Abstract
Pharmacologic treatment options for neonatal seizures have expanded over the last two decades and there is no consensus on optimal treatment strategy. We systematically reviewed the published literature to determine which medication(s) are most effective for treating neonatal seizures, by retrieving trials and observational investigations via PubMed (through August 2011) that focused on pharmacological seizure treatment of neonates (≤ 28 days old) and utilized continuous or amplitude-integrated EEG to confirm seizure diagnosis and cessation. Our search identified 557 initial articles and 14 additional studies after reference reviews, with 16 meeting inclusion criteria. Two were randomized trials and only three additional investigations included comparison groups. We found limited evidence regarding the best pharmacologic treatment for neonatal seizures, but were able to devise a treatment algorithm from available data. These findings have the potential to serve both as a clinical reference and inform the design of comparative effectiveness investigations for neonatal antiepileptics.
Keywords: neonatal seizures, seizure treatment, antiepileptic drugs, phenobarbital, phenytoin, levetiracetam, lidocaine, midazolam, lorazepam, thiopental, paraldehyde, systematic review
INTRODUCTION
Treatment options for neonatal seizures remain limited despite their relatively common occurrence (2–4 per 1000 North American births, 19–58 per 1000 in <1500 gram birth weight infants:),1–3 even with the introduction of several new antiepileptic medications over the last two decades. In addition, there is significant variation in approach to the treatment of neonatal seizures, both in medication choice and in when, or whether, to treat.4–7
Phenobarbital and phenytoin/fosphenytoin have traditionally been the most commonly used medications to treat neonatal seizures, despite only approximately 50% efficacy 8 In addition, concern exists for short-term side effects, medication interactions, the need for frequent blood-level monitoring, and potentially negative neurodevelopmental consequences. 9–11 Perhaps due to these limitations, the use of other antiepileptic medications is increasingly being reported. Silverstein and Ferriero’s 2007 survey of pediatric neurologists found that 73% reported use of levetiracetam and/or topiramate in the treatment of neonatal seizures. 7 Reasons for choosing these antiepileptics included fewer adverse effects and ease of use. 7
We conducted a systematic review to examine the published evidence regarding pharmacological therapy for neonatal seizures. Our objective was to determine which antiepileptic medication or medications are most effective for treating neonatal seizures, in order to guide development of an evidence-based treatment algorithm to reduce variation among providers, and to inform the design of future investigations to determine the comparative effectiveness of antiepileptics in neonates.
METHODS
The systematic review was conducted following the general principles published by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and the Institute of Medicine Standards for Systematic Reviews. 12,13 Although randomized controlled trial (RCT) study designs provide the most internal validity for examining treatment efficacy, previous Cochrane reviews have shown limited RCT evidence on this topic. 5 Given the lack of robust RCT evidence, we included observational investigations in our systematic review and focused on highlighting the strengths and methodological limitations of each included study.
We searched MEDLINE via PubMed from inception to August 2011. A librarian trained in literature search strategies assisted us in the design of our search terms, which were subsequently reviewed by a second librarian. The search was limited to humans and English language articles and included the specific search terms: National Library of Medicine Medical Subject Heading [MeSH] term "Seizures/therapy" AND ("Infant, Newborn"[Mesh] OR neonat*) AND (Humans[Mesh] AND English[lang]). Upon reviewing references from articles in the original search, we included additional relevant manuscripts that met our inclusion criteria.
Inclusion and Exclusion Criteria
We only included articles that focused on pharmacological seizure treatment of neonates, defined as infants less than or equal to 28 days postnatal age. Since neonatal seizures are often misdiagnosed by clinical impression alone, we required that seizure diagnosis and cessation of ongoing seizures be confirmed by continuous electrophysiologic monitoring (conventional electroencephalogram (EEG) or amplitude-integrated EEG (aEEG). 14–16 Case reports, review articles with no primary data, and non-peer reviewed studies including meeting abstracts were excluded, as were articles that did not include seizure cessation as an outcome. Whenever an investigation included other childhood age groups without neonatal-specific outcomes or failed to report medication dosages, it was also excluded. We did not include articles with a primary focus on seizures due to metabolic disorders (eg. pyridoxine deficiency, nonketotic hyperglycinemia), electrolyte disturbances (eg. correctable hypocalcemia, hypoglycemia), or opioid withdrawal since seizures secondary to these disorders have very specific treatments/prognoses. Studies that focused on benign neonatal convulsions (“fifth-day fits”) were not analyzed since short-term seizure cessation was not likely reflective of pharmacological effectiveness, but rather the natural disease course.
Data Extraction and Quality Assessment
Two authors independently reviewed each title and abstract to determine eligibility. Whenever the abstract did not reveal sufficient information about the study design, the full article was retrieved for review. All three authors reviewed all papers that were deemed potentially eligible following abstract review using a structured checklist to evaluate study design, methods, results, potential for study bias, and adverse events. When there was uncertainty about inclusion of an article it was discussed by all authors and a consensus was achieved.
Data Synthesis
The investigations were divided into two tiers based on their study design. Investigations that included a control/comparison group were placed into Tier I. Since the literature on neonatal seizure treatment is limited, we chose to construct and present a Tier II for investigations otherwise meeting the inclusion criteria, but without a comparison group against which the effectiveness of the treatment might be judged. No quantitative synthesis (meta-analysis) was attempted due to the large degree of heterogeneity and design weaknesses of included studies.
Ethics
The authors report no financial relationships or conflicts of interest relevant to this article. The Nationwide Children’s Hospital Institutional Review Board deemed the investigation non-human subjects research.
RESULTS
Our MEDLINE search identified 557 articles. After reviewing titles and abstracts and eliminating manuscripts that did not fulfill inclusion criteria, all three authors reviewed the full-texts of the 64 remaining articles. Review of the references from these publications, yielded 14 additional articles for review. Upon review of these 78 full-texts, a total of 16 investigations utilized EEG for seizure diagnosis and treatment monitoring and otherwise met all inclusion criteria. 8,17–31 Five studies contained control or comparison groups by design and were designated as Tier I investigations (Table 1). 8,17–20 Only two of these investigations, a comparison of phenobarbital to phenytoin by Painter et al8 and a comparison of lidocaine versus benzodiazepines as second-line therapy by Boylan et al17, were randomized trials. Eleven additional studies meeting inclusion criteria, but without comparison groups, were placed into Tier II (Table 2). 21–31
Table 1.
Studies of medications used to treat neonatal seizures designed with a control or comparison group (Tier 1), and with electrographic confirmation of seizure diagnosis and response to therapy.
| Study | Medications and doses studied |
Study design |
Study population |
EEG | Key findings | Additional findings | Short-term Adverse effects |
Study Limitations |
|---|---|---|---|---|---|---|---|---|
| Painter et al, 19998 |
Phenobarbital (goal free level 25 ug/mL) VS. Phenytoin (goal free level 3 ug/mL) |
Randomized, prospective trial, block design, with crossover if failure of initial treament. Single-blinded. |
N=59 (30 pheno-barbital, 29 phenytoin) Term and preterm |
cEEG | Phenobarbital and phenytoin were equally effective/ineffective Initial seizure control: phenobarbital group 13/30 (43%), phenytoin 13/29 (45%) (P=1) Seizures completely controlled in 57 % and 62% respectively in the two groups after crossover to 2nd drug. If consider “substantial improvement” (80% reduction in seizures), had overall response rates of 80% and 72% in each treatment group respectively. |
Successful seizure control inversely associated with seizure severity regardless of which treatment received | No serious adverse effects noted | Single center Potential for imbalance of unmeasured confounders, given imbalance in baseline variables Single-blinded. Physicians and hospital staff and EEG techs aware of treatment assignment |
| Boylan et al, 200417 |
Lidocaine 4mg/kg IV bolus then infusion of 2mg/kg/hr, could be increased to 4mg/kg/hr. VS. Midazolam 60 ug/kg IV (0.06mg/kg) bolus then infusion of 150ug/kg/hr, could be increased to 300ug/kg/hr (OR clonazepam rather than midazolam in preterms) 2nd line after failure of 40mg/kg phenobarbital. Clonazepam in preterms: dose not described. |
Randomized, prospective trial of lidocaine versus midazolam (clonazepam in preterms). Unblinded. 1year neuro-develop-mental follow up with Amiel-Tison and Griffiths assessments |
N=27 with EEG seizures, N=11 who got randomized to lidocaine or benzo-diazepines (nonrespon-ders to phenobarbital). N=5 for lidocaine group, N=6 for benzo-diazepines (3 clonazepam, 3 midazolam) Term and preterm |
cEEG | No responders (short term) to midazolam or clonazepam. 3/5 (60%) responded to lidocaine (two seizure-free, one with 80% reduction in seizures burden)- these did require maximum dose, and took about 12 hrs to respond Minimal difference ins 1-yr outcomes between lidocaine and benzodiazepine groups. 4 died, 1 lost to F/U, 1 mild abnormal, 2 moderate abnormal, 3 severe abnormal (the mild and mod abnormal were benzodiazepine group, but also had deaths in this group). |
11/22 (50%) responded to phenobarbital alone (protocol violations excluded). Those who responded to phenobarbital alone-: 4 died, 5 normal at F/U, 2 moderately abnormal. |
Not described | Small number of subjects Also routine midazolam sedation used in unit Not comparing lidocaine to ONE benzodiazepine, but to two, and unclear what dose of clonazepam used |
| Hellstrom-Westas et al, 198818 |
Lidocaine Loading doses 2mg/kg, VS. 1–1.5mg/kg, VS. no loading dose, then drip rate of 2–6mg/kg/hr. 2nd or 3rd line after failure of phenobarbital (10–15mg/kg) +/− diazepam 1–2mg/kg |
Prospective, comparison of 3 groups treated with differing loading doses of lidocaine, then maintenance drip. Unblinded. |
N=46 (11 no loading dose, 6 with 1–1.5mg/kg load, 29 with 2mg/kg load) Term and preterm |
aEEG and inter-mittent full EEG. | Overall: success in 38 cases (83%) – seizures stopped within 3–4 hours. Of 29 given a 2mg/kg loading dose, 26 responded within 30 min (and an additional 1 by 3–4 hrs). (27/29= 93% were responders) Of 11 cases with no loading dose, 5 did not respond til 1–4 hrs, and 4 with no response by 4 hrs. (4/11 nonresponders) |
Initial maintenance dose of 6mg/kg/hr abolished seizures completely in 23/25 (92%) of cases (who were started at that maintenance dose). Background aEEG amplitude often transiently depressed. |
7 infants with BP changes, 22 with HR changes (brady or tachycardic). No aggravation of seizures noted “No dangerous circulatory or cerebral side effects in this study”. |
No long-term F/U reported; did the use of lidocaine make a difference in the long run Variation in size of groups, and in maintenance dose for each group after the bolus. Difficult to make comparisons. |
| Castro Conde et al, 200519 |
Midazolam 0.15mg/kg bolus (up to 2 times) then drip rate from 1–18ug/kg/min (= 60–1080 ug/kg/hr). as 2nd line to phenobarbital (up to 40mg/kg phenobarbital). VS. historical cohort who received phenobarbital +/− phenytoin |
Retrospective cohort, with historical comparison **higher doses of midazolam than used in other studies |
N=45 with EEG-confirmed seizures Comparison of NONRESPONDERS (N=17) from historical cohort who got phenobarbital/ phenytoin only (Group 1 NONRESPONDERS), to the midazolam-treated (Group 2) (N=13). Term only |
cEEG for at least 4 hrs, then inter-mittent EEG’s at 24hr intervals | 100% responded to midazolam (10/13 with seizures controlled in 1st hour) No difference between overall outcomes at 1 year if look at whole Group 1 compared to group 2. But, better outcome (p<0.01) at 1 year for Group 1 NONRESPONDERS compared to Midazolam-treated group (53.9% vs 11.8% assessed as normal). |
Patients who had markedly abnormal EEG background, in either group, had poor outcome or died. With moderately abnormal background EEG, normal neurodevelopment more frequent in midazolam group. Only 46.9% response to phenobarbital +/− phenytoin treatment (similar to other studies) |
Hypotension in 2 infants with multiorgan failure due to severe HIE 4 infants with decreased consciousness following midazolam bolus (recovered in 10–15 min) |
Retrospective Potential for other advances in treatment to improve outcomes in midazolam group vs Group 1 nonresponders, since historical cohort Didn’t state details of their 1 yr outcomes; how assessed |
| Shany et al, 200720 |
Lidocaine 2mg/kg load, then 4–6mg/kg/hr drip VS. Midazolam 60–200ug/kg/hr without a load 2nd or 3rd line after up to two 20mg/kg phenobarbital doses and/or IV dose of diazepam or lorazepam. |
Retrospective cohort, comparison of two drugs, crossover to alternate agent in some nonresponders | N=67 who received 2nd line drugs, only 30 who fulfilled inclusion criteria. Lidocaine : N=22 Midazolam : N=8 >= 36wk gestational age, with HIE |
aEEG |
Lidocaine: 17/22 (77%) with some response. 11/22 (50%) cessation of all seizure activity > 6 hrs, 6/22 (27%) with decreased EEG seizures or cessation but then recurrence within 6 hrs Midazolam: 4/8 (50%) with partial response. None with >6 hr cessation. Lidocaine favored p=0.01 for >6hr seizures cessation No significant difference (p=0.2 on our secondary analysis) for number of patients with any response |
Complete control after crossover to 3rd-line agent: 5/5 (100%) midazolam nonresponders achieved control. 2/4 (50%) lidocaine nonresponders achieved >6hr cessation, and the other 2/4 (50%) partially responded. Overall response rates: 81% for lidocaine, 67% for midazolam |
Not described | Observational Smaller sample of pts receiving midazolam Generalizable to >36wk gestational age infants with seizures due to HIE |
Abbreviations: cEEG= continuous electroencephalography, aEEG= amplitude-integrated electroencephalography, BP = blood pressure, HR= heart rate, F/U = follow-up, IV = intravenous, HIE= hypoxic-ischemic encephalopathy
Table 2.
Studies of medications used to treat neonatal seizures without control or comparison groups (Tier II), but with electrographic confirmation of seizure diagnosis and response to therapy.
| Study | Medications and doses studied |
Study design |
Study population |
EEG monitoring |
Key findings | Additional findings | Short-term Adverse effects |
Study Limitations |
|---|---|---|---|---|---|---|---|---|
| Deshmukh et al, 198621 |
Lorazepam 0.05mg/kg IV 3rd line after phenobarbital (1st line) 15mg/kg, and phenytoin (2nd line) 15mg/kg |
Uncontrolled, experimental (prospective) | N=7 total, But only N=3 with cEEG All infants with HIE 5 term, 2 preterm |
cEEG, in only 3/7 infants. Does not state how long the EEG was continued after lorazepam dosing (implied by description of movements several hours later, that EEG was no longer ongoing) | 3/3 (100%) on EEG monitored infants had seizure cessation within 5 min 4/4 (100%) clinically monitored infants with seizure cessation within 5 min |
In EEG-monitored subjects- 1 of the 3 had bicycling movements recurring 8 hrs after lorazepam In clinically monitored kids, one had bicycling movements 8 hrs after lorazepam, 1 had recurrence of multifocal seizures 12 hrs after lorazepam, and 2 had occasional lipsmacking but no definite seizure recurrence |
No significant side effects observed In two instances, following lorazepam administration, the serum phenobarbital level increased into the “toxic range” |
No control/ comparison group Continuous EEG limited to only 3 patients, and unclear how long it was used. Lorazepam dose lower than typically used for seizure cessation in other age groups (e.g. 0.1mg/kg standard) |
| Connell et al, 198922 | Clinical seizures treated, in this order: Phenobarbital- (1st line)- 20mg/kg Paraldehyde- (2nd line)- 0.3mL/kg rectally or 1–3mL/kg/hr IV Phenytoin- (3rd line)- 20mg/kg IV Diazepam- (4th line)- 0.25mg/kg IV |
Prospective study of developmental outcomes in patients with neonatal seizures, treated (any antiepileptic drug) or untreated Partially blinded- neonatalogists were not given EEG results unless requested. Treatment decisions typically made on clinical basis without EEG info. |
N=55 with EEG seizures, only 31 treated with anti-convulsants (untreated group = 24) Term and preterm (note- 22 were under 32 wks gestational age) |
cEEG (but 2 channel only) | Complete response in 2/31 (6%) (with phenobarbital), equivocal response in 6/31 (19%) (2 phenobarbital, 4 paraldehyde). 13/31 (42%) had persistent EEG seizures, 10/31 (32%) showed no response (Definitions: Complete response: immediate and sustained cessation of EEG and clinical seizures Equivocal response = delayed response (but within 6 hrs) OR temporary response (recurred within 24hrs)) |
Neurologic outcome (range 6–24 months) of survivors- did not differ significantly between treated and untreated group. Outcomes: Normal: 2 treated, 2 untreated. Dystonia: 3 treated, 5 untreated Major abnormality: 6 treated, 7 untreated Death: 20 treated, 10 untreated Worse EEG background correlates with worse outcome- 27 infants with severe background EEG abnormalities died or had major neurologic abnormalities. |
Not described | No control/ comparison group Untreated infants were not recruited and randomized as a formal control group- consisted mostly of patients WITHOUT clinical manifestations of EEG seizure activity |
| Bonati et al, 199023 |
Thiopental 10mg/kg IV 2nd line after phenobarbital 20mg/kg IV |
Uncontrolled, experimental (prospective) | N=9 All ventilated, with severe HIE Term and preterm (>33wks) |
cEEG duration not clear |
9/9 (100%) with seizure cessation clinically and by EEG (“prompt and complete”) | All infants also kept on maintenance phenobarbital, with serum levels near 20. Recurrence of seizures in 1 infant 3 days later, stopped with a repeat dose. | Mild hypotension (mean systolic decrease of 27%) in 6/9 patients about 2 min after thiopental, requiring ethylephedrine dosing in all, dopamine in one, and volume explanion in two. Could not assess respiratory response, all infants mechanically ventilated |
No control/ comparison group Did not use more phenobarbital than 20mg/kg- why not? |
| Maytal et al, 199124 |
Lorazepam 0.05–0.15mg/kg IV 2nd line after phenobarbital 40mg/kg IV |
Prospective observational, no comparison group. Bolus doses of 0.05mg/kg given, repeated q 15 min up to total of 3 doses if still seizing | N=7 All but 1 were term Only 3 with EEG during lorazepam administration |
cEEG only in 3 subjects, for a short duration after lorazepam given- 17–46 min. Duration of response to lorazepam determined clinically. |
Complete cessation of seizures in 1–3 min in all but 1 patient (86%), who did have reduction of frequency and duration of seizures. “All patients responded clinically or on EEG recordings within 5 minutes”. |
Duration of clinical seizure freedom: recurred after 12–16 hrs in 2 pts; no recurrence for >24hrs in 4 pts though 1 of these had lip-smacking at 6 hrs and 1 had occasional myoclonic jerks. EEG amplitude attenuation seen in some, after lorazepam given |
No significant side effects observed | No control/ comparison group Small numbers, and did not really have consistent cEEG monitoring to determine full seizure cessation |
|
Bye and Flanagan 199525 |
Series of anticonvulsants: Phenobarbital (1st line) 20mg/kg IV, if failure then up to cumulative dose of 40mg/kg or level of 40. Phenytoin (2nd line), 15–20mg/kg Clonazepam (3rd line)-intermittent 50ug/kg |
Prospective, no comparison group for efficacy. Main purpose of study was to characterize the EEG seizures. | N=32 22 with seizure cessation during the monitoring period (data are unclear in 10- some died, 7 with seizure cessation but unclear if due to an anticonvulsant response). Term and preterm |
cEEG. Monitoring continued for 12 hrs after achieving seizure control. Strength- Defined seizure cessation as within 120 min (if >120 min, unsure if could attribute to the drug) |
Overall, seizure cessation in 15/22 (68%) within 120min. (12 within 30 min = 54%). 2 of these with <=20mg/kg phenobarbital, 6 with 21–30mg/kg phenobarbital, none with >30mg/kg phenobarbital, 5 with addition of phenytoin, 2 with addition of clonazepam. Amount of anticonvulsant required for an individual infant could not be predicted. 7 patients had seizures temporarily suspended after anticonvulsant doses but not completely controlled. |
Clinical manifestations decreased after sequential anticonvulsant doses (highest correlation of clinical to EEG seizure was BEFORE anticonvulsants given). Inadequacy of relying on clinical observation to tell if seizure free. Some neonates with very long interictal periods. Anticonvulsants affected seizure characteristics: reduced seizure duration, increased interictal periods, and reduced EEG spread. |
Not described | No control/ comparison group |
| Sheth et al, 199626 |
Midazolam 0.15mg/kg IV load, then 0.1–0.4mg/kg/hr 3rd line after 20–40mg/kg phenobarbital and usually 20mg/kg phenytoin |
Retrospective, observational, no control group | N=6 mechanically ventilated infants 5 term and one 30week preterm |
cEEG | 6/6 (100%) seizure free within 1 hr (4/6 = 67% had seizure cessation immediately after loading dose) |
No significant side effects observed, but all infants were already mechanically ventilated | No control/ comparison group Very small number Risk factors and illness severity different in infants who got long-term continuous EEG |
|
| Boylan et al, 200227 | Phenobarbital, 20mg/kg IV then additional loads up to 40mg/kg as needed | Secondary analysis of a prospective observational investigation | N=14 Term and preterm |
cEEG for at least 1 hr pre-treatment and continued at least 1–2 hrs post treatment, plus intermittent or continuous monitoring in subsequent days | 4/14 (29%) “responded” to 1st phenobarbita load Of the responders- 2 remained seizure free completely, 1 with a single seizure the next day then no others, and the last had 2 seizures (1 clinical, 1 electrographic) later after the phenobarbital. 2 additional babies had initial dramatic response but seizures were recurring by 24 hrs. |
Responders tended to be those with low seizure burden and normal or mildly abnormal EEG background Responders had normal neurologic outcome at 1 year in 2 subjects, moderate hearing impairment in another, and 1 died. Nonresponders had mostly moderate to severe neurologic outcomes or died. None normal. |
Not described | No control/ comparison group Potential for selection bias- only 14/33 infants with seizure in a 3 yr period had sufficient video-EEG data to meet inclusion criteria If electrographic seizures persisted, additional treament was not given until the following day |
| Van Leuven et al 200428 |
Midazolam 0.05mg/kg IV load, then 0.15mg/kg/hr 3rd line after phenobarbital 20mg/kg and then lidocaine |
Uncontrolled, experimental (prospective) aEEG reader blinded to final patient outcome |
N=15 Term infants with HIE |
aEEG | 11/15 (73%) with seizure cessation within 24 hrs 1/15 with reduced seizure frequency |
Brief, moderate aEEG background suppression × about 2 hours, in 4/15 patients | No significant side effects observed | No control/ comparison group 3 patients also received clonazepam during the midazolam infusion due to severe ongoing seizures |
| Malingre et al, 200629 |
Lidocaine 2mg/kg IV over 10 min, then 6mg/kg/hr × 12 hrs, then 4mg/kg/hr × 12 hrs, then 2mg/kg/hr × 12 hrs 3rd line after Phenobarbital (1st line), and midazolam or clonazepam (2nd line in term vs preterms) |
Uncontrolled, experimental (prospective) aEEG assessed blindly Second confirmatory study – 2nd group of 15 patients with slightly different dosing (6mg/kg/hr maintenance for 6 hours rather than 12) |
N=20 infants (21 treatments- one infant treated twice due to seizure recurrence) Term and preterm Followup study N=15, with 16 treatments) |
aEEG | 11/21 (52%) achieved seizure cessation, 5/21 (24%) had diminished seizure activity or cessation with later recurrence. So overall-76% with some effect. Secondary confirmatory study- some effect in 78% (similar) |
Main focus of manuscript was the development of an optimal dosing strategy without cardiac toxicity | No significant side effects observed | No control/ comparison group Time to seizure cessation not described in detail |
| Abend et al, 201130 |
Levetiracetam (1st, 2nd, or 3rd line) 10mg/kg IV then maintenance (mean 45mg/kg/day) divided BID Phenobarbital 1st line then phenytoin in some. |
Retrospective, observational, no control group | N=23 (2 as first line, 13 as 2nd line, 3 as 3rd line, 1 as 4th line) Term and late preterm |
cEEG EEG usually continued for 24hrs after seizure cessation |
8/23 (35%) with seizure improvement (>50% reduction) within 24 hrs (7/8 with termination of seizures). Another 4 had improvement in 24–72 hrs. Note that only 35% response is similar to what we get with phenobarbital or phenytoin, but many of these patients had already received at least a first line agent |
First-line: ½ with seizure cessation 2nd line: 4/13 (31%) with cessation in <24hrs, 2/13 with cessation in >24hrs, 1/13 with 50% reduction within 24 hrs, 1/13 with reduction > 24 hrs, and 5/13 with no improvement |
States that no serious cardio-pulmonary adverse events were recorded, and no serious or intolerable adverse effects | Observational, no comparison group, wide range in dosage, varies as to which other anticonvulsants had previously been received (1st, 2nd or 3rd line levetiracetam usage) |
|
Khan et al 201131 |
Levetiracetam 10–50mg/kg IV load (usually 2nd line to phenobarbital but sometimes 1st line), then maintenance. |
Retrospective, observational, no control group | N=22 20 of the 22 got loading dose of 50mg/kg (one got 10, one 20mg/kg) 19 of these maintained on 50mg/kg/day Term only |
cEEG “seizures were clinical at onset and then electroclinical on followup prolonged continuous EEG monitoring”. All patients had EEG correlation at time of seizure cessation. |
19/22 (86%) had immediate seizure cessation at 1 hr 7/22 (32%) with complete seizure cessation after loading dose, 14 (64%) by 24 hrs, 19 (86%) by 48 hrs, all (100%) by 72 hrs. (Most had already received phenobarbital and not completely responded). Defined seizure response at 1 hr after loading dose, and time to complete cessation. |
Of those followed til 6 mo (17)– 71% had achieved complete seizure freedom off anticonvulsants, 24% while still on levetiracetam were seizure free, and 1 with recurrence even on levetiracetam | No significant side effects observed One patient with irritability, improved on pyridoxine |
No control/ comparison group Half were receiving other anticonvulsants concomitantly with levetiracetam- though usually just for a few days |
Abbreviations: cEEG= continuous electroencephalography, aEEG= amplitude-integrated electroencephalography, hr=hour, min=minute, F/U = follow-up, IV = intravenous, HIE = hypoxic-ischemic encephalopathy
Of the Tier I investigations, Painter et al’s study8 was the only one to focus on phenobarbital and phenytoin. The well-constructed trial randomized 59 patients to receive phenobarbital to a goal free level of 25 micrograms/mL or phenytoin to a free level of 3 micrograms/mL, with crossover to the alternate therapy if the first failed. Continuous EEG was utilized. Seizures were initially controlled in 43% of term and preterm infants with phenobarbital and 45% with phenytoin (P=1), and ultimate seizure control was obtained after crossover in 57% and 62% respectively (P=0.67). Overall, phenobarbital and phenytoin were equally effective. If one considers “substantial improvement” (80% seizure reduction) rather than complete seizure cessation, these efficacy rates improve to 80% and 72% (P=0.3) respectively. No serious adverse events were observed. Although the patients were randomized, this study did have some minor limitations. It was a single center, there was some imbalance in baseline characteristics between groups, and only single-blinded, as physicians and EEG technicians were aware of treatment assignment.
Other Tier I investigations focused on lidocaine and/or midazolam as second- or third-line after phenobarbital failure.
Hellstrom-Westas et al, 1988, 18 compared different lidocaine regimens following phenobarbital (10–15mg/kg) failure and also second-line diazepam in some patients. Forty-six patients were compared prospectively in three groups, receiving loading doses of 2mg/kg, 1–1.5mg/kg, or no load, followed by a 2–6mg/kg/hour maintenance drip. Continuous aEEG monitoring with intermittent full EEG’s, demonstrated seizure cessation in 83% of cases within 3–4 hours. Notably, 26 of the 29 (90%) loaded with 2mg/kg responded within 30 minutes, whereas 4 of the 11 cases (36%) receiving no loading dose did not respond at all and 5 cases (45%) responded between 1–4 hours. No “dangerous” side effects were reported, although 7 of the 32 cases (22%) with arterial monitoring demonstrated blood pressure changes and 22 of 43 (51%) with cardiac monitoring showed heart-rate changes.
Boylan et al, 2004, 17 performed a randomized trial of lidocaine versus a benzodiazepine drip (midazolam: term cases, clonazepam: preterms), after phenobarbital failure (40mg/kg). Continuous EEG was used. Lidocaine was bolused at 4mg/kg then infused at 2–4mg/kg/hr. Midazolam boluses were 60 micrograms/kg followed by a 150–300 micrograms/kg/hr infusion. Clonazepam dosing was not described. A major limitation was its small size that prevented statistical analysis: 5 cases were randomized to the lidocaine group, 6 to benzodiazepines (3 midazolam, 3 clonazepam). There were no responders to benzodiazepines, and 3 of 5 patients respond to lidocaine (one with 80% seizure reduction, two seizure-free). However, one-year neurodevelopmental outcomes were equally poor between groups. No short-term adverse effects were described. Notably, about 50% of patients screened for inclusion, responded initially to phenobarbital, consistent with Painter et al’s8 rates.
Castro-Conde et al, 2005, 19 retrospectively analyzed and compared second-line midazolam use, after up to 40mg/kg phenobarbital, to a historical cohort of neonatal seizure patients treated only with phenobarbital and/or phenytoin. Continuous EEG was used for at least several hours, then intermittent EEG at 24-hour intervals to verify no seizure recurrence. Midazolam was bolused up to two times at 150 micrograms/kg (0.15mg/kg) if needed, then infused at 60–1080 micrograms/kg/hr (0.06–1.08 mg/kg), a notably higher dose than used in Boylan et al’s17 and other studies. There was a 100% response rate to midazolam, with seizure control within one hour for 10 of 13 in the treated group. However, there were no between-group differences in one-year neurodevelopmental outcomes. Interestingly, if Group 1 nonresponders (seizures continued after phenobarbital or phenytoin) were compared to the midazolam-treated group, there was a significant difference in the percentage of patients assessed as normal, in favor of the midazolam group. Since comparison was to an historical cohort, other practice-changes between the two time periods could have potentially biased the results. Side-effects included decreased consciousness for 10–15 minutes in 4 of 13 infants (31%) following the midazolam bolus and hypotension in two infants with multisystem organ failure due to severe hypoxic-ischemic injury (HIE) that was not clearly midazolam-related.
Shany et al, 2007, 20 used a retrospective cohort to compare second- or third-line treatment with lidocaine versus midazolam in term or near-term neonatal seizure patients with hypoxic-ischemic encephalopathy, after up to 40mg/kg of phenobarbital and/or IV diazepam or lorazepam single doses. Continuous amplitude-integrated EEG was used. Lidocaine boluses were 2mg/kg IV, followed by a 4–6mg/kg/hour infusion. Midazolam was infused at 60–200 micrograms/kg/hr without a loading dose. Some response was noted in 77% of the lidocaine group, although cessation of all seizure activity for > 6 hours occurred in only 50%. Only 50% of the midazolam-treated infants showed any response, and none maintained complete seizure cessation for >6 hours. A few non-responders were crossed over to the alternative agent. This led to an overall response rate of 81% to lidocaine, versus 67% to midazolam. No short-term adverse effects were observed. The absence of midazolam loading doses limits comparison with other investigations.
Investigations without comparison groups (Tier II)
In the eleven studies placed into Tier II (Table 2) for lack of a comparison group, a wide variety of agents were used, including phenobarbital, phenytoin, lorazepam, paraldehyde, thiopental, midazolam, lidocaine, and levetiracetam. In many cases, the agents under investigation were used second- or third-line. Some investigations only recorded continuous EEG for a portion of subjects. If grouped by evidence for each particular antiepileptic agent, the following results can be summarized.
Phenobarbital- Connell et al, 1989, 22 reported complete response in 2 of 31 (6%) patients given phenobarbital 20mg/kg as first-line treatment. Bye and Flanagan’s 1995 investigation25 to assess spatial and temporal characteristics of seizures, utilized a seizure-treatment protocol that began with phenobarbital up to 40mg/kg, or a serum level of 40ug/mL, and they documented sustained seizure cessation, achieved within 120 minutes in 8 of 32 (25%) patients, after phenobarbital alone. Boylan et al, 2002, 27 had 4 of 14 (29%) patients responding to a first phenobarbital load of 20mg/kg. Two had sustained seizure freedom, and two had brief seizure recurrence within a few days. They reported that responders tended to display low seizure burden and normal or only mildly abnormal EEG background.
Phenytoin- Bye and Flanagan’s 1995 study25 (above) followed phenobarbital (40mg/kg) with second-line phenytoin (15–20mg/kg) and seizure cessation was achieved in 5 of 32 patients (16%) within 120 minutes following the addition of phenytoin.
Paraldehyde- Connell et al, 198922 (second-line after phenobarbital). There were no cases of complete and sustained seizure cessation although four cases of equivocal response, defined as delayed or temporary response with seizure recurrence within 24 hours were noted.
Thiopental- Bonati et al, 1990, 23 administered second-line intravenous thiopental (10mg/kg) after phenobarbital (20mg/kg) in nine ventilated patients with severe HIE, noting “prompt and complete” seizure cessation in 100% after thiopental, followed by maintenance phenobarbital. Respiratory side-effects could not be assessed because all subjects were already ventilated.
Lorazepam- Deshmukh et al, 1986, 21 used a single third-line lorazepam dose (0.05mg/kg), following phenobarbital (15mg/kg) and (phenytoin 15mg/kg) in seven infants. Among the three with continuous EEG monitoring, all had seizure cessation within 5 minutes following lorazepam, although one had recurrence of bicycling movements (unclear if seizures) eight hours later. In two instances, after lorazepam administration, the serum phenobarbital level increased into the “toxic range”. Maytal et al, 1991, 24 used lorazepam (0.05–0.15 mg/kg) IV as second-line to phenobarbital 40mg/kg, in 7 subjects. Three subjects had continuous EEG for relatively short duration (maximum 46 minutes) following lorazepam. “Complete” seizure cessation was recorded in approximately 1–3 minutes in all but one patient. The only reported side-effect was amplitude attenuation on EEG in several cases following lorazepam.
Clonazepam- Bye and Flanagan, 1995 25 (above), also examined third-line clonazepam (50 micrograms/kg) after phenobarbital and phenytoin. Two patients who failed phenobarbital (>30mg/kg) and phenytoin (15–20mg/kg) had seizure cessation within 120 minutes after clonazepam administration.
Midazolam- Sheth et al, 1996, 26 reported third-line midazolam (0.15mg/kg load, then 0.1–0.4 mg/kg/hr) after phenobarbital and phenytoin in six mechanically ventilated patients with 100% seizure-free within 1 hour and 4 of 6 (67%) with an immediate post-load response. Van Leuven et al, 2004, 28 prospectively administered third-line midazolam (0.05mg/kg IV load followed by 0.15mg/kg/hr) in term infants with hypoxic-ischemic encephalopathy following phenobarbital and lidocaine with seizure cessation within 24 hours in 11 of 15 subjects (73%). No significant side effects were reported.
Lidocaine- Malingre et al, 2006, 29 prospectively treated 20 infants with lidocaine (dosing protocol: 2mg/kg load, 6mg/kg/hour for 12 hrs, 4mg/kg/hour for 12 hours, then 2mg/kg/hour for 12 hours) after failure of phenobarbital and either midazolam or clonazepam. Seizure reduction was seen in 76%, and 52% had complete seizure cessation. Since their goal was to develop optimal lidocaine dosing with minimal cardiac side-effects, a follow-up study performed with reduced dosing (6mg/kg/hour infusion for only 6 hours versus 12), produced a similar overall response rate (some effect in 78%). No significant side effects were observed.
Levetiracetam- Abend et al, 2011, 30 retrospectively reported levetiracetam use [10–20mg/kg load, then 10–80 mg/kg/day divided twice daily (mean dose: 45mg/kg/day)] in 23 neonates, as first-, second-, or third-line after phenobarbital and in some cases phenytoin. A greater than 50% seizure reduction within 24 hours of levetiracetam initiation was recorded in 35% of neonates, and 88% became seizure free. No serious adverse effects were reported. As stated in their discussion, some seizure cessation may have been secondary to the passage of time. Khan et al, 2011, 31 retrospectively reviewed 22 patients receiving first- or second-line levetiracetam [10–50mg/kg intravenous load (20 of 22 cases received 50mg/kg load), and 50 mg/kg/day maintenance dosing for most]. Seizure cessation occurred in 19 of 22 (86%) within 1 hour, although seizures recurred in some. Complete seizure cessation was recorded in 7 of 22 (32%) after the loading dose, in 14 (64%) by 24 hours, in 19 (86%) by 48 hours, and in all patients by 72 hours. The only reported side-effect was temporary irritability in one patient, which improved following pyridoxine supplementation.
DISCUSSION
There is limited evidence regarding the best pharmacologic treatment for neonatal seizures. Of the two randomized trials8,17 included in our review, only one was large enough to enable statistical analysis. 8 Only three other studies, including a prospective non-randomized experimental study18 and two retrospective cohort investigations19,20 allow comparison of between-group treatment effects.
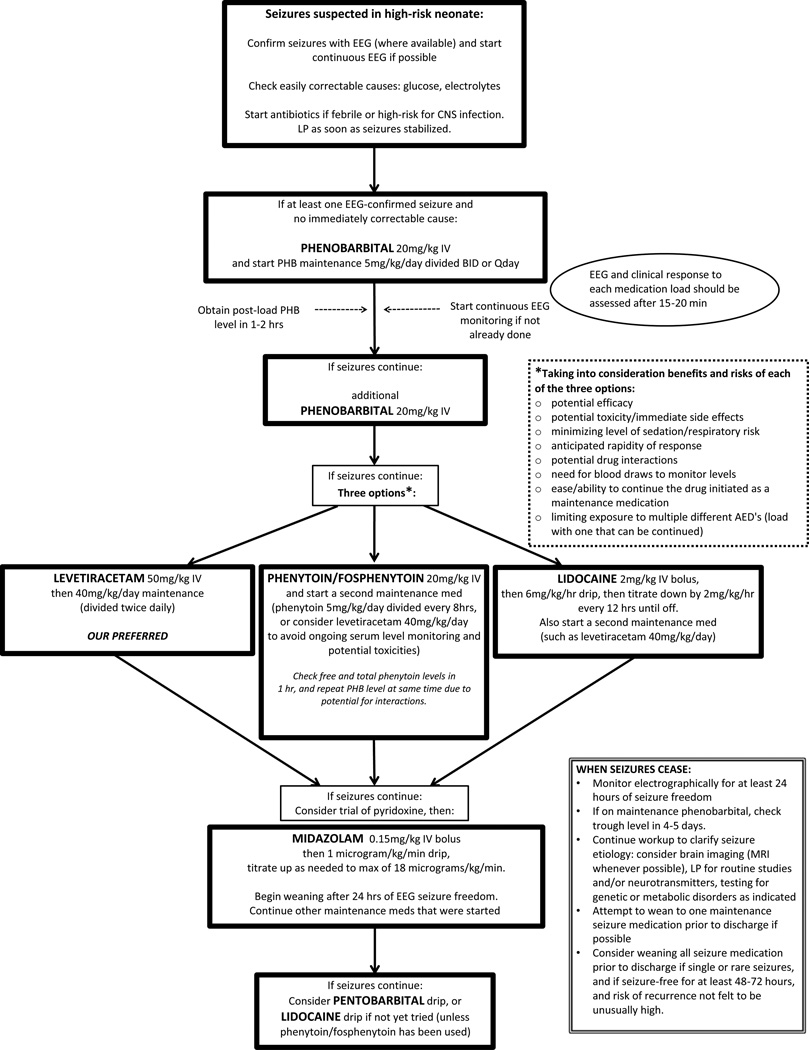
Previous investigations of neonatal seizure treatment were groundbreaking in their descriptions of the first exposures of seizing infants to novel medications. However, determining the risks and benefits of the anti-epileptic drugs from much of the existing data is limited by study design, including the lack of a comparison/control group. Despite this limited evidence, we present a hierarchy of treatment options based on the current literature (Figure 1). We include considerations of practicality and patient safety in our rankings.
Figure 1.
Suggested treatment algorithm for recurrent neonatal seizures. Solid arrow indicates next step if electrographically-confirmed seizures are continuing (clinical or subclinical). Abbreviations: CSF=cerebrospinal fluid, CNS=central nervous system, EEG= electroencephalogram, IV= intravenous, LP = lumbar puncture, MRI= magnetic resonance imaging, PHB= phenobarbital.
We recommend phenobarbital as first-line treatment given its inclusion in the only RCT of first-line treatment of neonatal seizure8, the fact that it is the most studied anti-epileptic medication in animals, 32 and its historical precedence as the first-line antiepileptic drug for neonates. 32 We caution that there is extremely limited evidence on the effect of phenobarbital on long-term neonatal neurodevelopment. Safety considerations severely limit its use in older populations and the U.S. Food and Drug Administration (FDA) has never approved phenobarbital for use in any patient population. 33 Nonetheless, no neonatal antiepileptic has been shown superior to phenobarbital in a well-designed investigation. Although phenytoin was found to be equally effective by Painter et al, 8 the potential for side-effects, the unpredictability of metabolism in neonates, and the need for frequent blood-level monitoring prevent us from recommending it as a first-line option.
Levetiracetam, phenytoin/fosphenytoin, and lidocaine all appear potentially effective as second-line treatments for neonatal seizures that are unresponsive to phenobarbital. Based on our systematic review findings alone, there is not strong evidence to recommend the use of any one of the medications over the others for second-line seizure control.
Lidocaine appears effective compared to benzodiazepines, but has a narrow therapeutic window and the potential to cause cardiac arrhythmias or hypotension, and can induce seizures at high doses. 34–36 Lidocaine should also not be given following fosphenytoin/phenytoin because they may have additive cardiodepressive effects. 37 Phenytoin/fosphenytoin was shown to only provide about a 10–15% increase in seizure control when given following phenobarbital failure. 8 It also requires frequent blood-level monitoring, and is not an ideal medication for maintenance at discharge given its erratic oral absorption, frequency of dosing (usually every 6–8 hours), and continuing drug metabolism changes in young infants. 8,38,39 Blood loss due to therapeutic drug monitoring may be benign in older patients, but is potentially harmful and increases the risk of transfusion and associated morbidities/mortality in neonates whose blood volumes average 80 ml/kg. 40–45
Levetiracetam appeared to acutely reduce seizure burden in two separate studies, but there were no within-study comparison groups. 30,31 It does not require blood-level monitoring, is easily continued as outpatient therapy, and is one of the few FDA-approved antiepileptics for children as young as one-month of age. 46 For these reasons, and given the limitations of lidocaine and fosphenytoin/phenytoin, it is often the preferred second-line treatment at our institution. However, we caution that that its efficacy and safety profile has not been adequately studied in term or preterm neonates within the first month of life. When used, we suggest dosing of 40–50mg/kg bolus in line with Khan et al’s study, 31 which showed good rapidity of response without any significant side effects.
Although Castro-Conde et al19 found better seizure-reduction with second-line midazolam than historically matched phenobarbital non-responders, Shany et al’s cohort study20 and Boylan et al’s trial17 found it inferior to lidocaine. A serious side-effect of benzodiazepines including midazolam is sedation, 19 potentially leading to respiratory depression and intubation. 47,48 Still, midazolam may be considered as a second- or third-line therapy choice, especially in already intubated neonates.
No articles on treatment options, including pentobarbital coma, for infants in status epilepticus met our inclusion criteria. Therefore, we have no reason to recommend against a trial of pentobarbital in intubated patients following failure of other pharmacological therapies. A trial of lidocaine may also be considered in this grave situation if phenytoin was not previously given. 37 We excluded pyridoxine-deficiency from our review, but remind the reader to consider pyridoxine challenge when other antiepileptics provide no response.
A key finding of our systematic review is that well-designed research investigations are clearly needed to determine which antiepileptic medication or medications are most effective for treating neonatal seizures. There is minimal data for many antiepileptics including topiramate, which is being increasingly used in neonates. Future studies should include verification of seizure diagnosis and cessation via conventional or amplitude-integrated EEG and, when feasible, focus on similar seizure etiologies and patients. 32,49
Randomized controlled trials provide the most unbiased estimates of treatment effects, but have proven demanding due to expense, the need for cooperation between multiple neonatal centers to ensure adequate statistical power, and reports of difficulty enrolling neonates in clinical trials of anticonvulsants, at least partially due to parental apprehension about randomization and blinding of treatment. 16,50 A comprehensive cohort design, in which infants whose parents decline randomization are still enrolled in a prospective cohort, is a potential solution that is being increasingly used by clinical investigators. 51 Existing collaborative multicenter networks have the potential to play a critical role in funding and conducting neonatal seizure treatment trials 52,53 and further collaboration among institutions remains imperative for trial success.
Although prone to confounding by indication, well-designed observational studies also have a critical role to play in neonatal seizure therapy research. Carefully constructed prospective and retrospective cohort investigations could discover treatment associations that will inform both clinical practice and the design of future trials. In addition, large observational databases are perhaps the ideal tool to study rare but potentially harmful drug side effects.54,55
Conclusion
There is limited evidence regarding the best pharmacologic treatment for neonatal seizures. We anticipate that this systematic review of neonatal seizure treatment will serve as a valuable reference that will aid clinicians and inform the design of future investigations to compare the effectiveness of neonatal antiepileptics. Further research is needed not only to determine which antiepileptic medication is most effective in neonates, but also to verify the safety of treating neonates with these medications.
Acknowledgements
We thank Nationwide Children’s librarians Lisa Blackwell, MLS and Linda DeMuro, MLS for their assistance in creating our literature search strategy.
Funding: Supported by KL2RR025754 (Jonathan Slaughter, PI) from the National Center For Research Resources, which is now at the National Center for Advancing Translational Sciences, Grant 8KL2TR000112-05. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of conflicting interests: All authors (LAS, ADP, JLS) have no conflicts of interest or commercial/financial relationships to disclose.
Author Contributions: All named authors meet the criteria for authorship as defined in the instructions for authorship. Specific contributions are outlined below:
Developed the idea for the study – L Slaughter, Patel, J Slaughter
Original Draft of manuscript– L Slaughter, Patel, J Slaughter
Data Extraction and Quality Assessment– L Slaughter, Patel, J Slaughter
Interpretation of Data- L Slaughter, Patel, J Slaughter
Revision of manuscript for important intellectual content – L Slaughter, Patel, J Slaughter
Final approval of draft for submission – L Slaughter, Patel, J Slaughter
REFERENCES
- 1.Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology. 1995;45:724–732. doi: 10.1212/wnl.45.4.724. [DOI] [PubMed] [Google Scholar]
- 2.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr. 1999;134:71–75. doi: 10.1016/s0022-3476(99)70374-4. [DOI] [PubMed] [Google Scholar]
- 3.Saliba RM, Annegers JF, Waller DK, et al. Incidence of neonatal seizures in Harris County, Texas, 1992–1994. Am J Epidemiol. 1999;150:763–769. doi: 10.1093/oxfordjournals.aje.a010079. [DOI] [PubMed] [Google Scholar]
- 4.Blume HK, Garrison MM, Christakis DA. Neonatal seizures: treatment and treatment variability in 31 United States pediatric hospitals. J Child Neurol. 2009;24:148–154. doi: 10.1177/0883073808321056. [DOI] [PubMed] [Google Scholar]
- 5.Booth D, Evans DJ. Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. 2004;(4) doi: 10.1002/14651858.CD004218.pub2. CD004218. [DOI] [PubMed] [Google Scholar]
- 6.Wirrell EC. Neonatal seizures: to treat or not to treat? Semin Pediatr Neurol. 2005;12:97–105. doi: 10.1016/j.spen.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39:77–79. doi: 10.1016/j.pediatrneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 9.Thorp JA, O'Connor M, Jones A, et al. Does perinatal phenobarbital exposure affect developmental outcome at age 2? Am J Perinatol. 1999;16:51. doi: 10.1055/s-2007-993836. [DOI] [PubMed] [Google Scholar]
- 10.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol. 2012;72:363–372. doi: 10.1002/ana.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine. Finding What Works in Health Care: Standards for Systematic Reviews. Natl Academy Pr; 2011. [PubMed] [Google Scholar]
- 14.Murray DM, Boylan GB, Ali I, et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–F191. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 15.Malone A, Anthony Ryan C, Fitzgerald A, et al. Interobserver agreement in neonatal seizure identification. Epilepsia. 2009;50:2097–2101. doi: 10.1111/j.1528-1167.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij LGM, Toet MC, van Huffelen AC, et al. Effect of Treatment of Subclinical Neonatal Seizures Detected With aEEG: Randomized, Controlled Trial. Pediatrics. 2010;125:e358–e366. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 17.Boylan G, Rennie J, Chorley G, et al. Second-line anticonvulsant treatment of neonatal seizures. Neurology. 2004;62:486–488. doi: 10.1212/01.wnl.0000106944.59990.e6. [DOI] [PubMed] [Google Scholar]
- 18.Hellström-Westas L, Westgren U, Rosen I, Svenningsen N. Lidocaine for treatment of severe seizures in newborn infants. Acta Paediatr Scand. 1988;77:79–84. doi: 10.1111/j.1651-2227.1988.tb10602.x. [DOI] [PubMed] [Google Scholar]
- 19.Castro-Conde JR, Borges AAH, Martinez ED, et al. Midazolam in neonatal seizures with no response to phenobarbital. Neurology. 2005;64:876–879. doi: 10.1212/01.WNL.0000152891.58694.71. [DOI] [PubMed] [Google Scholar]
- 20.Shany E, Benzaqen O, Watemberg N. Comparison of continuous drip of midazolam or lidocaine in the treatment of intractable neonatal seizures. J Child Neurol. 2007;22:255–259. doi: 10.1177/0883073807299858. [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh A, Wittert W, Schnitzler E, Mangurten HH. Lorazepam in the treatment of refractory neonatal seizures: a pilot study. Am J Dis Child. 1986;140:1042. doi: 10.1001/archpedi.1986.02140240088032. [DOI] [PubMed] [Google Scholar]
- 22.Connell J, Oozeer R, De Vries L, et al. Clinical and EEG response to anticonvulsants in neonatal seizures. Arch Dis Child. 1989;64:459–464. doi: 10.1136/adc.64.4_spec_no.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonati M, Marraro G, Celardo A, et al. Thiopental efficacy in phenobarbital-resistant neonatal seizures. Dev Pharmacol Ther. 1990;15:16. doi: 10.1159/000457614. [DOI] [PubMed] [Google Scholar]
- 24.Maytal J, Novak GP, King KC. Lorazepam in the treatment of refractory neonatal seizures. J Child Neurol. 1991;6:319–323. doi: 10.1177/088307389100600406. [DOI] [PubMed] [Google Scholar]
- 25.Bye A, Flanagan D. Electroencephalograms, clinical observations and the monitoring of neonatal seizures. J Paediatr Child Health. 1995;31:503–507. doi: 10.1111/j.1440-1754.1995.tb00872.x. [DOI] [PubMed] [Google Scholar]
- 26.Sheth RD, Buckley DJ, Gutierrez AR, et al. Midazolam in the treatment of refractory neonatal seizures. Clin Neuropharmacol. 1996;19:165. doi: 10.1097/00002826-199619020-00005. [DOI] [PubMed] [Google Scholar]
- 27.Boylan G, Rennie J, Pressler R, et al. Phenobarbitone, neonatal seizures, and video-EEG. Arch Dis Child Fetal Neonatal Ed. 2002;86:F165–F170. doi: 10.1136/fn.86.3.F165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Leuven K, Groenendaal F, Toet M, et al. Midazolam and amplitude-integrated EEG in asphyxiated full-term neonates. Acta Paediatrica. 2004;93:1221–1227. [PubMed] [Google Scholar]
- 29.Malingré MM, Van Rooij LGM, Rademaker CMA, et al. Development of an optimal lidocaine infusion strategy for neonatal seizures. Eur J Pediatr. 2006;165:598–604. doi: 10.1007/s00431-006-0136-x. [DOI] [PubMed] [Google Scholar]
- 30.Abend NS, Gutierrez-Colina AM, Monk HM, et al. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26:465–470. doi: 10.1177/0883073810384263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan O, Chang E, Cipriani C, et al. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44:265–269. doi: 10.1016/j.pediatrneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Clancy RR. Summary proceedings from the neurology group on neonatal seizures. Pediatrics. 2006;117:S23–S27. doi: 10.1542/peds.2005-0620D. [DOI] [PubMed] [Google Scholar]
- 33.Meadow M. The FDA Takes Action Against Unapproved Drugs. FDA Consumer Magazine. 2007 Jan-Feb; [PubMed] [Google Scholar]
- 34.Van Rooij LGM, Toet MC, Rademaker KMA, et al. Cardiac arrhythmias in neonates receiving lidocaine as anticonvulsive treatment. Eur J Pediatr. 2004;163:637–641. doi: 10.1007/s00431-004-1513-y. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein P, Dornbusch J, Shaywitz BA. Prolonged seizures associated with the use of viscous lidocaine. J Pediatr. 1982;101:461–463. doi: 10.1016/s0022-3476(82)80088-7. [DOI] [PubMed] [Google Scholar]
- 36.Resar LM, Helfaer MA. Recurrent seizures in a neonate after lidocaine administration. J Perinatol. 1998;18:193–195. [PubMed] [Google Scholar]
- 37.Wood RA. Sinoatrial arrest: an interaction between phenytoin and lignocaine. Br Med J. 1971;1:645. doi: 10.1136/bmj.1.5750.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loughnan PM, Greenwald A, Purton WW, et al. Pharmacokinetic observations of phenytoin disposition in the newborn and young infant. Arch Dis Child. 1977;52:302–309. doi: 10.1136/adc.52.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Painter M, Minnigh M, Gaus L, et al. Neonatal phenobarbital and phenytoin binding profiles. J Clin Pharmacol. 1994;34:312–317. doi: 10.1002/j.1552-4604.1994.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 40.Usher R, Shephard M, Lind J. The blood volume of the newborn infant and placental transfusion. Acta Paediatrica. 1963;52:497–512. doi: 10.1111/j.1651-2227.1963.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 41.Usher R, Lind J. Blood volume of the newborn premature infant. Acta Paediatrica. 1965;54:419–431. doi: 10.1111/j.1651-2227.1965.tb06397.x. [DOI] [PubMed] [Google Scholar]
- 42.Bauer K, Linderkamp O, Versmold H. Systolic blood pressure and blood volume in preterm infants. Arch Dis Child. 1993;69:521–522. doi: 10.1136/adc.69.5_spec_no.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.dos Santos AMN, Guinsburg R, de Almeida MFB, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr. 2011;159:371–376. doi: 10.1016/j.jpeds.2011.02.040. e3. [DOI] [PubMed] [Google Scholar]
- 44.Blau J, Calo JM, Dozor D, et al. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–409. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Paul DA, Mackley A, Novitsky A, et al. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–641. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 46.U.S. Dept. of Health and Human Services. [cited 2012 Oct 5];Food and Drug Administration Center for Drug Evaluation and Research. Application Number NDA 21505/S-026 [Internet] Available from: http://www.accessdata.fda.gov.
- 47.Blumer JL. Clinical pharmacology of midazolam in infants and children. Clin Pharmacokinet. 1998;35:37–47. doi: 10.2165/00003088-199835010-00003. [DOI] [PubMed] [Google Scholar]
- 48.Ng E, Klinger G, Shah V, Taddio A. Safety of benzodiazepines in newborns. Ann Pharmacother. 2002;36:1150–1155. doi: 10.1345/aph.1A328. [DOI] [PubMed] [Google Scholar]
- 49.Silverstein FS, Jensen FE, Inder T, et al. Improving the treatment of neonatal seizures: National Institute of Neurological Disorders and Stroke workshop report. J Pediatr. 2008;153:12–15. doi: 10.1016/j.jpeds.2008.01.041. e1. [DOI] [PubMed] [Google Scholar]
- 50.Guillet R University of Rochester. Prophylactic Phenobarbital After Neonatal Seizures (PROPHENO) ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. [cited 2012 Oct 5]. Available from: http://clinicaltrials.gov/ct2/show/NCT01089504?term=propheno&rank=1. [Google Scholar]
- 51.Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Center for Research Resources. Laparotomy vs. Drainage for Infants With Necrotizing Enterocolitis (NEST) ClinicalTrials.gov. October 2011. In: ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2012. [cited 2012 Oct 5]. Available from: http://clinicaltrials.gov/ct2/show/NCT01029353?term=nest&rank=1. [Google Scholar]
- 52.National Institues of Health; Eunice Kennedy Shriver National Institute of Child Health and Human Development. [cited 2012 Oct 5];Neonatal Research Network [Internet] Available from: http://neonatal.rti.org/
- 53.National Institutes of Health; National Institute of Neurological Disorders and Stroke. [cited 2012 Oct 5];NeuroNEXT: Network for Excellence in Neuroscience Clinical Trials [Internet] Available from: http://www.neuronext.org/
- 54.Vandenbroucke JP. What is the best evidence for determining harms of medical treatment? Can Med Assoc J. 2006;174:645–646. doi: 10.1503/cmaj.051484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou R, Aronson N, Atkins D, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health-care program. J Clin Epidemiol. 2010;63:502–512. doi: 10.1016/j.jclinepi.2008.06.007. [DOI] [PubMed] [Google Scholar]