doi: 10.1093/rheumatology/kem342. [DOI] [PubMed] [Google Scholar]
- 47.Petri MA, Mease PJ, Merrill JT, Lahita RG, Iannini MJ, Yocum DE, Ginzler EM, Katz RS, Gluck OS, Genovese MC, Van Vollenhoven R, Kalunian KC, Manzi S, Greenwald MW, Buyon JP, Olsen NJ, Schiff MH, Kavanaugh AF, Caldwell JR, Ramsey-Goldman R, St Clair EW, Goldman AL, Egan RM, Polisson RP, Moder KG, Rothfield NF, Spencer RT, Hobbs K, Fessler BJ, Calabrese LH, Moreland LW, Cohen SB, Quarles BJ, Strand V, Gurwith M, Schwartz KE. Effects of prasterone on disease activity and symptoms in women with active systemic lupus erythematosus. Arthritis Rheum. 2004;50:2858–2868. doi: 10.1002/art.20427. [DOI] [PubMed] [Google Scholar]
- 48.van Vollenhoven RF, Morabito LM, Engleman EG, McGuire JL. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. J. Rheumatol. 1998;25:285–289. [PubMed] [Google Scholar]
- 49.Chang DM, Lan JL, Lin HY, Luo SF. Dehydroepiandros-terone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind placebo-controlled trial. Arthritis Rheum. 2002;46:2924–2927. doi: 10.1002/art.10615. [DOI] [PubMed] [Google Scholar]
- 50.Crosbie D, Black C, McIntyre L, Royle PL. Thomas S Dehydroepiandrosterone for systemic lupus erythematosus, Cochrane Database Syst. Rev. 2007 doi: 10.1002/14651858.CD005114.pub2. 0CD005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cutolo M, Balleari E, Giusti M, Intra E, Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1–5. doi: 10.1002/art.1780340102. [DOI] [PubMed] [Google Scholar]
- 52.Hall GM, Larbre JP, Spector TD, Perry LA, Da Silva JA. A randomized trial of testosterone therapy in males with rheumatoid arthritis. Br. J. Rheumatol. 1996;35:568–573. doi: 10.1093/rheumatology/35.6.568. [DOI] [PubMed] [Google Scholar]
- 53.Margolis HM, Caplan PS. The effect of some steroids (testosterone propionate desoxycorticosterone acetate ascorbic acid, and 21-acetoxy delta-5-pregnenolone, artisone acetate Wyeth) in rheumatoid arthritis. Ann. Intern. Med. 1951;34:61–71. doi: 10.7326/0003-4819-34-1-61. [DOI] [PubMed] [Google Scholar]
- 54.Booji A, Biewenga-Booji CM, Huber-Bruning O, Cornelis C, Jacobs JW, Bijlsma JW. Androgens as adjuvant treatment in postmenopausal female patients with rheumatoid arthritis. Ann. Rheum. Dis. 1996;55:811–815. doi: 10.1136/ard.55.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straub RH, Harle P, Yamana S, Matsuda T, Takasugi K, Kishimoto T, Nishimoto N. Anti-interleukin-6 receptor antibody therapy favors adrenal androgen secretion in patients with rheumatoid arthritis: a randomized double-blind placebo-controlled study. Arthritis Rheum. 2006;54:1778–1785. doi: 10.1002/art.21826. [DOI] [PubMed] [Google Scholar]
- 56.Sicotte NL, Giesser BS, Tandon V, Klutch R, Steiner B, Drain AE, Shattuck DW, Hull L, Wang HJ, Elashoff RM, Swerdloff RS, Voskuhl RR. Testosterone treatment in multiple sclerosis: a pilot study. Arch. Neurol. 2007;64:683–688. doi: 10.1001/archneur.64.5.683. [DOI] [PubMed] [Google Scholar]
- 57.Gold SM, Chalifoux S, Giesser BS, Voskuhl RR. Immune modulation and increased neurotrophic factor production in multiple sclerosis patients treated with testosterone. J. Neuroinflammation. 2008;5:32. doi: 10.1186/1742-2094-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012;97:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J. Clin. Epidemiol. 1998;51:1271–1276. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 60.Jacobsen BK, Heuch I, Kvale G. Age atnatural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am. J. Epidemiol. 2003;157:923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 61.O’Connor KA, Holman DJ, Wood JW. Declining fecundity and ovarian ageing in natural fertility populations. Maturitas. 1998;30:127–136. doi: 10.1016/s0378-5122(98)00068-1. [DOI] [PubMed] [Google Scholar]
- 62.Burger HG. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur. J. Endocrinol. 1994;130:38–42. doi: 10.1530/eje.0.1300038. [DOI] [PubMed] [Google Scholar]
- 63.Ferrell RJ, O’Connor KA, Rodriguez G, Gorrindo T, Holman DJ, Brindle E, Miller RC, Schechter DE, Korshalla L, Simon JA, Mansfield PK, Wood JW, Weinstein M. Monitoring reproductive aging in a 5-year prospective study: aggregate and individual changes in steroid hormones and menstrual cycle lengths with age. Menopause. 2005;12:567–577. doi: 10.1097/01.gme.0000172265.40196.86. [DOI] [PubMed] [Google Scholar]
- 64.Ferrell RJ, Simon JA, Pincus SM, Rodriguez G, O’Connor KA, Holman DJ, Weinstein M. The length of peri-menopausal menstrual cycles increases later and to a greater degree than previously reported. Fertil. Steril. 2006;86:619–624. doi: 10.1016/j.fertnstert.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 65.O’Connor KA, Ferrell RJ, Brindle E, Shofer J, Holman DJ, Miller RC, Schechter DE, Singer B, Weinstein M. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol. Biomark. Prev. 2009;18:828–836. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Connor KA, Ferrell R, Brindle E, Trumble B, Shofer J, Holman DJ, Weinstein M. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16:1178–1187. doi: 10.1097/gme.0b013e3181aa192d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 68.Aschheim P. Results provided by heterochronic grafts of the ovaries in the study of the hypothalamo-hypophyso-ovarian regulation of senile rats. Gerontologia. 1964;10:65–75. [PubMed] [Google Scholar]
- 69.Neal-Perry G, Nejat E, Dicken C. The neuroendocrine physiology of female reproductive aging: an update. Maturitas. 2010;67:34–38. doi: 10.1016/j.maturitas.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw ND, Srouji SS, Histed SN, McCurnin KE, Hall JE. Aging attenuates the pituitary response to gonadotropin-releasing hormone. J. Clin. Endocrinol. Metab. 2009;94:3259–3264. doi: 10.1210/jc.2009-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hale GE, Hughes CL, Burger HG, Robertson DM, Fraser IS. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50–59. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 72.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J. Clin. Endocrinol. Metab. 1996;81:1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 73.Faas M, Bouman A, Moesa H, Heineman MJ, de Leij L, Schuiling G. The immune response during the luteal phase of the ovarian cycle: a Th2-type response. Fertil. Steril. 2000;74:1008–1013. doi: 10.1016/s0015-0282(00)01553-3. [DOI] [PubMed] [Google Scholar]
- 74.Shakhar K, Shakhar G, Rosenne E, Ben-Eliyahu S. Timing within the menstrual cycle sex and the use of oral contraceptives determine adrenergic suppression of NK cell activity. Br. J. Cancer. 2000;83:1630–1636. doi: 10.1054/bjoc.2000.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White HD, Crassi KM, Givan AL, Stern JE, Gonzalez JL, Memoli VA, Green WR, Wira CR. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J. Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- 76.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl. Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 77.Gameiro CM, Romao F, Castelo-Branco C. Menopause and aging: changes in the immune system—a review. Maturitas. 2010;67:316–320. doi: 10.1016/j.maturitas.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 78.Gameiro C, Romao F. Changes in the immune system during menopause and aging. Front Biosci. (Elite Ed) 2010;2:1299–1303. doi: 10.2741/e190. [DOI] [PubMed] [Google Scholar]
- 79.Straub RH, Hense HW, Andus T, Scholmerich J, Riegger GA, Schunkert H. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women: a population-based study. J. Clin. Endocrinol. Metab. 2000;85:1340–1344. doi: 10.1210/jcem.85.3.6355. [DOI] [PubMed] [Google Scholar]
- 80.Brooks-Asplund EM, Tupper CE, Daun JM, Kenney WL, Cannon JG. Hormonal modulation of interleukin-6 tumor necrosis factor and associated receptor secretion in postmen-opausal women. Cytokine. 2002;19:193–200. doi: 10.1006/cyto.2002.1963. [DOI] [PubMed] [Google Scholar]
- 81.Blum M, Zacharovich D, Pery J, Kitai E. Lowering effect of estrogen replacement treatment on immunoglobulins in men-opausal women. Rev. Fr. Gynecol. Obstet. 1990;85:207–209. [PubMed] [Google Scholar]
- 82.Costenbader KH, Feskanich D, Stampfer MJ, Karlson EW. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262. doi: 10.1002/art.22510. [DOI] [PubMed] [Google Scholar]
- 83.Font J, Pallares L, Cervera R, Lopez-Soto A, Navarro M, Bosch X, Ingelmo M. Systemic lupus erythematosus in the elderly: clinical and immunological characteristics. Ann. Rheum. Dis. 1991;50:702–705. doi: 10.1136/ard.50.10.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boddaert J, Huong DL, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 2004;83:348–359. doi: 10.1097/01.md.0000147737.57861.7c. [DOI] [PubMed] [Google Scholar]
- 85.Ho CT, Mok CC, Lau CS, Wong RW. Late onset systemic lupus erythematosus in southern Chinese. Ann. Rheum. Dis. 1998;57:437–440. doi: 10.1136/ard.57.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Namjou B, Scofield RH, Kelly JA, Goodmon E, Aberle T, Bruner GR, Harley JB. The effects of previous hysterectomy on lupus. Lupus. 2009;18:1000–1005. doi: 10.1177/0961203309104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mok CC, Lau CS, Ho CT, Wong RW. Do flares of systemic lupus erythematosus decline after menopause. Scand. J. Rheumatol. 1999;28:357–362. doi: 10.1080/03009749950155346. [DOI] [PubMed] [Google Scholar]
- 88.Sanchez-Guerrero J, Villegas A, Mendoza-Fuentes A, Romero-Diaz J, Moreno-Coutino G, Cravioto MC. Disease activity during the premenopausal and postmenopausal periods in women with systemic lupus erythematosus. Am. J. Med. 2001;111:464–468. doi: 10.1016/s0002-9343(01)00885-3. [DOI] [PubMed] [Google Scholar]
- 89.Urowitz MB, Ibanez D, Jerome D, Gladman DD. The effect of menopause on disease activity in systemic lupus erythematosus. J. Rheumatol. 2006;33:2192–2198. [PubMed] [Google Scholar]
- 90.Fernandez M, Calvo-Alen J, Alarcon GS, Roseman JM, Bastian HM, Fessler BJ, McGwin G, Jr, Vila LM, Sanchez ML, Reveille JD. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): Disease activity, XXI damage accrual and vascular events in pre- and postmenopausal women, Arthritis Rheum. 2005;52:1655–1664. doi: 10.1002/art.21048. [DOI] [PubMed] [Google Scholar]
- 91.Mok CC, Wong RW, Lau CS. Ovarian failure and flares of systemic lupus erythematosus. Arthritis Rheum. 1999;42:1274–1280. doi: 10.1002/1529-0131(199906)42:6<1274::AID-ANR26>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 92.Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS. Hormonal reproductive risk factors for development of systemic lupus erythematosus: results of a population-based, case-control study. Arthritis Rheum. 2002;46:1830–1839. doi: 10.1002/art.10365. [DOI] [PubMed] [Google Scholar]
- 93.Meier CR, Sturkenboom MC, Cohen AS, Jick H. Post-menopausal estrogen replacement therapy and the risk of developing systemic lupus erythematosus or discoid lupus. J. Rheumatol. 1998;25:1515–1519. [PubMed] [Google Scholar]
- 94.Buyon JP, Petri MA, Kim MY, Kalunian KC, Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcon GS, Manzi S, Belmont HM, Askanase AD, Sigler L, Dooley MA, Von Feldt J, McCune WJ, Friedman A, Wachs J, Cronin M, Hearth-Holmes M, Tan M, Licciardi F. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann. Intern. Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 95.Mok CC, Lau CS, Ho CT, Lee KW, Mok MY, Wong RW. Safety of hormonal replacement therapy in postmenopausal patients with systemic lupus erythematosus. Scand. J. Rheumatol. 1998;27:342–346. doi: 10.1080/03009749850154357. [DOI] [PubMed] [Google Scholar]
- 96.Arden NK, Lloyd ME, Spector TD, Hughes GR. Safety of hormone replacement therapy (HRT) in systemic lupus erythematosus (SLE) Lupus. 1994;3:11–13. doi: 10.1177/096120339400300104. [DOI] [PubMed] [Google Scholar]
- 97.Sanchez-Guerrero J, Gonzalez-Perez M, Durand-Carbajal M, Lara-Reyes P, Jimenez-Santana L, Romero-Diaz J, Cravioto MD. Menopause hormonal therapy in women with systemic lupus erythematosus. Arthritis Rheum. 2007;56:3070–3079. doi: 10.1002/art.22855. [DOI] [PubMed] [Google Scholar]
- 98.Bhattoa HP, Bettembuk P, Balogh A, Szegedi G, Kiss E. The effect of 1-year transdermal estrogen replacement therapy on bone mineral density biochemical markers of bone turnover in osteopenic postmenopausal systemic lupus erythematosus patients: a randomized double-blind placebo-controlled trial. Osteoporos. Int. 1990;15:396–404. doi: 10.1007/s00198-003-1553-6. [DOI] [PubMed] [Google Scholar]
- 99.Goemaere S, Ackerman C, Goethals K, De Keyser F, Van der Straeten C, Verbruggen G, Mielants H, Veys EM. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J. Rheumatol. 1990;17:1620–1622. [PubMed] [Google Scholar]
- 100.Merlino LA, Cerhan JR, Criswell LA, Mikuls TR, Saag KG. Estrogen and other female reproductive risk factors are not strongly associated with the development of rheumatoid arthritis in elderly women. Semin. Arthritis Rheum. 2003;33:72–82. doi: 10.1016/s0049-0172(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 101.Pikwer M, Bergstrom U, Nilsson JA, Jacobsson L, Turesson C. Early menopause is an independent predictor of rheumatoid arthritis. Ann. Rheum. Dis. 2012;71:378–381. doi: 10.1136/ard.2011.200059. [DOI] [PubMed] [Google Scholar]
- 102.Pikwer M, Nilsson JA, Bergstrom U, Jacobsson LT, Turesson C. Early menopause and severity of rheumatoid arthritis in women over 45 years of age. Arthritis Res. Ther. 2012;14:R190. doi: 10.1186/ar4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Turkcapar N, Demir O, Atli T, Kopuk M, Turgay M, Kinikli G, Duman M. Late onset rheumatoid arthritis: clinical and laboratory comparisons with younger onset patients. Arch. Gerontol. Geriatr. 2006;42:225–231. doi: 10.1016/j.archger.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Deal CL, Meenan RF, Goldenberg DL, Anderson JJ, Sack B, Pastan RS, Cohen AS. The clinical features of elderly-onset rheumatoid arthritis. A comparison with younger-onset disease of similar duration. Arthritis Rheum. 1985;28:987–994. doi: 10.1002/art.1780280905. [DOI] [PubMed] [Google Scholar]
- 105.Filipowicz-Sosnowska A, Rupinski R. Elderly onset rheumatoid arthritis. Pol. Arch. Med. Wewn. 2008;118:36–42. Suppl. [PubMed] [Google Scholar]
- 106.Kuiper S, van Gestel AM, Swinkels HL, de Boo TM, da Silva JA, van Riel PL. Influence of sex age and menopausal state on the course of early rheumatoid arthritis. J. Rheumatol. 2001;28:1809–1816. [PubMed] [Google Scholar]
- 107.Walitt B, Pettinger M, Weinstein A, Katz J, Torner J, Wasko MC, Howard BV. Effects of postmenopausal hormone therapy on rheumatoid arthritis: the women’s health initiative randomized controlled trials. Arthritis Rheum. 2008;59:302–310. doi: 10.1002/art.23325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hall GM, Daniels M, Huskisson EC, Spector TD. Arandomised controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal rheumatoid arthritis. Ann. Rheum. Dis. 1994;53:112–116. doi: 10.1136/ard.53.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study. Arthritis Rheum. 2004;50:3458–3467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 110.Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499–1505. doi: 10.1002/art.1780371014. [DOI] [PubMed] [Google Scholar]
- 111.MacDonald AG, Murphy EA, Capell HA, Bankowska UZ, Ralston SH. Effects of hormone replacement therapy in rheumatoid arthritis: a double blind placebo-controlled study. Ann. Rheum. Dis. 1994;53:54–57. doi: 10.1136/ard.53.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.D’Elia HF, Larsen A, Mattsson LA, Waltbrand E, Kvist G, Mellstrom D, Saxne T, Ohlsson C, Nordborg E, Carlsten H. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J. Rheumatol. 2003;30:1456–1463. [PubMed] [Google Scholar]
- 113.Forsblad-d’Elia H, Carlsten H. Hormone replacement therapy in postmenopausal women with rheumatoid arthritis stabilises bone mineral density by digital x-ray radiogrammetry in a randomised controlled trial. Ann. Rheum. Dis. 2011;70:1167–1168. doi: 10.1136/ard.2010.137133. [DOI] [PubMed] [Google Scholar]
- 114.Paty DW, Noseworthy JH, Ebers GC. Diagnosis of multiple sclerosis, in. In: Paty DW, Ebers GC, editors. Philadelphia, PA: Mult Scler, FA Davis Company; 1997. pp. 48–51. [Google Scholar]
- 115.Hooge JP, Redekop WK. Multiple sclerosis with very late onset. Neurology. 1992;42:1907–1910. doi: 10.1212/wnl.42.10.1907. [DOI] [PubMed] [Google Scholar]
- 116.Kis B, Rumberg B, Berlit P. Clinical characteristics of patients with late-onset multiple sclerosis. J. Neurol. 2008;255:697–702. doi: 10.1007/s00415-008-0778-x. [DOI] [PubMed] [Google Scholar]
- 117.Tremlett H, Devonshire V. Is late-onset multiple sclerosis associated with a worse outcome. Neurology. 2006;67:954–959. doi: 10.1212/01.wnl.0000237475.01655.9d. [DOI] [PubMed] [Google Scholar]
- 118.Noseworthy J, Paty D, Wonnacott T, Feasby T, Ebers G. Multiple sclerosis after age 50. Neurology. 1983;33:1537–1544. doi: 10.1212/wnl.33.12.1537. [DOI] [PubMed] [Google Scholar]
- 119.Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T. Effect of gender on late-onset multiple sclerosis. Mult. Scler. 2012;18:1472–1479. doi: 10.1177/1352458512438236. [DOI] [PubMed] [Google Scholar]
- 120.Filippi M, Wolinsky JS, Sormani MP, Comi G. Enhancement frequency decreases with increasing age in relapsing-remitting multiple sclerosis. Neurology. 2001;56:422–423. doi: 10.1212/wnl.56.3.422. [DOI] [PubMed] [Google Scholar]
- 121.Mowry EM, Pesic M, Grimes B, Deen S, Bacchetti P, Waubant E. Demyelinating events in early multiple sclerosis have inherent severity and recovery. Neurology. 2009;72:602–608. doi: 10.1212/01.wnl.0000342458.39625.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas. 2006;54:149–153. doi: 10.1016/j.maturitas.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 123.Smith R, Studd JW. A pilot study of the effect upon multiple sclerosis of the menopause hormone replacement therapy and the menstrual cycle. J. R. Soc. Med. 1992;85:612–613. doi: 10.1177/014107689208501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wundes A, Amtmann D, Brown T, Christian S. Menopause in women with multiple sclerosis, Int. J. MS Care. 2011;13:47. [Google Scholar]
- 125.Sammaritano LR. Menopause in patients with autoimmune diseases. Autoimmun. Rev. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]