Abstract
Normal mineral metabolism is critical for skeletal integrity and recently serum fibroblast Growth factor 23 (FGF23) levels were found to be directly related to overall fracture risk in elderly Swedish Men. To confirm this association, we performed a prospective case-cohort study to understand the relation of FGF23 and fracture risk in older Caucasian men enrolled in the Osteoporotic Fractures in Men (MrOS) Study.
Methods
In the cohort of 5994 men attending the baseline MrOS examination, we evaluated a subgroup of 387 men with incident nonvertebral fracture including 73 hip fractures and a sample of 1385 men randomly selected from the cohort with baseline mineral and calcium hormone measurements. FGF23 was measured in baseline serum samples by ELISA (Millipore, Billerica, MA). Modified Cox proportional hazards models that account for case cohort study design were used to estimate the relative hazards (RH) of fracture in men across quartiles of FGF23. Subjects were also stratified by renal function and RH per strata was estimated in men with the highest quartile of FGF23 compared to quartile 3, 2 and 1.
Results
Overall, there was no difference in risk of nonspine or hip fracture by baseline FGF23. However, associations differed by strata of eGFRCrCy. Among men with eGFRCrCys < 60ml/min/1.73m2 (n=73/313 non-spine fractures), the RH in the highest quartile of FGF23 compared to the rest was 2.02 (95% CI: 1.07-3.79), but in men with eGFRCrCy, > 60ml/min/1.73m2 (304/1370 fractures) the RH was 0.91 (95% CI: 0.66-1.25) after adjustment for age, clinic site, BMI, race, total hip BMD, vitamin D, PTH, alcohol use, physical activity, fracture history and serum phosphorus.
Summary
Serum FGF23 levels are not associated with incident fractures in elderly men overall. However, higher levels of serum FGF23 are associated with fracture risk in those with poor renal function.
Introduction
Fibroblast growth factor 23 (FGF23) is a known regulator of phosphate homeostasis, and the majority of this protein is produced by osteocytes that reside within the bone. The main target of FGF23 is the kidney where FGF23 reduces the expression or insertion of sodium phosphate transporters within renal proximal tubular membranes so that phosphate can be excreted (1). In addition, FGF23 is reported to inhibit renal 1 hydroxylase expression which reduces the production of 1,25 dihydroxyvitamin D which then reduces the gastrointestinal absorption of calcium and phosphate (2).
Recently, elevated serum levels of FGF23 have been reported to be associated with an increased risk of osteoporotic fractures in elderly Swedish men (3). The release of FGF23 by both young and old osteocytes may be a mechanism whereby the osteocytes can control mineralization and phosphate homeostasis. Osteocytes regulate FGF23 signaling and biomineralization through molecules produced by the osteocyte and include phosphate regulating gene with homologies to endopeptidase on X chromosome (PHEX), dentin matrix protein-1 (DMP1) and matrix extracellular phosphor-glycopoteins (MEPE) (4,5). Loss of either functional DMP1 or PHEX, results in elevated FGF23 levels both in osteocytes and in the circulation, phosphate excretion is elevated in the kidney, and the osteomalacia is present in the bones as is rickets (4, 5, 6). A number of clinical skeletal disorders that results in mineralization abnormalities and elevated FGF23 serum levels have been reported and include autosomal dominant hypophosphotemic rickets (ADHR), tumor induced osteomalacia (TIO), x-linked hypophosphotemic rickets (ARHR) (3,6). Moreover, mice overexpressing FGF23 have low cortical and trabecular bone mineral density.(7)
Recently, Mirza et al reported that elevated serum levels of FGF23 was associated with an elevated fracture risk in older Swedish men (3). We performed a prospective case-cohort study to better understand the relation of FGF23 and fracture risk in older Caucasian men enrolled in the Osteoporotic Fractures in Men (MrOS) study in the United States.
Methods
Study Population
5994 community-dwelling men at six clinical centers in the United States (Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California) enrolled in MrOS (From March 2000 through April 2002), a study of osteoporosis and fractures in elderly men. Eligible men were 65 years of age or older, without bilateral hip replacements, and able to walk without the assistance of another person. Details of the MrOS design and cohort have been published elsewhere (8,9,10). The Institutional Review Board (IRB) at each center approved the study protocol, and written informed consent was obtained from all participants.
Follow-up and Outcome Ascertainment
Tri-annual questionnaires were sent to the men to report any fractures. All non-spine fractures were verified by medical records and confirmed by blinded central adjudicators[11]. Pathologic fractures were excluded.
Major osteoporotic fractures were defined as incident fractures that occurred in the hip, spine, forearm (radius or ulna) or humerus. Incident nonspine fractures and spine, forearm and humerus fractures were ascertained by the methods mentioned above.
Incident vertebral fractures were identified from semi-quantitative (SQ) readings of lateral spine x-rays. A change of SQ grade > 1 at the second clinic visit for at least one vertebral level for a participant, regardless of SQ grade at baseline was considered a incident vertebral fracture (12). Given that we only selected nonspine fractures that occurred outside of the subcohort for FGF23 assays as part of the case-cohort study design, we were restricted to analyze major osteoporotic fractures that occurred in the random cohort only. Similarly incident vertebral fractures that occurred in the random cohort were considered for this study.
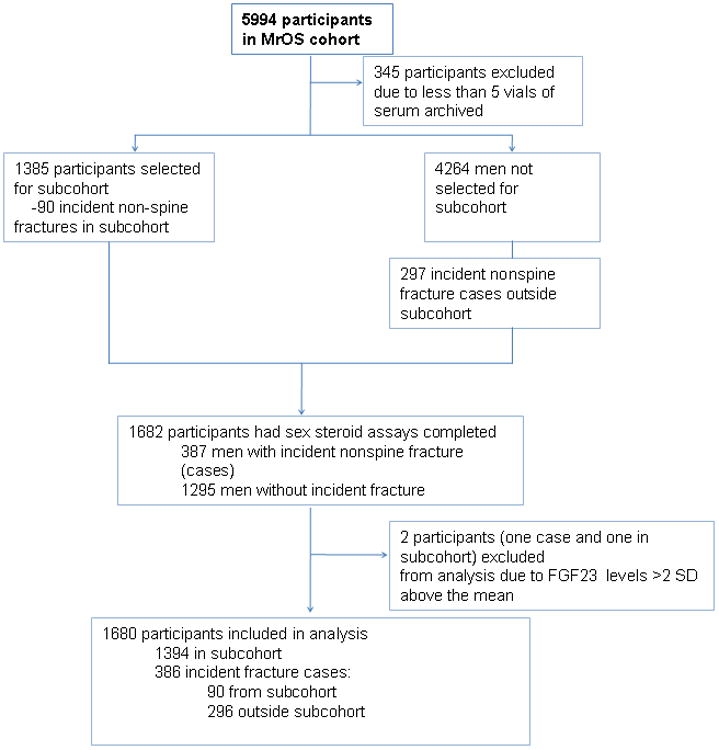
jCase-Cohort Study Design
The current study is a prospective case-cohort study of men nested within the MrOS. Men with 5 or more vials of archived serum were eligible for inclusion in the study. Hence of the 5649 eligible participants, we randomly selected 1385 men to serve as the sub-cohort. Over the 5.3 years of follow-up, 90 nonspine cases (including 16 hip fractures) arose from the randomly selected subcohort, and an additional 297 nonspine cases (including 57 hip fractures) were selected from the remaining MrOS cohort [9] resulting in a total of 1682 participants with FGF23 measures.. Two participants were excluded who had FGF23 levels >2 SD above the mean (18.9 pg/ml), one from the subcohort and one nonspine fracture case, resulting in 1384 men in the subcohort and 296 fracture cases outside of the cohort for this study. The total study sample for the nonspine fracture analyses was 1680 (figure 1) men. Similarly there were 73 hip fractures with 16 occurring in the subcohort and 57 hip fracture cases outside the cohort resulting in 1441 men for the hip fracture analysis.
Figure 1. Case-cohort design for the MrOS FGF-23 and fracture study.
Vitamin D, PTH and Cystatin C assays
Fasting morning blood was collected, sera protected from sunlight, prepared immediately after phlebotomy, and was stored at −70¡C until first thawed for vitamin D, PTH and cystatin C and creatinine assays. Measures for 25(OH)vitamin D2 (derived from ergocalciferol) and 25(OH) vitamin D3 (derived from cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described (13). Total 25(OH)Vitamin D was calculated by summing the values of 25(OH)vitamin D2 and 25(OH)vitamin D3. Total intact PTH was assessed at Columbia University using the 115 immunoradiometric assay from Scantibodies (3KG600). Serum cystatin C levels were determined using a BN100 nephelometer (Dade Behring Inc., Deerfield, IL) using a particle-enhanced immunonepholometric assay(14). Serum creatinine and phosphate concentrations were measured using a Roche COBAS Integra 800 analyzer (Roche Diagnostic Corporation, Indianapolis, IN) at the Portland VA Medical Center using an enzymatic method calibrated with materials assayed by isotope-dilution mass spectrometry (IDMS). A combined formula using standardized Cystatin C and creatinine -based estimated glomerular filtration rate (eGFRcysC) was computed using a CKD-EPI given that this was shown to be the most accuarate estimate of renal function in a recent publication (15,16,17).
FGF23 assays
Intact FGF23 was measured using an ELISA according to the manufacturer's protocol. This second generation polyclonal goat antibody ELISA has been found to recognize the biologically active intact FGF23 (18,19). The Millpore lowest limit of detection was 3.3pg/ml in our laboratory with an intrassay and interassay coefficient of variation of less than 11%. This was similar to the manufacturer's reported variation.
Other Measures
All covariates were assessed at baseline. Participants were mailed questionnaires to ascertain information on date of birth, race/ethnicity, self-rated health, alcohol consumption, fracture history, and history of falls. Physical activity was assessed using walk speed (time in seconds to walk 6 m at usual pace expressed as m/s). Bone mineral density (BMD) (g/cm2) of the total hip and its subregions (femoral neck; trochanter) was measured using fan-beam dual-energy x-ray absorptiometry (DXA) (QDR 4500W, Hologic Inc., Bedford, Mass). Details of this measure are published elsewhere (12,20,21).
Height (cm) was measured on Harpenden stadiometers, and weight (kg) was measured on standard balance beam or digital scales using standard protocols, with participants wearing light clothing without shoes. Body mass index (BMI) was calculated as kg/m2. At baseline, participants were asked to bring in all medications used within the last 30 days. All prescription medications recorded by the clinics were stored in an electronic medications inventory database (San Francisco Coordinating Center, San Francisco, CA). Each medication was matched to its ingredient(s) based on the Iowa Drug Information Service (IDIS) Drug Vocabulary (College of Pharmacy, University of Iowa, Iowa City, IA) (8,11).
Statistical Methods
Baseline characteristics were compared in the subcohort across quartiles of FGF23 using chi-square tests for categorical variables and ANOVA for continuous variables. Wilcoxon nonparametric tests were used for skewed covariates. Baseline characteristics were also compared between non-spine fracture cases and men without fractures. Baseline characteristics that were associated with non-spine fracture and FGF23 at p-value < 0.05 were considered confounders. Other covariates including hip and spine BMD, 25(OH) vitamin D PTH, fracture history after 50 years, and phosphorus known to be confounders from the literature were also selected. Associations between FGF23 and incident non-spine fractures and hip fractures were assessed in proportional hazards regression models modified for the case-cohort design. Associations for major osteoporotic fractures and incident vertebral fractures with FGF23 were assessed using traditional Cox proportional hazard models and logistic regression models respectively for fractures that occurred in the random cohort. All associations were first examined in our base model which included adjustment for age, clinic, race and BMI. Models were further adjusted for hip BMD for nonspine, hip and major osteoporotic fractures and total spine BMD for veterbral fractures in addition to vitamin D, PTH, alcohol use, walking speed fracture history and serum phosphorus.
Hazard ratios (HR) and 95% confidence intervals were calculated from the Cox proportional hazards models modified for case cohort analysis to test the association of non-spine and hip fractures across quartiles. FGF23 levels were divided into quartile categories defined on the basis of the distribution in the random cohort. The lowest quartile formed the referent group. Similarly, odds ratios (OR) and 95% CI were calculated for major osteoporotic fractures and incident vertebral fractures occurring in the random cohort across quartiles of FGF23.
FGF23 levels have been reported to be increased with chronic renal disease, therefore we tested the association between nonspine fractures and FGF23 in two strata of eGFR ; < 60 and > 60. Stratified analyses were not conducted for other fracture types due to small number of fractures in the eGFR < 60 strata. Hazard ratios (HR) and 95% confidence intervals for nonspine fractures were calculated for men in two strata; men with eGFRCrCysC <60 and men with eGFRCrCysC > 60. Analyses were performed comparing the highest quartile of FGF23 to the lower quartiles. Analysis was performed using SAS9.2 software.
Results
We evaluated the baseline characteristics of the study subjects based on quartiles of serum FGF23 (table 1). The mean age was 73 years, nearly 90% of the subjects were Caucasian: BMI and femoral neck BMD were similar across the quartiles of FGF23. Alcohol use and walking speed were significantly associated with FGF23, although the directions of associations appeared non-linear across quartiles. There were no significant differences in general health, falls over the past 12 months or 25 vitamin D or phosphorus levels across the quartiles of FGF23. However, serum creatinine and phosphorus were higher and eGFR was lower in the subjects in the higher FGF23 quartile compared to the other quartiles of FGF23 (p<0.01). There were 101 (8%) major osteoporotic fractures and 52 (4%) incident vertebral fractures in the random cohort and there were no differences in the proportion of men with these fractures across the quartiles of FGF23.
Table 1. Baseline Characteristics of Men across quartiles of FGF23.
| Quartiles of FGF23 (pg/ml) | ||||
|---|---|---|---|---|
| Baseline Characteristics | Q1: 4.2-12.0 (N= 346) | Q2: 12.1-16.6 (N= 346) | Q3: 16.6-22.4 (N= 345) | Q4: 22.4-111.1 (N= 347) |
| Demographic | ||||
| Age mean +/- SD y | 73.4 +/- 5.8 | 73.63 +/- 5.91 | 74.4 +/- 6.0 | 73.7 +/- 5.8 |
| Caucasian % | 309 (89.3) | 307 (88.73) | 317 (91.9) | 326 (94.0) |
| Anthropometry, mean and SD | ||||
| Height cm | 174.1 +/- 6.9 | 174.2 +/- 6.7 | 173.8 +/- 6.6 | 174.9 +/- 7.0 |
| Weight kg | 82.7 +/- 12.9 | 83.1 +/- 13 | 82.7 +/- 13.4 | 84.3 +/- 12.6 |
| BMI kg/cmˆ2 | 27.3+/- 3.8 | 27.4 +/- 3.8 | 27.4 +/- 3.8 | 27.5 +/- 3.5 |
| DXA BMD, mean g/cmˆ2 | ||||
| Total Hip BMD | 0.96 +/- 0.14 | 0.95 +/- 0.14 | 0.95 +/- 0.14 | 0.95 +/- 0.14 |
| Health Status n(%) | ||||
| Self Reported Health Status | 300 (86.7) | 304 (87.9) | 291 (84.4) | 280 (81.0) |
| History of fracture after age 50 | 80 (23.2) | 81 (23.5) | 91 (26.5) | 68 (19.7) |
| History of Falls within the past 12 months | 65 (18.8) | 68 (19.7) | 71 (20.6) | 75 (21.6) |
| Avg number of drinks/wk mean +/- SD* | 4.72 +/- 8.14 | 5.45 +/- 7.82 | 3.77 +/- 5.62 | 4.44 +/- 8.17 |
| Physical activity | ||||
| 6 meter Usual Pace m/sec mean +/- SD* | 1.26 +/- 0.25 | 1.24 +/- 0.25 | 1.21 +/- 0.23 | 1.25 +/- 0.24 |
| Serum measures, mean and SD | ||||
| 25(OH) total vitamin D | 25.9 +/- 8.5 | 24.5 +/- 7.2 | 24.8 +/- 8.1 | 25.6 +/- 8.1 |
| Total intact PTH | 31.4 +/- 13.1 | 32.9 +/- 12.4 | 34.9 +/- 43.4 | 35.1 +/- 24.1 |
| eGFRCrCysC using CDK-EPI definition** | 78 +/- 15.5 | 76.4 +/- 14.9 | 72.3 +/- 16.1 | 69.9 +/- 18.0 |
| Serum Phosphate mg/dl * | 3.19 +/- 0.41 | 3.13 +/- 0.44 | 3.18 +/- 0.42 | 3.2 +/- 0.5 |
| Creatinine mg/dl * | 0.98 +/- 0.21 | 1 +/- 0.2 | 1.04 +/- 0.24 | 1.1 +/- 0.4 |
| FGF23 pg/ml | 9.46 +/- 1.76 | 14.32 +/- 1.4 | 19.24 +/- 1.6 | 32.2 +/- 14.3 |
| Fractures | ||||
| Incident Vertebral Fractures | 18 (5.2) | 9 (2.6) | 9 (2.6) | 16 (4.6) |
| Major osteoporotic Fractures | 34 (31.2) | 23 (21.1) | 29 (26.6) | 23 (21.1) |
p<0.05
P<0.001
Men with nonspine fracture were older, mostly white, were more likely to have a history of falls and fracture after age of 50, had slower walking speed and consumed less alcohol compared to non cases. In addition, men with nonspine fractures had lower hip BMD and higher serum phosphorus levels.
Association of fracture and FGF23
The mean FGF23 in the study population was 18.82 pg/ml (range:4.2-111.1 pg/ml). There was no difference in the risk of incident nonspine, hip, major osteoporotic or incident vertebral fractures by quartile of FGF23 (Table 2) in base models adjusted for age, BMI, race and clinic site. Further adjustment for BMD, vitamin D, PTH, walking speed, alcohol use, fracture history and serum phosphorus did not change the results significantly. In addition, there were no association among those with the highest FGF23 level compared to the lower FGF23 levels with nonspine fractures (Base Model, Q4 vs. Q1,2,3 HR: 1.1 95%CI: 0.85,1.43). Similarly no associations were found among the other fracture types with FGF23.
Table 2. Association between Fracture type and Quartiles of FGF23.
| HR (95%CI) | OR(95% CI) | |||
|---|---|---|---|---|
|
|
||||
| FGF23 Quartiles | Nonspine fractures (N=386) | Hip Fractures (N=73) | † Major Osteoporotic fractures (N=109) | † Incident Vertebral Fractures (N=52) |
| N=386 | N=73 | N=109 | N=52 | |
| Q1 (ref) | 1.0 | 1.0 | 1.0 | 1.0 |
| Q2 | 1.2 0.09-1.6) | 1.6 (0.8-3.4) | 0.6 (0.4-1.1) | 0.47 (0.2-1.08) |
| Q3 | 1.1 (0.8-1.5) | 1.6 (0.7-3.4) | 0.8 (0.5-1.3) | 0.49 (0.21-1.14) |
| Q4 | 1.2 (0.9-1.7) | 1.6 (0.7-3.5) | 0.7 (0.4-1.2) | 0.81 (0.39-1.66) |
| P trend | 0.43 | 0.3 | 0.3 | 0.61 |
| Q4 vs rest | 1.1 (0.85-1.43) | 1.15 (0.66-2.02) | 0.91 (0.76-1.09) | 1.25 (0.67-2.33) |
Quartile cut-points 12.2, 16.6, 22.4 pg/ml
Models adjusted for age, race, clinic site and BMI
= fractures occurring in the random cohort only
FGF23 with Risk of Fracture by Level of Kidney Function
To determine if men with poor renal function and high FGF23 have an increased risk of fracture we stratified men into two strata; eGFRCrCysC <60 mL/min/1.73 m2 and eGFRCrCysC > 60 mL/min/1.73 m2. There were 73 nonspine fractures (33 in the Q4 of FGF23) among 313 men with eGFRCrCysC<60, and 304 nonspine fractures (68 in Q4 of FGF23) among 1345 men with eGFRCrCysC > 60. There were 22 men (9 nonspine fractures) who had missing creatinine measures and hence were not included in these analyses. We observed a 2 fold increased risk of nonspine fractures in the highest quartile of FGF23 compared to the rest among men with low eGFR (HR: 2.25, 95%CI: 1.26-4) in base models (Table 3). Further adjustment for hip BMD, vitamin D, PTH, alcohol use, walking speed, fracture history and serum phosphorus did not change the results significantly (HR: 2.02, 95%CI: 1.07-3.79). In contrast, among men with eGFRCrCysC > 60, there was no association between FGF23 and non-spine fracture (Base Model: HR 0.94, 95%CI 0.69-1.27) (Table 3). Adjusting for eGFR in the stratified analysis did not change the results significantly. Stratified analysis by eGFR strata was limited and not conducted due to small numbers of fractures within the <60 strata.
Table 3. Association of Highest quartile of FGF23 with nonspine fractures stratified beGFRCrCysC.
| HR (95%CI) | HR (95%CI) | |
|---|---|---|
|
|
||
| FGF23 Quartile 4 vs. Q3,2,1 | eGFR <60 | eGFR≥60 |
| Number of fractures/total N | 73/313 | 304/1345 |
| Unadjusted | 1.88 (1.12-3.14) | 0.93 (0.69-1.26) |
| Age adjusted | 2.06 (1.21-3.51) | 0.96 (0.71-1.3) |
| Base Model | 2.25 (1.26-4) | 0.94 (0.69-1.27) |
| MV | 2.02 (1.07-3.79) | 0.91 (0.66-1.25) |
Quartile cut-points 12.2, 16.6, 22.4 pg/ml
Base Model adjusted for age, race, BMI and clinic site
MV model adjusted for base model and vitamin D, PTH, walking speed, alcohol use, hip BMD, fracture history and serum phosphorus
Discussion
We determined that FGF23 levels were not associated with incident nonspine fractures. However, in individuals with eGFR < 60cc/min there was a two fold increased risk of nonspine fractures in the highest quartile of FGF23 compared to the lower quartiles. In clinical practice, the combination of bone mass and clinical risk factors has improved a clinician's ability to identify individuals at a high risk of fracture. However, the other variables such as bone turnover markers or calciotrophic hormones may further improve our ability to identify and treat high risk patients with bone active agents. In an attempt to identify novel biomarkers of bone strength and confirm another report of an associated of FGF23 with incident fractures (3), we measured serum FGF23 in elderly men and found that only among men with compromised renal function was there an association with nonspine fractures.
Our study results are in contrast to Mirza et al that reported higher serum FGF23 level was a novel independent predictor of overall fracture risk and vertebral fracture risk in older Swedish men. Mirza et al found the relationship was stronger in individuals with serum FGF23 levels greater than 55.7 pg/ml, and subjects in the highest quartile of FGF23 ( > 57.4pg/ml) were at a 63% increased risk for nonvertebral osteoporotic fractures compared to those in the lowest three quartiles, and the association remained significant after adjustment for BMD (Mirza). Based on the knowledge that FGF23 serum levels increase with renal insufficiency to control for the potential influence on renal function on fracture risk Mirza et al performed stratified analysis was performed in subjects above and below the median eGFR ( 71.5ml/min/1.73 m2) and the association was only significant for FGF23 and subjects with eGFR above the median with and OR of 1.3 (95% CI: 1.1-1.6). In contrast, a study of Japanese subjects with early CKD that were on average 73 years with an eGFR of 45 ml/min/1.73m2, and without treatment with either osteoporosis medications or phosphate binders, the odds ratio for vertebral fractures within five years of the FGF23 measurement was 4.4, and the optimal cutoff level of FGF23 for vertebral fracture was 56.8pg/ml (sensitivity, 0.82; specificity, 0.63) (22). Interestingly, when adjusted for age and eGFR the odds were nearly 8. Significantly positive associations were found for FGF23 and body mass index and serum phosphate levels and negative associations with eGFR. Interestingly, the serum levels of FGF23 reported by both studies were similar for the increased odds of both vertebral and nonvertebral fractures however Mirza only studied males (3).
Our finding of an association of serum FGF23 levels with nonvertebral fractures in men with compromised renal function provides support that that serum FGF23 levels may be a biomarker for metabolic abnormalities that can result in both fractures and other adverse patient outcomes. FGF23 is reported to increase in early renal insufficiency and prior to changes in serum PTH or 1,25 vitamin D. In the context of reduced renal function, elevated FGF23 levels predicted nonspine fractures in our study. However, whether or not FGF23 is a marker for reduced bone strength is not known. FGF23 serves as a phosphaturic factor synthesized by osteocytes and inhibits 1,25 (OH)2VitaminD3, production by the kidney to maintain the balance between phosphate homeostasis and skeletal mineralization (6). A recent in vitro study demonstrated that over-expression of FGF23 suppressed osteoblast differentiation and matrix mineralization (23). Another study evaluated the proteins associated with osteocytes and bone mineralization and found that FGF23 co-localized to the secondary spongiosa of the trabecular bone and areas of the cortical bone in areas where the osteocyte lacunar system was very mature, fully functional syncytium suggesting that the FGF23 produced by the osteocytes would then be part of the bone-renal axis that is central to proper mineral metabolism (24,25). Elevated levels of serum FGF23 have been found in individuals with autosomal hypophosphatemic rickets which carry mutations in the Dentin Matrix Protein-1, DMP-1, gene and other forms of rickets and osteomalacia have elevated levels of FGF23 despite normal calciuria (26). In contrast, mice with a Klotho gene deletion developed elevated DMP-1, hyperphosphatemia and low FGF23 levels (27). Also, overexpression of FGF23 in primary rat calvaria cell cultures suppressed matrix mineralization (23) and increased FGF23 expression in ovine callus was associated with delayed fracture healing in one pilot study (28). One other potential mechanism may be that subjects with chronic kidney disease have decreased renal acid excretion that leads to metabolic acidosis which can directly affect bone cell activity. Kreiger et al reported an increase in mRNA and protein levels of FGF23 in osteoblasts from mice with renal insufficiency and metabolic acidosis (29).
In kidney proximal tubular cells FGF23 inhibits phosphate reabsorption and leads to decreased synthesis and enhanced catabolism of 1,25(OH)(2) D(3). Elevated levels of FGF23 cause renal phosphate wasting and suppress circulating 1,25(OH)(2) D(3) levels and this phenomenon has been reported in a in a number of several hereditary hypophosphatemic disorders with skeletal abnormalities, including X-linked hypophosphatemic rickets (XLH) and autosomal recessive hypophosphatemic rickets (ARHR). Currently, therapeutic approaches to these diseases are limited to treatment with activated vitamin D analogues and phosphate supplementation, often merely resulting in partial correction of the skeletal aberrations. However, a recent study by Wšhrle S (30), reported that the use of and FGFR inhibitor in two different hypophosphatemic mouse models, Hyp and Dmp1-null mice, resembling the human diseases XLH and ARHR, found that that pharmacological inhibition of FGFRs efficiently reduced FGF23 signaling and normalized the hypophosphatemic and hypocalcemic conditions of these mice. Also the continued FGFR inhibition in Hyp mice leads to enhanced bone growth, increased mineralization and reorganization of the disturbed growth plate structure suggesting that elevated levels of FGF23 reduced the ability of the bone to normally mineralize and inhibited bone formation (30). Taken together, these data suggest that elevated FGF23 may alter the normal mineralization of bone and reduce bone formation and bone strength. Additional studies will need to be performed to elucidate these findings.
This study has a number of strengths including a well characterized longitudinal cohort of elderly men with information on both risk factors and radiographic confirmation of fractures and over 97% of the enrolled subjects have complete follow-up. However, there are a few shortcomings that include the availability of serum FGF23 levels at the baseline visit and effects of change on FGF23 levels on fracture risk are unknown. In addition, we performed our analysis on baseline serum that had thawed and refrozen at least one other time and this may have influenced our results. However, we utilized the intact FGF23 ELISA assay, and the mean values of serum FGF23 were similar to other reports (32,33). Also, we only report results on older men, therefore these results are not generalizable to older women or younger individuals. Our cohort of men were recruited from the community and not clinical practices such that the sample was not selected for comorbid diseases that might influence serum FGF23 levels. The lack of association with major osteoporotic, hip and vertebral fractures could be a result of small number of fractures available for analysis. In addition, we were unable to determine the specific non-spine fracture type that was responsible for the strong association of FGF23 and CKD. In the stratified analysis, however, fractures are continually ascertained on a tri annual basis in the MrOS cohort and further research can determine this association in the future.
In summary, we found no association with serum FGF23 levels and nonspine fractures in elderly men, however in men with eGFR <60, there was a significant increase risk of nonspine fracture with high levels of FGF23 compared to lower levels. In contrast in men with eGFR≥60, there was no association of nonspne fracture risk and FGF23. Studies that further elucidate the relation between, serum FGF23 levels, renal insufficiency and fractures are warranted.
Acknowledgments
All authors of this publication participated many aspect of this research including composing the study question NEL, WY, JC, MC), preparing the samples for the assay, performing the assays (MC), reviewing the data and writing the manuscripts (all authors).
This work was supported by grants K24-AR04884 and The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140.
References
- 1.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiolo. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a poent regulator of vitamin D metabolism and phosphonate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 3.Mirza MA, Karlsson MK, Mellstrom D, Orwoll E, Ohlsson C, Ljunggren O, Larsson TE. Serum fibroblast growth factor-23 (FFGF-23) and fracture risk in elderly men. J Bone Mineral Res. 2011;26:857–864. doi: 10.1002/jbmr.263. [DOI] [PubMed] [Google Scholar]
- 4.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP 1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2013;28(4):563–8. doi: 10.1007/s00467-012-2309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonewald LF. In: Osteocytes. Marcus DFR, Nelson D, Rosen C, editors. Elsevier; Amsterdam: 2007. pp. 169–190. [Google Scholar]
- 7.Larsson T, Marsell R, Schipani E, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha I collagen promoter exhibit growth retardation, osteomalacia and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 8.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Prentice R. A case-control design for epidemiologic cohort studies and disease preveintion trials. Biometricka. 1986;73:1–11. [Google Scholar]
- 11.Lewis CE, Ewing SK, Taylor BC, et al. Predictors of nonspine fracture in elderly men: the MrOS study. J Bone Miner Res. 2007;22(2):211–219. doi: 10.1359/jbmr.061017. [DOI] [PubMed] [Google Scholar]
- 12.Black D, Cummings SR, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res. 1996;11:984–996. doi: 10.1002/jbmr.5650110716. [DOI] [PubMed] [Google Scholar]
- 13.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. Journal of Clinical Endocrinology & Metabolism. 2006;91(8):3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 15.Inker LA, Eckfeldt J, Levey AS, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58:682–684. doi: 10.1053/j.ajkd.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.TJ Weber TJ, Liu S, Indridason OS, Quarles RD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone and Miner Res. 2003;18(7):1237–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz Mahmut I, Sonmez Alper, Saglam Mutlu, Yaman Halil, Kilic Selim, Demirkaya Erkan, Eyileten Tayfun, Caglar Kayser, Oguz Yusuf, Vural Abdulgaffar, Yenicesu Mujdat, Zoccali Carmine. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010;78:679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 20.Ensrud KE, Taylor BC, Paudel ML, et al. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee CG, Boyko EJ, Nielson CM, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isakova T, Wahl P, Vargas GS, GutiŽrrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Yoshiko Y, Yamamoto R, Minamizaki T, Kozai K, Tanne K, Aubin JE, Maeda N. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23(6):939–48. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 24.Ubaidus S, Li M, Sultana S, de Freitas PH, Oda K, Maeda T, Takagi R, Amizuka N. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009;58(6):381–392. doi: 10.1093/jmicro/dfp032. [DOI] [PubMed] [Google Scholar]
- 25.Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18(7):1227–34. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Zhou J, Tang W, Menard R, Feng JQ, Quarles LD. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295(2):E254–61. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goebel S, Lienau J, Rammoser U, Seefried L, Wintgens KF, Seufert J, Duda G, Jakob F, Ebert R. FGF23 is a putative marker for bone healing and regeneration. J Orthop Res. 2009;27(9):1141–6. doi: 10.1002/jor.20857. [DOI] [PubMed] [Google Scholar]
- 29.Krieger NS, Culbertson CD, Kyker-Snowman K, Bushinsky DA. Metabolic acisois increase fibroblast growth factor 23 in neomatula mouse bone. Am J Physiol Renal Physiol. 2012 Aug 1;303(3):F431–6. doi: 10.1152/ajprenal.00199.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wšhrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus Porta D. Pharmacological inhibition of FGFR signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res. 2012;28(4):899–911. doi: 10.1002/jbmr.1810. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Esliger A, Hurley MM. Nuclear fibroblast growth factor 2 (FGF2) isoforms inhibit bone marrow stromal cell mineralization through FGF23/FGFR/MAPK in vitro. J Bone Miner Res. 2013;28(1):35–45. doi: 10.1002/jbmr.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazama JJ, Sato F, Omori K, Hama H, Yamamoto S, Maruyama H, Narita I, Gejyo F, Yamashita T, Fukumoto S, Fukagawa M. Pretreatment serum FGF-23 levels predict the efficacy of calcitriol therapy in dialysis patients. Kidney Int. 2005;67:1120–1125. doi: 10.1111/j.1523-1755.2005.00178.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 33.Koiwa F, Kazama JJ, Tokumoto A, Onoda N, Kato H, Okada T, Nii-Kono T, Fukagawa M, Shigematsu T. Sevelamer hydrochloride and calcium bicarbonate reduce serum fibroblast growth factor 23 levels in dialysis patients. Ther Apher Dial. 2005;9:336–339. doi: 10.1111/j.1744-9987.2005.00293.x. [DOI] [PubMed] [Google Scholar]


