Abstract
Research has elucidated causal links between stress exposure and the development of anxiety disorders, but due to the limited use of female or sex-comparative animal models, little is known about the mechanisms underlying sex differences in those disorders. This is despite an overwhelming wealth of evidence from the clinical literature that the prevalence of anxiety disorders is about twice as high in women compared to men, in addition to gender differences in severity and treatment efficacy. We here review human gender differences in generalized anxiety disorder, panic disorder, posttraumatic stress disorder and anxiety-relevant biological functions, discuss the limitations of classic conflict anxiety tests to measure naturally occurring sex differences in anxiety-like behaviors, describe sex-dependent manifestation of anxiety states after gestational, neonatal, or adolescent stressors, and present animal models of chronic anxiety states induced by acute or chronic stressors during adulthood. Potential mechanisms underlying sex differences in stress-related anxiety states include emerging evidence supporting the existence of two anatomically and functionally distinct serotonergic circuits that are related to the modulation of conflict anxiety and panic-like anxiety, respectively. We discuss how these serotonergic circuits may be controlled by reproductive steroid hormone-dependent modulation of crfr1 and crfr2 expression in the midbrain dorsal raphe nucleus and by estrous stage-dependent alterations of γ-aminobutyric acid (GABAergic) neurotransmission in the periaqueductal gray, ultimately leading to sex differences in emotional behavior.
Keywords: Anxiety, Gender/sex, Stress, Panic disorder, PTSD, Estrous cycle
Human gender differences in anxiety and emotional disorders
Similar to the increased prevalence of depression in women [194, 197, 387] the US National Institute of Mental Health reports that the lifetime prevalence of an anxiety disorder is 60 % higher in women than in men [198, 215, 247, 269] and that the onset, severity, clinical course, and treatment response of anxiety disorders differ significantly in women [293]. According to the current Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR), anxiety disorders are categorized into generalized anxiety disorder (GAD), panic disorder with or without agoraphobia, agoraphobia without history of panic disorder, posttraumatic stress disorder (PTSD), acute stress disorder, obsessive–compulsive disorder, social anxiety disorder (social phobia), anxiety secondary to a medical condition, substance-induced anxiety disorder, and stimulus-specific phobias [16, 189]. For this review, GAD, panic disorder, PTSD, and to a certain extent, acute stress disorder are of particular relevance because acute, repeated, or chronic stress exposures are common triggers for these psychiatric disorders [273, 286], because women may have an inherently increased stress vulnerability, and because key symptoms of these disorders have been successfully modeled in animals.
Generalized anxiety disorder
GAD is characterized by constant, nonspecific, often irrational worry and increased arousal in generally safe situations or interactions, resulting in significant impairment of everyday functionality. In developed, but not in developing, countries, women are two to three times more likely than men to suffer from GAD and have higher self-reported anxiety scores [90, 138, 215, 396]. In light of evolution, different rates of game-togenesis, number of gamete availability, and partner selection may have predestined females to display a “more carefully assessing,” selective, or anxious behavioral spectrum than men, with the exception of pregnancy, peripartum period, and lactation, times when more aggressive and less anxious behaviors are beneficial in order to protect the offspring [265]. Accordingly, naturally higher anxiety scores in females commonly disappear during the peripartum period and lactation in both women [254, 280, 360] and rodents [49, 265].
Panic disorder
Panic disorder patients suffer from sudden brief periods of intense fear, hypervigilance, and distress, including autonomic symptoms like tachycardia, difficulty breathing, or nausea, without significant hypothalamic–pituitary–adrenal (HPA) axis stress responses [135]. Panic attacks can be, but do not have to be, triggered by specific stimuli, and the prevalence for panic disorder is two to three times as high in women as in men [127, 199, 396]. Also, in an 8-year longitudinal study Yonkers et al. [396] reported a threefold higher incidence of relapse in women compared to men. Kelly et al. [192] found that, among healthy test subjects, both men and women exposed to 20 % CO2, a stimulus that elicits responses comparable to a spontaneous panic attack in panic patients, display similar autonomic responses (heart rate, electrodermal response, and frontalis muscle tension), while the subjective experience of fear and panic is far greater in women, indicating differences in how a panicogenic stimulus is perceived or processed in the female brain. To dissect such gender differences, research must also take the estrous cycle stage into account. The female brain must have mechanisms in place to cope with the monthly fluctuations of sex steroids, many of which are neuroactive [157, 252, 325], and it is probably only when such adaptive mechanisms are disturbed that psychiatric diseases manifest themselves. Anxiety sensitivity, e.g., the fearful belief that certain bodily sensations or the experience of anxiety itself may indicate undetected illness [248], is an established cognitive risk factor for the development of panic disorder [233, 268, 323]. During the premenstrual phase (days 24–28) both women with panic disorder and women with high anxiety sensitivity scores display a greater electro-dermal response magnitude to auditory anxiety-provoking stimuli than healthy controls, while baseline recordings (in the absence of auditory stimulation) are the same among all groups and estrous phases [333, 334]. Similarly, women suffering from panic disorder are more likely to experience a panic attack after a laboratory CO2 challenge during their premenstrual phase (days 23–28), compared to their intermenstrual phase (days 8–22) or healthy controls in either phase. The fact that a panicogenic challenge with CO2 [132, 196] or intravenous sodium lactate [113, 318] causes premenstrual dysphoric disorder (PMDD) patients to display panic attacks at about the same rate as in panic disorder patients further indicates a common underlying psychobiology [368]. Rodent as well as human research [190, 260, 274, 333] now proposes a three-factor interaction between (a) the rate at which progesterone and its anxiolytic metabolite allopregnanolone drop during the late luteal phase (humans) or during late diestrus (comparable phase in rodents) [221, 222, 314], (b) γ-aminobutyric acid (GABA)A receptor sensitivity, kinetics, and subunit assembly in stress-coping circuitries including the amygdala and the periaqueductal gray (PAG) [143, 147], and (c) external stressors [94, 95, 340] as a model of sex-dependent predisposition for panic disorder [268].
Posttraumatic stress disorder
According to Olff [275], Breslau [60, 62], and Cohen and Yehuda [80], women are also more likely than men to develop acute stress disorder or PTSD, but controversy exists on whether this is due to inherently increased stress vulnerability or an earlier average age of trauma exposure, different types of PTSD-inducing events (e.g., sexual vs. combat-related assaults), or increased societal victimization of women combined with a different perception of the PTSD-inducing event [80, 275]. In contrast to GAD, PTSD diagnosis requires the experience of one or a series of psychologically traumatic events that result in flashback memories and nightmares as well as avoidance of stimuli associated with that event, in combination with typical anxiety symptoms such as hypervigilance. HPA axis dysfunction in PTSD is proposed for both genders [395], but a recent meta-analysis revealed that especially female PTSD patients appear to have lower circulating cortisol concentrations, compared to healthy controls [251].
Anxiety-relevant physiological and psychological gender differences
In healthy individuals, research over the past decade has identified several physiological and neurological gender differences that are relevant to stress responsiveness and anxiety. In the Trier Social Stress Test (TSST) [205], an anxiogenic, social-evaluative laboratory setting that reliably activates the human HPA axis, many studies report lower salivary cortisol responses in women compared to men [186, 212, 213, 324], while others find no difference [193, 388], yet women report overall more irritability and distress after the test [193]. Overall, these human HPA axis response results are puzzling because rodent studies robustly find the opposite sex difference, meaning higher increases of corticosterone secretion in females following various types of stressors [156, 327, 365, 381]. Because estrogens have been shown to positively regulate the expression of the human corticotropin-releasing factor (CRF) gene [363], which does not only orchestrate HPA axis activity, but is also expressed in anxiety-related brain circuits [139, 216, 335, 375] and facilitates anxiety-like behaviors and lasting anxiety states [107, 328], differences in central actions of CRF may be more relevant to the female bias in anxiety disorders. This notion is supported by recent animal studies [28, 244, 346].
Imaging studies have now revealed several structural or functional gender differences in anxiety-relevant brain regions, such as the prefrontal cortex (PFC), hippocampus, and extended amygdala complex [348]. For example, the central part of the bed nucleus of the stria terminalis (BNST) is smaller in women compared to men [399], and a meta-analysis found that negative emotions were consistently associated with a stronger activation of the left central amygdala (CE) in women, whereas positive emotions activated the left CE more in men compared to women [348].
Interestingly, women have greater pain sensitivity than men [119, 142], a phenomenon that is due to thalamocortical processing or emotional appraisal of the stimulus, not spinal nociceptive activity [130], and that disappears when trait anxiety is controlled for [130, 300]. This likely depends on an interaction of sex steroid fluctuation and stress exposure because rodent studies have demonstrated that such sex-dependent hyperalgesia only occurs after a mild anxiogenic stressor during late diestrus, but not during other estrous stages [93].
Thus, while some gender discrepancies in emotional disorders might be skewed by the fact that women tend to report and seek help more readily than men [43, 199, 235], understanding the true biological determinants of anxiety disorders in both women and men is of therapeutic and economic importance.
Significance and socioeconomic impact of gender differences in anxiety
In Western civilizations, the lifetime prevalence for anxiety disorders amounts to approximately 18 % of the population [198], the average onset age for anxiety disorders is 11 years of age [269], and while the overall costs of anxiety disorders in the USA were an estimated $42 billion/year during the 1990s [141], mental health care costs, including those for anxiety disorders, are currently outgrowing those of heart disease and cancer [270]. In women, more so than in men, anxiety disorders are also often identified as a preexisting condition before the onset of a major depressive episode [63, 64, 163, 281, 282], and anxiety often remains a major comorbidity with depression [61, 77, 106, 246, 258]. Recent studies have also shown that anxiety disorders occurring as early as childhood and adolescence are strong predictors of later depressive episodes [20] and, in girls, of later suicide attempts [65]. Generally, anxiety disorders are much more common in girls than in boys [20, 267], and adolescent anxiety is associated with increased rumination in girls but not in boys [158]. This highlights the ethical and socioeconomic need to prevent or treat anxiety disorders as early as possible under the reasonable assumption that preventing the manifestation of an early anxiety disorder may also reduce the risk of later affective disorders.
While research acknowledges existing gender differences in anxiety disorders, treatment remains largely indifferent towards those facts [37, 78]. Revealing biological substrates and mechanisms relevant to the etiology of anxiety disorders in females compared to males would relieve some of the economic and personal burden originating from ineffective treatment strategies.
Major questions and challenges for animal models
Key questions are whether sex differences in anxiety disorders originate mainly because of sex-chromosomal gene expression [89], sexually dimorphic developmental differentiation of brain regions and stress-response systems (organizational effects of sex steroids), female reproductive hormone fluctuations postpuberty, protective or vulnerability-inducing effects of reproductive hormones in adulthood (activational effects of sex steroids), or differences in stressor perception and emotional appraisal. Since the Y chromosome contains a very limited number of genes and the Barr body (second X chromosome in females) remains largely inactive in healthy cells of the female body [73, 74], the impact of chromosomal differences in the classic sense [19, 299] on anxiety-related behavior is probably small in comparison to developmental or adult neuroendocrine sex differences, but not negligible [35, 89, 241]. The role of the maternally inherited mitochondrial genome and its effects on energy balance should also not be ignored because a higher predisposition for anxiety and depressive disorders is detected in mothers and matrilineal relatives of children with maternally inherited mitochondrial diseases [48], and clinical in vitro fertilization studies among offspring of genetically related or unrelated mothers suggest that, in many cases, affective and anxiety-linked genetic traits may be inherited from the mother [38, 307, 308]. It also to be expected that anxiety-relevant biological sex differences exist with regard to stress vulnerability, meaning how the female brain perceives and processes stressful events, and that some of these differences should be detectable on a molecular level.
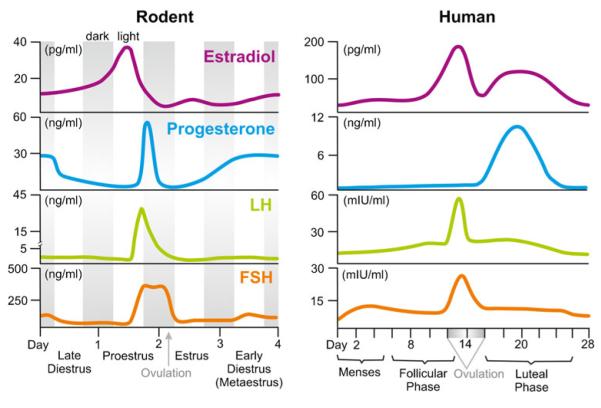
While neuroanatomy and physiology are very similar between female rodents and women, discrepancies in reproductive cycle duration (4 days in rodents vs. 28 days in humans), cycle pattern of estradiol and progesterone, and hormone amplitude differences [35, 120] exist. For a detailed comparison of the hormonal fluctuations across the rodent estrous cycle and the human menstrual cycle, please refer to Fig. 1.
Fig. 1.
Schematic comparison of the 4-day rodent reproductive cycle and the 28-day human menstrual cycle. Depicted are average fluctuations of the circulating hormones 17-beta-estradiol, progesterone, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) in a female rat (left panel) vs. a female human (right panel). Gray bars in the left panel depict the dark phases. Data for individual hormones were adapted from [120, 339, 353]
Additional challenges are increased costs of studies comparing both sexes or analyzing all four stages of the rodent cycle (proestrus, estrus, early/late diestrus), instead of using ovariectomized or hormone-replaced females, and conceptual issues such as interpreting detected sex differences properly. Not every (neuro)biological sex difference indicates vulnerability, and many responses to acute or even chronic stressors may be adaptive not maladaptive [8]. For example, rodent research may find more acute stress-induced expression of the immediate early gene c-fos in several brain regions of male rats, but not proestrus or estrus female rats [45, 117], or increased memory loss in male rats compared to female rats [225] after chronic stress, but it remains to be determined whether such differences represent adaptive or maladaptive coping mechanisms. This leads to the importance of pairing neuroendocrine or neuroanatomical studies with appropriate behavioral tests. Since we cannot inquire about subjective states of anxiety in a rodent, we instead employ tests that have face validity (e.g., classic tests of conflict anxiety) and correlate those with biological measures (e.g., neuronal activation or gene expression). Useful behavioral tests thus either translate the human concept into a test situation that is evolutionarily comparable and relevant to the rodent without anthropomorphizing the animal’s behavior or are based on known neurocircuits or neurotransmitter systems within the body.
While animal models cannot mimic societal injustice that still exists towards women in parts of the world or address how societal issues and gender-dependent reinforcement are processed by males vs. females, animal models can inform on underlying biological differences, sex-dependent symptomology, and coping mechanisms in stress-related anxiety disorders and may lead to the identification of sex-specific targets for pharmaceutical treatment. In the following sections, we focus on sex differences in naturally occurring trait anxiety and sex differences in anxiety states induced by acute, repeated, or chronic stress exposure.
Sex differences in conflict anxiety and current animal models
Naturally occurring sex differences in classic anxiety tests
One behavioral symptom of GAD with face validity in rodents is conflict anxiety, meaning an inhibited approach when placed in an ambiguous situation that involves both potential reward and potential danger or punishment [140, 249, 250]. In other words, the rodent’s inherent avoidance behavior (e.g., towards open/exposed/brightly lit areas, a novel object, or towards an aversive stimulus) competes with the natural explorative drive, for example, to seek a reward such as food or water. Similarly, human anxiety is maladaptive when it prevents the individual from participating in normal daily activities and interactions or from seeking reward due to an unrealistic, exaggerated fear of failure, social scrutiny, or punishment. Unconditioned behavioral paradigms such as the light–dark (white/black) box [256, 302], elevated plus maze (EPM) [170, 288], open field (OF) [298, 378], novelty-suppressed feeding [46, 161], or Vogel punished drinking [369] are classic approaches to test inherent conflict anxiety (for a schematic overview, see Fig. 2). The elevated T-maze (ETM) [397] evaluates both conflict anxiety, with longer latencies to enter the open arm of the maze as a measure of inhibitory avoidance, and panic-like escape responses, with shorter latencies to escape from the open arm of the maze as an index of increased panic-like behavior. The social interaction (SI) test [118], using a novel adult conspecific of the same age, weight, and sex, and the juvenile social exploration test [75], using a novel adolescent conspecific of the same sex, evaluate anxiety in the context of rodent-specific, nonaggressive social identification, contact, and play behaviors initiated by the experimental rat.
Fig. 2.

Schematic overview of commonly used tests of unconditioned conflict anxiety in adult rodents and of neonatal ultrasonic vocalization. Shades of gray represent darker (or, in case of the OF and SI test, more protected) zones of the respective test paradigm. Thick black lines designate walls of the test apparatus or cage (in the case of novelty-suppressed feeding/neophagia). In the OF, light–dark (white/black) box, EPM, and ETM, an increased latency to enter into and less time spent in the brighter (or more exposed) zones designates anxiety-like behavior. Similarly, decreased SI behavior with an age-matched, weight-matched, and sex-matched conspecific, decreased numbers of shock-punished licks (1 shock every 20 licks) at the drinking bottle after 16–24 h of water deprivation in the Vogel punished drinking test, and suppressed consumption of food in a brightly lit, novel cage or arena also indicate an anxiety-like behavioral state. Frequency and duration of neonatal ultrasonic vocalizations at 40–50 kHz are used to screen for anxiolytic drugs and are strongly correlated with adult trait anxiety
Interestingly, most murine rodent models report lower anxiety-like behavior in females, compared to males. Table 1 lists examples of adult sex differences, as well as age-dependent and estrous stage-dependent female behavior in classic conflict anxiety paradigms. We also list sex differences in ultrasonic vocalization of isolated neonates (40–50 kHz) because it has proven to be positively correlated with adult trait anxiety [68, 389, 392] and is used to screen for anxiolytic drugs [168, 255], such as 5-hydroxytryptamine receptor type 1A (5-HT1A) agonists or benzodiazepines [121], targeting GABAA receptors. A major advantage of assessing the duration or frequency of ultrasonic vocalization is that it is an objective behavioral endpoint, easily quantifiable, automated, locomotion-independent, and requires no conditioning procedure. Early studies using adult rodents [182, 195] often entirely failed to distinguish between anxiety-like behavior and locomotion or general activity, which is commonly higher in females [115], but even in more careful evaluations, it is impossible to evaluate anxiety-like variables distinctly from locomotor activity because locomotion is the driving force underlying the variables of interest [102]. In contrast, the SI and Vogel punished drinking tests tend to detect a sex bias towards increased anxiety-like behavior in females [182, 343]. However, male–male vs. female–female SI may have inherently different components, and more avoidance in the Vogel punished drinking paradigm might be compromised by enhanced female pain sensitivity [119]. Sex differences in anxiety-like behavior also depend on the species (with monogamous, alloparenting species such as Mongolian gerbils [66] and prairie voles [26] potentially proving to be better rodent models for sex differences in anxiety than mice and rats), strain [13, 309, 358], age [110, 175], and whether female data were pooled for all estrous stages or not. In fact, estrous stage appears to be a major determinant of conflict anxiety, with diestrus females acting more anxious than males or estrus, metaestrus, and proestrus females [125, 133, 234, 261]. Also, circadian testing time, light or dark phase, light intensity, and other methodological differences, such as pretesting conditions or test order, can profoundly alter outcome variables [72, 172, 302]. Conclusively, it seems necessary to evaluate existing paradigms more carefully, e.g., using principal component analyses [13, 115] or z-scoring computation [146], to develop novel behavioral tests that are not driven by anxiety-irrelevant behaviors or physiology and to identify reliable, easily measurable correlates of conflict anxiety in order to properly address and quantify sex differences in rodent emotionality models.
Table 1.
Examples of naturally occurring sex differences and estrous stage-dependent female behavior in unconditioned, classic rodent tests of adult conflict anxiety and neonatal ultrasonic vocalization
| Test paradigm | Species and strain | Sex difference or major finding | Reference |
|---|---|---|---|
| Light–dark box | Mouse, FVB/NHsd | ↓ anxiety in females vs. males | [370] |
| Rat, Lewis | ↓ anxiety in females vs. males | [303] | |
| Mongolian gerbils | ↑ anxiety (dark-side entries, but not dark-side time) in females throughout all estrous stages vs. males |
[66] | |
| Elevated plus maze | Mouse, DAB/2 | ↓ anxiety in females vs. males | [309] |
| Rat, Wistar | ↓ anxiety in 90-day-old females vs. males | [175] | |
| Rat, Long Evans | ↓ anxiety in proestrus females vs. males and other estrous stage females | [125] | |
| Rat, Wistar | ↑ anxiety in diestrus vs. proestrus females | [234] | |
| Mouse, 129S2/SvHsd× C57BL/6J |
↓ anxiety in females vs. males | [370] | |
| Rat, Lewis | ↓ anxiety in females vs. males | [303] | |
| Rat, Wistar | ↑ anxiety in early diestrus females vs. males and other estrous stage females | [97] | |
| Mongolian gerbils | ↑ anxiety in females, throughout all estrous stages vs. males | [66] | |
| Prairie voles | ↑ anxiety in females (two times more time in closed relative to open arms) vs. males |
[26] | |
| Rat, Wistar | ↓ anxiety in 60-day-old females vs. males | [110] | |
| Rat, Long Evans | ↑ anxiety in senescent vs. reproductively competent females | [372] | |
| Mouse, C57BL/6J | ↑ anxiety in females vs. males | [13] | |
| Elevated T-maze | Rat, Sprague Dawley | ↓ avoidance in females vs. males | [5] |
| Rat, Lewis | ↓ avoidance in females vs. males | [303] | |
| Rat, Wistar | ↑ avoidance and decreased escape in diestrus females vs. males | [133] | |
| Novelty-suppressed feeding |
Rat, Sprague Dawley | ↑ anxiety in diestrus females vs. females in other estrous stages | [261] |
| Open field | Rat, Long Evans | ↓ anxiety in proestrus females vs. males | [125] |
| Mouse, C57BL/6J | ↑ anxiety in females vs. males | [13] | |
| Social interaction | Rat, Lister hooded | ↓ social interaction in females vs. males | [182] |
| Rat, Long Evans | ↑ social interaction in proestrus females vs. males and females in other estrous stages |
[125] | |
| Mongolian gerbils | ↓ (aggressive) social interaction in females, throughout all estrous stages vs. males | [66] | |
| Rat, Sprague Dawley | ↓ social interaction in proestrus and diestrus females vs. males | [343] | |
| Mouse, C57BL/6J | ↑ social interaction in females vs. males | [13] | |
| Ultrasound vocalization |
Rat, Wistar | ↑ vocalization in male pups when female pups in litter vs. male-only litters | [264] |
| Mouse, various strains | ↑ vocalization in male pups on postnatal days 2–6 vs. females | [149] | |
| Vogel punished drinking |
Rat, Lister hooded | ↓ punished licks in females vs. males | [182] |
| Rat, Long Evans | ↓ punished licks in senescent vs. reproductively competent females | [372] |
Adult anxiety states induced by adverse early life experience
A large body of literature exists on the development of adult anxiety states following adverse early life experience [315], an effect that is likely dependent on epigenetic modifications that result in long-lasting alterations of brain physiology and stress-coping strategies [171], and many of these models report sex differences in anxiety-like behaviors. To discuss this literature in detail is beyond the scope of this review, but it is worth mentioning several anxiety-relevant sex differences discovered in a variety of rodent models using manipulations of maternal diet, other gestational stressors, neonatal lipopolysaccharide (LPS) exposure, maternal separation (MS) or low maternal care, and adolescent stressors to investigate resilience-inducing or vulnerability-inducing effects of early life adversity. For a review of comparable developmental ages in rodents [14] vs. humans, refer to Eiland and Romeo [108], and for a species-comparative table listing the developmental windows relevant for the formation of anxiety-relevant mesolimbocortical brain regions, see Weinstock et al. 2001 [380].
Maternal high-fat diet
Obesity rates are alarmingly high in Western societies. A recent survey concluded that, by 2008, 68 % of all adult Americans were overweight [122], and the percentage is likely to have increased since then. While genetic predisposition is estimated to contribute < 2 % to the body mass index variation between individuals [219], sedentary lifestyle and high-fat dietary choices are likely to explain many obesity cases. In addition to the numerous physiological and mental comorbidities [210], including increased risk for anxiety and depression [310], that are associated with obesity, animal studies recently revealed that poor nutritional choices by the mother may also imprint the offspring to suffer from a disadvantageous energy balance [57, 367], increased susceptibility to metabolic disorders [184], increased brain inflammation [41], and anxiety-like behavior [41, 287, 350]. Specifically, Bilbo et al. [41] fed rat dams a high-fat diet (HFD; both a saturated-fat diet and a trans-fat diet had similar consequences) for 4 weeks prior to mating and throughout pregnancy and lactation. Upon weaning, rat pups were raised on standard rat chow. After reaching adulthood, male, but not female, HFD offspring displayed increased anxiety on the EPM. In contrast, female, but not male, juveniles born to Japanese macaques that were fed an HFD for up to 4 years, including pregnancy and lactation, displayed increased anxiety when presented with a novel object task in a study by Sullivan et al. [350], while the mRNA expression of both tph2, encoding tryptophan hydroxylase 2 (Tph2, the rate-limiting enzyme for serotonin synthesis), and htr1a, encoding the inhibitory 5-HT1A autoreceptor, were increased about twofold within the serotonergic dorsal raphe nucleus (DR) in fetuses (third trimester) of both sexes. This indicates maternal diet-induced alterations of brain serotonergic systems, which may become further dysregulated during puberty when reproductive hormones become active. Sullivan’s nonhuman primate HFD model is consistent with the human sex bias in anxiety disorders and may, to date, be one of the best animal models for anxiety and depression, as TPH2 mRNA and TPH protein expression have repeatedly been shown to be increased in depressed suicide victims [21, 22, 47, 359] and because the association between obesity and affective as well as anxiety disorders is 1.5-fold to 2-fold stronger in women than men [92].
Other gestational stressors
A variety of other gestational disturbances, including malnutrition, maternal exposure to psychological (e.g., repeated restraint) or social stressors (e.g., defeat by a lactating, highly aggressive dam), or increased maternal inflammatory milieu due to bacterial or viral infections, have been shown to alter brain development and increase adult-life anxiety in the offspring, as reviewed by Markham and Koenig [236]. Some of these behavioral alterations may reflect adaptive emotional coping strategies from an evolutionary standpoint [315]. If you are born into a stressful world, increased vigilance and avoidance may save your life while you are vulnerable and developing, and only become maladaptive when applied to safe or potentially rewarding situations later on. Interestingly, prenatal stress appears to partially reverse extremes of genetically inbred trait anxiety, with prenatal stress reducing anxiety in the offspring of high-anxiety-behavior rats and increasing it in the offspring of low-anxiety-behavior rats [50]. With regard to sex differences due to gestational stressors, outcomes depend on the choice of stressor and anxiety test. Gestational malnutrition (6 % vs. normal 25 % protein content) increases open-arm exploration of female offspring tested on the EPM (an anxiolytic effect), but significantly decreases social exploration in both sexes in the SI test due to increased rearing behavior, possibly indicative of increased explorative escape behavior, vigilance, and impulsivity at the expense of social behaviors [6, 7, 209]. A similar hypervigilance, together with faster escape behavior, was detected in female offspring of a prenatal stress model by Louvart et al. [220]. Schulz et al. [326] exposed pregnant rats to daily unpredictable stressors during the last gestational week, resulting in paradigm-dependent elevated anxiety-related behaviors in male (decreased SI time) and female offspring (less time in the open compartments of the elevated zero maze, an open/closed-arm paradigm that lacks the ambiguous center square of the EPM). In contrast, prenatal restraint stress was found to selectively increase OF anxiety-like behavior of female offspring [55].
Maternal separation
MS appears to be a reliable way to induce a chronic anxiety state in male, but not female, offspring. MS protocols, consisting of several hours of litter isolation from the dam, typically between postnatal days 2 to 14 (while maintaining adequate temperature conditions), often produce a bidirectional sexually dimorphic effect on later-life anxiety, with exaggerated anxiety-like behavior in male offspring, but less intense [390] or even anxiolytic outcomes in females tested in conflict anxiety paradigms such as the OF, EPM, and ETM [245, 263, 305, 311, 338, 390]. MS also increases startle and adult (20– 28 kHz) ultrasonic vocalization responses to acoustic stimulation in males, but not females [188]. Sexually dimorphic sensitivity to reduced sensory stimulation (tactile, olfactory, and auditory) during MS may also exist because neonatal tactile stimulation can reverse MS-induced increases in contextual fear-conditioned freezing to that of non-isolated controls in females, but not in males [174]. Similarly to MS, early weaning from maternal care also exerts sex-dependent anxiogenic effects in both mice [200, 201] and rats [208], with a strong bias towards pronounced and longer-lasting male vulnerability.
A recent animal study found that same-strain cross-fostered male Fischer 344 (but not Sprague Dawley) rats scored higher on adult anxiety-related behaviors in the SI and novelty-suppressed feeding tasks than controls [358]. Female offspring were also cross-fostered in this study, but excluded from behavioral assessment. This suggests that, in addition to classic MS models, adoption may also increase the risk to develop anxiety states later in life [148].
Neonatal lipopolysaccharide
Several research groups have demonstrated that an immune challenge early in life, for example, with the endotoxin LPS (a pyrogenic cell wall component of gram-negative bacteria), can result in a long-lasting anxiety-like state throughout adulthood and even senescence [58, 374], in particular in “double-hit” models, including a second immune response activation [351] or psychological stress exposure [376] during adulthood. Indicating a similar male-biased trend of early life disturbances as observed in MS models, Tenk et al. describe that neonatally LPS-challenged male rats are more anxious in the OF test 2 h after a second homotypic immune challenge in adulthood [351] than females, and that neonatal LPS (without a second challenge during adulthood) actually decreases anxiety in female rats tested in the light–dark box [352]. Walker et al. [376] used a “hide box/OF” setup, offering rats a protected box within the OF to retreat into, to reveal that neonatal LPS together with adult restraint stress causes increased risk assessment and overall vigilance in both sexes, but that neonatally LPS-challenged males are more susceptible to anxiety-like behavior in the OF and EPM after restraint during adulthood than females. Interestingly, the anxiety state induced by neonatal LPS persists into the F2 offspring generation when male or female LPS-group rats are mated with control rats, potentially due to epigenetic changes in the paternal line [373]. In the maternal line, however, this anxiety inheritance depends on maternal lactation patterns and maternal care and is reversible by cross-fostering F2 pups with saline mothers [373].
Adolescent stressors
Adolescence is the somewhat ill-defined phase between nutritional independence and adulthood, defined by the endocrine, neural, and behavioral events of sexual maturation [336], and is accompanied by both increased social stressors and a need for social buffering as attention naturally shifts from parent to peer interactions [202]. During this period, social behaviors, such as aggression, risk taking, social play, dominance establishment, and mating, as well as stress-responsive limbic [312] and cortical brain regions [185, 237] take on adult patterns. In fact, a certain amount of stress exposure or socio-environmental stimulation is probably necessary for normal development and exerts long-term stress-protective, anxiolytic, and antidepressant effects later in life [240, 277, 355, 356], especially if the adolescent stressor remains controllable [211]. Following the murine postweaning phase (postnatal days 21–30), rat/mouse adolescent days 31 to 60 include puberty and are roughly commensurate with human ages of 10 to 18 years [108]. However, rodent offspring are weaned from maternal care before adolescence, while humans remain under parental care for much longer. Research now attempts to parse out stress-related and anxiety-related sex differences and to match prepubertal and postpubertal endocrine profiles between the species throughout different developmental stages within adolescence [67].
Compared to other early life stressors discussed previously, postweaning and/or adolescent stress models are a reliable way to produce long-lasting alterations in female emotional behavior. Male rats are especially prone to develop a chronic, long-lasting anxiety state when the stressor, such as social isolation, is initiated in preadolescence [283, 386, 393], while male social isolation initiated during adolescence may be anxiolytic [17, 18, 354, 382] or have no effect [59, 217]. With the exception of Weiss et al., who found anxiogenic effects of social isolation in male, but not female [386] rats, social and nonsocial stressors during both preadolescent and periadolescent time windows seem to result in elevated anxiety states of female rats during adulthood [18, 217, 242, 297, 382]. Bourke and Neigh [52] chose a mixed-modality adolescent stress paradigm (restraint, isolation, and social defeat) in Long Evans rats of both sexes (using retired breeder males or ovariectomized females for social defeat) that also increased adult anhedonic behavior in the sucrose preference test and reactive stress coping (increased immobility) in the forced swim test (FST) in females, but not males. Also, only female adolescence-stressed rats of this study displayed increased adult locomotor activity, rears, and overall vigilance on the EPM (without altering open-arm time) and three times as many escape-seeking dives during the FST than unstressed controls, indicating a complex alteration of context-dependent risk assessment and coping strategies (active or reactive) that may closely resemble the behavioral symptoms of panic disorder or PTSD. Postweaning isolation protocols in female rats result in increased adult anxiety, as measured by deficits in SI behavior, OF exploration, and novelty-suppressed feeding [161], and increased vigilance and arousal upon injection of an anxiogenic pharmacological compound [229]. These chronic anxiety states have been associated with altered central serotonergic [227, 229], GABAergic [226], and glutamatergic functions [161].
Adult anxiety states induced by acute or chronic stress
While only a few acute stressors have been shown to produce long-lasting anxiety-like states in adult rodents, a variety of chronic stress conditions, some employing an array of unpredictable heterotypic stressors [145, 146, 257, 319] and others using repeated exposure to a homotypic stressor such as restraint [54, 172, 203], result in chronically increased anxiety-like behavior and commonly also depressive-like behavior. To avoid an exhaustive description of such models, we here solely discuss learned helplessness and psychosocial stress models in light of the female-specific physiology and behavioral outcomes. Glucocorticoid (GC)-mediated or CRF/urocortin-mediated adult anxiety states are integrated into the “Potential mechanisms for sex differences in stress-related anxiety states” section.
Inescapable stress and learned helplessness
Responding to and coping with acute or temporary stressors belongs to the normal repertoire of mammals. However, severe, traumatic, or uncontrollable stressors are capable of inducing a depression-like and anxiety-like state for up to 48 h, also termed “learned helplessness,” in which the individual then also perceives controllable situations as uncontrollable. Very few studies use female rodents as subjects, but in male rats, 1 session of 100 inescapable tail shocks (about 1 h 40 min total duration) is sufficient to induce a 48-h-long anxiety-like and depressive-like behavioral phenotype [231]. Learned helplessness is thought to be due to a prolonged release of serotonin within the DR and its target regions during and after stress exposure, resulting in functional desensitization of 5-HT1A autoreceptors [313] and subsequent exaggerated serotonin release within fore-brain structures controlling anxiety-like behaviors [10].
Acutely, inescapable shock activates stress-induced serotonin release from local axon collaterals [238] and induces the expression of the immediate early gene c-fos [137] in a mid-brain serotonergic region that is also activated by urocortin 2 [152, 344], a CRF-like peptide that preferably binds to CRF type II receptors (CRFR2), certain anxiogenic pharmacologic compounds [1], and anxiogenic environmental stimuli [126, 341]. This subdivision has been identified as the dorsal and caudal DR (DRD/DRC; see Fig. 3). In contrast, escapable tail shock, in other words experiencing control by being able to terminate each shock, while receiving exactly the same physical stressor as a yoked rat receiving inescapable tail shock, prevents the behavioral and neurobiological phenotype of learned helplessness and even renders the individual resilient towards subsequent inescapable stress [11]. Projections from the medial PFC to the DRD/DRC region mediate some of the behavioral manifestations of controllability [31]. Both the PFC and the DRD/DRC are sensitive targets for fluctuating concentrations of reproductive hormones, especially estrogens [116, 364].
Fig. 3.
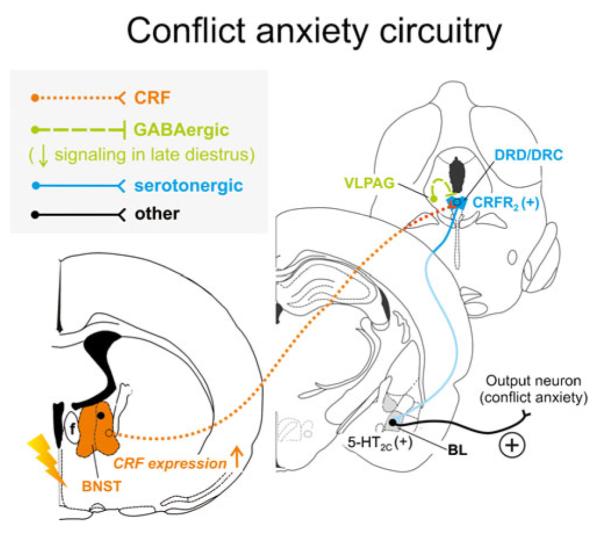
Hypothetical model of how stress-induced increases in corticotropin-releasing factor (CRF) expression and signaling from the bed nucleus of the stria terminalis (BNST) may interact with decreased GABAergic inhibition from the ventrolateral periaqueductal gray (VLPAG) during late diestrus to enhance serotonergic output in the conflict anxiety-related dorsal and caudal DR (DRD/DRC). During late diestrus in rodents, declining circulating concentrations of progesterone and its neuroactive metabolite allopregnanolone cause increased expression of α4, β1, and δ subunits of the γ-aminobutyric acid (GABA) receptor type A within the PAG [143, 144], including the VLPAG, ultimately resulting in attenuated ongoing GABAergic inhibitory signaling [221]. Attenuated activity of GABAergic neurons from the VLPAG render serotonergic neurons in the DRD/DRC more active [183], and stress induces CRF expression in the conflict anxiety-related BNST [232, 331, 375]. Enhanced CRF release from BNST projections further activates serotonergic neurons through CRF receptor type 2 (CRFR2) [204] within the DRD/DRC [335]. Together with stress-induced desensitization of autoinhibitory 5-HT1A receptors on DRD/ DRC serotonin neurons [313], late diestrus-enhanced hyperactivity of the DRD/DRC causes increased serotonergic output to distal target sites controlling conflict anxiety-like behavior, in particular through actions on excitatory 5-HT2C receptors [76] in the BL [12, 150]. Neuronal projections are drawn unilaterally solely for simplicity and do not imply functional laterality
Whether inescapable shock-induced learned helplessness also manifests itself in females remains to be determined, although recent evidence suggests that female rats maintain their escape-seeking behavior, interpretable as a sign of resilience [87, 332]. In humans, on the other hand, overly active escape-seeking or hypervigilance may be symptomatic of panic or agoraphobia. Bland et al. [45] was one of the first to use females in the learned helplessness model and found that, immediately following inescapable tail shock, male rats display a greater increase of both c-fos and bdnf expression (encoding the presumably neuroprotective brain-derived neurotrophic factor [BDNF]) in the PFC than females, compared to home cage controls. In contrast, expression levels of both genes were either similar in both sexes or increased in females, compared to males, 60 min after inescapable shock. These findings suggest differential temporal response patterns in males vs. females, while the behavioral consequences and neuronal effects within downstream target sites of the female PFC remain to be determined.
Psychosocial stress models
Since female rodents do not display the same aggressive, territorial, and hierarchy-establishing behaviors as males, only few relevant and effective psychosocial stress models exist. Among those, the resident–intruder test, exposing the experimental female to an aggressive lactating dam after temporary removal of her pups [266], and novel chronic psychosocial stress mouse models based on disruption of the animal’s social stability, such as social isolation (single housing) or rotation of cage mates [279, 322], are most promising. Schmidt et al. [322], for example, rotated the group composition of four female mice per cage twice a week for a total of 7 weeks from adolescence throughout young adulthood, resulting in increased anxiety in the novelty-suppressed feeding task. There is a clear need for more psychosocial stress models in females because results from both human TSST studies [193] and rodent models [177] indicate that females perceive socially stressful situations as much more fear-inducing and distressing than males despite similar or comparably low GC responses. Because social stressors are most pervasive to humans and are key contributors to the etiology of anxiety and mood disorders, stress models that are derived from socially important and evolutionary meaningful contexts from the rodent’s perspective currently offer the best face, construct, and predictive validity [153, 154, 162] and an alternative to socially irrelevant or painful stress procedures.
Sex differences in animal models of panic disorder
To our knowledge, no physiologically and behaviorally validated female rodent models for panic disorder exist to date. However, there are well-characterized models in male rodents that could easily be replicated in females while monitoring estrous cycle stage through hormonal profiling or vaginal smears [35] and would contribute important information on sex differences in panic-like behaviors and panic-like physiological responses. Also, many behavioral and physiological characteristics of chronic anxiety states in rodent models, as described previously, may pertain to panic-specific biological symptomology.
Clinically relevant sex differences may exist in response to stimulation with panicogenic agents. Genest et al., for example, report more hypercapnia-induced tachypnea (increased respiratory rate), as measured in a plethysmography chamber, only in maternally separated female rats, whereas neonatally separated males hyperventilated less than controls [129]. Estrous stage-dependent variations in reproductive hormones may be a critical factor for altering the sensitivity of cardiovascular and respiratory control centers to a panic-inducing stimulus because intravenous administration of the synthetic, panicogenic peptide pentagastrin has been shown to cause increased tachycardia and tachypnea during diestrus compared to proestrus in anesthetized female rats [56]. Concordant with Klein’s “false suffocation alarm” hypothesis of panic disorder [206], hypercapnia is a useful tool to compare human (see the “Panic disorder” section) to rodent responses [34, 178]. Similar to increased CO2 sensitivity and predisposition to panic disorder in humans from unstable parental environments [33], recent studies have shown that female and male rodents exposed to neonatal MS in infancy or to an unstable cross-fostering environment show an increased hypercapnic ventilation response to the panicogenic agent CO2 as adults [86, 104]. An equally relevant method to detect panic-related physiology and behavior in both humans and rodents is intravenous infusion of sodium lactate. Sodium lactate infusions, through central actions of sodium rather than changes in osmolarity or lactate [259], are sufficient to induce panic attacks in panic disorder patients, but not in healthy controls [84, 131, 290], and likewise cause panic-like responses in animal models of panic disorder [301, 316]. Sodium lactate infusion into male control rats activates serotonergic neurons in the “lateral wings” of the DR, the so-called ventrolateral DR/ventrolateral periaqueductal gray (DRVL/VLPAG), while male rats that have been rendered panic-prone (through disinhibition of the medial hypothalamus with the GABA synthesis inhibitor l-allylglycine) fail to activate these neurons [179]. Five days of subthreshold priming of CRFR1 with the CRF-like peptide urocortin 1 locally within the basolateral amygdala (BL) also affects the DRVL/VLPAG region, causing an increase in tph2 mRNA and reduced SI in male rats [99]. Rats of the same intra-BL priming model react with panic-like physiological and behavioral responses to sodium lactate infusion, while controls do not [301]. Serotonergic DRVL/VLPAG projections are likely to travel through the periventricular tract to innervate the dorsal periaqueductal gray (DPAG) [36, 349]. This region controls a spectrum of defensive behaviors ranging from freezing to escape, depending on the perception of how close or imminent a threat is [44, 296], and controls autonomic responses to stress in mammals [134, 173, 191, 249]. Serotonergic signaling is capable of inhibiting those responses, for example, through actions on postsynaptic 5-HT1A [296] and 5-HT2A receptors [295]. The serotonergic DRVL/VLPAG is thus ideally positioned to control escape behaviors and panic-like responses. Failure to activate the DRVL/VLPAG, in contrast, may result in increased vulnerability to stress and facilitate escape-like and panic-like responses. The questions that remain are whether these panic models can be reproduced in females and if they would reveal (as we expect) sex-dependent or estrous stage-dependent (re) activity of the DRVL/VLPAG.
Another new rodent model of panic disorder uses a noninvasive ultrasound stimulus to induce panic-like responses in adult rats, but also has yet to be tested in females. Namely, Lister hooded rats respond with tachycardia and hyperthermia to a noninvasive 22-kHz (typical frequency for an adult rat) ultrasound stressor without altering HPA axis function, and this is associated with increased c-fos activation of the DPAG/dorsolateral periaqueductal gray (DLPAG) [207]. This is of interest because one core characteristic of a classic panic attack in humans is the activation of autonomic, but not neuroendocrine, stress-response systems [135]. Concordantly, excitatory stimulation of the DPAG/DLPAG in primates [191] and (electrically) in awake non-panic disorder human patients [173] causes panic-like emotional and autonomic responses (e.g., tachycardia and hyperventilation) and, when increased in intensity, a shift from reactive freezing behavior to active escape in rodents [321].
Sex differences in animal models of PTSD
Rodent models of PTSD are hard to validate, and only little substantiated evidence for sex differences exist. PTSD rodent models generally strive to avoid chronic or repeated exposure to homotypic stressors in order to prevent habituation, but instead try to mimic the isolated, traumatic, and life-threatening nature of the inducing stressor(s), ideally in a species-relevant context, such as exposure to a predator or to predator smell [4]. Such protocols appear to be most successful in modeling increased vulnerability in females. While 10 min of protected exposure to an actual cat produces a long-lasting anxiety state in both sexes of C57BL/6J mice, only females are just as susceptible to the feline odor by itself [3]. As a hint towards serotonergic involvement in this sex difference, male serotonin transporter knockout mice (SERT–/–) take on the same vulnerability as females by also developing a long-lasting anxiety state upon exposure to cat odor alone [2]. An observation of potential relevance for wild-type mice as well is that 5-HT1A receptor functionality appears to be particularly impaired in SERT–/– females [51, 112], probably as a result of estrogenic downregulation of 5-HT1A autoinhibitory functionality that can be reversed by ovariectomy [51, 239, 276]. A rise in 17-beta-estradiol during the late diestrus and early proestrus could thus impair autoinhibition of serotonergic neurons, rendering them more active. Classic inescapable and uncontrollable stress paradigms (see the “Inescapable stress and learned helplessness” section) are also often interpreted as PTSD models [80, 394] and are certainly useful to elucidate the inescapability aspect of a trauma-inducing event, but often fail to induce behavioral expression of learned helplessness in females [80, 96].
Potential mechanisms for sex differences in stress-related anxiety states
Many sexually dimorphic characteristics, including neuro-transmitter systems [15, 103, 164, 362], neuroactive peptides [85, 244, 337], actions of reproductive steroid hormones within the mesolimbocortical system [98, 383, 384, 398], functionality of the GC receptor and HPA axis negative feedback [27, 42, 53, 70, 342, 366], neonatal microRNA spectrum [262], BDNF polymorphisms and PFC expression of bdnf [32, 45, 109], and immunological/inflammatory responses [159, 253, 278], have emerged as candidates for mechanisms underlying sex differences in anxiety states. To discuss all of them is beyond the scope of this review. However, in an attempt to integrate interactions of reproductive hormones, GCs, CRF-related signaling, and brain serotonergic systems, we here describe models for neural circuits controlling conflict anxiety (Fig. 3) and panic-like anxiety (Fig. 4) that, based on recent evidence, may be more vulnerable to stress-induced disturbances in females than in males.
Fig. 4.
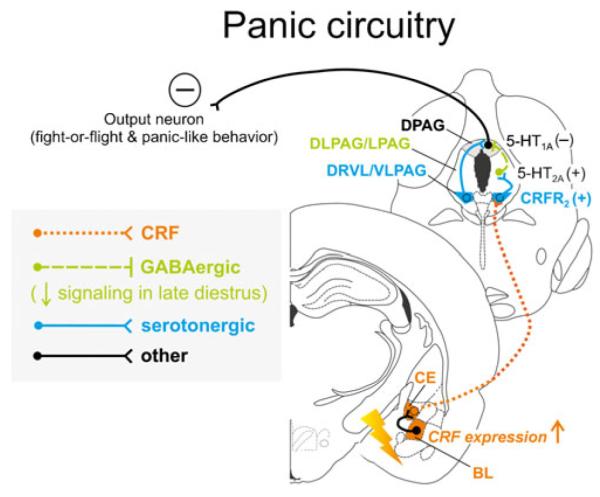
Hypothetical model of how stress-induced increases in corticotropin-releasing factor (CRF) expression and signaling from the basolateral (BL) and central (CE) amygdaloid complex likely function to activate serotonergic output from the “lateral wings” of the dorsal raphe nucleus (DR) and may interact with decreased GABAergic inhibition from the periaqueductal gray (PAG) during late diestrus to increase panic-like responses. During late diestrus in rodents, declining circulating concentrations of progesterone and its neuroactive metabolite allopregnanolone cause increased expression of α4, β1, and δ subunits of the γ-aminobutyric acid (GABA) receptor type A within the PAG [143, 144], ultimately resulting in attenuated ongoing GABAergic inhibitory signaling [221] within the panic-related dorsal PAG (DPAG). Stress-induced elevation of CRF within the BL leads to increased CRF release from CE projections that target the “lateral wings” of the DR, namely, the ventrolateral portions of the DR and PAG (DRVL/VLPAG), either acting on CRF receptor type 2 (CRFR2) directly on serotonergic neurons [204] or indirectly via CRFR2-mediated inhibition of nonserotonergic neurons [289]. This normally leads to increased activation of the DRVL/VLPAG, increased serotonergic output to distal target sites, and postsynaptic 5-HT1A-mediated and/or 5-HT2A-mediated inhibition of panic-like responses. During late diestrus, however, decreased GABAergic signaling disinhibits the DPAG, facilitating fight-or-flight and panic/escape-like responses [134]. Neuronal projections are drawn unilaterally solely for simplicity and do not imply functional laterality
Sex differences within a neural circuit controlling conflict anxiety
A conflict anxiety-related serotonergic region that may be particularly vulnerable in females is the midbrain DRD/DRC. In a rat model of violence in intimate relationships (females cohabitating with aggressive males, inducing a long-lasting anxiety state in the females), Cordero et al. [83] recently found selective hyperactivation of the DRD/DRC, a serotonergic system that appears to control conflict anxiety-like behavior (see Fig. 3), upon subsequent exposure to an unfamiliar male. Interestingly, this effect persisted into the F1 generation even when rearing conditions were controlled for. DRD/DRC hyperactivation is likely dependent on CRF overexpression in a specific region of the extended amygdala complex. Mechanistic studies in male rodents suggest that projections from the BNST specifically target the DRD/DRC region because overexpression of CRF within the lateral BNST [335] has recently been reported to enhance the expression of contextual fear (similar to conflict anxiety) after conditioning and to alter CRFR2 receptor density selectively within the DRD. The DRD/DRC responds with increased c-fos expression in response to various anxiogenic stimuli, such as threatening situations, anxiogenic drugs, or urocortin 2 [1, 126, 152, 285, 344, 345]. Additional support for an important role for the BNST in controlling DRD/DRC serotonergic neurons comes from the observations that learned helplessness [231] depends on BNST functionality [155], that inescapable, relative to escapable, tail shock specifically activates serotonergic neurons in the DRD/DRC region [9], and that the delivery of only two foot shocks 24 h following inescapable tail shock produces a markedly increased release of serotonin within limbic target areas of the DRD/DRC, for example, the BL [10]. Desensitization of 5-HT1A autoreceptors within the DRD/DRC appears to facilitate this effect [313]. Importantly, antagonism of 5-HT2C serotonergic receptors in the BL is sufficient to block the DRD/DRC-mediated learned helplessness effects of inescapable tail shock 24 h later, as measured in the juvenile SI test [76]. Other prominent anxiety-related or fear-related distal target sites of DRD/DRC projections are the BNST itself [292] and a conflict anxiety network that includes the BL [150, 151] and the ventral hippocampus [284] and is activated by exposure to an OF, a comparably mild conflict anxiety-inducing stressor [151]. The ventral hippocampus is an important region for fear conditioning and memory consolidation of fearful events [79, 114], but also serves to assess risk in typical conflict anxiety situations [249].
It is unclear what mechanisms may cause this conflict anxiety circuit to be overly responsive or active following stress, but the DRD/DRC system has been demonstrated to be more sensitive to estrogens than other DR regions. Local wax pellet implants of 17-beta-estradiol flanking the DR of ovariectomized female rats increase tph2 expression in the DRD/DRC [98] and in the DRC when 17-beta-estradiol is given systemically [166]. In contrast to the topographic match of altered tph2 expression, the anxiety-related behavioral effects of estrogen treatment in the aforementioned studies, however, were inconclusive. Five days of local delivery of 17-beta-estradiol or of an estrogen receptor beta (ERβ)-specific compound within the immediate surround of the DR did not alter anxiety-like behaviors [98] of ovariectomized female rats, whereas systemic administration of 17-beta-estradiol to ovariectomized female rats can have anxiolytic or anxiogenic effects, with the latter being correlated with decreased expression of the gene encoding the autoinhibitory 5-HT1B receptor in the ventromedial DR [98, 165, 167]. These seemingly contradictory effects may partially be due to the expression pattern of the two ER systems within the brain. While only very few serotonergic neurons express the androgen receptor in either sex [329], ERα is found primarily in nonserotonergic neurons (e.g., in GABA neurons in the inhibitory surround of the DR) and ERβ appears to be predominately localized within serotonergic cell nuclei [272, 329]. ERα is generally accepted to exert anxiogenic actions, while ERβ has repeatedly been shown to display anxiolytic function [357, 371, 383], but where exactly within the brain those dichotomous ER receptor systems are exerting these effects and whether they contribute to the development of female-specific anxiety states remains subject to further investigation [383–385]. Luine reported in 1993 that the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) increases by 580 % in the DR within a 5-h period of the proestrus afternoon, while no such increase happens during diestrus [224]. Another group found that female rats, independent of estrous stage, display a much higher ratio of 5-HIAA to serotonin (a measure of serotonergic metabolism, which is often correlated with serotonergic activity) than male rats [97], but only in the DR, not in the median raphe nucleus (MnR). Interestingly, a single exposure to the EPM conflict anxiety paradigm in the same study, lastingly and sex-specifically decreased the 5-HIAA/serotonin ratio in the DR of females (but not males) and in the MnR of males (but not females) for almost 2 weeks.
We have determined that chronic administration of corticosterone via the drinking water [100] creates a conflict anxiety-like behavioral phenotype and elevated tph2 expression in anxiety-related serotonergic systems of male rats, but have yet to determine if similar effects are evident in females or how tph2 expression and enzyme activity respond to estrous stage. Animal models of chronic stress [243] or chronic GC exposure during adulthood [82, 88, 100] are likely to be mediated through GC-induced elevation of CRF expression within the BNST [232, 330] and reliably result in increased conflict anxiety-like behavior as a major behavioral outcome, associated with increased tph2 expression (see supplemental material to [100]) and in vivo tryptophan hydroxylase activity [101] specifically in the DRD/DRC region.
Recent results from female nonhuman primate studies suggest that elevation of reproductive steroids, namely 17-beta-estradiol, may sensitize CRF receptor systems, in a female-specific manner. In support of estrogen-induced alteration of female-specific stress responsiveness within the DRD/DRC, 28 days of 17-beta-estradiol replacement in ovariectomized female macaques markedly increased crfr2 expression within laser-captured serotonin neurons of the DR in comparison to ovariectomized controls without hormone replacement or combined estradiol/progesterone administration [317]. In addition, both estradiol and combined estradiol/progesterone treatment in ovariectomized female macaques altered the composition of cell adhesion molecules within serotonergic neurons and thus their synapse assembly [40]. How may this specifically affect the DRD/DRC system? CRF is likely to act directly in the DRD/DRC, via actions on CRFR2 receptors, to increase serotonergic neuronal firing rates. The DRD/DRC densely expresses crfr2/CRFR2 [91, 335], CRFR2 is expressed directly on serotonergic neurons of the DRD/DRC [230], and CRF elicits CRFR2-mediated currents in ex vivo electrophysiological studies of DR serotonergic neurons [204]. Thus, female vulnerability of the DRD/DRC circuitry may be increased, depending on fluctuating concentrations of estradiol.
Sex differences within a neural circuit controlling panic and escape
An anxiety-related neurocircuit that includes the BL, CE, and serotonergic DRVL/VLPAG [99] probably exerts inhibitory control over panic-like and escape-like autonomic and behavioral responses originating in the DPAG and may also display reproductive steroid-dependent sex differences, especially with respect to developmental manipulations. In an early life adversity model, Lukkes et al. [229] recently discovered that adult female, but not male (unpublished data), rats that underwent social isolation during adolescence display increased vigilance behavior and decreased basal tph2 expression in the panic-related “lateral wing” region of the DR (DRVL/VLPAG; see Fig. 4). Expression of panic-like anxiety is thought to depend on CRFR1 signaling within the BL [319]. Five days of sub-threshold intra-BL priming of CRFR1 with urocortin 1, a CRF-like peptide, for example, decreases SI time and renders male rats prone to sodium lactate-induced tachycardia, hypertension, and hyperventilation [301]. Conversely, the anxiogenic effects of intra-BL priming with urocortin 1 are reversible when a CRFR1 antagonist is locally infused into the BL prior to assessment of behavior in the SI test [128]. In a recent collaboration with the Shekhar laboratory [99], we also found that the same intra-BL urocortin 1 priming model selectively alters tph2 expression in the DRVL/VLPAG. Serotonin neurons within the DRVL/VLPAG may be required to suppress panic attacks [136]. When activated, serotonergic projections to the DPAG are thought to inhibit panic-like autonomic and behavioral responses [36, 296, 349]. Only control, but not panic-prone, rats respond with increases in c-fos expression in DRVL/VLPAG serotonergic neurons following sodium lac-tate infusion [179]. Failure to activate DRVL/VLPAG neurons may not only decrease inhibitory serotonergic signaling from the DRVL/VLPAG to the DPAG [296], but also to the rostral ventrolateral medulla (RVLM), a brainstem region exerting sympathoexcitatory control over physiological responses including heart rate, blood pressure, and respiration [23, 24, 223], and to the perifornical region of the hypothalamus, from where orexin/hypocretin neurons control arousal and vigilance behaviors [180, 181]. Both CRFR1 and CRFR2 are expressed within the DR and in the immediate inhibitory surround of the DR [91]. During stress, CRF from CE-derived afferents likely binds to CRFR1 on GABAergic interneurons within the DRVL/VLPAG to facilitate and maintain appropriate inhibitory control of DR serotonergic neurons [204]. Meanwhile, intra-DR activation of CRFR2-expressing serotonergic neurons, possibly within the DRVL/VLPAG, appears to facilitate anti-panic/anti-escape behaviors such as freezing [123]. Likewise, local blockade of CRFR2 prevents serotonin release in distal target regions [124, 228]. Interestingly, crfr1 expression within (laser-captured) DR serotonin neurons is highest in ovariectomized female macaques without hormone replacement compared to female 17-beta-estradiol-treated or estradiol/progesterone-treated ovariectomized animals [39, 317], and combined estradiol/progesterone treatment (28 days of estradiol/progesterone during the last 14 days) reduces both crfr1 and crfr2 expression [317]. Estrous cycle stages of low estradiol and progesterone may thus exaggerate CRFR1-mediated inhibition of DRVL/VLPAG serotonergic neurons. Developmentally, our data [229] and findings from other groups [283, 386, 393] support the idea that females are particularly vulnerable to the anxiety-inducing effects of social isolation during adolescence and that this may be a good model for panic disorder because it results in decreased basal tph2 mRNA expression in the DRVL/VLPAG [229]. Decreased inhibitory serotonergic signaling from serotonin neurons in this region to distal autonomic target sites would consequently be expected to disinhibit escape behaviors and arousal systems [136, 296].
Sex differences in CRF signaling and receptor expression within anxiety and arousal systems
Aside from reproductive hormone-dependent crfr1 and crfr2 expression within DR serotonergic neurons, other sex differences in CRF systems may also pertain to sexually dimorphic stress perception, response, and adaptation and thus contribute to the sex-specific prevalence of chronic conflict-like or panic-like anxiety states. Rat research, for example, has shown that females typically express more CRF than males in certain brain regions, including the paraventricular hypothalamic nucleus, but also the CE and BNST [105, 176, 347, 365]. Receptor binding studies furthermore revealed that, throughout puberty, more CRFR2 becomes expressed within the CE, BL, and medial amygdala of male, but not female, rats, whereas adult females display more CRFR1 in the posteroventral medial amygdala and in the BL [214, 379]. Adult males of two monogamous and two promiscuous prairie vole species are also reported to have elevated CRFR2 expression in the BNST compared to their female counterparts [218]. The fact that these sex differences do not occur until puberty together with findings of response elements to reproductive steroids in the promoter region of CRF receptor genes suggest a key role for gonadal hormone regulation. Abnormal stress hormone exposure during certain developmental windows, in contrast, may lead to a “feminized” expression pattern of CRF receptors in the brain. Consistent with the idea that, in some forebrain regions, the anxiogenic effects of CRF are mediated via CRFR1, while CRFR2 counteracts these effects, a prenatal stress model [69] that only causes anxiety in adult male, but not female, offspring demonstrated that increased anxiety in male offspring was correlated with a higher crfr1/crfr2 expression ratio in the amygdala. Also, only prenatally stressed males had more crfr1 mRNA in the BL and CE, but lower crfr2 expression in the BL. It remains to be determined how different ratios of CRFR1/CRFR2 [25] within other mesolimbocortical brain regions of relevance, such as the DR, may contribute sex differences in anxiety-like behaviors.
Immunoprecipitation studies of Bangasser, Valentino, and colleagues recently revealed sex differences in the intersection of stress and the noradrenergic arousal center of the locus coeruleus (LC), especially with regard to sexually dimorphic intracellular signaling and trafficking of the CRFR1 receptor [27, 30, 361]. In unstressed control rats, the CRFR1 receptor associated more strongly with its GTP-binding protein Gs (signaling through the cyclic adenosine monophosphate/protein kinase A pathway) in females than it did in males [28], and only male rats displayed CRFR1 coupling with β-arrestin after stress exposure, an integral step of receptor internalization. The latter finding is consistent with electrophysiological evidence of stress-induced desensitization to CRF in males, but excessive or nondesensitizing CRF-mediated activation of LC neurons in females [85]. Consistently, a genetic mouse model of (global) CRF overexpression found equally increased CRF fiber innervation of the LC in both males and females, but a much higher discharge rate of female LC slices in vitro, in concert with a preponderance of membrane-localized (not internalized) CRFR1 immunostaining in females compared to more cytoplasmic CRFR1 in males [29]. These findings offer an entirely new perspective on sex-dependent stress adaptation of CRFR1-mediated signaling, not only in the LC, but possibly also in other brain regions.
Estrous stage-dependent variations in GABAergic neurotransmission
The elegant work of Lovick and colleagues [221] has demonstrated that GABAergic neurotransmission in the PAG is highly sensitive to estrous stage-related hormonal fluctuations. Due to its value as a pharmacological target for anxiolytic drugs, such as benzodiazepines [255, 391], or as a site of action for anxiogenic compounds, such as FG-7142 [111, 291], the GABAA receptor has been a major focus of anxiety research. During late diestrus (late luteal phase in humans), concentrations of progesterone and its anxiolytic metabolite allopregnanolone [400, 401] rapidly drop, and this decrease in allopregnanolone alters the subunit composition of the GABAA receptor, meaning a shift towards decreased expression of the α1 subunit and increased expression of the α4, β1, and δ subunits [143]. This reduces the ongoing inhibitory output of the GABA neurons within the PAG [222] and is correlated with increased anxiety-like behaviors in diestrus rats, but not proestrus, metaestrus, or estrus rats [93, 94]. Decreased inhibitory output from GABAergic PAG neurons during diestrus is likely to disinhibit the DPAG and thus reduce the suppression of panic-like responses. Indeed, stimulation of the PAG during late diestrus, either via a local electrode within the PAG [320] or through systemic administration of the panicogenic agent pentagastrin [56], lowers the threshold for escape behaviors and enhances autonomic responsiveness. Interestingly, both allopregnanolone and BDNF are found to be decreased in corticolimbic brain regions of PTSD and depressed patients, but in socially isolated mice, a rodent model of PTSD-like behavioral deficits, antidepressant treatment with fluoxetine reduces anxiety-like behavior and restores corticolimbic levels of allopregnanolone and BDNF at lower doses than those required for serotonin reuptake inhibition [271, 294].
Integrative model of sex differences in conflict anxiety vs. panic
Taken together, estrous stage, estradiol, progesterone/allopregnanolone, different expression patterns of CRF receptor systems, and inherent differences in female vs. male CRF receptor signaling and CRF receptor-dependent stress adaptation may all contribute to sexually dimorphic serotonergic activity within anxiety-related circuitries.
In the conflict anxiety circuitry (Fig. 3), evidence from male rats suggests that stress-induced increases in CRF signaling from the BNST [375] act on CRFR2 either directly on serotonergic neurons [335] or indirectly via inhibition of local GABAergic interneurons [289] to activate serotonin neurons in the DRD/DRC. During late diestrus in females, however, stress-induced increases in CRF expression and signaling from the BNST may interact with decreased GABAergic inhibition from the VLPAG to enhance serotonergic output from the conflict anxiety-related DRD/DRC [183]. Declining circulating concentrations of progesterone and its neuroactive metabolite allopregnanolone during late diestrus cause increased expression of α4, β1, and δ subunits of the GABA receptor type A within the PAG [143, 144], including the VLPAG, ultimately resulting in attenuated ongoing GABAergic inhibitory activity towards serotonergic target neurons [221]. Attenuated activity of GABAergic neurons from the VLPAG would subsequently render serotonergic neurons in the DRD/DRC more active [183]. During a conflict anxiety-relevant stress exposure, CRF expression in the BNST [232, 331] and CRF release from BNST projections would become enhanced and further activate serotonergic neurons through CRFR2 [204] within the DRD/DRC [335]. In fact, CRF overexpression in the lateral BNST has been shown to alter CRFR2 receptor density specifically in the DRD [335]. Together with possible stress-induced desensitization of autoinhibitory 5-HT1A receptors on DRD/DRC serotonin neurons [313], this hyperactivity of the DRD/DRC is likely to cause increased serotonergic output to distal target sites controlling conflict anxiety-relevant behavioral parameters, mainly through actions of serotonin on postsynaptic 5-HT2C receptors in the BL [12, 76, 150]. Other relevant target sites of the DRD/DRC are the anxiety-related BNST [187, 292, 306, 375], the fear-related CE [81, 377], and the ventral hippocampus, an area that is involved in risk assessment during conflict anxiety [151] and facilitates memory consolidation of stressful situations [79, 114].
In the panic circuitry (Fig. 4), several sexually dimorphic, CRF-mediated mechanisms may occur. Based on our working hypothesis, stress-induced increases in CRF signaling from the CE, a region with volume reductions in panic disorder patients [160], may act directly on CRFR2-expressing serotonin neurons of the DRVL/VLPAG to increase their inhibitory actions within the DPAG in both females and males. Pharmacological and electrophysiological studies suggest that DRVL/VLPAG serotonergic neurons exert their panic-suppressive actions directly via postsynaptic, inhibitory 5-HT1A receptors on local “pro-panic” glutamatergic and DPAG output neurons or indirectly via excitatory actions on 5-HT2A receptors located on GABAergic interneurons within the PAG [295, 296, 304]. Conversely, blockade of 5-HT1A or 5-HT2A receptors within the PAG promotes panic-like escape behaviors [295]. During late diestrus, however, GABAergic inhibitory tone from PAG interneurons towards the DPAG decreases [221] in females, thus rendering the system more vulnerable to stress and panicogenic situations. Lastly, it is also possible that females display a steroid hormone-dependent increase in crfr1 expression on GABAergic DRVL/VLPAG interneurons during late diestrus (similar to the high crfr1 expression in serotonergic neurons of ovariectomized macaques [39, 40, 317]) or (comparable to the sexually dimorphic CRF receptor signaling and trafficking principles discovered in the LC [27]) a prolonged CRFR1-mediated activation of GABAergic DRVL/VLPAG interneurons compared to males. These mechanisms would lead to an overly pronounced inhibition of DRVL/VLPAG serotonergic neurons and, consequently, to hyperactive or hyperresponsive panicogenic output neurons within the DPAG. Overall, a failure to properly activate these DRVL/VLPAG serotonergic neurons would facilitate escape-like fight-or-flight responses [134] via the DPAG, sympathoexcitation and hypertension [23, 24, 223] via the RVLM, and hyperarousal and hypervigilance due to hyperactive orexin/hypocretin neurons in the perifornical region of the hypothalamus [181].
Conclusions
Considering the hormonal fluctuations in premenopausal women, not even considering pregnancies, one may deem female physiology as quite stress-resilient and the 2:1 gender ratio of emotional disorders as surprisingly small. At the same time, this is exactly the biological interface where research can discover which mechanisms maintain resilience or how dysregulation of specific systems creates increased vulnerability. Future studies should address questions like: what keeps some individuals from experiencing PMDD or increased anxiety during certain estrous cycle stages or what protects most maternal brains from developing postpartum blues? To assess which sex differences are worth in-depth research, two separate considerations may aid the decision: first, does the sex difference have clinical relevance or does it pertain to translational studies? If yes, then we have an ethical obligation to pursue the matter and use the opportunity to develop sex-specific preventive, diagnostic, and therapeutic tools. Second, how can a sex difference help us better understand a certain biological system? Longitudinal human studies can now identify elevated trait anxiety, and genetic, behavioral, or physiological parameters, such as anxiety sensitivity [268] or baseline proinflammatory state [169] as early markers of inherent vulnerability (allowing for a chance for prevention or early intervention), jointly with the characterization of acquired state anxiety later on, for example, due to stressful or traumatic life events. Also, to date, no comprehensive literature exists on the interaction of biological sex and gender identity regarding anxiety disorders in transgender men and women [71, 399] before and after hormone therapy, an area of research that may contribute valuable insight on the relative impact of sex chromosomes, reproductive hormones, as well as neuronal and psychosocial aspects. Anxiety-relevant research on sex differences within central autonomic control of sympathetic and parasympathetic pathways, too, are chronically under-researched and deserve more attention [30, 362], and much work still lies ahead to increase the validity and translational value of behavioral endpoints in rodent models that differentiate not only between the expression of male vs. female anxiety states, but also between conflict-relevant, panic-relevant, or PTSD-relevant anxiety-like behaviors.
In summary, the sex bias in anxiety and affective disorders provides reason enough to acknowledge, instead of ignore, the complexity introduced by the female menstrual cycle and to intensify research on sexually dimorphic developmental programming of the brain and on sex-dependent stress coping mechanisms in adulthood.
Footnotes
Conflicts of interest None of the authors report conflicts of interest related to the submitted manuscript.
References
- 1.Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Adamec R, Burton P, Blundell J, Murphy DL, Holmes A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav. 2006;88:12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: sex, serotonin and other factors-relevance to PTSD. Neurosci Biobehav Rev. 2008;32:1287–1292. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects avoidance but not escape behavior in the elevated T-maze test. Physiol Behav. 1996;60:191–195. doi: 10.1016/0031-9384(95)02209-0. [DOI] [PubMed] [Google Scholar]
- 6.Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects exploratory behavior of female rats in the elevated plus-maze test. Physiol Behav. 1996;60:675–680. doi: 10.1016/s0031-9384(96)80047-3. [DOI] [PubMed] [Google Scholar]
- 7.Almeida SS, Tonkiss J, Galler JR. Prenatal protein malnutrition affects the social interactions of juvenile rats. Physiol Behav. 1996;60:197–201. doi: 10.1016/0031-9384(95)02236-8. [DOI] [PubMed] [Google Scholar]
- 8.Altemus M. Sex differences in depression and anxiety disorders: potential biological determinants. Horm Behav. 2006;50:534–538. doi: 10.1016/j.yhbeh.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 9.Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- 10.Amat J, Matus-Amat P, Watkins LR, Maier SF. Escapable and inescapable stress differentially alter extracellular levels of 5-HT in the basolateral amygdala of the rat. Brain Res. 1998;812:113–120. doi: 10.1016/s0006-8993(98)00960-3. [DOI] [PubMed] [Google Scholar]
- 11.Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of later uncontrollable stress: role of the ventral medial prefrontal cortex. J Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 13.An XL, Zou JX, Wu RY, Yang Y, Tai FD, Zeng SY, Jia R, Zhang X, Liu EQ, Broders H. Strain and sex differences in anxiety-like and social behaviors in C57BL/6J and BALB/cJ mice. Exp Anim. 2011;60:111–123. doi: 10.1538/expanim.60.111. [DOI] [PubMed] [Google Scholar]
- 14.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 15.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–1498. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 16.APA . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing (APP); Arlington: 2000. [Google Scholar]
- 17.Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behav Process. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Arakawa H. Ontogenetic interaction between social relationships and defensive burying behavior in the rat. Physiol Behav. 2007;90:751–759. doi: 10.1016/j.physbeh.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: sex chromosomes and brain sexual differentiation. Endocrinology. 2004;145:1057–1062. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 20.Aune T, Stiles TC. The effects of depression and stressful life events on the development and maintenance of syndromal social anxiety: sex and age differences. J Clin Child Adolesc Psychol. 2009;38:501–512. doi: 10.1080/15374410902976304. [DOI] [PubMed] [Google Scholar]
- 21.Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- 22.Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13(507–513):465. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bago M, Dean C. Sympathoinhibition from ventrolateral periaqueductal gray mediated by 5-HT(1A) receptors in the RVLM. Am J Physiol Regul Integr Comp Physiol. 2001;280:R976–R984. doi: 10.1152/ajpregu.2001.280.4.R976. [DOI] [PubMed] [Google Scholar]
- 24.Bago M, Sprtel BM, Dean C. Modulation of sympathetic nerve activity by microinjection of the 5-HT1A receptor agonist 8-OH-DPAT into the rostroventrolateral medulla. J Auton Nerv Syst. 1999;76:127–134. doi: 10.1016/s0165-1838(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 25.Bale TL, Lee KF, Vale WW. The role of corticotropin-releasing factor receptors in stress and anxiety. Integr Comp Biol. 2002;42:552–555. doi: 10.1093/icb/42.3.552. [DOI] [PubMed] [Google Scholar]
- 26.Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- 27.Bangasser DA. Sex differences in stress-related receptors: “micro” differences with “macro” implications for mood and anxiety disorders. Biol Sex Differ. 2013;4:2. doi: 10.1186/2042-6410-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(877):896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2013;18:166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103:342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baratta MV, Zarza CM, Gomez DM, Campeau S, Watkins LR, Maier SF. Selective activation of dorsal raphe nucleus-projecting neurons in the ventral medial prefrontal cortex by controllable stress. Eur J Neurosci. 2009;30:1111–1116. doi: 10.1111/j.1460-9568.2009.06867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bath KG, Chuang J, Spencer-Segal JL, Amso D, Altemus M, McEwen BS, Lee FS. Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage-specific expression of anxiety-like behavior in female mice. Biol Psychiatry. 2012;72:499–504. doi: 10.1016/j.biopsych.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Battaglia M, Bertella S, Politi E, Bernardeschi L, Perna G, Gabriele A, Bellodi L. Age at onset of panic disorder: influence of familial liability to the disease and of childhood separation anxiety disorder. Am J Psychiatry. 1995;152:1362–1364. doi: 10.1176/ajp.152.9.1362. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia M, Perna G. The 35 % CO2 challenge in panic disorder: optimization by receiver operating characteristic (ROC) analysis. J Psychiatr Res. 1995;29:111–119. doi: 10.1016/0022-3956(94)00045-s. [DOI] [PubMed] [Google Scholar]
- 35.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 36.Beitz AJ. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- 37.Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–S193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- 38.Bergemann ER, Boles RG. Maternal inheritance in recurrent early-onset depression. Psychiatr Genet. 2010;20:31–34. doi: 10.1097/YPG.0b013e3283351153. [DOI] [PubMed] [Google Scholar]
- 39.Bethea CL, Reddy AP. The effect of long-term ovariectomy on midbrain stress systems in free ranging macaques. Brain Res. 2012;1488:24–37. doi: 10.1016/j.brainres.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bethea CL, Reddy AP. Effect of ovarian steroids on gene expression related to synapse assembly in serotonin neurons of macaques. J Neurosci Res. 2012;90:1324–1334. doi: 10.1002/jnr.23004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010;24:2104–2115. doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- 42.Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 43.Bjerkeset O, Romundstad P, Gunnell D. Gender differences in the association of mixed anxiety and depression with suicide. Br J Psychiatry. 2008;192:474–475. doi: 10.1192/bjp.bp.107.045203. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard RJ, Flannelly KJ, Blanchard DC. Defensive behavior of laboratory and wild Rattus norvegicus. J Comp Psychol. 1986;100:101–107. [PubMed] [Google Scholar]
- 45.Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and bdnf mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 46.Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacol (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- 47.Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 48.Boles RG, Burnett BB, Gleditsch K, Wong S, Guedalia A, Kaariainen A, Eloed J, Stern A, Brumm V. A high predisposition to depression and anxiety in mothers and other matrilineal relatives of children with presumed maternally inherited mitochondrial disorders. Am J Med Genet B Neuropsychiatr Genet. 2005;137B:20–24. doi: 10.1002/ajmg.b.30199. [DOI] [PubMed] [Google Scholar]
- 49.Bosch OJ. Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm Behav. 2011;59:202–212. doi: 10.1016/j.yhbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Bosch OJ, Kromer SA, Neumann ID. Prenatal stress: opposite effects on anxiety and hypothalamic expression of vasopressin and corticotropin-releasing hormone in rats selectively bred for high and low anxiety. Eur J Neurosci. 2006;23:541–551. doi: 10.1111/j.1460-9568.2005.04576.x. [DOI] [PubMed] [Google Scholar]
- 51.Bouali S, Evrard A, Chastanet M, Lesch KP, Hamon M, Adrien J. Sex hormone-dependent desensitization of 5-HT1A autoreceptors in knockout mice deficient in the 5-HT transporter. Eur J Neurosci. 2003;18:2203–2212. doi: 10.1046/j.1460-9568.2003.02960.x. [DOI] [PubMed] [Google Scholar]
- 52.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bourke CH, Raees MQ, Malviya S, Bradburn CA, Binder EB, Neigh GN. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowman RE, Kelly R. Chronically stressed female rats show increased anxiety but no behavioral alterations in object recognition or placement memory: a preliminary examination. Stress. 2012;15:524–532. doi: 10.3109/10253890.2011.645926. [DOI] [PubMed] [Google Scholar]
- 55.Bowman RE, MacLusky NJ, Sarmiento Y, Frankfurt M, Gordon M, Luine VN. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]
- 56.Brack KE, Jeffery SM, Lovick TA. Cardiovascular and respiratory responses to a panicogenic agent in anaesthetised female Wistar rats at different stages of the oestrous cycle. Eur J Neurosci. 2006;23:3309–3318. doi: 10.1111/j.1460-9568.2006.04881.x. [DOI] [PubMed] [Google Scholar]
- 57.Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Mol Cell Endocrinol. 2001;185:73–79. doi: 10.1016/s0303-7207(01)00634-7. [DOI] [PubMed] [Google Scholar]
- 58.Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–438. doi: 10.1006/brbi.2001.0642. [DOI] [PubMed] [Google Scholar]
- 59.Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behav Brain Res. 2009;197:125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 60.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 61.Breslau N, Chilcoat H, Schultz LR. Anxiety disorders and the emergence of sex differences in major depression. J Gend Specif Med. 1998;1:33–39. [PubMed] [Google Scholar]
- 62.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 63.Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54:81–87. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- 64.Breslau N, Schultz L, Peterson E. Sex differences in depression: a role for preexisting anxiety. Psychiatry Res. 1995;58:1–12. doi: 10.1016/0165-1781(95)02765-o. [DOI] [PubMed] [Google Scholar]
- 65.Brezo J, Barker ED, Paris J, Hebert M, Vitaro F, Tremblay RE, Turecki G. Childhood trajectories of anxiousness and disruptiveness as predictors of suicide attempts. Arch Pediatr Adolesc Med. 2008;162:1015–1021. doi: 10.1001/archpedi.162.11.1015. [DOI] [PubMed] [Google Scholar]
- 66.Bridges NJ, Starkey NJ. Sex differences in Mongolian gerbils in four tests of anxiety. Physiol Behav. 2004;83:119–127. doi: 10.1016/j.physbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 67.Brown GR, Spencer KA. Steroid hormones, stress and the adolescent brain: a comparative perspective. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.12.016. doi:10.1016/j.neuroscience.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 68.Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV) Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- 69.Brunton PJ, Donadio MVF, Russell JA. Sex differences in prenatally programmed anxiety behaviour in rats: differential corticotropin-releasing hormone receptor mRNA expression in the amygdaloid complex. Stress. 2011;14:634–643. doi: 10.3109/10253890.2011.604750. [DOI] [PubMed] [Google Scholar]
- 70.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 71.Burgess D, Tran A, Lee R, van Ryn M. Effects of perceived discrimination on mental health and mental health services utilization among gay, lesbian, bisexual and transgender persons. J LGBT Health Res. 2007;3:1–14. doi: 10.1080/15574090802226626. [DOI] [PubMed] [Google Scholar]
- 72.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 73.Carone DM, Lawrence JB. Heterochromatin instability in cancer: from the Barr body to satellites and the nuclear periphery. Semin Cancer Biol. 2013;23:99–108. doi: 10.1016/j.semcancer.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chadwick BP, Willard HF. Chromatin of the Barr body: histone and non-histone proteins associated with or excluded from the inactive X chromosome. Hum Mol Genet. 2003;12:2167–2178. doi: 10.1093/hmg/ddg229. [DOI] [PubMed] [Google Scholar]
- 75.Christianson JP, Paul ED, Irani M, Thompson BM, Kubala KH, Yirmiya R, Watkins LR, Maier SF. The role of prior stressor controllability and the dorsal raphe nucleus in sucrose preference and social exploration. Behav Brain Res. 2008;193:87–93. doi: 10.1016/j.bbr.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Christianson JP, Ragole T, Amat J, Greenwood BN, Strong PV, Paul ED, Fleshner M, Watkins LR, Maier SF. 5-Hydroxytryptamine 2C receptors in the basolateral amygdala are involved in the expression of anxiety after uncontrollable traumatic stress. Biol Psychiatry. 2010;67:339–345. doi: 10.1016/j.biopsych.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cimpean D, Drake RE. Treating co-morbid chronic medical conditions and anxiety/depression. Epidemiol Psychiatr Sci. 2011;20:141–150. doi: 10.1017/s2045796011000345. [DOI] [PubMed] [Google Scholar]
- 78.Clayton AH, Stewart RS, Fayyad R, Clary CM. Sex differences in clinical presentation and response in panic disorder: pooled data from sertraline treatment studies. Arch Wom Ment Health. 2006;9:151–157. doi: 10.1007/s00737-005-0111-y. [DOI] [PubMed] [Google Scholar]
- 79.Cleren C, Tallarida I, Guiniec EL, Janin F, Nachon O, Canini F, Spennato G, Moreau JL, Garcia R. Low-frequency stimulation of the ventral hippocampus facilitates extinction of contextual fear. Neurobiol Learn Mem. 2013;101:39–45. doi: 10.1016/j.nlm.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 80.Cohen H, Yehuda R. Gender differences in animal models of posttraumatic stress disorder. Dis Markers. 2011;30:141–150. doi: 10.3233/DMA-2011-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Commons KG, Connolley KR, Valentino RJ. A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology. 2003;28:206–215. doi: 10.1038/sj.npp.1300045. [DOI] [PubMed] [Google Scholar]
- 82.Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cordero MI, Poirier GL, Marquez C, Veenit V, Fontana X, Salehi B, Ansermet F, Sandi C. Evidence for biological roots in the transgenerational transmission of intimate partner violence. Transl Psychiatry. 2012;2:e106. doi: 10.1038/tp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cowley DS, Dunner DL. Response to sodium lactate in panic disorder: relationship to presenting clinical variables. Psychiatry Res. 1988;25:253–259. doi: 10.1016/0165-1781(88)90096-0. [DOI] [PubMed] [Google Scholar]
- 85.Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 86.D’Amato FR, Zanettini C, Lampis V, Coccurello R, Pascucci T, Ventura R, Puglisi-Allegra S, Spatola CA, Pesenti-Gritti P, Oddi D, Moles A, Battaglia M. Unstable maternal environment, separation anxiety, and heightened CO2 sensitivity induced by gene-by-environment interplay. PLoS One. 2011;6:e18637. doi: 10.1371/journal.pone.0018637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2008;33:1559–1569. doi: 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- 88.David DJ, Samuels BA, Rainer Q, Wang J-W, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux J-P, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and - independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Davies W, Wilkinson LS. It is not all hormones: alternative explanations for sexual differentiation of the brain. Brain Res. 2006;1126:36–45. doi: 10.1016/j.brainres.2006.09.105. [DOI] [PubMed] [Google Scholar]
- 90.Davis MC, Matthews KA, Twamley EW. Is life more difficult on Mars or Venus? A meta-analytic review of sex differences in major and minor life events. Ann Behav Med. 1999;21:83–97. doi: 10.1007/BF02895038. [DOI] [PubMed] [Google Scholar]
- 91.Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, alpha 1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, gamma-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desai RA, Manley M, Desai MM, Potenza MN. Gender differences in the association between body mass index and psychopathology. CNS Spectr. 2009;14:372–383. doi: 10.1017/s1092852900023026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Devall AJ, Liu ZW, Lovick TA. Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology. 2009;34:587–596. doi: 10.1016/j.psyneuen.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 94.Devall AJ, Lovick TA. Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats. Neuropsychopharmacology. 2010;35:1174–1185. doi: 10.1038/npp.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Devall AJ, Santos JM, Lovick TA. Estrous cycle stage influences on neuronal responsiveness to repeated anxiogenic stress in female rats. Behav Brain Res. 2011;225:334–340. doi: 10.1016/j.bbr.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 96.Diehl LA, Silveira PP, Leite MC, Crema LM, Portella AK, Billodre MN, Nunes E, Henriques TP, Fidelix-da-Silva LB, Heis MD, Goncalves CA, Quillfeldt JA, Dalmaz C. Long lasting sex-specific effects upon behavior and S100b levels after maternal separation and exposure to a model of post-traumatic stress disorder in rats. Brain Res. 2007;1144:107–116. doi: 10.1016/j.brainres.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 97.Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80:203–210. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 98.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–718. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress Anxiety. 2012;29:307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donner NC, Montoya CD, Lukkes JL, Lowry CA. Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology. 2012;37:645–661. doi: 10.1016/j.psyneuen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donner NC, Siebler PH, Mani S, Lowry CA. Chronic glucocorticoid intake increases basal- and stress-induced tryptophan hydroxylase activity in anxiety-, sleep/wake-, and memory-related serotonergic systems. Abstract for the Annual Meeting of the Society for Neuroscience; New Orleans, LA. 2012. Abstract 900.05. [Google Scholar]
- 102.Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 103.Duchesne A, Dufresne MM, Sullivan RM. Sex differences in corticolimbic dopamine and serotonin systems in the rat and the effect of postnatal handling. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:251–261. doi: 10.1016/j.pnpbp.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 104.Dumont FS, Biancardi V, Kinkead R. Hypercapnic ventilatory response of anesthetized female rats subjected to neonatal maternal separation: insight into the origins of panic attacks? Respir Physiol Neurobiol. 2011;175:288–295. doi: 10.1016/j.resp.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 105.Duncko R, Kiss A, Skultetyova I, Rusnak M, Jezova D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. 2001;26:77–89. doi: 10.1016/s0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- 106.Dunlop BW, Davis PG. Combination treatment with benzodiazepines and SSRIs for comorbid anxiety and depression: a review. Prim Care Companion J Clin Psychiatry. 2008;10:222–228. doi: 10.4088/pcc.v10n0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 108.Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.10.048. doi:10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Epperson CN, Bale TL. BDNF Val66Met polymorphism and brain-derived neurotrophic factor levels across the female life span: implications for the sex bias in affective disorders. Biol Psychiatry. 2012;72:434–436. doi: 10.1016/j.biopsych.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Estanislau C, Morato S. Behavior ontogeny in the elevated plus-maze: prenatal stress effects. Int J Dev Neurosci. 2006;24:255–262. doi: 10.1016/j.ijdevneu.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 111.Evans AK, Lowry CA. Pharmacology of the beta-carboline FG-7,142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- 113.Facchinetti F, Romano G, Fava M, Genazzani AR. Lactate infusion induces panic attacks in patients with premenstrual syndrome. Psychosom Med. 1992;54:288–296. doi: 10.1097/00006842-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 114.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandes C, Gonzalez MI, Wilson CA, File SE. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav. 1999;64:731–738. doi: 10.1016/s0091-3057(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 116.Ferreira-Nuno A, Overstreet DH, Morales-Otal A, Velazquez-Moctezuma J. Masculine sexual behavior features in the Flinders sensitive and resistant line rats. Behav Brain Res. 2002;128:113–119. doi: 10.1016/s0166-4328(01)00313-8. [DOI] [PubMed] [Google Scholar]
- 117.Figueiredo HF, Dolgas CM, Herman JP. Stress activation of cortex and hippocampus is modulated by sex and stage of estrus. Endocrinology. 2002;143:2534–2540. doi: 10.1210/endo.143.7.8888. [DOI] [PubMed] [Google Scholar]
- 118.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 119.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 121.Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek KA. Distress vocalizations in maternally separated mouse pups: modulation via 5-HT(1A), 5-HT(1B) and GABA(A) receptors. Psychopharmacol (Berl) 2000;149:277–285. doi: 10.1007/s002130000370. [DOI] [PubMed] [Google Scholar]
- 122.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 123.Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006;141:1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 124.Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ. Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. Eur J Neurosci. 2008;28:299–310. doi: 10.1111/j.1460-9568.2008.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 126.Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136:181–191. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- 127.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–413. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 128.Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol. 2005;509:145–153. doi: 10.1016/j.ejphar.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 129.Genest SE, Gulemetova R, Laforest S, Drolet G, Kinkead R. Neonatal maternal separation induces sex-specific augmentation of the hypercapnic ventilatory response in awake rat. J Appl Physiol. 2007;102:1416–1421. doi: 10.1152/japplphysiol.00454.2006. [DOI] [PubMed] [Google Scholar]
- 130.Goffaux P, Michaud K, Gaudreau J, Chalaye P, Rainville P, Marchand S. Sex differences in perceived pain are affected by an anxious brain. Pain. 2011;152:2065–2073. doi: 10.1016/j.pain.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 131.Gorman JM, Goetz RR, Dillon D, Liebowitz MR, Fyer AJ, Davies S, Klein DF. Sodium d-lactate infusion of panic disorder patients. Neuropsychopharmacology. 1990;3:181–189. [PubMed] [Google Scholar]
- 132.Gorman JM, Kent J, Martinez J, Browne S, Coplan J, Papp LA. Physiological changes during carbon dioxide inhalation in patients with panic disorder, major depression, and premenstrual dysphoric disorder: evidence for a central fear mechanism. Arch Gen Psychiatry. 2001;58:125–131. doi: 10.1001/archpsyc.58.2.125. [DOI] [PubMed] [Google Scholar]
- 133.Gouveia A, Jr, dos Santos UD, Felisbino FE, de Afonseca TL, Antunes G, Morato S. Influence of the estrous cycle on the behavior of rats in the elevated T-maze. Behav Process. 2004;67:167–171. doi: 10.1016/j.beproc.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 134.Graeff FG. Serotonin, the periaqueductal gray and panic. Neurosci Biobehav Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Graeff FG. Anxiety, panic and the hypothalamic–pituitary–adrenal axis. Rev Bras Psiquiatr. 2007;29:3–6. doi: 10.1590/s1516-44462007000500002. [DOI] [PubMed] [Google Scholar]
- 136.Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- 137.Grahn RE, Will MJ, Hammack SE, Maswood S, McQueen MB, Watkins LR, Maier SF. Activation of serotonin-immunoreactive cells in the dorsal raphe nucleus in rats exposed to an uncontrollable stressor. Brain Res. 1999;826:35–43. doi: 10.1016/s0006-8993(99)01208-1. [DOI] [PubMed] [Google Scholar]
- 138.Grant B, Hasin D, Stinson F, Dawson D, June R, Goldstein R, Smith S, Saha T, Huang B. Prevalence, correlates, comorbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA. Psychol Med. 2005;35:1747–1759. doi: 10.1017/S0033291705006069. [DOI] [PubMed] [Google Scholar]
- 139.Gray TS, Bingaman EW. The amygdala: corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol. 1996;10:155–168. doi: 10.1615/critrevneurobiol.v10.i2.10. [DOI] [PubMed] [Google Scholar]
- 140.Gray JA, McNaughton N. The neuropsychology of anxiety: reprise. Nebr Symp Motiv. 1996;43:61–134. [PubMed] [Google Scholar]
- 141.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, Ballenger JC, Fyer AJ. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–435. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 142.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Griffiths J, Lovick T. Withdrawal from progesterone increases expression of alpha4, beta1, and delta GABA(A) receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol. 2005;486:89–97. doi: 10.1002/cne.20540. [DOI] [PubMed] [Google Scholar]
- 144.Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–466. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 145.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 146.Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31. doi: 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gulinello M, Orman R, Smith SS. Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci. 2003;17:641–648. doi: 10.1046/j.1460-9568.2003.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: associations with stress reactivity and regulation in 10–12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hahn ME, Karkowski L, Weinreb L, Henry A, Schanz N, Hahn EM. Genetic and developmental influences on infant mouse ultrasonic calling. II. Developmental patterns in the calls of mice 2–12 days of age. Behav Genet. 1998;28:315–325. doi: 10.1023/a:1021679615792. [DOI] [PubMed] [Google Scholar]
- 150.Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience. 2008;157:733–748. doi: 10.1016/j.neuroscience.2008.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Shekhar A, Lowry CA. Exposure to an open-field arena increases c-Fos expression in a distributed anxiety-related system projecting to the basolateral amygdaloid complex. Neuroscience. 2008;155:659–672. doi: 10.1016/j.neuroscience.2008.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hale MW, Stamper CE, Staub DR, Lowry CA. Urocortin 2 increases c-Fos expression in serotonergic neurons projecting to the ventricular/periventricular system. Exp Neurol. 2010;224:271–281. doi: 10.1016/j.expneurol.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haller J, Baranyi J, Bakos N, Halasz J. Social instability in female rats: effects on anxiety and buspirone efficacy. Psychopharmacol (Berl) 2004;174:197–202. doi: 10.1007/s00213-003-1746-x. [DOI] [PubMed] [Google Scholar]
- 154.Haller J, Fuchs E, Halasz J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 1999;50:33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 155.Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 156.Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 157.Handa RJ, Ogawa S, Wang JM, Herbison AE. Roles for oestrogen receptor beta in adult brain function. J Neuroendocrinol. 2012;24:160–173. doi: 10.1111/j.1365-2826.2011.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hankin BL. Rumination and depression in adolescence: investigating symptom specificity in a multiwave prospective study. J Clin Child Adolesc Psychol. 2008;37:701–713. doi: 10.1080/15374410802359627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hauser G, Tkalcic M, Stimac D, Milic S, Sincic BM. Gender related differences in quality of life and affective status in patients with inflammatory bowel disease. Coll Antropol. 2011;35(Suppl 2):203–207. [PubMed] [Google Scholar]
- 160.Hayano F, Nakamura M, Asami T, Uehara K, Yoshida T, Roppongi T, Otsuka T, Inoue T, Hirayasu Y. Smaller amygdala is associated with anxiety in patients with panic disorder. Psychiatry Clin Neurosci. 2009;63:266–276. doi: 10.1111/j.1440-1819.2009.01960.x. [DOI] [PubMed] [Google Scholar]
- 161.Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Herzog CJ, Czeh B, Corbach S, Wuttke W, Schulte-Herbruggen O, Hellweg R, Flugge G, Fuchs E. Chronic social instability stress in female rats: a potential animal model for female depression. Neuroscience. 2009;159:982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- 163.Hettema JM, Kuhn JW, Prescott CA, Kendler KS. The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychol Med. 2006;36:789–795. doi: 10.1017/S0033291706007367. [DOI] [PubMed] [Google Scholar]
- 164.Higley JD, Suomi SJ, Linnoila M. CSF monoamine metabolite concentrations vary according to age, rearing, and sex, and are influenced by the stressor of social separation in rhesus monkeys. Psychopharmacol (Berl) 1991;103:551–556. doi: 10.1007/BF02244258. [DOI] [PubMed] [Google Scholar]
- 165.Hiroi R, McDevitt RA, Morcos PA, Clark MS, Neumaier JF. Overexpression or knockdown of rat tryptophan hyroxylase-2 has opposing effects on anxiety behavior in an estrogen-dependent manner. Neuroscience. 2011;176:120–131. doi: 10.1016/j.neuroscience.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 167.Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hodgson RA, Guthrie DH, Varty GB. Duration of ultrasonic vocalizations in the isolated rat pup as a behavioral measure: sensitivity to anxiolytic and antidepressant drugs. Pharmacol Biochem Behav. 2008;88:341–348. doi: 10.1016/j.pbb.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 169.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- 170.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 171.Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 172.Huynh TN, Krigbaum AM, Hanna JJ, Conrad CD. Sex differences and phase of light cycle modify chronic stress effects on anxiety and depressive-like behavior. Behav Brain Res. 2011;222:212–222. doi: 10.1016/j.bbr.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 173.Iacono RP, Nashold BS., Jr Mental and behavioral effects of brain stem and hypothalamic stimulation in man. Hum Neurobiol. 1982;1:273–279. [PubMed] [Google Scholar]
- 174.Imanaka A, Morinobu S, Toki S, Yamamoto S, Matsuki A, Kozuru T, Yamawaki S. Neonatal tactile stimulation reverses the effect of neonatal isolation on open-field and anxiety-like behavior, and pain sensitivity in male and female adult Sprague-Dawley rats. Behav Brain Res. 2008;186:91–97. doi: 10.1016/j.bbr.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 175.Imhof JT, Coelho ZM, Schmitt ML, Morato GS, Carobrez AP. Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res. 1993;56:177–180. doi: 10.1016/0166-4328(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 176.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 177.Jacobson-Pick S, Audet MC, McQuaid RJ, Kalvapalle R, Anisman H. Stressor exposure of male and female juvenile mice influences later responses to stressors: modulation of GABAA receptor subunit mRNA expression. Neuroscience. 2012;215:114–126. doi: 10.1016/j.neuroscience.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 178.Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, Lowry CA. Induction of c-Fos in ‘panic/de-fence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25:26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- 179.Johnson P, Lowry C, Truitt W, Shekhar A. Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol. 2008;22:642–652. doi: 10.1177/0269881107082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson PL, Truitt WA, Fitz SD, Lowry CA, Shekhar A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology. 2008;33:2093–2107. doi: 10.1038/sj.npp.1301621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiol Behav. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 183.Jolas T, Aghajanian GK. Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 1997;755:229–245. doi: 10.1016/s0006-8993(97)00103-0. [DOI] [PubMed] [Google Scholar]
- 184.Jones AP, Dayries M. Maternal hormone manipulations and the development of obesity in rats. Physiol Behav. 1990;47:1107–1110. doi: 10.1016/0031-9384(90)90359-c. [DOI] [PubMed] [Google Scholar]
- 185.Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann N Y Acad Sci. 2004;1021:431–435. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- 186.Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 187.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 189.Karsnitz D, Ward S. Spectrum of anxiety disorders: diagnosis and pharmacologic treatment. J Midwifery Womens Health. 2011;56:266–281. doi: 10.1111/j.1542-2011.2011.00045.x. [DOI] [PubMed] [Google Scholar]
- 190.Kask K, Gulinello M, Backstrom T, Geyer MA, Sundstrom-Poromaa I. Patients with premenstrual dysphoric disorder have increased startle response across both cycle phases and lower levels of prepulse inhibition during the late luteal phase of the menstrual cycle. Neuropsychopharmacology. 2008;33:2283–2290. doi: 10.1038/sj.npp.1301599. [DOI] [PubMed] [Google Scholar]
- 191.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 192.Kelly MM, Forsyth JP, Karekla M. Sex differences in response to a panicogenic challenge procedure: an experimental evaluation of panic vulnerability in a non-clinical sample. Behav Res Ther. 2006;44:1421–1430. doi: 10.1016/j.brat.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 193.Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J Behav Ther Exp Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–1251. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- 195.Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 196.Kent JM, Papp LA, Martinez JM, Browne ST, Coplan JD, Klein DF, Gorman JM. Specificity of panic response to CO(2) inhalation in panic disorder: a comparison with major depression and premenstrual dysphoric disorder. Am J Psychiatry. 2001;158:58–67. doi: 10.1176/appi.ajp.158.1.58. [DOI] [PubMed] [Google Scholar]
- 197.Kessler R. The epidemiology of depression among women. In: Keyes C, Goodman S, editors. Women and depression. Cambridge University Press; New York: 2006. pp. 22–37. [Google Scholar]
- 198.Kessler R, Chiu W, Demler O, Walters E. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 200.Kikusui T, Kiyokawa Y, Mori Y. Deprivation of mother–pup interaction by early weaning alters myelin formation in male, but not female, ICR mice. Brain Res. 2007;1133:115–122. doi: 10.1016/j.brainres.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 201.Kikusui T, Mori Y. Behavioural and neurochemical consequences of early weaning in rodents. J Neuroendocrinol. 2009;21:427–431. doi: 10.1111/j.1365-2826.2009.01837.x. [DOI] [PubMed] [Google Scholar]
- 202.Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361:2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim KS, Han PL. Optimization of chronic stress paradigms using anxiety- and depression-like behavioral parameters. J Neurosci Res. 2006;83:497–507. doi: 10.1002/jnr.20754. [DOI] [PubMed] [Google Scholar]
- 204.Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28:12927–12937. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 206.Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Arch Gen Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- 207.Klein S, Nicolas LB, Lopez-Lopez C, Jacobson LH, McArthur SG, Grundschober C, Prinssen EP. Examining face and construct validity of a noninvasive model of panic disorder in Lister-hooded rats. Psychopharmacol (Berl) 2010;211:197–208. doi: 10.1007/s00213-010-1882-z. [DOI] [PubMed] [Google Scholar]
- 208.Kodama Y, Kikusui T, Takeuchi Y, Mori Y. Effects of early weaning on anxiety and prefrontal cortical and hippocampal myelination in male and female Wistar rats. Dev Psychobiol. 2008;50:332–342. doi: 10.1002/dev.20289. [DOI] [PubMed] [Google Scholar]
- 209.Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 210.Kopelman P. Health risks associated with overweight and obesity. Obes Rev. 2007;8(Suppl 1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 211.Kubala KH, Christianson JP, Kaufman RD, Watkins LR, Maier SF. Short- and long-term consequences of stressor controllability in adolescent rats. Behav Brain Res. 2012;234:278–284. doi: 10.1016/j.bbr.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 213.Kumsta R, Entringer S, Hellhammer DH, Wust S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 214.Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- 215.Leach LS, Christensen H, Mackinnon AJ, Windsor TD, Butterworth P. Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Soc Psychiatry Psychiatr Epidemiol. 2008;43:983–998. doi: 10.1007/s00127-008-0388-z. [DOI] [PubMed] [Google Scholar]
- 216.Lee Y, Fitz S, Johnson PL, Shekhar A. Repeated stimulation of CRF receptors in the BNST of rats selectively induces social but not panic-like anxiety. Neuropsychopharmacology. 2008;33:2586–2594. doi: 10.1038/sj.npp.1301674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62:22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- 218.Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Comp Neurol. 2005;487:75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Loos RJ. Recent progress in the genetics of common obesity. Br J Clin Pharmacol. 2009;68:811–829. doi: 10.1111/j.1365-2125.2009.03523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Louvart H, Maccari S, Darnaudery M. Prenatal stress affects behavioral reactivity to an intense stress in adult female rats. Brain Res. 2005;1031:67–73. doi: 10.1016/j.brainres.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 221.Lovick TA. Estrous cycle and stress: influence of progesterone on the female brain. Braz J Med Biol Res. 2012;45:314–320. doi: 10.1590/S0100-879X2012007500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lovick TA, Devall AJ. Progesterone withdrawal-evoked plasticity of neural function in the female periaqueductal grey matter. Neural Plast. 2009;2009:730902. doi: 10.1155/2009/730902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lowry C, Hale M. Serotonin and the neurobiology of anxious states. In: Mueller C, Jacobs B, editors. Handbook of behavioral neuroscience. Elsevier; Amsterdam: 2010. pp. 379–397. [Google Scholar]
- 224.Luine VN. Serotonin, catecholamines and metabolites in discrete brain areas in relation to lordotic responding on proestrus. Neuroendocrinology. 1993;57:946–954. doi: 10.1159/000126458. [DOI] [PubMed] [Google Scholar]
- 225.Luine V. Sex differences in chronic stress effects on memory in rats. Stress. 2002;5:205–216. doi: 10.1080/1025389021000010549. [DOI] [PubMed] [Google Scholar]
- 226.Lukkes JL, Burke AR, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation attenuates c-Fos expression in GABAergic interneurons in the basolateral amygdala of adult female rats. Physiol Behav. 2012;107:719–725. doi: 10.1016/j.physbeh.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lukkes JL, Engelman GH, Zelin NS, Hale MW, Lowry CA. Post-weaning social isolation of female rats, anxiety-related behavior, and serotonergic systems. Brain Res. 2012;1443:1–17. doi: 10.1016/j.brainres.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lukkes J, Kopelman J, Donner N, Hale M, Lowry C. Development×environment interactions control tph2 mRNA expression. Neuroscience. 2013;237C:139–150. doi: 10.1016/j.neuroscience.2013.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lukkes JL, Staub DR, Dietrich A, Truitt W, Neufeld-Cohen A, Chen A, Johnson PL, Shekhar A, Lowry CA. Topographical distribution of corticotropin-releasing factor type 2 receptor-like immunoreactivity in the rat dorsal raphe nucleus: co-localization with tryptophan hydroxylase. Neuroscience. 2011;183:47–63. doi: 10.1016/j.neuroscience.2011.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 232.Makino S, Gold PW, Schulkin J. Effects of corticosterone on crh mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- 233.Maller R, Reiss S. Anxiety sensitivity in 1984 and panic attacks in 1987. J Anxiety Disord. 1992;6:241–247. [Google Scholar]
- 234.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 235.Mariu KR, Merry SN, Robinson EM, Watson PD. Seeking professional help for mental health problems, among New Zealand secondary school students. Clin Child Psychol Psychiatry. 2012;17:284–297. doi: 10.1177/1359104511404176. [DOI] [PubMed] [Google Scholar]
- 236.Markham JA, Koenig JI. Prenatal stress: role in psychotic and depressive diseases. Psychopharmacol (Berl) 2011;214:89–106. doi: 10.1007/s00213-010-2035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 238.Maswood S, Barter JE, Watkins LR, Maier SF. Exposure to inescapable but not escapable shock increases extracellular levels of 5-HT in the dorsal raphe nucleus of the rat. Brain Res. 1998;783:115–120. doi: 10.1016/s0006-8993(97)01313-9. [DOI] [PubMed] [Google Scholar]
- 239.Maswood S, Stewart G, Uphouse L. Gender and estrous cycle effects of the 5-HT1A agonist, 8-OH-DPAT, on hypothalamic serotonin. Pharmacol Biochem Behav. 1995;51:807–813. doi: 10.1016/0091-3057(95)00038-x. [DOI] [PubMed] [Google Scholar]
- 240.Mathews IZ, Wilton A, Styles A, McCormick CM. Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav Brain Res. 2008;190:33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 241.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 242.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–238. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 243.McEuen JG, Beck SG, Bale TL. Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. J Neurosci. 2008;28:8169–8177. doi: 10.1523/JNEUROSCI.0004-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.McEuen JG, Semsar KA, Lim MA, Bale TL. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology. 2009;150:3709–3716. doi: 10.1210/en.2008-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.McIntosh J, Anisman H, Merali Z. Short- and long-periods of neonatal maternal separation differentially affect anxiety and feeding in adult rats: gender-dependent effects. Brain Res Dev Brain Res. 1999;113:97–106. doi: 10.1016/s0165-3806(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 246.McLaughlin KA, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behav Res Ther. 2011;49:186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.McLean CP, Anderson ER. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin Psychol Rev. 2009;29:496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 248.McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–946. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- 249.McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 250.McNaughton N, Zangrossi H., Jr . Theoretical approaches to the modeling of anxiety in animals. In: Blanchard RJ, Blanchard DC, Griebel G, Nutt D, editors. Handbook of anxiety and fear. Elsevier; Oxford: 2008. pp. 11–28. [Google Scholar]
- 251.Meewisse ML, Reitsma JB, de Vries GJ, Gersons BP, Olff M. Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br J Psychiatry. 2007;191:387–392. doi: 10.1192/bjp.bp.106.024877. [DOI] [PubMed] [Google Scholar]
- 252.Melcangi RC, Panzica G, Garcia-Segura LM. Neuroactive steroids: focus on human brain. Neuroscience. 2011;191:1–5. doi: 10.1016/j.neuroscience.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 253.Menzies V, Lyon DE, Elswick RK, Jr, Montpetit AJ, McCain NL. Psychoneuroimmunological relationships in women with fibromyalgia. Biol Res Nurs. 2013;15:219–225. doi: 10.1177/1099800411424204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Mezzacappa ES, Katlin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychol. 2002;21:187–193. [PubMed] [Google Scholar]
- 255.Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacol (Berl) 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- 256.Miller SM, Piasecki CC, Lonstein JS. Use of the light-dark box to compare the anxiety-related behavior of virgin and post-partum female rats. Pharmacol Biochem Behav. 2011;100:130–137. doi: 10.1016/j.pbb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 50258.Mittal D, Fortney JC, Pyne JM, Edlund MJ, Wetherell JL. Impact of comorbid anxiety disorders on health-related quality of life among patients with major depressive disorder. Psychiatr Serv. 2006;57:1731–1737. doi: 10.1176/ps.2006.57.12.1731. [DOI] [PubMed] [Google Scholar]
- 259.Molosh AI, Johnson PL, Fitz SD, Dimicco JA, Herman JP, Shekhar A. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacology. 2010;35:1333–1347. doi: 10.1038/npp.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Monteleone P, Luisi S, Tonetti A, Bernardi F, Genazzani AD, Luisi M, Petraglia F, Genazzani AR. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–273. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- 261.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 262.Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ. 2012;3:22. doi: 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 264.Naito H, Tonoue T. Sex difference in ultrasound distress call by rat pups. Behav Brain Res. 1987;25:13–21. doi: 10.1016/0166-4328(87)90041-6. [DOI] [PubMed] [Google Scholar]
- 265.Neumann ID. Brain mechanisms underlying emotional alterations in the peripartum period in rats. Depress Anxiety. 2003;17:111–121. doi: 10.1002/da.10070. [DOI] [PubMed] [Google Scholar]
- 266.Neumann ID, Kromer SA, Bosch OJ. Effects of psychosocial stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30:791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 267.Nielsen TA, Laberge L, Paquet J, Tremblay RE, Vitaro F, Montplaisir J. Development of disturbing dreams during adolescence and their relation to anxiety symptoms. Sleep. 2000;23:727–736. [PubMed] [Google Scholar]
- 268.Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clin Psychol Rev. 2011;31:1183–1191. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.NIMH (National Institute of Mental Health) Any anxiety disorder among adults. 2012 Available at http://www.nimh.nih.gov/statistics/1ANYANX_ADULT.shtml.
- 270.NIMH (National Institute of Mental Health) Total expenditures for the five most costly medical conditions (1996 vs. 2006) 2012 Available at http://www.nimh.nih.gov/statistics/4TOT_MC9606.shtml.
- 271.Nin MS, Martinez LA, Pibiri F, Nelson M, Pinna G. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front Endocrinol (Lausanne) 2011;2:73. doi: 10.3389/fendo.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nomura M, Akama K, Alves S, Korach K, Gustafsson J, Pfaff D, Ogawa S. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 273.Nugent NR, Tyrka AR, Carpenter LL, Price LH. Gene–environment interactions: early life stress and risk for depressive and anxiety disorders. Psychopharmacol (Berl) 2011;214:175–196. doi: 10.1007/s00213-010-2151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Nyberg S, Wahlstrom G, Backstrom T, Sundstrom Poromaa I. Altered sensitivity to alcohol in the late luteal phase among patients with premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29:767–777. doi: 10.1016/S0306-4530(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 275.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 276.Osterlund MK, Halldin C, Hurd YL. Effects of chronic 17beta-estradiol treatment on the serotonin 5-HT(1A) receptor mRNA and binding levels in the rat brain. Synapse. 2000;35:39–44. doi: 10.1002/(SICI)1098-2396(200001)35:1<39::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 277.Oztan O, Aydin C, Isgor C. Stressful environmental and social stimulation in adolescence causes antidepressant-like effects associated with epigenetic induction of the hippocampal BDNF and mossy fibre sprouting in the novelty-seeking pheno-type. Neurosci Lett. 2011;501:107–111. doi: 10.1016/j.neulet.2011.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Painsipp E, Wultsch T, Shahbazian A, Edelsbrunner M, Kreissl MC, Schirbel A, Bock E, Pabst MA, Thoeringer CK, Huber HP, Holzer P. Experimental gastritis in mice enhances anxiety in a gender-related manner. Neuroscience. 2007;150:522–536. doi: 10.1016/j.neuroscience.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav. 2001;73:411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- 280.Papinczak TA, Turner CT. An analysis of personal and social factors influencing initiation and duration of breastfeeding in a large Queensland maternity hospital. Breastfeed Rev. 2000;8:25–33. [PubMed] [Google Scholar]
- 281.Parker G, Hadzi-Pavlovic D. Is any female preponderance in depression secondary to a primary female preponderance in anxiety disorders? Acta Psychiatr Scand. 2001;103:252–256. doi: 10.1034/j.1600-0447.2001.00375.x. [DOI] [PubMed] [Google Scholar]
- 282.Parker G, Hadzi-Pavlovic D. Is the female preponderance in major depression secondary to a gender difference in specific anxiety disorders? Psychol Med. 2004;34:461–470. doi: 10.1017/s0033291703001181. [DOI] [PubMed] [Google Scholar]
- 283.Parker V, Morinan A. The socially-isolated rat as a model for anxiety. Neuropharmacology. 1986;25:663–664. [Google Scholar]
- 284.Pasquier DA, Reinoso-Suarez F. The topographic organization of hypothalamic and brain stem projections to the hippo-campus. Brain Res Bull. 1978;3:373–389. doi: 10.1016/0361-9230(78)90106-5. [DOI] [PubMed] [Google Scholar]
- 285.Paul ED, Hale MW, Lukkes JL, Valentine MJ, Sarchet DM, Lowry CA. Repeated social defeat increases reactive emotional coping behavior and alters functional responses in serotonergic neurons in the rat dorsal raphe nucleus. Physiol Behav. 2011;104:272–282. doi: 10.1016/j.physbeh.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Pego JM, Sousa JC, Almeida OF, Sousa N. Stress and the neuroendocrinology of anxiety disorders. Curr Top Behav Neurosci. 2010;2:97–117. doi: 10.1007/7854_2009_13. [DOI] [PubMed] [Google Scholar]
- 287.Peleg-Raibstein D, Luca E, Wolfrum C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav Brain Res. 2012;233:398–404. doi: 10.1016/j.bbr.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 288.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 289.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–1311. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, Wilkinson CW, Raskind MA. Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol Psychiatry. 1998;44:1007–1016. doi: 10.1016/s0006-3223(98)00053-5. [DOI] [PubMed] [Google Scholar]
- 291.Petersen EN, Paschelke G, Kehr W, Nielsen M, Braestrup C. Does the reversal of the anticonflict effect of phenobarbital by beta-CCE and FG 7142 indicate benzodiazepine receptor-mediated anxiogenic properties? Eur J Pharmacol. 1982;82:217–221. doi: 10.1016/0014-2999(82)90517-9. [DOI] [PubMed] [Google Scholar]
- 292.Petit JM, Luppi PH, Peyron C, Rampon C, Jouvet M. VIP-like immunoreactive projections from the dorsal raphe and caudal linear raphe nuclei to the bed nucleus of the stria terminalis demonstrated by a double immunohistochemical method in the rat. Neurosci Lett. 1995;193:77–80. doi: 10.1016/0304-3940(95)11669-n. [DOI] [PubMed] [Google Scholar]
- 293.Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621–672. vi–vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 294.Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pobbe RL, Zangrossi H., Jr 5-HT(1A) and 5-HT(2A) receptors in the rat dorsal periaqueductal gray mediate the antipanic-like effect induced by the stimulation of serotonergic neurons in the dorsal raphe nucleus. Psychopharmacol (Berl) 2005;183:314–321. doi: 10.1007/s00213-005-0196-z. [DOI] [PubMed] [Google Scholar]
- 296.Pobbe RL, Zangrossi H, Jr, Blanchard DC, Blanchard RJ. Involvement of dorsal raphe nucleus and dorsal periaqueductal gray 5-HT receptors in the modulation of mouse defensive behaviors. Eur Neuropsychopharmacol. 2011;21:306–315. doi: 10.1016/j.euroneuro.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci. 2007;121:462–474. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- 298.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 299.Quinn JJ, Hitchcott PK, Umeda EA, Arnold AP, Taylor JR. Sex chromosome complement regulates habit formation. Nat Neurosci. 2007;10:1398–1400. doi: 10.1038/nn1994. [DOI] [PubMed] [Google Scholar]
- 300.Racine M, Tousignant-Laflamme Y, Kloda LA, Dion D, Dupuis G, Choiniere M. A systematic literature review of 10 years of research on sex/gender and pain perception—part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153:619–635. doi: 10.1016/j.pain.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 301.Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Ramos A. Animal models of anxiety: do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 303.Ramos A, Kangerski AL, Basso PF, Da Silva, Santos JE, Assreuy J, Vendruscolo LF, Takahashi RN. Evaluation of Lewis and SHR rat strains as a genetic model for the study of anxiety and pain. Behav Brain Res. 2002;129:113–123. doi: 10.1016/s0166-4328(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 304.Reimer AE, de Oliveira AR, Brandao ML. Glutamatergic mechanisms of the dorsal periaqueductal gray matter modulate the expression of conditioned freezing and fear-potentiated startle. Neuroscience. 2012;219:72–81. doi: 10.1016/j.neuroscience.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 305.Renard GM, Suarez MM, Levin GM, Rivarola MA. Sex differences in rats: effects of chronic stress on sympathetic system and anxiety. Physiol Behav. 2005;85:363–369. doi: 10.1016/j.physbeh.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 306.Resstel LB, Alves FH, Reis DG, Crestani CC, Correa FM, Guimaraes FS. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience. 2008;154:869–876. doi: 10.1016/j.neuroscience.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 307.Rice F, Harold GT, Boivin J, Hay DF, van den Bree M, Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proc Natl Acad Sci USA. 2009;106:2464–2467. doi: 10.1073/pnas.0808798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2010;40:335–345. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Rodgers RJ, Cole JC. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol Behav. 1993;54:729–736. doi: 10.1016/0031-9384(93)90084-s. [DOI] [PubMed] [Google Scholar]
- 310.Rofey DL, Kolko RP, Iosif AM, Silk JS, Bost JE, Feng W, Szigethy EM, Noll RB, Ryan ND, Dahl RE. A longitudinal study of childhood depression and anxiety in relation to weight gain. Child Psychiatry Hum Dev. 2009;40:517–526. doi: 10.1007/s10578-009-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 312.Romeo RD, Sisk CL. Pubertal and seasonal plasticity in the amygdala. Brain Res. 2001;889:71–77. doi: 10.1016/s0006-8993(00)03111-5. [DOI] [PubMed] [Google Scholar]
- 313.Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Saavedra M, Contreras CM, Azamar-Arizmendi G, Hernandez-Lozano M. Differential progesterone effects on defensive burying and forced swimming tests depending upon a gradual decrease or an abrupt suppression schedules. Pharmacol Biochem Behav. 2006;83:130–135. doi: 10.1016/j.pbb.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 315.Sachser N, Hennessy MB, Kaiser S. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci Biobehav Rev. 2011;35:1518–1533. doi: 10.1016/j.neubiorev.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 316.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 317.Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sandberg D, Endicott J, Harrison W, Nee J, Gorman J. Sodium lactate infusion in late luteal phase dysphoric disorder. Psychiatry Res. 1993;46:79–88. doi: 10.1016/0165-1781(93)90010-e. [DOI] [PubMed] [Google Scholar]
- 319.Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior—modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28:1836–1848. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- 320.Santos JM, Lovick TA, Brandau ML. Effects of acute treatment with fluoxetine on the neural substrates of fear of the periaqueductal gray matter in female rats. Abstract for the Annual Meeting of the Society for Neuroscience; Washington, DC. 2011. Abstract 281.01. [Google Scholar]
- 321.Schenberg LC, Bittencourt AS, Sudre EC, Vargas LC. Modeling panic attacks. Neurosci Biobehav Rev. 2001;25:647–659. doi: 10.1016/s0149-7634(01)00060-4. [DOI] [PubMed] [Google Scholar]
- 322.Schmidt MV, Scharf SH, Liebl C, Harbich D, Mayer B, Holsboer F, Muller MB. A novel chronic social stress paradigm in female mice. Horm Behav. 2010;57:415–420. doi: 10.1016/j.yhbeh.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 323.Schmidt NB, Zvolensky MJ, Maner JK. Anxiety sensitivity: prospective prediction of panic attacks and axis I pathology. J Psychiatr Res. 2006;40:691–699. doi: 10.1016/j.jpsychires.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 324.Schoofs D, Wolf OT. Are salivary gonadal steroid concentrations influenced by acute psychosocial stress? A study using the Trier Social Stress Test (TSST) Int J Psychophysiol. 2011;80:36–43. doi: 10.1016/j.ijpsycho.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 325.Schule C, Eser D, Baghai TC, Nothdurfter C, Kessler JS, Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 326.Schulz KM, Pearson JN, Neeley EW, Berger R, Leonard S, Adams CE, Stevens KE. Maternal stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol Behav. 2011;104:340–347. doi: 10.1016/j.physbeh.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic–pituitary–adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16:989–998. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- 328.Shekhar A, Truitt W, Rainnie D, Sajdyk T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 2005;8:209–219. doi: 10.1080/10253890500504557. [DOI] [PubMed] [Google Scholar]
- 329.Sheng Z, Kawano J, Yanai A, Fujinaga R, Tanaka M, Watanabe Y, Shinoda K. Expression of estrogen receptors (alpha, beta) and androgen receptor in serotonin neurons of the rat and mouse dorsal raphe nuclei; sex and species differences. Neurosci Res. 2004;49:185–196. doi: 10.1016/j.neures.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 330.Shepard JD, Chambers CO, Busch C, Mount A, Schulkin J. Chronically elevated corticosterone in the dorsolateral bed nuclei of stria terminalis increases anxiety-like behavior. Behav Brain Res. 2009;203:146–149. doi: 10.1016/j.bbr.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 331.Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res. 2006;174:193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 332.Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sigmon ST, Dorhofer DM, Rohan KJ, Hotovy LA, Boulard NE, Fink CM. Psychophysiological, somatic, and affective changes across the menstrual cycle in women with panic disorder. J Consult Clin Psychol. 2000;68:425–431. doi: 10.1037//0022-006x.68.3.425. [DOI] [PubMed] [Google Scholar]
- 334.Sigmon S, Fink C, Rohan K, Hotovy L. Anxiety sensitivity and mentrual cycle reactivity: psychological and self-report differences. Journal of Anxiety Disorders. 1996;10:393–410. [Google Scholar]
- 335.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2013;18:308–319. doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 337.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacology. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 338.Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KC. Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain Res. 2006;1097:123–132. doi: 10.1016/j.brainres.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 339.Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 340.Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacol (Berl) 2006;186:323–333. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Spannuth BM, Hale MW, Evans AK, Lukkes JL, Campeau S, Lowry CA. Investigation of a central nucleus of the amygdala/dorsal raphe nucleus serotonergic circuit implicated in fear-potentiated startle. Neuroscience. 2011;179:104–119. doi: 10.1016/j.neuroscience.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Speert DB, McClennen SJ, Seasholtz AF. Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology. 2002;143:4730–4741. doi: 10.1210/en.2002-220556. [DOI] [PubMed] [Google Scholar]
- 343.Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Staub DR, Evans AK, Lowry CA. Evidence supporting a role for corticotropin-releasing factor type 2 (CRF2) receptors in the regulation of subpopulations of serotonergic neurons. Brain Res. 2006;1070:77–89. doi: 10.1016/j.brainres.2005.10.096. [DOI] [PubMed] [Google Scholar]
- 345.Staub DR, Spiga F, Lowry CA. Urocortin 2 increases c-Fos expression in topographically organized subpopulations of serotonergic neurons in the rat dorsal raphe nucleus. Brain Res. 2005;1044:176–189. doi: 10.1016/j.brainres.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 346.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Elliott E, Chen A, Peeters BW, Roubos EW, Kozicz T. Chronic stress induces sex-specific alterations in methylation and expression of corticotropin-releasing factor gene in the rat. PLoS One. 2011;6:e28128. doi: 10.1371/journal.pone.0028128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sterrenburg L, Gaszner B, Boerrigter J, Santbergen L, Bramini M, Roubos EW, Peeters BW, Kozicz T. Sex-dependent and differential responses to acute restraint stress of corticotropin-releasing factor-producing neurons in the rat paraventricular nucleus, central amygdala, and bed nucleus of the stria terminalis. J Neurosci Res. 2012;90:179–192. doi: 10.1002/jnr.22737. [DOI] [PubMed] [Google Scholar]
- 348.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 349.Stezhka VV, Lovick TA. Inhibitory and excitatory projections from the dorsal raphe nucleus to neurons in the dorsolateral periaqueductal gray matter in slices of midbrain maintained in vitro. Neuroscience. 1994;62:177–187. doi: 10.1016/0306-4522(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 350.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, Smith MS, Coleman K, Grove KL. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci. 2010;30:3826–3830. doi: 10.1523/JNEUROSCI.5560-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Tenk CM, Kavaliers M, Ossenkopp KP. Sexually dimorphic effects of neonatal immune system activation with lipopolysaccharide on the behavioural response to a homotypic adult immune challenge. Int J Dev Neurosci. 2008;26:331–338. doi: 10.1016/j.ijdevneu.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 352.Tenk CM, Kavaliers M, Ossenkopp KP. Neonatal treatment with lipopolysaccharide differentially affects adult anxiety responses in the light–dark test and taste neophobia test in male and female rats. Int J Dev Neurosci. 2013;31:171–180. doi: 10.1016/j.ijdevneu.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 353.Thorneycroft IH, Mishell DR, Jr, Stone SC, Kharma KM, Nakamura RM. The relation of serum 17-hydroxyprogesterone and estradiol-17-beta levels during the human menstrual cycle. Am J Obstet Gynecol. 1971;111:947–951. doi: 10.1016/0002-9378(71)90951-3. [DOI] [PubMed] [Google Scholar]
- 354.Thorsell A, Slawecki CJ, El Khoury A, Mathe AA, Ehlers CL. The effects of social isolation on neuropeptide Y levels, exploratory and anxiety-related behaviors in rats. Pharmacol Biochem Behav. 2006;83:28–34. doi: 10.1016/j.pbb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 355.Toledo-Rodriguez M, Pitiot A, Paus T, Sandi C. Stress during puberty boosts metabolic activation associated with fear-extinction learning in hippocampus, basal amygdala and cingu-late cortex. Neurobiol Learn Mem. 2012;98:93–101. doi: 10.1016/j.nlm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 356.Toledo-Rodriguez M, Sandi C. Stress during adolescence increases novelty seeking and risk-taking behavior in male and female rats. Front Behav Neurosci. 2011;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Uchida S, Hara K, Kobayashi A, Otsuki K, Hobara T, Yamagata H, Watanabe Y. Maternal and genetic factors in stress-resilient and -vulnerable rats: a cross-fostering study. Brain Res. 2010;1316:43–50. doi: 10.1016/j.brainres.2009.11.070. [DOI] [PubMed] [Google Scholar]
- 359.Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 360.Uvnas-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- 361.Valentino RJ, Bangasser D, Van Bockstaele E. Sex biased stress signaling: the corticotropin-releasing factor receptor as a model. Mol Pharmacol. 2013;83:737–745. doi: 10.1124/mol.112.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Velazquez-Zamora DA, Garcia-Segura LM, Gonzalez-Burgos I. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm Behav. 2012;61:512–517. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 365.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 366.Viau V, Meaney MJ. Variations in the hypothalamic–pituitary–adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 367.Vickers MH, Ikenasio BA, Breier BH. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–3973. doi: 10.1210/endo.142.9.8390. [DOI] [PubMed] [Google Scholar]
- 368.Vickers K, McNally RJ. Is premenstrual dysphoria a variant of panic disorder? A review. Clin Psychol Rev. 2004;24:933–956. doi: 10.1016/j.cpr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 369.Vogel JR, Beer B, Clody DE. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 370.Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- 371.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Walf AA, Paris JJ, Frye CA. Nociceptive and anxiety-like behavior in reproductively competent and reproductively senescent middle-aged rats. Gend Med. 2009;6(Suppl 2):235–246. doi: 10.1016/j.genm.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Walker AK, Hawkins G, Sominsky L, Hodgson DM. Transgenerational transmission of anxiety induced by neonatal exposure to lipopolysaccharide: implications for male and female germ lines. Psychoneuroendocrinology. 2012;37:1320–1335. doi: 10.1016/j.psyneuen.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 374.Walker FR, March J, Hodgson DM. Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav Brain Res. 2004;154:63–69. doi: 10.1016/j.bbr.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 375.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 377.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 378.Walsh R, Cummins R. The open-field test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 379.Weathington JM, Cooke BM. Corticotropin-releasing factor receptor binding in the amygdala changes across puberty in a sex-specific manner. Endocrinology. 2012;153:5701–5705. doi: 10.1210/en.2012-1815. [DOI] [PubMed] [Google Scholar]
- 380.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 381.Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci. 1998;16:289–295. doi: 10.1016/s0736-5748(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 382.Weintraub A, Singaravelu J, Bhatnagar S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010;1343:83–92. doi: 10.1016/j.brainres.2010.04.068. [DOI] [PubMed] [Google Scholar]
- 383.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic–pituitary–adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 387.Weissman MM, Bland R, Joyce PR, Newman S, Wells JE, Wittchen HU. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 388.Wiemers US, Schoofs D, Wolf OT. A friendly version of the trier social stress test does not activate the HPA axis in healthy men and women. Stress. 2013;16:254–260. doi: 10.3109/10253890.2012.714427. [DOI] [PubMed] [Google Scholar]
- 389.Wigger A, Loerscher P, Weissenbacher P, Holsboer F, Landgraf R. Cross-fostering and cross-breeding of HAB and LAB rats: a genetic rat model of anxiety. Behav Genet. 2001;31:371–382. doi: 10.1023/a:1012222402346. [DOI] [PubMed] [Google Scholar]
- 390.Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- 391.Wilson MA. GABA physiology: modulation by benzodiazepines and hormones. Crit Rev Neurobiol. 1996;10:1–37. doi: 10.1615/critrevneurobiol.v10.i1.10. [DOI] [PubMed] [Google Scholar]
- 392.Woehr M, Schwarting RK. Maternal care, isolation-induced infant ultrasonic calling, and their relations to adult anxiety-related behavior in the rat. Behav Neurosci. 2008;122:310–330. doi: 10.1037/0735-7044.122.2.310. [DOI] [PubMed] [Google Scholar]
- 393.Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- 394.Yamamoto S, Morinobu S, Takei S, Fuchikami M, Matsuki A, Yamawaki S, Liberzon I. Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26:1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 395.Yehuda R. Advances in understanding neuroendocrine alterations in PTSD and their therapeutic implications. Ann N Y Acad Sci. 2006;1071:137–166. doi: 10.1196/annals.1364.012. [DOI] [PubMed] [Google Scholar]
- 396.Yonkers KA, Bruce SE, Dyck IR, Keller MB. Chronicity, relapse, and illness—course of panic disorder, social phobia, and generalized anxiety disorder: findings in men and women from 8 years of follow-up. Depress Anxiety. 2003;17:173–179. doi: 10.1002/da.10106. [DOI] [PubMed] [Google Scholar]
- 397.Zangrossi H, Jr, Graeff FG. Behavioral validation of the elevated T-maze, a new animal model of anxiety. Brain Res Bull. 1997;44:1–5. doi: 10.1016/s0361-9230(96)00381-4. [DOI] [PubMed] [Google Scholar]
- 398.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Zhou JN, Hofman MA, Gooren LJ, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378:68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 400.Zimmerberg B, Brunelli SA, Fluty AJ, Frye CA. Differences in affective behaviors and hippocampal allopregnanolone levels in adult rats of lines selectively bred for infantile vocalizations. Behav Brain Res. 2005;159:301–311. doi: 10.1016/j.bbr.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 401.Zimmerberg B, Kajunski EW. Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav. 2004;78:465–471. doi: 10.1016/j.pbb.2004.03.021. [DOI] [PubMed] [Google Scholar]