Abstract
The amygdala is a key region in emotion processing. Particularly, fMRI studies have demonstrated that the amygdala is active during the viewing of emotional faces. Previous research has consistently found greater amygdala responses to fearful faces as compared to neutral faces in adults, convergent with a focus in the animal literature on the amygdala's role in fear processing. Studies have found that the amygdala also responds differentially to other facial emotion types in adults. Yet, the literature regarding when this differential amygdala responsivity develops is limited and mixed. Thus, the goal of current study was to examine amygdala responses to emotional and neutral faces in a relatively large sample of healthy school age children (N = 52). While the amygdala was active in response to emotional and neutral faces, the results do not support the hypothesis that the amygdala responds differentially to emotional faces in 7 – 12 year old children. Nonetheless, amygdala activity was correlated with the severity of subclinical depression symptoms and emotional regulation skills. Additionally, sex differences were observed in frontal, temporal, and visual regions as well as effects of pubertal development in visual regions. These findings suggest important differences in amygdala reactivity in childhood.
Keywords: amygdala, childhood, emotional processing, face processing, fMRI
1. Introduction
Early investigations of the amygdala's functional role in humans consistently noted that the amygdala is significantly involved in the processing of fear-related stimuli (e.g., Adolphs et al., 1995; Bishop et al., 2004; Broks et al., 1998; Calder, 1996; LaBar et al., 1998; LeDoux, 2003; Morris et al., 1998; Phillips et al., 1997). More recent studies have indicated that the amygdala is responsive to other emotions as well. For example, meta-analytic work has indicated that emotional stimuli are significantly more likely to elicit amygdala activity than neutral stimuli, for a variety of both negative and positive emotions (e.g. fear, disgust, sadness, humor, happiness, etc.; Costafreda et al., 2008). However, while amygdala responsivity to emotional stimuli in adults has been well studied, relatively little is known about when differential amygdala responsivity to different emotionally evocative stimuli arises during development. The existing studies in the developmental literature (looking at healthy samples or investigating healthy groups within studies of psychopathology) have provided mixed results, with some studies finding increased amygdala activity to emotional faces as compared to neutral faces (e.g. Britton et al., 2010; Guyer et al., 2008; Monk et al., 2003) and others failing to detect a differential response (e.g. Thomas et al., 2001b; Tottenham et al., 2011; Viding et al., 2012). Many of these studies employed relatively small samples sizes across a wide age range, which may have contributed to conflicting findings. Thus, the goal of this study was to examine whether the amygdala shows differential activity to emotional and neutral faces in a relatively large sample of healthy children between the ages of 7 and 12.
1.1 Differential Amygdala Responses to Emotional Faces in Adults
There is clear evidence that the amygdala is more active in response to fearful faces than neutral faces in adults (Breiter et al., 1996; Bryant et al., 2008; Pessoa et al., 2002; Surguladze et al., 2003; Wright et al., 2001), but mixed evidence as to whether amygdala responses to fearful faces are stronger than responses to other types of emotional faces. Some studies have found greater amygdala activity to fearful compared to happy faces (Morris et al., 1998; Whalen et al., 1998) or disgusted faces (Phillips et al., 2004), and a meta-analysis found that amygdala activity to fear stimuli was significantly more likely than activity to sad and happy stimuli (Phan et al., 2002). However, other studies have shown amygdala responsivity to a wide variety of emotional stimuli in healthy adults (Breiter et al., 1996; Iidaka et al., 2001; Pessoa et al., 2002; Surguladze et al., 2003; Wright et al., 2001), and a more recent meta-analysis showed consistent amygdala activity in response to happy, sad, and fearful faces (Fusar-Poli et al., 2009), though this meta-analysis also provided some evidence for greater amygdala responsivity to fearful faces compared to either happy or sad faces. Thus, the literature provides robust evidence for greater amygdala activity to emotional as compared to neutral faces in adults, with somewhat more mixed evidence as to whether this activity is greater for fearful compared to others types of emotional faces.
1.2 Differential Amygdala Responses to Emotional Faces Across Development
The ability to discriminate facial emotions develops in early childhood and improves at differential rates for different emotion types through school age into adulthood (De Sonneville et al., 2002; Herba et al., 2006; Thomas et al., 2007; Vicari et al., 2007). Similarly, the neural circuitry for emotional processing develops over early childhood and adolescence. It has been suggested that amygdala responses to emotional stimuli, including faces, follow an inverted U-shaped developmental trajectory, where adolescents show stronger emotional responses than children or adults (Moore et al., 2012). This may be due to the differential developmental trajectories of the amygdala and prefrontal cortex (PFC) control regions, where the amygdala and other sub-cortical regions reach maturity earlier than cortical regions that contribute to their regulation (Casey et al., 2010; Somerville and Casey, 2010). Several studies using fMRI emotional processing tasks provide support for heightened emotional reactivity during childhood and adolescence as compared to adulthood (Guyer et al., 2008; Monk et al., 2003) and in adolescence greater than adulthood or childhood (Hare et al., 2008). However, other studies have found different relationships between amygdala activity and age. For example, in a study of children and adolescents (9-17 years old), age negatively correlated with left amygdala response to fearful faces, particularly in females (Killgore et al., 2001).
Further, the age at which differential amygdala responsivity to emotional and neutral facial expressions emerges is unclear. Examining the few studies focused on amygdala reactivity in healthy children and adolescents, in addition to studies on psychopathology that report results from healthy control samples, the data on when differential amygdala reactivity to emotional and neutral faces arises are very mixed. Two studies of children/adolescents that included a wide age range (9-17 years old, N = 31; N =17) showed significantly greater amygdala responses to fearful as compared to neutral faces (Guyer et al., 2008; Monk et al., 2003). Similarly, the healthy controls (mean age 13 years old, N = 17) in a study of pediatric obsessive-compulsive disorder showed significant right amygdala activity for fearful vs. neutral faces and significant bilateral amygdala responses to the combined contrast of fearful, happy, and disgusted faces vs. neutral faces (Britton et al., 2010). On the other hand, studies that have included or focused on younger children have not consistently shown greater amygdala responses to fearful or emotional faces as compared to neutral faces. Two such studies examining emotion processing in late school age children found the amygdala showed deactivation to fearful faces or significantly less activity to fearful faces compared to neutral faces (Lobaugh et al., 2006 [10 to 12 year olds, N = 10]; Thomas et al., 2001a [healthy controls: mean age 11 years old, N = 12]). The healthy control children in three other studies (8 to 15 year olds, N = 15; mean age 10 years old, N = 27; 10-16 year boys, N = 16), exhibited no difference in amygdala responses to fearful faces compared to neutral/calm faces (Tottenham et al., 2011). Yet, one study using magnetoencephalography did find differential activity in the left amygdala for fearful/happy faces vs. neutral faces in younger children (7-10 year olds, N = 10), but did not find this differential activity in older children and adults when it is typically observed (Hung et al., 2012). Thus, the previous literature exploring emotion processing in school age children does not provide clear developmental norms. This may be due to the differing age ranges of the participants included in the previous studies (7-17 years old). Studies including older children more often find adult-like differentiation in amygdala responses to different emotions, while studies including mostly younger children find more mixed evidence for this. Inconsistencies in the literature may also be in part due to the small samples sizes of healthy participants used in some studies (range 10-31, median of 16 subjects). As such, a study with a relatively large sample size and a relatively restricted age range is needed to clarify whether or not there is differential amygdala activity to different emotional faces in healthy school-age children to inform developmental norms.
1.3 Sex and Pubertal Influences on Amygdala Response to Emotional Faces
A number of studies have also examined sex differences in amygdala responsivity during emotional processing. In adult males, the amygdala is larger than in adult females, even when adjusting for whole brain volume (Hamann, 2005). In addition, males and females show different patterns of laterality in the amygdala responses that predict subsequent memory for emotionally evocative stimuli (Cahill, 2010; 2004), with right amygdala activity predicting subsequent memory in males, and left amygdala activity in females. Yet, sex differences in amygdala responsivity to emotional faces have been less clear. Early meta-analytic work did not find clear effects of sex on amygdala activity (Wager et al., 2003). However, a more recent meta-analysis by Fusar-Poli et al. (2009) found significantly greater right amygdala activity in adult males than females, with a recent large-scale study confirming these findings in adolescents (Schneider et al., 2011). There has been little examination of sex differences in amygdala responsivity to emotional faces in school age children.
Puberty may play a role in the inverted U-shaped trajectory of amygdala responses described above. It has been noted that pubertal development positively correlates with the higher magnitude of responses in the amygdala (and thalamus and extrastriate visual cortex) to emotional faces during passive viewing. This relationship was observed for viewing angry, fearful, happy, sad, and neutral faces in 13-year-old children, but the same positive relationship between pubertal development and amygdala responsivity was only observed for viewing neutral faces in children 10 years of age (Moore et al., 2012). Greater left amygdala activation has also been found for neutral faces as compared to non-face control stimuli in pre/early pubertal as compared to mid/late pubertal adolescents (Forbes et al., 2011). Based on these findings puberty has been characterized as a developmental period with heightened amygdala activation to emotions.
1.4 Amygdala Hyperactivity in Depression
The amygdala is also hypothesized to be a key structure in the pathology of depression. Elevated amygdala activation during emotion processing tasks has been clearly shown in depressed adults, adolescents, and children (e.g. Barch et al., 2012; Beesdo et al., 2009; Gaffrey et al., 2011; Surguladze et al., 2005; Yang et al., 2010). Amygdala hyperactivity has also been shown to be reduced after antidepressant treatment in adults (Sheline et al., 2001) and this reduction predicts better treatment outcomes among adult depressed patients (Canli et al., 2005; Siegle et al., 2006) potentially suggesting that amygdala response to faces may be a correlate of an underlying disruption or bias in emotion processing and is impacted by treatment of depression. Interestingly, amygdala hyperactivity to emotional faces is also observed in individuals at risk for developing depression (Monk et al., 2008; Zhong et al., 2011). Importantly, even subclinical levels of depression in otherwise psychiatrically healthy adults have been shown to correlate positively with amygdala reactivity to negative vs. neutral words (Laeger et al., 2012) and to the expectation of negative pictures (Abler et al., 2010). Yet, it is unclear whether subclinical levels of depression (or normative depressive emotions) may relate to amygdala reactivity in psychiatrically healthy children. Thus, another goal of the current study was to examine the relationship between self and parent reports of subclinical depressive symptoms in school-age children and amygdala reactivity to emotionally valenced faces.
1.5 Current Study
In summary, it is unclear from the developmental literature whether children exhibit differential amygdala responses to different emotionally evocative and neutral faces. Discrepant findings in the literature may be due to the small samples sizes of healthy children and the wide age range of participants in some studies. As such, the goal of the current study was to assess amygdala responsivity during facial emotion processing in a relatively large sample of healthy children (7-12 years old) using fMRI in an attempt to inform developmental norms. We tested the hypothesis that children show greater activity for emotional than neutral faces and differential amygdala activity to different facial emotion expressions (happy, angry, sad, fearful, and neutral) during a gender judgment fMRI task. Additionally, we tested the hypotheses that amygdala reactivity to emotional faces would increase as a function of sex (greater amygdala activity in males), age (greater amygdala activity as children approach adolescence), and pubertal development (greater amygdala activity as children progress through puberty). Further, given the link between depression and elevated amygdala reactivity to emotional vs. neutral faces, we tested the hypotheses that amygdala reactivity would increase as a function of subclinical depression symptom severity and emotional regulation impairments in healthy children. To better characterize normative emotion processing in this sample of healthy school age children, we also followed the current emphasis in the field on providing whole brain results in addition to focused a priori region of interest analyses and tested for effects of emotion type, sex, puberty, and a relationship with participant age at a whole-brain level. By employing a relatively large sample of school-age children who have been thoroughly screened for any psychiatric diagnoses for several years prior to scanning, the results of the study can provide key insights into normative emotion processing in 7-12 year old children. Particularly, our findings can clarify discrepancies in the literature by testing whether children in this age range show adult-like differentiation in amygdala reactivity to emotional faces. Additionally, it is pivotal to understand amygdala reactivity to emotional faces in a psychiatrically healthy population to serve as a foundation for understanding findings in childhood psychopathology.
2. Materials and Methods
2.1 Participants
The participants represented a subsample of children enrolled in the Preschool Depression Study (PDS), a prospective longitudinal study of preschool age children and their families conducted at the Washington University School of Medicine Early Emotional Development Program (WUSM EEDP) in St. Louis. The broad goals of the PDS were to explore clinical and neural outcomes relating to preschool-onset major depression. As such, 3- to 5-year-old children and their primary caregivers were recruited from local daycares, preschools, and primary care sites in the St. Louis metropolitan area oversampling for children expressing symptoms of depression while also recruiting healthy children who did not meet diagnostic criteria for any psychiatric conditions (see Luby et al., 2009) for further recruitment details). The children were assessed annually and then participated in a neuroimaging session between the ages of 7 and 12. An additional 40 psychiatrically healthy children were recruited into the PDS when they were between the ages of 7 and 12, at the time of scanning. The in-depth clinical assessments involved in the PDS allowed us to identify a normative and psychiatrically healthy population of school age children to include in the current analyses. Thus, the current subsample represents 52 children who did not meet criteria for any psychiatric diagnosis at the initial or any subsequent assessment timepoints up to the time of scan (N = 22) and psychiatrically healthy participants recruited into the PDS at the time of scan (N = 30). All participants in this subsample participated in the neuroimaging phase of the PDS (see Table 1 for demographic details). A history of head trauma, neurological disease, developmental delay, and use of psychoactive medications were exclusionary for the current analyses. Additionally, only participants who met stringent criteria for neuroimaging data quality were included in this analysis (of the 67 psychiatrically healthy children who participated in the neuroimaging session, 52 met data quality criteria, further details in section 2.7 Movement Correction). Parental written consent and child assent were obtained prior to study participation and the Institutional Review Board at Washington University approved all experimental procedures.
Table 1.
Demographic characteristics of the sample by sex
| Male (N=28) | Female (N = 24) | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Age at Scan (years)* | 28 | 10.71 | 1.25 | 24 | 9.86 | 1.27 |
| Ethnicity | 28 | 24 | ||||
| % Caucasian | 64% | 58% | ||||
| % African American | 36% | 29% | ||||
| % Other Ethnicity | 0% | 13% | ||||
| % Right Handed | 28 | 89% | 24 | 79% | ||
| CDI-C | 27 | 40.48 | 5.51 | 22 | 40.23 | 3.56 |
| CDI-P* | 28 | 39.04 | 4.01 | 22 | 44.27 | 7.32 |
| CEMS-S | 22 | 21.09 | 3.10 | 17 | 21.41 | 3.10 |
| Tanner Pubertal Status | 28 | Mean Age | SD | 24 | Mean Age | SD |
| Stage I* | 46% | 9.99 | 1.17 | 58% | 8.96 | 0.63 |
| Stage II* | 32% | 10.89 | 0.73 | 8% | 10.21 | 0.06 |
| Stage III/IV/V | 21% | 12.03 | 0.87 | 33% | 11.35 | 0.76 |
The total sample size for each sex group is listed above and the N column for each sex represents the number of participants with data available on each measure.
CDI = Children's Depression Inventory, t-scores (C = child report, P = parent report), CEMS-S = Children's Emotion Management Scale - Sadness
group t-test by sex significant at p < 0.05, significant differences in age by Tanner Status ANOVA p < 0.05 for full sample and by sex I < II < III/IV/V
2.2 Diagnostic Assessment
Trained staff from the WUSM EEDP conducted up to four in-person assessment interviews with participants and their parents/guardians between the baseline assessment and the time of scan. Before the age of 8, children were assessed using the Preschool-Age Psychiatric Assessment (PAPA), a reliable and age appropriate semi-structured parent-report diagnostic interview (based on DSM-IV) widely used in research on preschool-age psychopathology (Egger et al., 2003) and has been validated for children up to the age of 8. Once study participants reached the age of 8, the Childhood and Adolescent Psychiatric Assessment (CAPA) was used. The CAPA also examines symptoms of various psychiatric disorders but, unlike the PAPA, also makes use of report from the child or adolescent in addition to report from the primary caregiver (Angold and Costello, 2000). These interviews were audiotaped, reviewed for reliability, and calibrated for accuracy, as previously reported (Luby et al., 2009). These assessments were used to select a subset of participants from the PDS who did not meet criteria for any psychiatric disorder at any time point from baseline to the time of scan to assure examination of a psychiatrically healthy population.
2.3 Additional Measures
Several other factors were assessed at the time of scan (see Table 1 for details). The severity of depression symptoms at the time of scan was assessed using the child- and parent-report versions of the Children's Depression Inventory (CDI-C and CDI-P, respectively; Kovacs, 1985). The scores reported here represent standardized t-scores totaled across the subscales of the CDI. A t-score of 50 represent an average score while a score above 65 may identify a potentially depressed individual (consistent with the fact that no children met criteria for MDD during any PAPA/CAPA interview, all children scored below 65 on the CDI).
The Children's Emotion Management Scale for Sadness (CEMS-S; Zeman et al., 2001) was used to assess the participants’ behavioral responses to sadness. This consists of 15 statements, each assessed on a 1-3 Likert scale. These questions have been shown to probe three dimensions of sadness management: inhibition, dysregulation, and coping. Higher scores on the inhibition and dysregulation subscales are endorsement of more frequent use of ineffective management strategies (e.g. “I do things like mope when I am sad”; previous results show positive correlations between the dysregulation and inhibition subscales and CDI scores Zeman et al., 2001). The coping subscale was reverse-scored so that higher scores also indicate less effective emotion management. A total score across these three subscales was used for the current analyses, with higher scores indicating less effective sadness management skills.
Parental report on the Tanner Staging Questionnaire (Tanner, 1955) was used to assess pubertal status. Children under 10 years old were not assessed using the Tanner Staging Questionnaire due to the sensitive nature of the questions. All children under 10 years old were assigned to the Stage I group to represent presumed pre-pubertal status. Due to the young age of the sample, few participants were identified as being in the later (IV and V) stages of puberty and thus stages III, IV, and V were collapsed into one late-puberty group. Pubertal status was assessed as a 3 level factor with pre (I), early (II) and late (III-V) puberty groups.
2.4 Facial Emotion Processing Task
The facial emotion processing task represents one segment of a longer scanning session that the children in the PDS completed, which included high resolution structural imaging, diffusion imaging, and resting state functional connectivity. This task is the only aspect of the scanning session used for the current analysis. During the task, children were shown a series of faces and were asked to judge the gender of the face, responding by button press to indicate whether the face was male or female. This task was chosen as previous research has indicated that the heightened amygdala responses to emotional faces associated with MDD may become more apparent with an implicit processing task rather than one requiring explicit emotion processing (Fales et al., 2008; Monk et al., 2008). This task was also preferable to a passive viewing task as it helps to ensure engagement with the visual stimuli. The face stimuli were drawn from the MacArthur Network Face Stimuli Set, a validated stimulus set containing images of 43 different actors from different ethnic backgrounds (Tottenham et al., 2009). Children saw faces with neutral, sad, angry, happy, and fearful expression from 10 of the individuals in this stimulus set. One goal of the PDS was to probe potential emotional biases relating to preschool-onset depression apparent with varying intensity of emotional facial expressions. To this end, we created intermediate sad, angry, happy, and fearful expressions by morphing the neutral expression for each individual with each emotional expression resulting in a face halfway between the neutral and each emotional face (MorphAge software). Yet, as this was not of direct interest in the current analysis, we collapsed across the full and half intensity faces for each emotion type to increase our power to find effects of emotion type. Preceding this task, all children underwent a mood induction paradigm in the scanner based on prior work (Gotlib et al., 2005). Previous work has shown that negative mood induction can reactivate affective processing biases (Scher et al., 2005) and neural changes in affective processing (Ramel et al., 2007) specifically in patients with a history of depression while not introducing any affective biases in individuals who have not experienced a history of depression. Given that the goals of the PDS were to explore the effects of a history of preschool-onset major depression on the brain, the mood induction was included for all children to reactivate any affective processing biases in children with a history of preschool-onset major depression who were depressed at the time of scan. The mood induction was short clip from the film “My Girl” (Gazer and Zieff, 1991) that portrays a child's loss of a close friend followed by verbal instructions to elaborate on any negative affect induced. A positive mood repair clip was shown at the end of the MRI session. Because we are only examining children in this subsample who were psychiatrically healthy at all assessments through the time of scan, we did not expect this mood induction to introduce any bias in the current results. To confirm this, we used the children's report of their mood before and after the induction (on a 5-point Likert-type scale; 1= very sad to 5 = very happy) to test whether post-induction mood or change in reported mood correlated with amygdala activity to rule out any induced bias.
Participants performed 2 runs, each with 45 stimuli, totaling 90 trials for each participant (18 from each of the 5 emotion types). Each stimulus was presented for 2250 ms, followed by an inter-trial interval of 250 ms, 2750 ms, or 5250 ms (each occurring at equal frequency). Gender judgment button responses were acquired during the stimulus presentation period via a fiber optic button box. Images were projected onto a computer screen behind the participant's head within the imaging chamber viewed by a mirror attached above the participant's face.
2.5 fMRI scanning
Structural and functional imaging data were collecting using a 3.0 Tesla TIM TRIO Siemens whole body system at Washington University in St. Louis. T1-weighted structural images were acquired in the sagittal plane using an MPRAGE 3D sequence (TR = 2400 ms, TE = 3.16 ms, flip angle = 8°, slab = 176 mm, 176 slic es, matrix size = 256×256, field of view (FOV) = 256 mm, voxel size = 1×1×1 mm). Functional images were collected during the face processing task with a 12-channel head coil using a T2*-weighted gradient-echo echo-planar sequence in the axial plane (TR = 3000 ms, TE = 27 ms, flip angle = 90°, FOV = 256 mm, voxel size = 4×4×4 mm). T2-weighted images were collected for registration purposes using a 3D SPACE acquisition (TR = 3200ms, TE = 497 ms, 160 slices, FOV = 256, voxel size = 1×1×1 mm).
2.6 fMRI pre-processing
Imaging data were preprocessed using the following steps: (1) correction for slice-dependent time shifts; (2) removal of first 4 images of each run to allow BOLD signal to reach steady state; (3) elimination of odd/even slice intensity differences due to interpolated acquisition; (4) realignment of data acquired from each participant within and across runs to compensate for rigid body motion (Ojemann et al., 1997); (5) image intensity normalization to a whole-brain mode value of 1000; (6) registration of the 3D structural volume (T1) to the atlas template in the Talairach coordinate system (Talairach and Tournoux, 1988) using a 12-parameter affine transform and re-sampling to 1 mm cubic representation (Buckner et al., 2004; Ojemann et al., 1997); (7) co-registration of the 3D fMRI volume to the T2, and the T2 to the participant's structural image; (8) transformation of the fMRI data to 3×3×3 mm voxel atlas space using a single affine 12-parameter transform; and (9) spatial smoothing using a 6 mm full-width half-maximum Gaussian filter.
2.7 Movement Correction
Stringent data quality criteria were used for participant inclusion in the current analyses. The signal-to-noise ratio (SNR: mean signal/standard deviation across each BOLD run, computed for each slice and then averaged across all slices) for each of the two task runs was calculated using in-house software following preprocessing. Only participants with an SNR for each of the task runs above 200 were included in the current analyses (mean SNR for first run: 579.83 ± 196.35, minimum = 223; mean SNR for second run: 510.35 ± 185.15, minimum = 230). Out of 67 psychiatrically healthy children who met all other inclusion criteria, 52 met these further criteria (i.e. 15 were excluded for not completing both runs of the task or for having an SNR > 200 on at least one run). After the pre-processing steps described above, we applied previously validated corrections for head motion, termed “motion scrubbing”, adapted for fMRI data (Power et al., 2012). The motion scrubbing procedure assesses frame-wise displacement based on the movement parameters used in pre-processing step 4. For any given frame (i.e. timepoint), this represents the differential head motion from the previous frame summing across linear (x,y,z) and rotational displacements (yaw, pitch, roll, where degrees of rotation are converted to millimeters of movement by calculating displacement on the surface of a sphere with a radius of 50mm). A temporal mask was created to remove any frame with a sum displacement greater than 0.5mm. This threshold was selected to be stringent and remove any spikes in head motion while still maintaining the majority of the data. Details on the validity and efficacy of this procedure and on the number of frames removed by the motion scrubbing are presented in the Supplementary Materials section.
2.8 fMRI Analysis
Analysis of fMRI data was performed using in-house software (FIDL analysis package, http://www.nil.wustl.edu/~fidl/). A voxel-wise general linear model (GLM) approach was used, which incorporated regressors for linear trend and baseline shifts. We did not assume a hemodynamic response shape for this analysis because of concerns about potential age-related differences in the shape or timing of this response (e.g. Huettel et al., 2001; Jacobs et al., 2008; Richter and Richter, 2003). Instead, a finite impulse response approach was used where 7 frames (7 2-second TRs per trial) were modeled for each trial. Only those trials on which the participant made a correct gender judgment were included in the analysis, though there were overall very few incorrect trials (mean error rate = 4.5%, between 0-7 errors/emotion type across subjects, mean 1.7 errors per emotion type). We performed region of interest (ROI) analyses focusing on the amygdala and then exploring effects at the whole-brain level. Both the primary ROI-based and whole-brain analyses consisted of a repeated-measures ANOVA with time (7 timepoints per trial) as the repeated-measure, emotion (5 facial expression types: neutral, sad, angry, happy, fearful) as a within-subject factor, and sex and pubertal status as between-subject factors. With this analysis, a significant main effect of time indicates that there are differences in activity across timepoints (testing the null hypothesis that activity is equal at all timepoints). We did not focus on main effects of emotion, sex, or puberty (which test for mean differences in these factors averaged across timepoints) as these often can indicate baseline shifts or poor modeling by the GLM in certain regions and are less meaningful than interactions with time. Any interactions with time indicate a significant difference in activity across conditions over at least some timepoints (e.g., a difference in peak amplitude or in shape). We did not attempt to interpret any four-way interactions (time × emotion × sex × pubertal) because of the complexity of such effect and their likely lack of robustness. However, we did examine up to the three-way interactions.
2.8.1 Amygdala ROI Analyses
Amygdala ROIs were defined in two ways: functionally and anatomically. The functionally defined ROIs were isolated from the whole-brain main effect of time from the ANOVA described above and in section 2.8.2 Whole-Brain Analyses. These ROIs selected voxels that showed significant activation to the task at the group level, but may not clearly represent the anatomical boundaries of the amygdala for individual participants. Thus, as an alternative approach, to define amygdala ROIs with greater anatomical specificity, we used FreeSurfer v4.5.0 (Fischl et al., 2002; 2004) to segment each participant's T1 anatomical image. Amygdala ROIs were extracted for each participant, down-sampled to match the functional resolution of the atlas space (3×3×3mm), and registered to the common atlas space using each participant's whole-brain transformation parameters from preprocessing. Mean timecourses for each emotion type were extracted for each participant from the group-level functional ROIs and from his or her individually defined anatomical amygdala ROIs. The ANOVA described above was performed on this data using IBM SPSS Statistics 18.0 (SPSS Inc., Chicago, Illinois). Additionally, correlation analyses were performed to explore the relationship between amygdala activity and age, CDI-C, CDI-P, and CEMS-S scores. Control correlations were also performed with post-induction mood and change in reported mood due to the mood induction to affirm that this prior task had no effect on the current results. These factors were correlated with the average activity from the anatomically defined amygdala ROIs for each emotion type, as well as the comparison of fearful faces to neutral faces and of sad faces to neutral faces.
2.8.2 Whole-Brain Analyses
The repeated-measures ANOVA described above was performed using the FIDL analysis package to examine effects of time, emotion, sex, and pubertal status at the whole-brain level. Based on Monte Carlo simulations implemented with in-house software, a significance threshold of 13 contiguous voxels with z-values >3.0 was used to obtain a whole-brain false positive rate of 0.05. The resulting significance maps were separated into ROI clusters using in-house peak finding scripts which identify peaks in z-scores at least 15mm apart from each other. These ROIs were used to examine the timecourse of the hemodynamic response and for post-hoc contrasts to parse the source of significant effects (Bonferroni corrected for the number of post-hoc tests performed for each ROI). Additionally, participant age was correlated at every voxel with the average activity for each emotion type. Based on Monte Carlo simulations, a significance threshold of 17 contiguous voxels with z-values >3.0 was used to obtain a whole-brain false positive rate of 0.05.
3. Results
3.1 Demographic Characteristics
Table 1 presents the demographic characteristics of the sample. Given the interest in potential effects of sex in emotional processing, we explored sex differences in the distribution of the other demographic factors. There was no significant difference in ethnic distribution as a function of sex (χ2(2, N = 52) = 3.74, p = 0.15). Most of the participants were right handed (44/52 participants) and there was no significant difference as a function of sex (χ2(1, N = 52) = 1.66, p = 0.44). The males in this sample were significantly older than the females (t(51) = 2.41, p = 0.02). However, no significant difference in the distribution of pubertal status was found as a function of sex (χ2(2, N = 52) = 4.50, p = 0.11), though the males were significantly older than females in Stages I (t(25) = 2.81, p = 0.01) and II (t(9) = 8.42, p = 0.02). Additionally, there was a significant relationship between pubertal status and age (Spearman's ρ(50) = 0.75, p < 0.001) No significant sex differences were found for scores on the CDI-C (t(48) = 0.19, p = 0.85) or CEMS-S (t(38) = 0.57, p = 0.57). However, the females in this sample showed higher t-scores on the CDI-P than the males (t(49) = 3.22, p = 0.002), though both groups were still in the non-depressed range (< 65).
3.2 Amygdala ROI Results
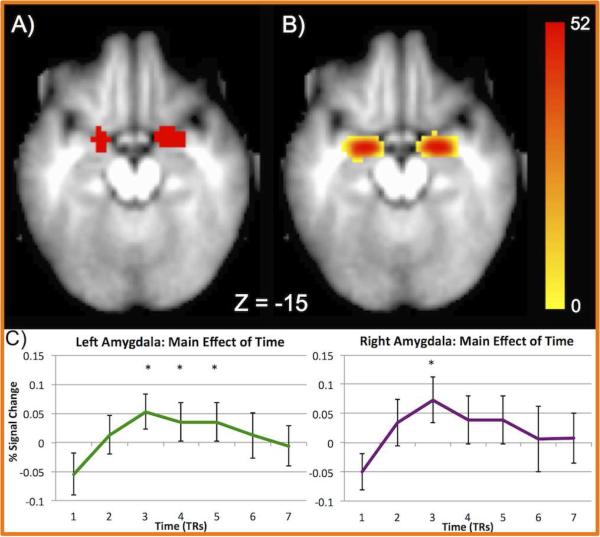
3.2.1 Amygdala ROI ANOVA
Table 2 presents the results of the ANOVA for the functionally and anatomically defined amygdala ROIs (Figure 1 shows these ROIs and the average timecourses for the anatomically defined ROIs). These results demonstrate that the amygdala is active during face viewing, given the significant main effect of timepoint found for both the bilateral amygdala, defined both functionally and anatomically (e.g. anatomical left amygdala ROI: F(3.46) = 5.63, p < 0.001, partial η2 = 0.11). However, no other effect reached significance in either the functionally or anatomically defined amygdala ROIs. While it is difficult to draw inferences with certainty from null results, we feel that our sample size provided sufficient power to isolate even a small effect of emotion type. Specifically, using G*Power (Faul et al., 2007), we derived post-hoc estimates of our achieved power to find significant effects of a within-subject condition (i.e. emotion type) with a repeated-measures ANOVA at the small effect sizes observed in these data. With 52 participants, we had power of 0.62 to detect a small effect with a partial eta2 of 0.01 and power over 0.99 to detect an effect with a partial eta2 of 0.03 or greater. We conducted three additional analyses to confirm these results: 1) a repeated-measures ANOVA including all 7 timepoints including only time and emotion type as factors, given that including pubertal status and sex as between-subject factors could lower power to detect effects of emotion; 2) a repeated-measures ANOVA with only emotion type as a factor and using only timepoint 3 (the peak timepoint of the average timecourses); and 3) a repeated-measures ANOVA with only emotion type as a within-subject factor using data modeled with an assumed hemodynamic response (SPM canonical function). The results of these three additional ANOVAs were essentially identical to those observed in Table 2 (i.e. significant amygdala responsivity to all face types, but no significant effect of emotion × time in both the left or right amygdala) and are presented in Supplementary Table 1 and Supplementary Figure 4. Additionally, to assure that including half-intensity emotional faces did alter the results, we also ran an ANOVA including only the full-intensity faces and still found no effect of emotion type (ps > 0.78).
Table 2.
Amygdala ROI ANOVA results from functionally and anatomically defined ROIs
| Functional ROIs | Anatomical ROIs | |||||||
|---|---|---|---|---|---|---|---|---|
| Left Hemisphere | df^ | F | p | Partial η2 | df | F | p | Partial η2 |
| Time* | 3.04 | 5.32 | 2E-03 | 0.10 | 3.46 | 5.63 | 1E-03 | 0.11 |
| Emotion × Time | 9.04 | 1.04 | 0.41 | 0.02 | 11.26 | 0.73 | 0.72 | 0.02 |
| Sex × Time | 3.04 | 1.16 | 0.33 | 0.03 | 3.46 | 1.16 | 0.33 | 0.03 |
| Emotion × Sex × Time | 9.04 | 0.77 | 0.64 | 0.02 | 11.26 | 0.40 | 0.96 | 0.01 |
| Puberty × Time | 6.08 | 0.41 | 0.88 | 0.02 | 6.92 | 0.83 | 0.56 | 0.04 |
| Emotion × Puberty × Time | 18.09 | 0.61 | 0.90 | 0.03 | 22.53 | 0.56 | 0.95 | 0.02 |
| Sex × Puberty × Time | 6.08 | 0.43 | 0.86 | 0.02 | 6.92 | 0.63 | 0.73 | 0.03 |
| Right Hemisphere | df | F | p | Partial η2 | df | F | p | Partial η2 |
|---|---|---|---|---|---|---|---|---|
| Time* | 3.70 | 9.49 | 1E-06 | 0.17 | 2.99 | 4.85 | 3E-03 | 0.10 |
| Emotion × Time | 10.24 | 0.88 | 0.55 | 0.02 | 10.50 | 0.72 | 0.72 | 0.02 |
| Sex × Time | 3.70 | 0.77 | 0.54 | 0.02 | 2.99 | 0.75 | 0.53 | 0.02 |
| Emotion × Sex × Time | 10.24 | 0.73 | 0.70 | 0.02 | 10.50 | 0.43 | 0.94 | 0.01 |
| Puberty × Time | 7.40 | 0.78 | 0.61 | 0.03 | 5.97 | 0.78 | 0.59 | 0.03 |
| Emotion × Puberty × Time | 20.47 | 0.65 | 0.88 | 0.03 | 21.00 | 0.53 | 0.96 | 0.02 |
| Sex × Puberty × Time | 7.40 | 0.69 | 0.69 | 0.03 | 5.97 | 0.50 | 0.81 | 0.02 |
This table represents the results of repeated-measures ANOVAs of left and right amygdala activity from functionally and anatomically defined ROIs. Emotion (5 face types) is a within-subject factor, time (7 timepoints/trial) is the repeated measure, and sex and pubertal status (3 groups) are between-subject factors.
Mauchly's Test was used to test the assumption of sphericity. df values represent numerator degrees of freedom adjusted for non-sphericity by Greenhouse-Geisser correction.
Figure 1. Functionally and anatomically defined amygdala ROIs.
A) Functionally defined amygdala ROIs isolated from the whole-brain main effect of time B) Anatomically defined amygdala ROIs isolated by FreeSurfer segmentation. Heat map values indicate the number of participants with overlapping ROIs at each voxel. C) Timecourses (average of all face types) for left and right anatomically defined ROIs with 95% confidence intervals. * = significant difference from zero at a given timepoint (p < 0.05)
3.2.2 Amygdala ROI Correlations
Table 3 presents the correlations between average activity for the different emotion types from the anatomically defined amygdala ROIs and age, CDI-C, CDI-P, and CEMS-S scores. In addition, we examined the differences in activity between sad and neutral faces and between fearful and neutral faces. To control false positives due to multiple comparisons, we used a false discovery rate (FDR) correction within measure and hemisphere. Partial correlations were used to control for the effect of sex in the age and CDI-P correlations, given that the boys and girls showed significant mean differences on these variables. No significant correlations were found between age and amygdala activity to any emotion type. CDI-C scores were not normally distributed in this sample (Kolmogorov-Smirnov test of normality: Z(47) = 1.59, p = 0.013), and as such, non-parametric correlations with CDI-C were calculated (Spearman's ρ). Significant negative correlations were found between bilateral amygdala activity to neutral faces and CDI-C scores, though only the correlation with left amygdala activity survived FDR correction. Significant positive correlations were found between parent report CDI scores and activity to angry, happy, and fearful faces, although only correlations with angry and happy face activity survived FDR correction. Finally, CEMS-S scores were positively correlated with right amygdala activity to sad faces as well as activity to sad–neutral faces and fearful–neutral faces, though only the correlation with fearful-neutral faces survived FDR correction. Post-induction mood and change in reported mood due to negative mood induction were not correlated with average activity, peak activity, or magnitude estimates with a modeled hemodynamic response function for any emotion type, sad-neutral faces, or fear-neutral faces (all ps > 0.1).
Table 3.
Correlations of average activity from anatomical amygdala ROIs with age, CDI-C, CDI-P, and CEMS-S scores
| Condition | Side | Neutral | Angry | Sad | Happy | Fear | Sad - Neutral | Fear - Neutral |
|---|---|---|---|---|---|---|---|---|
| Age (sex) | Left | 0.027 | 0.114 | -0.186 | 0.143 | -0.040 | -0.171 | -0.084 |
| N = 52 | Right | -0.001 | 0.121 | -0.132 | -0.025 | -0.103 | -0.202 | -0.076 |
| CDI-C | Left | -.450*** | 0.066 | -0.091 | 0.081 | -0.191 | 0.259 | 0.172 |
| N = 49 | Right | -.366** | -0.072 | -0.233 | -0.017 | -0.198 | 0.173 | 0.211 |
| CDI-P (sex) | Left | 0.250 | 0.416** | -0.019 | 0.407** | 0.286* | -0.257 | -0.063 |
| N = 50 | Right | 0.225 | 0.228 | 0.025 | 0.366** | 0.144 | -0.186 | -0.071 |
| CEMS-S | Left | -0.052 | 0.111 | 0.276 | -0.155 | 0.060 | 0.224 | 0.100 |
| N = 39 | Right | -0.222 | 0.262 | .355* | 0.194 | 0.296 | 0.496** | .374* |
All correlation values represent Pearson's r values, except non-parametric correlations with CDI-C (Spearman's ρ.) Partial correlations controlling for sex were used for age and CDI-P correlations. Cells shaded in gray survived FDR correction.
p < 0.05
p < 0.01
p < 0.001
Our analyses of puberty categorized individuals into pre, early, and late puberty groups. However, other work has examined correlations across a five level puberty range (Moore et al. 2012). To try to replicate these prior findings more directly, we performed Spearman correlations between pubertal status using the 5 Tanner stages and amygdala responses in addition to the ANOVAs reported. We found that right amygdala responses to sad faces and the sad – neutral faces contrast negatively correlated with pubertal status (sad: ρ(50) = -0.285, p = 0.041, sad – neutral: ρ(50) = -0.330, p = 0.017), though these effects did not pass correction for multiple comparisons.
3.3 Whole-Brain Results
3.3.1 Whole-Brain ANOVA
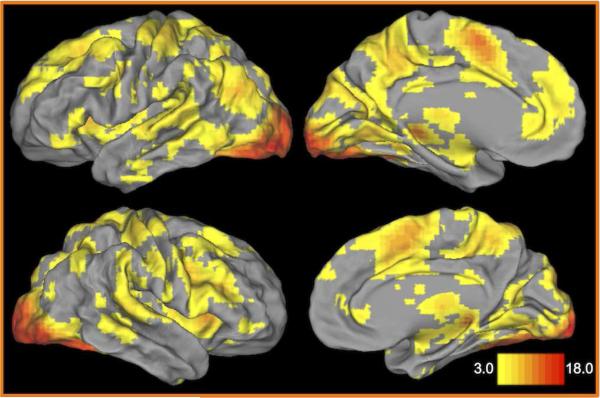
Figure 2 shows the whole-brain ANOVA results for the main effect of time (projected to the surface of the brain rendering). This encompasses a wide range of regions including occipital cortex regions, the fusiform gyrus, response-related motor regions, anterior and posterior cingulate regions, prefrontal regions, and a number of limbic regions, including the amygdala and hippocampus. The interaction between time and face emotion type was significant in several areas shown in Table 4. These areas included the left superior temporal gyrus, regions of the cerebellum, the cuneus, and the inferior parietal lobule. To clarify the source of these interactions, we computed post-hoc contrasts by examining whether there was an emotion × time interaction when each of the 4 emotional face types was compared to neutral faces (repeated-measures ANOVA with 2 emotion types and 7 timepoints). The results of these post-hoc contrasts are shown in Table 4 (significant comparisons are noted, Bonferroni corrected). Supplementary Figure 5 shows timecourses for each emotion type separately for two example regions. Notably, the amygdala was not among the regions showing a time × emotion type interaction.
Figure 2. Brain regions showing a main effect of time (timepoint within trial).
Surface renderings of the thresholded main effect of time from the whole-brain ANOVA results. The color scale indicates z-score values.
Table 4.
Emotion × Time, Sex × Time, and Emotion × Sex × Time interactions from the whole-brain ANOVA
| Effect | xa | y | z | Voxels | Fb | p-valuec | Region | BA | Post-Hoc |
|---|---|---|---|---|---|---|---|---|---|
| Emotion × Time | -44 | -75 | -37 | 13 | 2.81 | 8.60E-06 | Inferior Semi-Lunar Lobule | A,H,F vs. N* | |
| Emotion × Time | -10 | -87 | 6 | 23 | 2.52 | 7.34E-05 | Cuneus | 17 | A,H vs. N |
| Emotion × Time | 52 | -29 | 32 | 19 | 2.50 | 8.65E-05 | Inferior Parietal Lobule | 40 | A,S,H,F vs. N |
| Emotion × Time | 33 | -10 | 9 | 18 | 2.37 | 2.36E-04 | Claustrum | S,F vs. N | |
| Emotion × Time | -31 | 2 | -9 | 13 | 2.27 | 4.51E-04 | Superior Temporal Gyrus | 38 | S vs. N |
| Sex × Time | 22 | -90 | -2 | 72 | 13.63 | 1.50E-13 | Lingual Gyrus | 17 | F > M act# |
| Sex × Time | 35 | -75 | -11 | 46 | 9.07 | 4.67E-09 | Fusiform Gyrus | 19 | F > M act |
| Sex × Time | 39 | -59 | -13 | 42 | 9.05 | 4.88E-09 | Fusiform Gyrus | 37 | F > M act |
| Sex × Time | 27 | -53 | 51 | 59 | 6.62 | 1.47E-06 | Precuneus | 7 | F > M act; M:ns |
| Sex × Time | 65 | -8 | 5 | 10 | 4.09 | 6.03E-04 | Superior Temporal Gyrus | 22 | F > M,act; M:ns |
| Sex × Time | -42 | -62 | 28 | 24 | 7.12 | 4.51E-07 | Middle Temporal Gyrus | 39 | F > M,act; M:ns |
| Sex × Time | 64 | -23 | 3 | 25 | 5.06 | 6.09E-05 | Superior Temporal Gyrus | 22 | F > M,act; M:ns |
| Sex × Time | 27 | -83 | 41 | 15 | 6.95 | 6.83E-07 | Precuneus | 19 | F > M deact; M: ns |
| Sex × Time | -17 | -87 | 40 | 39 | 7.05 | 5.32E-07 | Cuneus | 19 | F > M deact; M: ns |
| Sex × Time | -5 | -57 | 6 | 25 | 6.58 | 1.65E-06 | Posterior Cingulate | 30 | F > M deact; M: ns |
| Sex × Time | -5 | 36 | 7 | 102 | 4.18 | 4.79E-04 | Anterior Cingulate | 24 | F > M deact; M: ns |
| Sex × Time | -9 | -49 | 26 | 36 | 4.76 | 1.24E-04 | Posterior Cingulate | 31 | F > M deact; M: ns |
| Sex × Time | -42 | -75 | 38 | 22 | 9.52 | 1.62E-09 | Precuneus | 19 | F > M deact |
| Sex × Time | -55 | -60 | 18 | 21 | 3.31 | 3.69E-03 | Superior Temporal Gyrus | 39 | F > M deact; M: ns |
| Sex × Time | -40 | 34 | -8 | 41 | 5.37 | 2.87E-05 | Inferior Frontal Gyrus | 47 | F > M deact; M: ns |
| Sex × Time | -50 | 10 | 45 | 13 | 3.22 | 4.54E-03 | Middle Frontal Gyrus | 6 | M > F act; F: ns |
| Sex × Time | 16 | -76 | -12 | 27 | 4.73 | 1.32E-04 | Declive of Cerebellum | M > F act | |
| Sex × Time | 26 | 4 | 1 | 25 | 3.77 | 1.26E-03 | Putamen | M > F,act; F: ns | |
| Emotion × Sex × Time | 45 | 45 | -1 | 15 | 3.01 | 1.73E-06 | Inferior Frontal Gyrus | 10 | H,F: S×T^ |
Coordinates for peak of cluster
F value at peak coordinates
p-value at peak coordinates, BA= Brodmann Area
Results of post-hoc contrasts comparing each emotion face type to neutral faces (A = angry, S = sad, H = happy, F= fearful, N =neutral)
Results of post-hoc contrasts examining main effects of time within males (M) and females (F) separately (act = activation, deact = deactivation, ns = not significant main effect within sex)
Results of post-hoc contrasts examining Sex × Time interactions (S×T) within each emotion face type separately
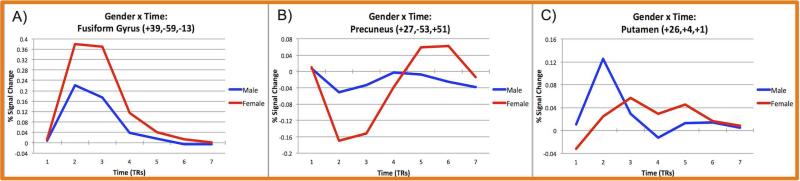
Table 4 (and Supplementary Figure 6) shows the regions demonstrating a significant interaction of sex and time. These regions showed several different timecourse patterns, as shown in Figure 3. First, there were several regions that showed greater activation in females as compared to males (in table 4: F>M act.), including the right lingual gyrus, right fusiform gyrus, right precuneus, and superior and middle temporal gyri. Several other regions showed greater deactivation in females as compared to males (F>M deact.), including the right and left precuneus, the left cuneus, left anterior and posterior cingulate, left inferior frontal gyrus, and left superior temporal gyrus. Post-hoc ANOVAs (7 timepoints as the repeated measure) testing for a main effect of time for each sex separately indicated that most of these regions were active in females but not males, as noted in Table 4. The middle frontal gyrus, declive, and putamen show greater activity in males than females, with no significant main effect of time in these regions for females (M>F act, F:ns).
Figure 3. Example timecourses in brain regions showing three different types of interactions between sex and time.
Lines indicate average timecourses for each sex. A) Fusiform gyrus shows greater activity in females than males (F>M,act). B) Precuneus shows deactivation in females and no significant effect of time in males (F>M deact; M:ns). C) Putamen shows activation in males and no significant effect of time in females (M>F act; F:ns).
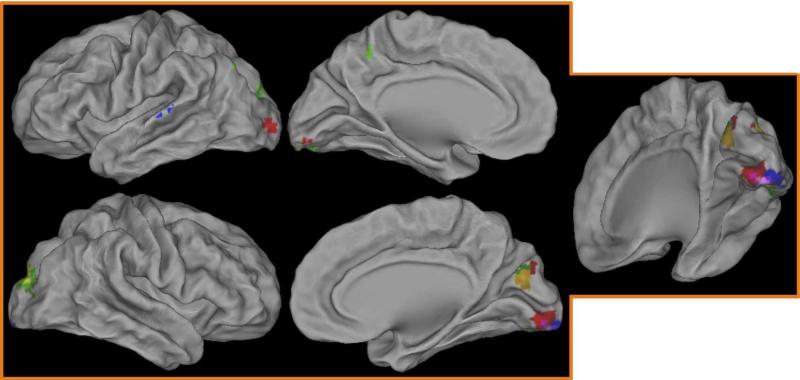
As indicated in Table 4, only one region showed a significant emotion type × sex × time interaction. This right inferior frontal gyrus region is shown in Figure 4. To understand the source of this interaction, we conducted post-hoc repeated-measures ANOVAs separately for each emotion type with time as the repeated-measure (7 timepoints) and sex as a between-subject factor. These post-hoc contrasts indicated a significant sex × time interaction only for happy (F(4.88)=3.49, p = 0.005) and fearful faces (F(4.15)=3.17, p = 0.014, though this effect did not pass Bonferroni correction). As shown in Figure 4, males tend to show earlier peaks in activity to both happy and fearful faces than females.
Figure 4. Timecourse for inferior frontal gyrus region showing emotion × sex × time interaction.
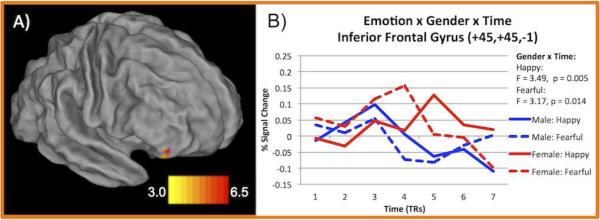
A) Surface rendering of region showing emotion × sex × time interaction. Color scale represents z-score values from whole-brain ANOVA. B) Lines indicate average timecourse for happy and fearful face trials (the only emotion types showing sex × time interaction, F and p-values indicated) split by sex.
Table 5 and Figure 5 show the ANOVA results for the interactions with pubertal status. As seen in Figure 5, the regions showing significant interactions with puberty are mainly located in primary and secondary visual cortex regions. As indicated by the post-hoc tests in Table 5 and the timecourses in Supplementary Figure 7, the regions with a significant puberty × time interaction show increased activity in the late and/or early puberty groups as compared to the pre-pubertal group including the lingual, fusiform, and inferior occipital gyri. The cuneus and middle occipital gyrus show this increased late vs. pre-pubertal group activity as well as differentiation between the late and early groups. Several regions show an emotion × puberty × time interaction, including the fusiform gyrus, lingual gyrus, and transverse and superior temporal gyri. A variety of visual regions also show a puberty × sex × time interaction. Post-hoc tests showed that the interaction in most regions was driven by puberty × time interactions in the females where the early puberty females show increased activity. The lingual gyrus and precuneus also show differential activity in the males across puberty groups where the males in late puberty show elevated activity.
Table 5.
Puberty × Time, Emotion × Puberty × Time, and Puberty × Sex × Time interactions from the whole-brain ANOVA
| Effect | xa | y | z | Voxels | Fb | p-valuec | Region | BA | Post-Hoc |
|---|---|---|---|---|---|---|---|---|---|
| Puberty × Time | 10 | -84 | -5 | 88 | 7.89 | 1.16E-12 | Lingual Gyrus | 18 | P<E=L* |
| Puberty × Time | 29 | -89 | 20 | 52 | 7.14 | 2.45E-11 | Middle Occipital Gyrus | 19 | P=E<L |
| Puberty × Time | 6 | -77 | 26 | 81 | 6.46 | 3.88E-10 | Cuneus | 18 | P=L<E |
| Puberty × Time | 21 | -101 | 10 | 31 | 5.32 | 4.26E-08 | Middle Occipital Gyrus | 18 | P=E<L |
| Puberty × Time | -25 | -90 | 0 | 18 | 4.38 | 2.11E-06 | Cuneus | 18 | P<E<L |
| Puberty × Time | -36 | -52 | -9 | 17 | 4.34 | 2.50E-06 | Fusiform Gyrus | 37 | P<E=L |
| Puberty × Time | 27 | -87 | -15 | 35 | 7.20 | 1.92E-11 | Inferior Occipital Gyrus | 18 | P<E=L |
| Emotion × Puberty × Time | 26 | -93 | -13 | 93 | 3.39 | 3.45E-13 | Fusiform Gyrus | 18 | F:P×T# |
| Emotion × Puberty × Time | -35 | -87 | -18 | 24 | 2.92 | 3.55E-10 | Declive of Cerebellum | - | |
| Emotion × Puberty × Time | 8 | -94 | -8 | 81 | 2.92 | 3.69E-10 | Lingual Gyrus | 18 | - |
| Emotion × Puberty × Time | -63 | -18 | 8 | 30 | 2.68 | 1.15E-08 | Transverse Temporal Gyrus | 42 | - |
| Emotion × Puberty × Time | -13 | -95 | -17 | 16 | 2.70 | 9.03E-09 | Lingual Gyrus | 17 | - |
| Emotion × Puberty × Time | -62 | -35 | 12 | 8 | 2.12 | 2.01E-05 | Superior Temporal Gyrus | 22 | P:E×T |
| Puberty × Sex × Time | 22 | -86 | 20 | 140 | 14.65 | 1.27E-23 | Cuneus | 18 | E,L:S×T; F:P×T^ |
| Puberty × Sex × Time | 24 | -81 | -6 | 91 | 8.74 | 3.95E-14 | Lingual Gyrus | 18 | E:S×T; M,F:P×T |
| Puberty × Sex × Time | 10 | -72 | 22 | 41 | 6.15 | 1.41E-09 | Precuneus | 31 | E:S×T; F:P×T |
| Puberty × Sex × Time | -7 | -91 | 29 | 52 | 6.47 | 3.84E-10 | Cuneus | 19 | E,L:S×T; F:P×T |
| Puberty × Sex × Time | -6 | -100 | 9 | 40 | 5.54 | 1.75E-08 | Cuneus | 18 | E:S×T; F:P×T |
| Puberty × Sex × Time | -17 | -87 | -15 | 29 | 4.96 | 1.95E-07 | Lingual Gyrus | 18 | E,L:S×T; M,F:P×T |
| Puberty × Sex × Time | -18 | -93 | 19 | 65 | 4.81 | 3.56E-07 | Cuneus | 18 | E,L:S×T; F:P×T |
| Puberty × Sex × Time | -40 | -84 | -20 | 16 | 4.18 | 4.81E-06 | Declive of Cerebellum | L:S×T; F:P×T | |
| Puberty × Sex × Time | -41 | -71 | 37 | 18 | 3.93 | 1.35E-05 | Precuneus | 19 | M:P×T |
| Puberty × Sex × Time | -4 | -48 | 45 | 14 | 3.24 | 2.22E-04 | Precuneus | 7 | P:S×T; M:P×T |
Coordinates for peak of cluster
F value at peak coordinates
p-value at peak coordinates, BA =Brodmann Area
Results of post-hoc contrasts comparing pre (P), early (E), and late (L) pubertal status children.
Results of post-hoc contrasts examining interactions of Puberty and Time (P×T) separately for each emotion or Emotion and Time (E×T) for each pubertal group
Results of post-hoc contrasts examining interactions of Sex × Time (S×T) separately for each puberty group (P = pre, E = early, L = late), or interactions of Puberty × Time (P×T) separately for males (M) and females (F)
Figure 5. Brain regions showing interactions with pubertal status.
Colors indicate interaction type. Inset shows ventral posterior right hemisphere to highlight region of greatest overlap. Red: puberty × time; Green: puberty × sex × time; Blue: emotion × puberty × time; Yellow: overlap of puberty × sex × time and puberty × time; Purple: overlap of emotion × puberty × time and puberty × time; Pink: overlap between all three contrasts
3.3.3 Whole-Brain Correlations
Table 6 shows the results of whole-brain partial correlations with age controlling for sex. All of the identified regions showed a negative correlation with age, such that older children exhibit less activity to the indicated emotion type. There was a negative correlation between left supramarginal gyrus activity to neutral faces and age and between left precentral gyrus activity to sad faces and age. Additionally, the responses to fearful faces in the cerebellum, parahippocampal gyrus, insula, cuneus, middle occipital gyrus, and cingulate gyrus were negatively correlated with age.
Table 6.
Whole-brain partial correlations with age controlling for effects of sex
| Emotion | xa | y | z | r | t-valueb | p-valuec | Region | BA |
|---|---|---|---|---|---|---|---|---|
| Neutral | -58 | -60 | 30 | -0.52 | -4.35 | 6.70E-05 | Supramarginal Gyrus | 40 |
| Sad | -22 | -21 | 48 | -0.51 | -4.15 | 1.30E-04 | Precentral Gyrus | 4 |
| Fear | 14 | -48 | -3 | -0.57 | -4.89 | 1.09E-05 | Culmen of Cerebellum | |
| Fear | 8 | -39 | 3 | -0.48 | -3.90 | 2.87E-04 | Parahippocampal Gyrus | 30 |
| Fear | 34 | -27 | 18 | -0.46 | -3.66 | 6.03E-04 | Insula | 13 |
| Fear | -10 | -87 | 18 | -0.48 | -3.87 | 3.17E-04 | Cuneus | 18 |
| Fear | 10 | -93 | 18 | -0.60 | -5.25 | 3.17E-06 | Middle Occipital Gyrus | 18 |
| Fear | -2 | 0 | 45 | -0.46 | -3.65 | 6.20E-04 | Cingulate Gyrus | 24 |
Coordinates for peak of cluster
t value at peak coordinates
p-value at peak coordinates, BA = Brodmann Area
4. Discussion
Research exploring emotion processing in humans has shown that the amygdala is central to the processing of fear (e.g. Adolphs et al., 1995; Bishop et al., 2004; Broks et al., 1998; Calder, 1996; LaBar et al., 1998; LeDoux, 2003; Morris et al., 1998; Phillips et al., 1997), though it also shows increased activity to other emotional stimuli (for meta-analyses, see Costafreda et al., 2008; Phan et al., 2002). Developmental studies of amygdala activity suggest that emotional responses show an inverted U-shaped developmental trajectory, where amygdala activity to emotional stimuli is stronger in adolescents than in children or adults (Guyer et al., 2008; Monk et al., 2003; Moore et al., 2012). However, the available literature offers mixed results and has been limited in its ability to inform whether children show differential activity to emotionally evocative faces vs. neutral faces due to small sample sizes and broad age ranges (e.g. Britton et al., 2010; Lobaugh et al., 2006; Thomas et al., 2001a; 2001b; Tottenham et al., 2011).
4.1 Amygdala Response to Emotional and Neutral Faces
Consistent with the large and established literature on this, we found that the bilateral amygdala was active in response to face processing in children. However, we found no evidence that the amygdala showed differential activity to emotionally valenced faces or neutral faces, either in ROI-based analyses or in whole-brain analyses. As such, our findings are consistent with prior results suggesting that differential amygdala responses to specific facial emotions observed in adults arise later in development, during adolescence and into early adulthood. Additionally, undifferentiated amygdala responses to emotional faces are consistent with studies of normative emotion development in childhood demonstrating that the ability to discern and understand the emotions of others increases in sophistication through school age and into adolescence. That is, the ability to react to and fully experience differences in emotions expressed by others is developing during this period (Durand et al., 2007; Gross and Ballif, 1991) and this may be reflected in the overall activity to faces but lack of differentiation to different emotion types in these data. These findings may be important for understanding emotion processing in children and across development. Particularly, these data indicate that differential amygdala responses to different emotional faces have not fully developed in this school-age range suggesting that peak of the proposed inverted U-shaped curve of emotional development occurs after this 7-12 year old range. Additionally, understanding the normative pattern of amygdala responses to emotional faces is key to further study of the neural correlates of emotion processing and related psychopathology in this age range. However, a crucial next step is to explore amygdala responses to emotion faces longitudinally, following the same individuals from childhood across adolescence and into adulthood to fully characterize the developmental trajectory of amygdala activity. Characterizing the normative developmental patterns of amygdala activity in healthy children is key to understanding emotion processing in child psychopathology.
We also examined whether the degree of amygdala response was associated with either age or puberty status, but did not find any significant relationships. This differs from previous results finding that pubertal development positively correlated with amygdala responses to emotional and neutral faces in 13-year-old children and with responses to neutral faces in 10-year-old children (Moore et al., 2012). The differences in results across studies may be due to differences in sample properties or in the analytic approach, although even when we used a similar analytical approach, the findings of Moore et al. 2012 were not replicated in this sample. In addition, a previous study of children and adolescents (9-17 years old) did find a negative correlation between age and left amygdala response to fearful faces (Killgore et al., 2001), which was not evident in our sample. However, the age range in the Killgore et al., (2001) study extended much later into adolescence than in our sample and the direction of the correlation in that study was different than what would be predicted by some other studies comparing amygdala responses in children, adolescents, and adults. In the current study, we did not detect a strong relationship between pubertal status and amygdala activity. However, most of the study participants were either pre-pubertal or in early pubertal stages so further study of these participants later in puberty would be important to further elucidate this issue.
Emotional face processing tasks tapping amygdala reactivity have been used as a key method to study depression and other psychiatric conditions. In these studies, adult and adolescent patients with major depressive disorder tend to show increased amygdala responses to emotional faces as compared to healthy controls (Beesdo et al., 2009; Peluso et al., 2009; Yang et al., 2010). The degree of amygdala over-activity has been shown to predict a more severe clinical course in MDD patients over eight months (Canli et al., 2005). Thus, we were also interested in examining whether the degree of amygdala response to any face type was associated with subclinical depression symptom severity (CDI) or the ability to regulate sadness (CEMS-S) in a non-clinical sample. We found that child reported CDI scores negatively correlated with left amygdala responses to neutral faces. In addition, parent reported CDI scores were positively correlated with left amygdala responses to angry, fearful and happy faces. Interestingly, differences between the child and parent report versions of the CDI in their relation to emotion-related brain activity were also noted in previous work with children from this same sample in a different task (Pagliaccio et al., 2011). While the parent and child versions of the CDI are related, they may access different aspects of MDD symptomology and potentially different aspects of emotion processing, as the parent report may be more representative of external indicators of mood, while the child report maybe more representative of internal experiences. Yet, increasing scores on both parent- and child-report versions of the CDI may be consistent with greater amygdala reactivity to emotional vs. neutral faces, typically seen in depression, as CDI-C scores negatively correlated with reactivity to neutral faces and CDI-P scores positively correlated with emotional face activity. It was also of interest that right amygdala activity to sad faces and to the contrasts of sad – neutral and fear – neutral faces positively correlated with CEMS-S scores (positive scores indicate increasingly maladaptive emotion management skills). In other words, children with poor sadness management skills showed greater amygdala response to sad and fearful faces as compared to neutral faces. A study exploring amygdala reactivity in 4-6 year old children with and without preschool-onset major depression noted a similar relationship between poor emotion regulation skills and amygdala reactivity to emotional and neutral faces (Gaffrey et al., under review).
4.2 Differential Responses to Emotion Types Outside of the Amygdala
Outside of the amygdala, several regions did show differential responses as a function of emotional face type. Particularly, the inferior semi-lunar lobule of the cerebellum, cuneus, inferior parietal lobule, claustrum, and superior temporal gyrus differentiated between several emotional face types and neutral faces, though the exact patterns differed across regions. These findings provide clues to what other regions of the brain, outside of the amygdala, are differentially involved in processing emotional and neutral faces. Other studies have found differential activity to emotional vs. neutral faces in a range of regions outside of the amygdala. In a meta-analyses, similar regions as noted here have been related to different emotion types though not necessarily in the pattern observed in this pediatric sample (Fusar-Poli et al., 2009). Overall, these patterns of results outside of the amygdala were not highly indicative of any strong conclusions about emotion processing and should be examined further in future work.
4.3 Sex Differences in Regions Outside of the Amygdala
We also found a number of regions that showed interactions with sex. These results may indicate important sex differences in attention to or engagement with emotional face stimuli, with girls showing greater responses in typical face and object processing regions (e.g. the fusiform gyrus and superior and middle temporal gyri) and deactivations in regions associated with the default network (e.g. anterior and posterior cingulate) that were not observed among boys. In contrast, the pattern of results in the boys could suggest that they use a more “cognitively” based strategy for processing faces in this task, as they showed greater activation of regions more typically associated with cognitive processing (e.g. middle frontal gyrus and the putamen). There was only a single region that showed a further interaction of sex with emotion type, in inferior frontal cortex. This sex difference seemed to primarily reflect differences in the timing of activation rather than the degree of activation, with boys showing early peaks than girls to both fearful and happy faces. A similar region also exhibited sex differences in activity evoked by emotionally valenced images in a previous study (Wrase et al., 2003). Additionally, this inferior frontal region has been associated with emotional perspective taking and observing facial expression making (Hynes et al., 2006; Jabbi and Keysers, 2008).
4.4 Effects of Puberty Outside of the Amygdala
We also examined influences of pubertal status on regions involved in facial emotion processing outside of the amygdala. The early and late puberty groups showed increased activity relative to the pre-pubertal group in the middle occipital gyrus, lingual gyrus, cuneus, and fusiform gyrus. A range of largely overlapping visual cortex regions also exhibited emotion × puberty × time and/or puberty × sex × time interactions. The overlap between these interactions with pubertal status suggests the importance of development on visual responses to emotional faces. Interestingly, Moore et al. (2012) also noted positive correlations between pubertal status and extrastriate visual cortex activity to both emotional and neutral faces. Though it cannot be determined specifically from these results, it is possible that this effect may indicate increased attention to the face stimuli in the children later in puberty. However, it is difficult to fully interpret the source of these pubertal effects with the given task design. Using a gender judgment task yields no direct measure of identification of or engagement with emotions. The hypothesis of a differential attentional focus or bias as a function of sex or pubertal status could thus be explored through other tasks with explicit measures of attention or engagement.
4.5 Limitations
One of the primary limitations of the current study is that one of the main findings (lack of differential amygdala responsivity to emotional faces) is a null result. Again, while the amygdala showed significant main effects of time across the 5 face types, there was no significant effect of emotion × time, indicating that the amygdala is active to all face types but likely does not show differential activation patterns to different emotions in healthy 7-12 year old children. Though it is difficult to draw inferences from null result, we feel that our sample size yields more than sufficient power to identify even small potential effects. Additionally, the effect sizes observed for the emotion × time interactions were very small indicating little to no difference between conditions. These results are also consistent at the whole-brain and ROI level (both anatomically and functionally defined) and when examining the magnitude of amygdala activity with an assumed hemodynamic response function, helping to indicate that amygdala activity in children 7 to 12 years old does not differentiate between facial emotion types.
Another limitation to note is that all children underwent a negative mood induction prior to the emotional face processing task. This was included in the scan session based on the goals of the PDS and though not of interest in the current analyses, we also do not believe that this prior task would introduce any bias into the current analyses. Previous work has shown that mood induction can reactivate negative affective biases specifically in previously depressed individual but not in never depressed individuals (Scher et al., 2005). As all children in this sample were assessed as psychiatrically healthy through the time of scan, we did not expect this mood induction to influence the current task. This was confirmed in control analyses showing that post-induction mood and change in mood due to negative mood induction were not correlated with amygdala response to any face type. Additionally, should the mood induction have had an effect, we would have expected it to increase the salience of negative facial expressions, which was not apparent in our null effect of emotion type.
4.6 Conclusion
Overall, we did not find evidence for differential amygdala responses to different emotional face types in 7-12 year old children but did find that increased amygdala activity was related to the severity of subclinical depression symptoms as rated by parents and to sadness regulation skills. Therefore, while amygdala responses to emotional and neutral faces may be sensitive to differences related to depression symptom severity and emotion management skills, it is important to consider this overall amygdala response to faces but null effect of emotion type when investigating psychopathology in school-age children. In contrast to findings in the amygdala, other regions of the brain did show differential activity to different emotion types, with influences of sex in frontal, temporal, and visual regions and of pubertal development, particularly in visual cortex regions. Future work will be needed to determine whether these sex and/or pubertal influences on activity in visual processing regions reflect differential attention to or processing of emotional face stimuli in children. Further study of amygdala processing in healthy children could be aided by the inclusion of targeted high-resolution functional scanning of the amygdala during this type of emotion processing task to assure the best quality signal, though studies in adults have found differential amygdala reactivity with whole-brain scans, and to potentially explore differences among amygdala sub-regions. Longitudinal follow-up will also be key to further understanding the developmental of differential amygdala responses to emotional faces, allowing for assessment of within-subject trajectories from childhood to adolescence.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Mental Health (MH64769 to J.L.L. and MH090786 to J.L.L., D.M.B., K.N.B.). Dr. Belden's work on this manuscript was supported by a grant from the National Institute of Mental Health (1K01MH090515-01). The NIMH had no further role in the design and conduct of the study (collection, management, analysis, and interpretation of data) or preparation, review, or approval of the manuscript. Author DP had full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
We thank all participants and their families that provided time and effort to making this study possible.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Abler B, Hofer C, Walter H, Erk S, Hoffmann H, Traue HC, Kessler H. Habitual emotion regulation strategies and depressive symptoms in healthy subjects predict fMRI brain activation patterns related to major depression. Psychiatry Research: Neuroimaging. 2010;183:105–113. doi: 10.1016/j.pscychresns.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. The journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. The Child and Adolescent Psychiatric Assessment (CAPA). JAAC. 2000;39:39–48. doi: 10.1097/00004583-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Barch DM, Gaffrey MS, Botteron KN, Belden AC, Luby JL. Functional Brain Activation to Emotionally Valenced Faces in School-Aged Children with a History of Preschool-Onset Major Depression. BPS. 2012:1–8. doi: 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Lau J, Guyer A, McClure-Tone E, Monk C, Nelson E, Fromm S, Goldwin M, Wittchen H, Leibenluft E. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of general psychiatry. 2009;66:275. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. The journal of Neuroscience. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Buckner R, Strauss M, Hyman S, Rosen B. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Britton JC, Stewart SE, Killgore WDS, Rosso IM, Price LM, Gold AL, Pine DS, Wilhelm S, Jenike MA, Rauch SL. Amygdala activation in response to facial expressions in pediatric obsessive-compulsive disorder. Depression and Anxiety. 2010;27:643–651. doi: 10.1002/da.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broks PP, Young AWA, Maratos EJE, Coffey PJP, Calder AJA, Isaac CLC, Mayes ARA, Hodges JRJ, Montaldi DD, Cezayirli EE, Roberts NN, Hadley DD. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum. Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Cahill L. Sex-Related Hemispheric Lateralization of Amygdala Function in Emotionally Influenced Memory: An fMRI Investigation. Learning & Memory. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Sex influences on brain and emotional memory: the burden of proof has shifted. Progress in Brain Research. 2010;186:29–40. doi: 10.1016/B978-0-444-53630-3.00003-8. [DOI] [PubMed] [Google Scholar]
- Calder AJ. Facial Emotion Recognition after Bilateral Amygdala Damage: Differentially Severe Impairment of Fear. Cognitive Neuropsychology. 1996;13:699–745. [Google Scholar]
- Canli T, Cooney R, Goldin P, Shah M, Sivers H, Thomason M, Whitfield-Gabrieli S, Gabrieli J, Gotlib I. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport. 2005;16:1267. doi: 10.1097/01.wnr.0000174407.09515.cc. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Cohen MM. Adolescence: What Do Transmission, Transition, and Translation Have to Do with It? Neuron. 2010;67:749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CHY. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58:57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- De Sonneville LMJ, Verschoor CA, Njiokiktjien C, Op het Veld V, Toorenaar N, Vranken M. Facial Identity and Facial Emotions: Speed, Accuracy, and Processing Strategies in Children and Adults. Journal of Clinical and Experimental Neuropsychology (Neuropsychology, Development and Cognition: Section A) 2002;24:200–213. doi: 10.1076/jcen.24.2.200.989. [DOI] [PubMed] [Google Scholar]
- Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin J-Y. The development of facial emotion recognition: The role of configural information. J Exp Child Psychol. 2007;97:14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Egger H, Ascher B, Angold A. The Preschool Age Psychiatric Assessment: Version 1.4. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Duke University Medical Center; Durham, NC: 2003. [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl BB, Salat DHD, van der Kouwe AJWA, Makris NN, Ségonne FF, Quinn BTB, Dale AMA. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Phillips ML, Silk JS, Ryan ND, Dahl RE. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev Neuropsychol. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of psychiatry & neuroscience: JPN. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Singer J, Shenoy R, Luby JL. Disrupted amygdala reactivity as an early biomarker of depression: an fMRI study of depressed 4-6 year old children. under review. [DOI] [PMC free article] [PubMed]
- Gaffrey MS, Luby JL, Belden AC, Hirshberg JS, Volsch J, Barch DM. Association between depression severity and amygdala reactivity during sad face viewing in depressed preschoolers: an fMRI study. Journal of Affective Disorders. 2011;129:364–370. doi: 10.1016/j.jad.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazer B, Zieff H. My Girl [Motion picture] Columbia Pictures; United States: 1991. [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JDE, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Gross AL, Ballif B. Children's understanding of emotion from facial expressions and situations: A review. Developmental Review. 1991;11:368–398. [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, Fromm SJ, Leibenluft E, Pine DS, Ernst M. A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Sex Differences in the Responses of the Human Amygdala. neuroscientist. 2005 doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- Hare T, Tottenham N, Galvan A, Voss H, Glover G, Casey B. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herba CM, Landau S, Russell T, Ecker C, Phillips ML. The development of emotion-processing in children: effects of age, emotion, and intensity. J Child Psychol Psychiatry. 2006;47:1098–1106. doi: 10.1111/j.1469-7610.2006.01652.x. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Singerman JD, McCarthy G. The effects of aging upon the hemodynamic response measured by functional MRI. NeuroImage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- Hung YY, Lou ML Smith M, Taylor MJM. Development of ACC-amygdala activations in processing unattended fear. NeuroImage. 2012;60:8–8. doi: 10.1016/j.neuroimage.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Iidaka TT, Omori MM, Murata TT, Kosaka HH, Yonekura YY, Okada TT, Sadato NN. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. Journal of Cognitive Neuroscience. 2001;13:1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hawco C, Kobayashi E, Boor R, LeVan P, Stephani U, Siniatchkin M, Gotman J. Variability of the hemodynamic response as a function of age and frequency of epileptic discharge in children with epilepsy. NeuroImage. 2008;40:601–614. doi: 10.1016/j.neuroimage.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. Neuroreport. 2001;12:427. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children's Depression, Inventory (CDI). Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Laeger I, Dobel C, Dannlowski U, Kugel H, Grotegerd D, Kissler J, Keuper K, Eden A, Zwitserlood P, Zwanzger P. Amygdala responsiveness to emotional words is modulated by subclinical anxiety and depression. Behavioural Brain Research. 2012;233:508–516. doi: 10.1016/j.bbr.2012.05.036. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17:215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- Luby J, Si X, Belden A, Tandon M, Spitznagel E. Preschool depression: Homotypic continuity and course over 24 months. Archives of general psychiatry. 2009;66:897. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Moore WE, Pfeifer JH, Masten CL, Mazziotta JC, Iacoboni M, Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Social Cognitive and Affective Neuroscience. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J, Friston K, Büchel C, Frith C. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998 doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Ojemann JGJ, Akbudak EE, Snyder AZA, McKinstry RCR, Raichle MEM, Conturo TET. Anatomic Localization and Quantitative Analysis of Gradient Refocused Echo-Planar fMRI Susceptibility Artifacts. NeuroImage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby J, Gaffrey M, Belden A, Botteron K, Gotlib IH, Barch DM. Anomalous functional brain activation following negative mood induction in children with pre-school onset major depression. Developmental Cognitive Neuroscience. 2011 doi: 10.1016/j.dcn.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso M, Glahn D, Matsuo K. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Research. 2009 doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Phan K, Wager T, Taylor S, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Williams LM, Heining M, Herba CM, Russell T, Andrew C, Bullmore ET, Brammer MJ, Williams SCR, Morgan M, Young AW, Gray JA. Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage. 2004;21:1484–1496. doi: 10.1016/j.neuroimage.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, McQuaid JR. Amygdala Reactivity and Mood-Congruent Memory in Individuals at Risk for Depressive Relapse. Biological Psychiatry. 2007;61:231–239. doi: 10.1016/j.biopsych.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Richter W, Richter M. The shape of the fMRI BOLD response in children and adults changes systematically with age. NeuroImage. 2003;20:1122–1131. doi: 10.1016/S1053-8119(03)00347-1. [DOI] [PubMed] [Google Scholar]
- Scher CDC, Ingram RER, Segal ZVZ. Cognitive reactivity and vulnerability: Empirical evaluation of construct activation and cognitive diatheses in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Menz MM, Miedl SF, Loth E, Banaschewski T, Barbot A, Barker G, Conrod PJ, Dalley JW, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Mallik C, Mann K, Artiges E, Paus T, Poline JB, Rietschel M, Reed L, Smolka MN, Spanagel R, Speiser C, Ströhle A, Struve M, Schumann G, Büchel C, consortium TI. Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. NeuroImage. 2011;56:1847–1853. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Sheline Y, Barch D, Donnelly J, Ollinger J, Snyder A, Mintun M. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle G, Carter C, Thase M. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. American Journal of Psychiatry. 2006;163:735. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey B. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20:236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SCR, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. BPS. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SCR, Phillips ML. A preferential increase in the extrastriate response to signals of danger. NeuroImage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. 1988 (1988) [Google Scholar]
- Tanner J. Growth at Adolescence. BlackwellScientific; Oxford,England: 1955. [Google Scholar]
- Thomas K, Drevets W, Whalen P, Eccard C, Dahl R, Ryan N, Casey B. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001a;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of general psychiatry. 2001b;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas LAL, De Bellis MDM, Graham RR, LaBar KSK. Development of emotional facial recognition in late childhood and adolescence. Developmental Sci. 2007;10:547–558. doi: 10.1111/j.1467-7687.2007.00614.x. [DOI] [PubMed] [Google Scholar]
- Todd RM, Evans JW, Morris D, Lewis MD, Taylor MJ. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Social Cognitive and Affective Neuroscience. 2011;6:12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research: Neuroimaging. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S, Reilly JS, Pasqualetti P, Vizzotto A, Caltagirone C. Recognition of facial expressions of emotions in school-age children: the intersection of perceptual and semantic categories. Acta Paediatrica. 2007;89:836–845. [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whalen P, Rauch S, Etcoff N, McInerney S, Lee M, Jenike M. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. The journal of Neuroscience. 1998;18:411. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Fischer H, Whalen P, McInerney S, Shin L, Rauch S. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12:379. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP. Adolescents With Major Depression Demonstrate Increased Amygdala Activation. JAAC. 2010b;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman J, Shipman K, Penza-Clyve S. Development and initial validation of the Children's Sadness Management Scale. Journal of Nonverbal Behavior. 2001;25:187–205. [Google Scholar]
- Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.