Abstract
Negative glucocorticoid feedback is essential for preventing the deleterious effects of excessive hypothalamic pituitary adrenal axis axis activation, with an important target being CRH transcription in the hypothalamic paraventricular nucleus. The aim of these studies was to determine whether glucocorticoids repress CRH transcription directly in CRH neurons, by examining glucocorticoid effects on glucocorticoid receptor (GR)–CRH promoter interaction and the activation of proteins required for CRH transcription. Immunoprecipitation of hypothalamic chromatin from intact or adrenalectomized rats subjected to either stress or corticosterone injections showed minor association of the proximal CRH promoter with the GR compared with that with phospho-CREB (pCREB). In contrast, the Period-1 (Per1, a glucocorticoid-responsive gene) promoter markedly recruited GR. Stress increased pCREB recruitment by the CRH but not the Per1 promoter, irrespective of circulating glucocorticoids. In vitro, corticosterone pretreatment (30 minutes or 18 hours) only slightly inhibited basal and forskolin-stimulated CRH heteronuclear RNA in primary hypothalamic neuronal cultures and CRH promoter activity in hypothalamic 4B cells. In 4B cells, 30 minutes or 18 hours of corticosterone exposure had no effect on forskolin-induced nuclear accumulation of the recognized CRH transcriptional regulators, pCREB and transducer of regulated CREB activity 2. The data show that inhibition of CRH transcription by physiological glucocorticoids in vitro is minor and that direct interaction of GR with DNA in the proximal CRH promoter may not be a major mechanism of CRH gene repression. Although GR interaction with distal promoter elements may have a role, the data suggest that transcriptional repression of CRH by glucocorticoids involves protein-protein interactions and/or modulation of afferent inputs to the hypothalamic paraventricular nucleus.
Normal activity of the hypothalamic-pituitary-adrenal (HPA) axis leading to adrenal glucocorticoid production is essential for homeostasis and for survival during severe stress situations. Activation of the HPA axis is initiated by release of CRH produced in the hypothalamic paraventricular nucleus (PVN) into the pituitary portal circulation (1, 2). Episodes of CRH release during stress are usually associated with increases in CRH transcription, as evidenced by rapid and transient increases in primary transcript or heteronuclear RNA (hnRNA). Inappropriate transcriptional regulation with consequent deficient or excessive CRH expression can lead to HPA axis dysregulation and pathological conditions, such as depression and immune and metabolic disorders (3–5). A major mechanism for limiting HPA axis activation is negative feedback by glucocorticoids at the pituitary corticotroph and several sites in the brain, including hypothalamic CRH neurons in the PVN (6, 7).
A body of evidence indicates that glucocorticoids negatively regulate CRH expression. Removal of endogenous glucocorticoids by adrenalectomy increases CRH expression in the PVN and potentiates responses to stress (8–11). Glucocorticoid administration, systemic or directly in the PVN region, has the converse effect (12–15). In addition, glucocorticoid receptor (GR) deficiency increases CRH release into the median eminence (16), and the presence of GRs in CRH neurons (17, 18) further supports a direct inhibitory effect of glucocorticoids at the CRH neuron level.
A number of in vitro studies using cell lines transfected with CRH promoter reporter genes have shown direct effects of glucocorticoids on CRH promoter activity (19–21). There is no classic glucocorticoid response element (GRE) in the CRH promoter, but conserved and functionally defined negative GRE half-sites have been described (20, 22–24). However, others have shown that none of the GR-interacting sites in the proximal CRH promoter have functional activity and that the repressor effect of glucocorticoids requires the CRH promoter cAMP responsive element (CRE) (19, 21, 25), suggesting an effect through protein-protein interactions. The aim of the in vivo and in vitro studies described here was to further characterize the molecular mechanisms mediating glucocorticoid suppression of CRH gene transcription by examining the effects of altering glucocorticoid levels within the physiological range on the interaction of GRs with the CRH promoter and on the activation of transcription factors involved in CRH gene expression. Our results demonstrate that in vivo elevation of circulating glucocorticoids does not increase GR binding to the proximal CRH promoter, including the region containing putative negative GRE sites (−278 to −249) and additional regions up to −2000 bp. Furthermore, corticosterone does not inhibit in vitro forskolin-induced nuclear accumulation of the CRH transcriptional regulators phospho-CREB (pCREB) and transducer of regulated CREB activity 2 (TORC2) in hypothalamic 4B cells, suggesting that glucocorticoids suppress CRH transcription primarily through indirect pathways such as modulation of CRH neuron function.
Materials and Methods
Animals and in vivo procedures
Adult male Sprague-Dawley rats weighing 275 to 325 g were housed 3 per cage on a 14:10-hour light/dark cycle with food and water available ad libitum, for at least 1 week before experimentation. To determine whether the interaction of the GR with the proximal CRH promoter is part of the negative feedback by glucocorticoids on CRH transcription, we performed chromatin immunoprecipitation (ChIP) assays in hypothalamic chromatin of intact or 4-day adrenalectomized rats subjected to restraint stress or a single injection of cyclodextrin-encapsulated corticosterone (2-hydroxypropyl-β-cyclodextrin (HBC)–corticosterone; Sigma-Aldrich) at 1 or 10 mg/rat ip, dissolved in 300 μL of sterile saline. Rats were subjected to adrenalectomy under ketamine/xylazine anesthesia by the dorsal route and given tap water and normal saline ad libitum as drinking fluid. Restraint stress was performed by placing rats into plastic restrainers (2.5 × 6 in.) for up to 1 hour. Groups of rats were killed by decapitation at 0.5 or 1 hour after restraint or 0.5 or 2 hours after HBC-corticosterone injection. These time points were chosen based on the observed time course of changes in plasma corticosterone after stress and corticosterone injection, and the fact that stress-stimulated CRH hnRNA levels return to basal levels by 60 minutes of restraint stress. Control rats were removed from the cages and killed within 30 seconds. Brains were immediately removed, and the hypothalamic region was microdissected from coronal sections between the optic chiasma and 1 mm rostral from the mammillary bodies. Sections were placed flat on a chilled rubber cork and cut at the top and 1-mm lateral at each side of the third ventricle. After removal of an additional 1-mm from the bottom, hypothalamic sections were frozen in 1.5-mL microtubes on dry ice. The whole procedure from decapitation to freezing of the tissue was performed in about 3 minutes.
Trunk blood was collected in ice-chilled plastic tubes containing 5 mg of EDTA and 500 trypsin inhibitor units of aprotinin. Plasma was separated by centrifugation and stored at −80°C for corticosterone determination. Corticosterone levels were measured using the rat corticosterone Coat-A-Count Kit (Diagnostic Products Corporation), according to the manufacturer's instructions. All experiments were performed in the morning with rats killed between 9:00 and 11:00 am. All procedures and experimental protocols were performed according to National Institutes of Health guidelines and approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Animal Care and Use Committee.
Cell culture
The hypothalamic cell line 4B was provided by Dr John Kasckow (Cincinnati, OH). This cell line contains endogenous GR, and it was originally described as expressing CRH mRNA and immunoreactive peptide. However, 4B cells do not show rapid regulation of CRH hnRNA by cAMP, as seen in vivo or in primary cultures of hypothalamic neurons (26), and therefore they are not suitable for studying endogenous CRH transcription. On the other hand, 4B cells have been proven to be useful for studies using reporter gene assays (26–29). Cells were cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, 10% horse serum, and 100 U/mL penicillin and 100 μg/mL streptomycin (26).
Primary cultures of hypothalamic neurons were obtained from fetal Sprague-Dawley rats at embryonic day 18. Fetal rats were rapidly removed from 18-day pregnant rats after CO2 sedation and decapitation. Fetuses were decapitated, and hypothalamic tissue was dissected and collected in ice-cold buffer (pH 7.4, containing 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4, 25 mM HEPES buffer, and 100 μg/mL gentamicin). Tissues were then digested for 1.5 hours with collagenase type 2 (1 mg/mL) (Worthington) dissolved in the above buffer, supplemented with 1 mg/mL glucose, 4 mg/mL BSA, and 0.2 mg/mL DNase. After filtration through a 40-μm cell strainer (BD Falcon), cells were pelleted by centrifugation at 200 × g for 10 minutes. Cells were washed in plating media (DMEM/F12, 100 μg/mL gentamicin, and 10% heat-inactivated fetal bovine serum) and plated at a density of 1 × 106 cells per well in 6-well plates coated with poly-l-lysine. After 48 hours of culture, medium was changed to Neurobasal medium (Gibco/Invitrogen), supplemented with B27 (Invitrogen) to support neuron growth, and 100 μg/mL gentamicin. From days 5 to 9 (ie, for 5 days), 5 μM cytosine arabinoside (Sigma-Aldrich), a selective inhibitor of DNA synthesis, was added to prevent glial proliferation. On day 10, neuronal cultures were changed into Neurobasal medium containing 10 mM HEPES and 100 μg/mL gentamicin, supplemented with a glucocorticoid- and progesterone-free mixture (site 3 supplement (Sigma-Aldrich), 0.5 mM l-glutamate, 100 mM sodium pyruvate, 25 mM glucose, 60 pM triiodo-l-thyronine, 10 μM putrescine, and 0.2% BSA). On day 11, cells were incubated in supplement-free Neurobasal medium containing 0.1% BSA for 2 hours before treatment with forskolin. HBC-corticosterone at a concentration of 100 nM was added to duplicate wells either 18 hours or 30 minutes before stimulation with forskolin. After 45 minutes of incubation with forskolin, medium was removed, and cells were lysed for RNA preparation by addition of 1 mL of TRIzol (Invitrogen). The content of the wells was transferred to 1.5-mL microfuge tubes and frozen at −80°C or immediately processed for RNA isolation.
RNA Isolation and real-time PCR for CRH hnRNA
Total RNA was extracted from primary cultures of hypothalamic neurons using TRIzol reagent, followed by purification using RNeasy minikit reagents and column DNase digestion (QIAGEN) to remove genomic DNA contamination. cDNA was reverse transcribed from 0.3 to 0.75 μg of total RNA, and CRH primary transcript levels (hnRNA) were measured using primer sequences designed to amplify the intron as described previously (26). Power SYBR Green PCR mix (Applied Biosystems) was used for the amplification mixture with each primer at a final concentration of 200 nM and 1.5 μL of cDNA for a total reaction volume of 12.5 μL. PCRs were performed on a spectrofluorometric thermal cycler (7900 HT Fast Real-Time PCR System; Applied Biosystems) as described previously (29). Levels of hnRNA were normalized to GAPDH mRNA as determined in separate real-time-quantitative (q) PCR reactions. The absence of RNA detection when the reverse transcription step was omitted indicated the lack of genomic DNA contamination in the RNA samples. The sequence of the PCR primers for CRH hnRNA were as follows: forward 5′-TCAATCCAATCTGCCACTCA-3′ and reverse 5′-TAAGCTATTCGCCCGCTCTA-3′.
Luciferase assays
Cultures of 4B cells were transfected by electroporation using a Nucleofector device (Amaxa) and Solution V purchased from the manufacturer. The CRH promoter-driven luciferase reporter gene (pGL3-CRHp) was a 613-bp restriction fragment containing the CRH promoter (−498 to +115 bp relative to the proximal transcription start point) of the CRH gene clone prCRHBglII (provided by Dr. Audrey Seasholtz, Ann Arbor, Michigan), cloned into pGL3Basic (Promega) (28). Aliquots of 5 million 4B cells were transfected with 2.5 μg of pGL3-CRHp plasmid and 75 ng of Renilla luciferase construct to normalize for transfection efficiency. After transfection, cells were resuspended in DMEM containing 10% horse serum and 10% fetal bovine serum and plated onto 48-well culture plates, at a density of 50,000 cells per well. Six hours later when cells had become adherent, the medium was changed to culture medium supplemented with 10% charcoal-dextran–stripped fetal bovine serum. After overnight culture, cells were preincubated for 1 hour in serum-free medium containing 0.1% BSA before addition of 3 μM forskolin or vehicle. Corticosterone at 100 nM (HBC-corticosterone) was added 18 hours or 30 minutes before addition of forskolin. After 6 hours of incubation, medium was removed, and cells were lysed by addition of 100 μL of passive lysis buffer (Promega). Luciferase activity in cell lysates was determined using reagents from Promega (Dual Luciferase Assay System).
ChIP assays
To determine whether GR is recruited by the proximal CRH promoter during increases in circulating glucocorticoids, we performed ChIP assays using kit reagents from the ChIP-IT Express Kit (Active Motif), according to the manufacturer's protocol with some modifications. In brief, pooled hypothalamic tissue from 3 to 4 rats was homogenized in 1 mL of 4% formaldehyde using a handheld motorized homogenizer (Bio-Gen PRO200; Pro Scientific), for 10 seconds on setting 2 and incubated for 10 minutes at room temperature to cross-link the DNA-protein complexes. After termination of the cross-linking reaction with glycine, homogenates were centrifuged and washed 3 times in cold PBS containing protease and phosphatase inhibitors. Pellets were then resuspended in 300 μL of lysis buffer (50 mM Tris-HCl, pH 8.1, 10 mM EDTA, and 0.5% SDS) with protease/phosphatase inhibitors, incubated on ice for 10 minutes, and sonicated by 10 cycles (30 seconds on and 30 seconds off) at high-level output in a Bioruptor (Diagenode) to generate 0.2- to 1-kb DNA fragments of chromatin. After 2 hours of preclearing using Protein A/G Plus beads (Santa Cruz Biotechnology), immunoprecipitation was performed using 13 to 15 μg of chromatin and an anti-GR antibody cocktail (3 μg each of GR PA1–511A and GR MA-510 [Thermo/Pierce] and GR M-20 [Santa Cruz Biotechnology]) or pCREB antibody (Millipore/Upstate) or rabbit IgG for the negative control in the presence of ChIP-IT Protein G magnetic beads (Active Motif) at 4°C under rotation, in a total volume of 200 μL, following the manufacturer's protocol (Active Motif). Before addition, Protein G magnetic beads were preincubated with 1 μg of herring sperm DNA per immunoprecipitation reaction to reduce the background. After overnight incubation Protein G magnetic beads containing the DNA-protein immunocomplexes were separated and washed consecutively once in 800 μL of ChIP buffer 1 and twice in ChIP buffer 2. Beads were then resuspended in 50 μL of elution buffer and reverse cross-linked with proteinase K according to the kit's protocol. Immunoprecipitated DNA fragments were quantified by real-time PCR using primers designed to amplify different regions of the CRH promoter or the period 1 (Per1) promoter as shown in Table 1. The amplification efficiency of the primers was usually about 100%, ranging from 90 to 110%.
Table 1.
Primers Used for qPCR Detection of the CRH and Per1 Promoters
| Primer Sequence | Product, bp | Promoter and Region |
|---|---|---|
| F: 5′-TCAGTATGTTTTCCACACTTGGAT-3′ | 112 | CRHp; −206 to 318 |
| R: 5′-TTTATCGCCTCCTTGGTGAC-3′ | ||
| F: 5′-TAATGCACACAGCTCACCGT-3′ | 257 | CRHp; − 593 to − 850 |
| R: 5′-AGCTCCTTAGTCTTCCCAAGAGCA-3′ | ||
| F: 5′-CCCAGGCACTTCCCTTTCTT-3′ | 107 | CRHp; − 1692 to − 1798 |
| R: 5′-TCTAACCCCTTCTCTGCCCA-3′ | ||
| F: 5′-CCAAGGCTGAGTGCATGTC-3′ | 6bp | Per1p; − 3391 to − 3458 |
| R: 5′-GCGGCCAGCGCACTA-3′ |
Abbreviations: F, forward; R, reverse.
Western blot
Cytosolic and nuclear proteins from 4B cells, after incubations with forskolin, with and without glucocorticoids, were prepared using kit reagents from NE-PER Nuclear and Cytoplasmic Extraction Reagent (Thermo/Pierce) as described previously (26). The protein concentration was quantified by spectrophotometry using the BCA Protein Assay (Thermo/Pierce). For Western blotting, 15 μg of cytoplasmic or nuclear extract was loaded and separated in a 10% Tris-glycine gel (Invitrogen) for GAPDH and histone deacetylase 1 (HDAC1) (Santa Cruz Biotechnology) or a 6% Tris-glycine gel (Invitrogen) for TORC2 and GR. Proteins were transferred to a polyvinylidene difluoride membrane (GE Amersham Biosciences), incubated with 5% nonfat milk in 1 × TBST (Tris-buffered saline plus 0.05% Tween 20) for 1 hour, and incubated overnight at 4°C with rabbit antibodies against TORC2 (Calbiochem/EDM Chemicals) at 1:6000 dilution or GR (H-300, Santa Cruz Biotechnology) at a 1:1000 dilution. After washing in 1 × TBST, membranes were incubated for 1 hour at room temperature with a horseradish peroxidase–conjugated donkey anti-rabbit IgG at a dilution of 1:10,000. Detection of immunoreactive bands was performed using ECL Plus reagents (GE Amersham Biosciences) followed by exposure to BioMax MR film (Eastman Kodak). After film exposure, blots were stripped and assayed for HDAC1 in the nucleus and GAPDH in cytoplasm as loading controls. The intensity of the bands was quantified using the computer image analysis system ImageJ (National Institutes of Health). Results are expressed as fold change over the values in control rats after correction for protein loading using HDAC1 for the nucleus and GAPDH for cytoplasm.
Statistical analysis
Data are presented as means ± SEM from the values in the number of experiments indicated in the legends to the figures. In vivo experiments were repeated at least 3 times, using pooled hypothalamic tissue of 3 rats per experimental group. Differences between groups were analyzed by 1- or 2-way ANOVA followed by the Fisher least significant difference post hoc test when appropriate. Statistical significance was set at P < .05.
Results
Effect of restraint stress on GR and pCREB recruitment by the CRH promoter
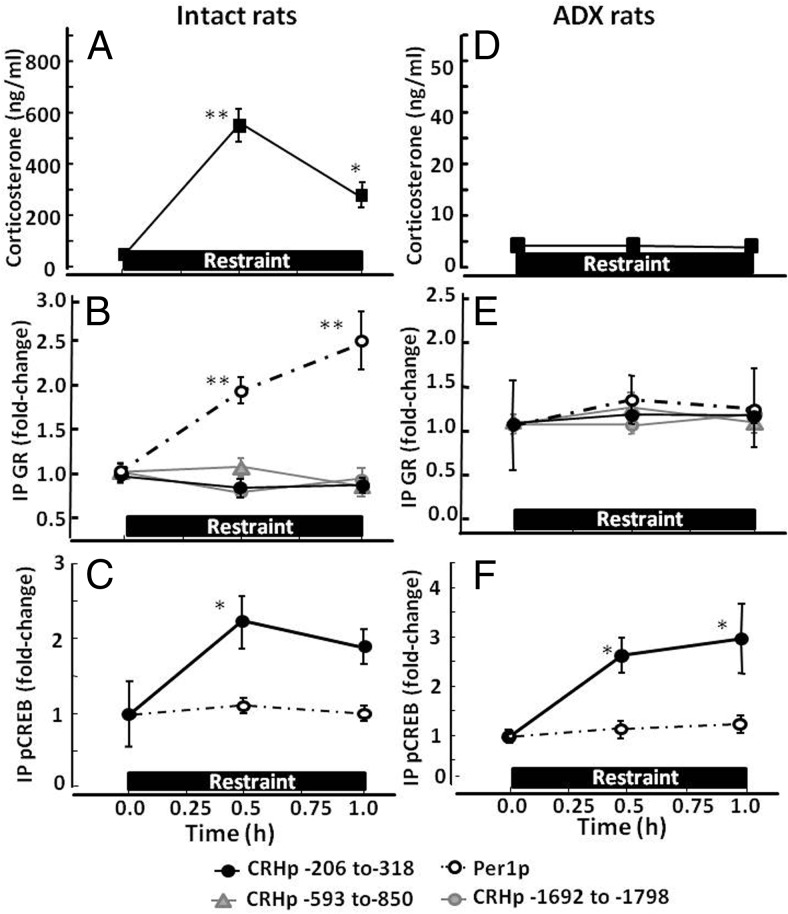
To determine whether the increases in plasma glucocorticoid levels during restraint stress are associated with GR recruitment by the proximal CRH promoter, GR ChIP assays were performed in hypothalamic chromatin of intact and adrenalectomized rats subjected to restraint stress. As shown in Figure 1A, plasma corticosterone levels increased markedly, from 30.1 ± 9.2 to 545 ± 55 ng/mL, at 30 minutes (P < .001, n = 12) and remained significantly elevated at 60 minutes of restraint stress (280 ± 45 ng/mL; P < .01). As expected in adrenalectomized rats, plasma corticosterone levels were barely detectable and remained unchanged during restraint (Figure 1D).
Figure 1.
Effect of restraint stress on GR and pCREB recruitment by the CRH and Per1 promoters in intact (A, B, and C) and adrenalectomized (Adx) (D, E, and F) rats. Plasma corticosterone measurements (A and D) and ChIP assays on hypothalamic chromatin for GR (B and E) and pCREB (C and F) were performed in basal conditions (time 0) and at 30 and 60 minutes during stress. Data points are the means and SE of the results of 4 and 3 experiments (using pooled hypothalamic tissue from 3 rats per experimental group) for intact and adrenalectomized rats, respectively. The dashed lines correspond to the Per1 promoter, and solid lines show different regions of the CRH promoter as indicated by the key at the bottom of the figure. The restraint stress period is shown by the horizontal boxes above the x-axis. **, P < .001 vs respective basal; *, P < .05 vs respective basal.
ChIP assays showed no effect of restraint stress on CRH promoter pulldown in hypothalamic chromatin immunoprecipitated with GR antibody either at 30 or 60 minutes in intact or adrenalectomized rats (Figure 1, B and E). In basal conditions, levels of CRH promoter associated with GR were similar in intact and adrenalectomized rats. The percentage of input pulled down by the GR antibody was 0.05 ± 0.03% and 0.06 ± 0.04% for intact and adrenalectomized rats, respectively (not shown). Similarly, qPCR using primers directed to upstream regions of the CRH promoter (−593 to −850 and −1692 to −1798) yielded very low levels, which were unchanged by stress (Figure 1, B and E). In contrast, Per1 promoter immunoprecipitated with GR antibody increased by 1.9 ± 0.15-fold at 30 minutes (P < .01) and 2.5 ± 0.35-fold (P < .01) by 1 hour of restraint in intact (Figure 1B) but not in adrenalectomized rats (Figure 1E). Basal levels of Per1 promoter associated with GR in hypothalamic chromatin were significantly higher in intact than in adrenalectomized rats, with percentages of input pulldowns of 0.32 ± 0.08 (n = 4) and 0.17 ± 0.03% (n = 3), respectively (P < .05).
In contrast to the lack of CRH promoter association with GR during restraint, ChIP using pCREB antibody showed significant increases in CRH promoter pulldown of 2.3 ± 0.5-fold over the basal levels at 30 minutes (P < .03) and 1.9 ± 0.3-fold at 1 hour (P < .05, n = 3) of restraint stress in intact rats (Figure 1C). Similar changes in pCREB recruitment by the CRH promoter were found in adrenalectomized rats with increases of 2.7 ± 0.4- and 2.9 ± 0.75-fold over the basal levels at 30 minutes and 1 hour, respectively (P < .03, n = 3 at both time points) (Figure 1F). No significant differences were found between the percent CRH promoter pulldown with pCREB antibody in hypothalamic chromatin from intact and adrenalectomized rats (0.05 ± 0.01% and 0.06 ± 0.02% of the input in intact and adrenalectomized rats, respectively). Levels of Per1 promoter pulldown by the pCREB antibody were low and did not change during restraint (Figure 1, C and F).
Effect of glucocorticoid injection on GR and pCREB recruitment by the CRH promoter
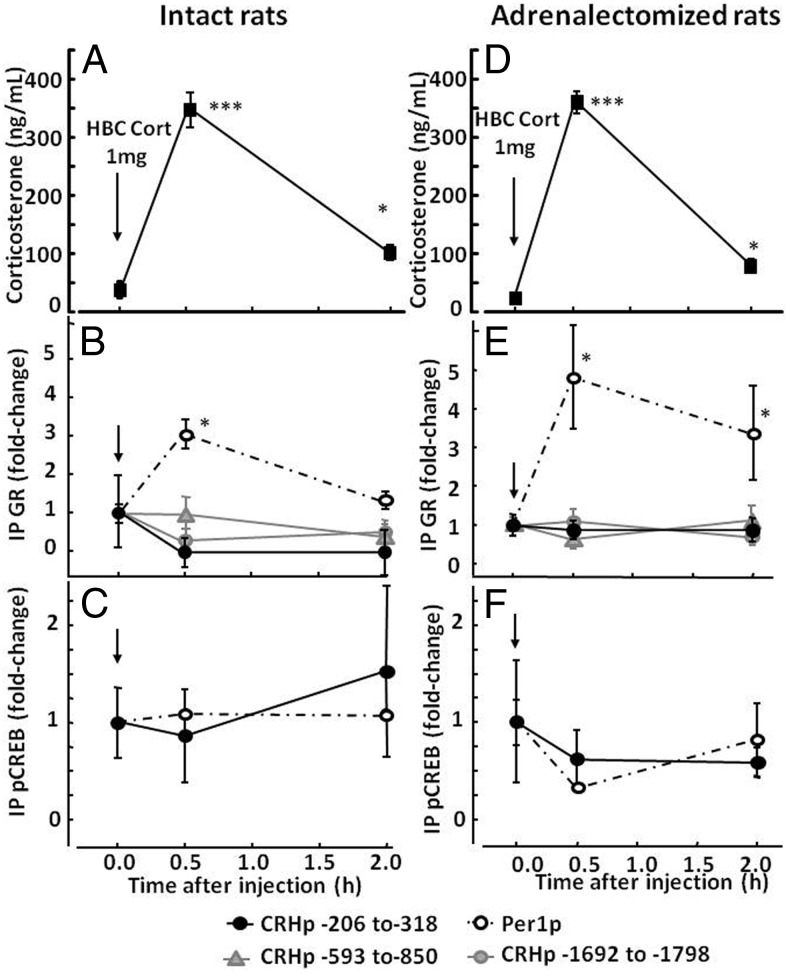
Injection of HBC-corticosterone (1 mg/rat ip) in intact rats increased plasma corticosterone from basal levels of 24.4 ± 17.6 to 350.4 ± 3.4 ng/mL by 30 minutes (P < .001, n = 4). Similar changes from 6.4 ± 3.4 to 379 ± 4.6 by 30 minutes were observed in adrenalectomized rats (P < .01, n = 3). At 2 hours, levels had decreased markedly but remained significantly higher than basal values in both intact and adrenalectomized rats (56.7 ± 8.7 and 59.7 ± 7.1, respectively) (Figure 2, A and D).
Figure 2.
Effect of corticosterone injection on GR and pCREB recruitment by the CRH and Per1 promoters in intact (A, B, and C) and adrenalectomized (D, E, and F) rats. Intact and adrenalectomized rats received an ip injection of 1 mg of HBC-corticosterone (HBC-Cort) and were killed in basal conditions (time 0) and at 30 minutes and 2 hours after injection for measurement of plasma corticosterone (A and D) and ChIP assays on hypothalamic chromatin for GR (B and E) and pCREB (C and F). Data points are the means and SE of the results of 4 and 3 experiments (using pooled hypothalamic tissue from 3 rats per group in each experiment) for intact and adrenalectomized rats, respectively. The dashed lines correspond to the Per1 promoter, and solid lines show different regions of the CRH promoter as indicated by the key at the bottom of the figure. The time of injection is indicated by the arrow. ***, P < .001 vs respective basal; **, P < .01 vs respective basal; *, P < .05 vs respective basal.
Similar to the observations during restraint stress, elevations of plasma corticosterone levels induced by HBC-corticosterone injection had no significant effect on CRH promoter pulldown by the GR antibody either in intact (Figure 2B) or adrenalectomized rats (Figure 2E). As with restraint stress, primers directed to CRH promoter regions −593 to −850 and −1692 to −1798 also failed to detect significant changes after corticosterone injection (Figure 2, B and E). Again, elevations in plasma corticosterone increased Per1 promoter pulldown by the GR antibody. In 4 experiments in intact rats, Per1 promoter pulldown increased by 2.9 ± 0.4-fold over basal values at 30 minutes (P < .05) and had returned to near basal levels by 2 hours (1.2 ± 0.16-fold over the basal levels). More marked Per1 promoter pulldown by the GR antibody after HBC-corticosterone injection was observed in adrenalectomized rats, with a 4.9 ± 1.3-fold increase at 30 minutes (P < .03) and remaining 3.4 ± 1.1-fold over the basal values 2 hours after injection (P < .05). ChIP using the pCREB antibody showed no significant changes in CRH or Per1 promoter pulldown after 1 mg of HBC-corticosterone injection (Figure 2, C and F).
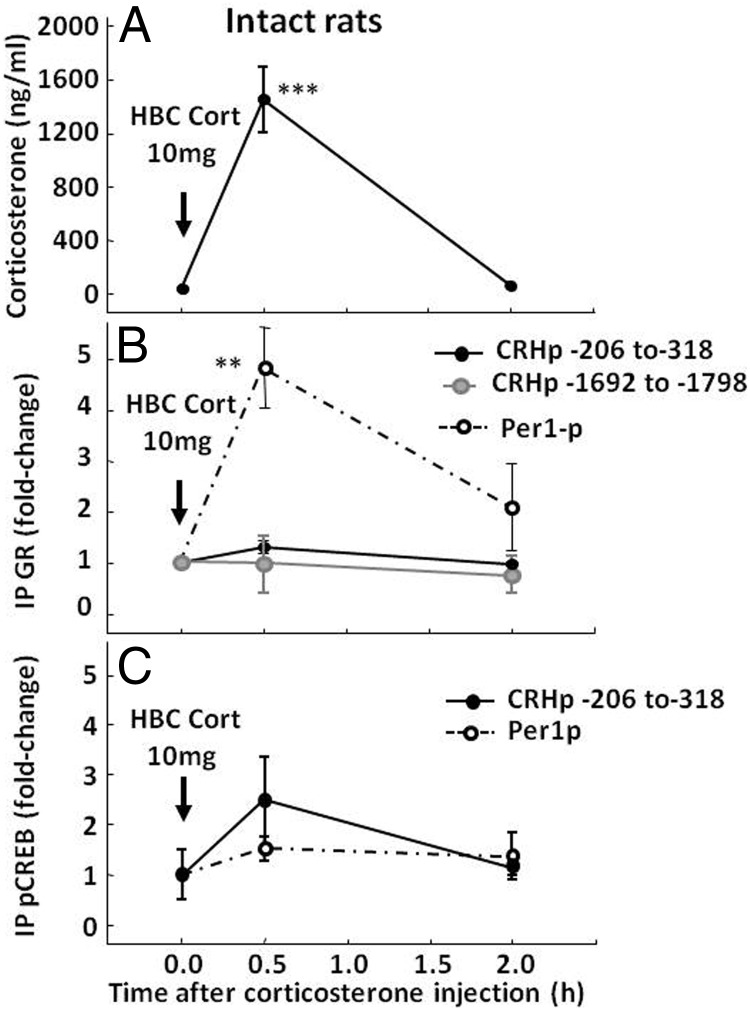
Because previous reports showing marked decreases in stress-induced CRH hnRNA used higher doses of corticosterone in intact rats (12), we examined GR interaction with the CRH promoter in groups of intact rats receiving injections of 10 mg of HBC-corticosterone. In these rats, plasma corticosterone levels increased from basal levels of 12.2 ± 2.3 to 1389.7 ± 143 and 87.0 ± 39.4 ng/mL at 30 minutes and 2 hours after injection, respectively (Figure 3A). As shown in Figure 3B, these larger increases in plasma corticosterone were equally ineffective in increasing CRH promoter pulldown by the GR antibody, using either primers against the GRE/CRE region (1.2-fold over the basal levels; P = .07, n = 2) or against the −1692 to −1798 region. However, similar to our findings from the stress and lower corticosterone dose experiments, 10 mg of HBC-corticosterone induced marked increases in Per1 promoter pulldown (4.8 ± 0.8 at 30 minutes; P < .01, n = 2). Two hours after corticosterone injection, Per1 promoter pulldown values had returned near to basal levels.
Figure 3.
Effect of a supraphysiological dose of corticosterone on GR and pCREB recruitment by the CRH and Per1 promoters in intact rats. Rats received an ip injection of 10 mg of HBC-corticosterone (HBC-Cort) and were killed either in basal conditions (time 0) or at 30 minutes and 2 hours after injection for measurement of plasma corticosterone (A) and ChIP assays on hypothalamic chromatin for GR (B) and pCREB (C). Data points are the means and SE of the results of 2 experiments using pooled hypothalamic tissue from 3 rats per group in each experiment. The dashed lines correspond to the Per1 promoter, and solid lines show 2 regions of the CRH promoter. Black circles, CRE/GRE region (−206 to 318); gray circles, −1692 to −1798. The time of injection is indicated by the arrow. ***, P < .001 vs respective basal; **, P < .01 vs respective basal.
Similar to the results with the low dose of corticosterone, ChIP using pCREB antibody in chromatin from rats receiving the 10-mg HBC-corticosterone injection showed no significant changes in CRH or Per1 promoter pulldown either at 30 minutes or 2 hours after injection (Figure 3C).
Effect of glucocorticoids on endogenous CRH transcription in primary cultures of hypothalamic cells
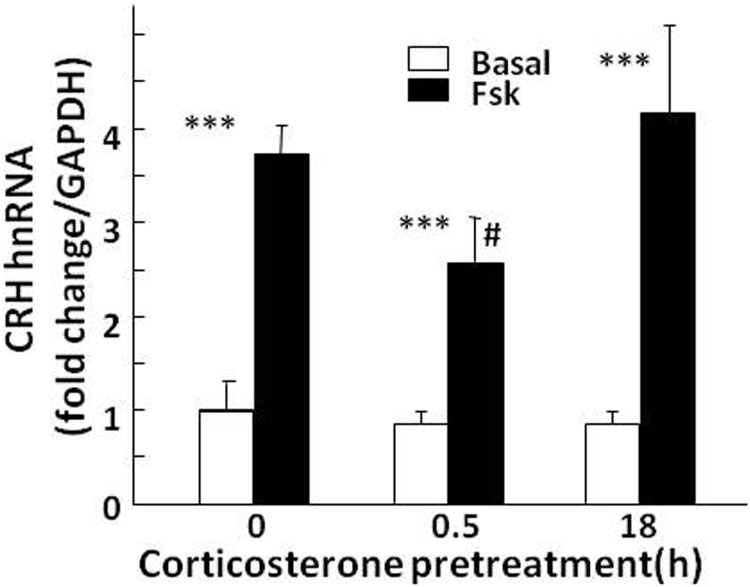
To determine whether glucocorticoids can inhibit CRH transcription directly in CRH neurons, we examined the effect of glucocorticoid exposure for 18 hours or 30 minutes on the increases of CRH hnRNA production induced by the adenylate cyclase stimulator, forskolin, in primary cultures of hypothalamic neurons. Incubation of control cultures with 1 μM forskolin for 45 minutes increased CRH hnRNA production by 3.82 ± 0.5-fold over the basal levels (P < 0. 001, n = 4) (Figure 4). Addition of 100 nM corticosterone 30 minutes before forskolin addition caused a 32.3 ± 8.6% decrease in forskolin-stimulated CRH hnRNA production, which was statistically significant only after log transformation of the data (P < .05, n = 4). Addition of corticosterone 18 hours before forskolin had no significant effect on the increase in CRH hnRNA stimulated by forskolin. Analysis by 2-way ANOVA after log transformation of the data showed a significant effect of forskolin (F = 56.9; P < .001) and an interaction between glucocorticoids and forskolin (F = 4.2; P < .05).
Figure 4.
Effect of corticosterone on forskolin-stimulated CRH hnRNA in primary cultures of hypothalamic neurons. At day 10 of culture, fetal rat hypothalamic neuronal cultures were exposed to 100 nM corticosterone for 18 hours or 30 minutes before addition of forskolin (Fsk) for an additional 45 minutes before RNA preparation. Data points are the means and SE of CRH hnRNA levels, normalized to GAPDH mRNA in 4 experiments. ***, P < .001 compared with basal; # P < .05 lower than forskolin at 0 minutes. Significance was calculated after log transformation of the data (only the effect of forskolin was significant without log transformation).
Effect of glucocorticoids on CRH promoter activity and CREB signaling in 4B cells
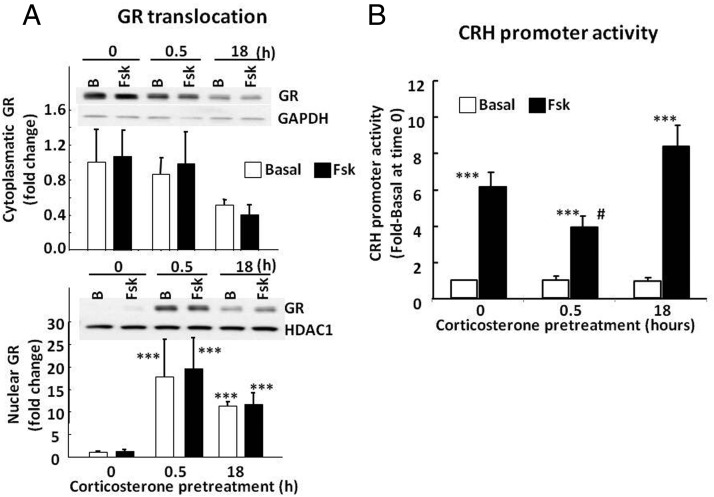
As shown in Figure 5A, endogenous GR levels were clearly detectable by Western blot in cytoplasmic and nuclear proteins from 4B cells. In basal conditions, GR levels were very low in the nucleus and increased markedly after 30 minutes of incubation with 100 nM corticosterone (P < .001, n = 3). Incubation with corticosterone for 18 hours tended to decrease GR levels in the cell, but nuclear levels were still elevated (P < .001) and not significantly different from nuclear levels at 30 minutes. Forskolin treatment for 30 minutes had no significant effect on GR trafficking in the presence or absence of glucocorticoids.
Figure 5.
Effect of corticosterone on GR translocation (A) and CRH promoter activity (B) in 4B cells transfected with a CRH promoter-driven luciferase reporter gene Cells were incubated with 100 nM corticosterone for 18 hours or 30 minutes (0.5 hour) with and without forskolin (Fsk) for 30 minutes before protein extraction for Western blot or treatment with forskolin for 6 additional hours for luciferase assays. Western blot images are representative of 3 experiments. Data are expressed as fold change after normalization for GAPDH in the cytoplasm or HDAC in the nucleus. Data points are the means and SE of values obtained in 4 experiments. After log transformation of the data: ***, P < .001 vs basal at time 0; #, P < .05 compared with forskolin no corticosterone control (time 0) or 18-hour preincubation with corticosterone. Only the effect of forskolin was significant without log transformation of the data. B, basal.
In 4B cells transfected with the CRH promoter-driven luciferase construct, incubation of the cells with forskolin caused marked increases in CRH promoter activity (6.1 ± 0.9-fold over the basal values; P < .001, n = 4). Addition of 100 nM corticosterone at 30 minutes before forskolin resulted in a 36.3 ± 7.4% decrease in forskolin-stimulated CRH promoter activity (3.9 ± 0.7-fold over the basal values), which was statistically significant only after log transformation of the data (P < .05, n = 4). Incubation of the cells with corticosterone for 18 hours before addition of forskolin had no effect on the stimulatory action of forskolin or basal CRH promoter activity (Figure 5B). Two-way ANOVA analysis of the log-transformed data showed a marked effect of forskolin (F = 76.9; P < .001) and a significant interaction between forskolin and corticosterone (F = 3.9; P < .04).
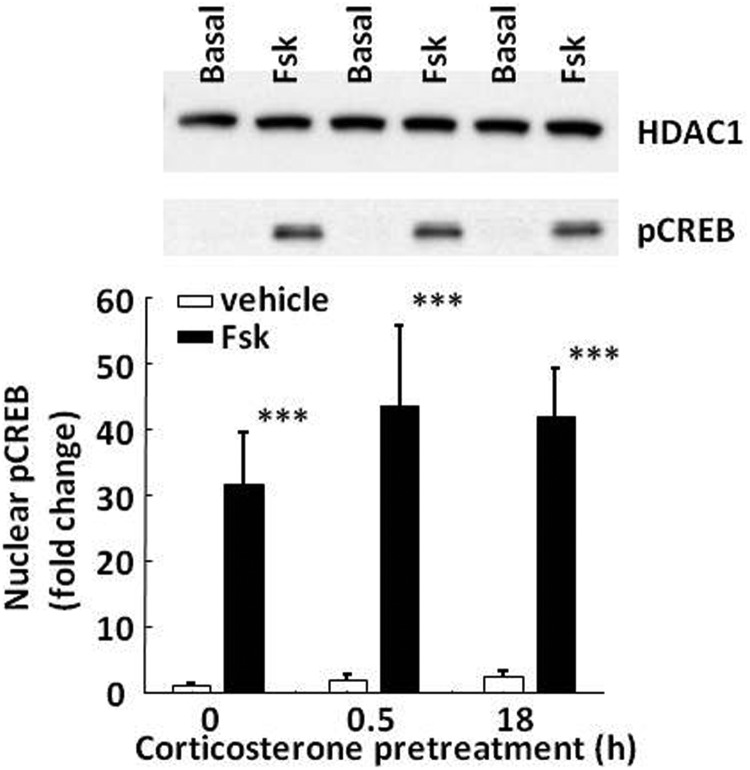
To determine whether glucocorticoids can alter the activation of key proteins required for CRH transcription, nuclear accumulation of CREB and TORC2 was measured by Western blot in 4B cells stimulated with forskolin in the presence or absence of 100 nM corticosterone. In basal conditions, pCREB levels were undetectable in the cytoplasm (not shown) and very low in the nucleus (Figure 6). Incubation of the cells with forskolin resulted in a marked increase (31.7 ± 7.9-fold) in nuclear pCREB levels (P < .001, n = 3). pCREB levels remained barely detectable in the cytoplasm (not shown). Exposure to corticosterone either for 30 minutes or 18 hours before forskolin addition had no significant effect on forskolin-induced pCREB accumulation in the nucleus (Figure 6). Two-way ANOVA showed a strong effect of forskolin increasing nuclear pCREB (F = 18.7; P < .002) and no effect of corticosterone (F = 0.; P > .05).
Figure 6.
Effect of corticosterone on nuclear accumulation of pCREB in 4B cells. Cells were exposed to 100 nM corticosterone for 30 minutes (0.5 hour) or 18 hours before treatment with forskolin (Fsk) for 30 additional minutes before protein extraction for Western blotting. The image is representative of 3 experiments. Bars represent the means and SE of data obtained from 3 experiments. Data are expressed as fold change after normalization for HDAC levels. ***, P < .001 compared with vehicle.
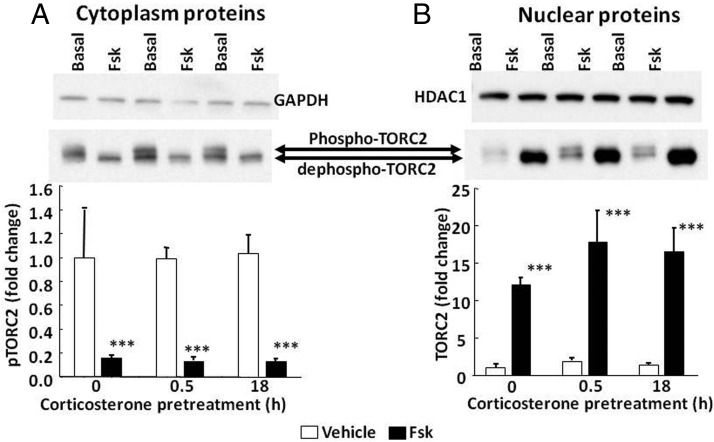
Similarly, 100 nM corticosterone had no effect on nuclear translocation of the CREB coactivator, TORC2. In basal conditions, the typical 2 bands corresponding to the phosphorylated and dephosphorylated forms of TORC2 were observed in the cytoplasm (Figure 7A). After incubation with forskolin for 30 minutes, the upper band (phosphorylated TORC2) decreased to near undetectable levels. Whereas in nuclear proteins the intensity of both TORC2 bands was weak in basal conditions, 30 minutes of incubation with forskolin induced marked increases in the fastest migrating band, corresponding to dephosphorylated TORC2 (P < .001, n = 3) (Figure 7B). Exposure of the cells to 100 nM corticosterone 30 minutes or 18 hours after addition of forskolin had no significant effect on the changes in cytoplasmic or nuclear levels of TORC2 induced by forskolin (Figure 7, A and B). Two-way ANOVA showed a strong effect of forskolin increasing nuclear dephospho-TORC2 (F = 15.1; P < .002) and no effect of corticosterone (F = 0.3; P > .05).
Figure 7.
Effect of corticosterone on nuclear translocation of TORC2 in 4B cells. Cells were exposed to 100 nM corticosterone for 30 minutes (0.5 hour) or 18 hours before treatment with forskolin for 30 additional minutes before protein extraction for Western blotting. Images are representative of 3 experiments. The arrows indicate the 2 TORC2 bands corresponding to phospho-TORC (slower migrating band evident in the cytoplasm in the absence of forskolin) and dephospho-TORC. Bars represent the means and SE of data obtained from 3 experiments. Data are expressed as fold change after normalization for GAPDH in the cytoplasm or HDAC in the nucleus. ***, P < .001 compared with vehicle.
Discussion
Although it is well accepted that a key component of negative glucocorticoid feedback on HPA axis activity is the inhibition of CRH transcription, the molecular mechanisms mediating this inhibition are still unclear (1, 30). Because current knowledge of the molecular mechanisms of repression of CRH transcription by glucocorticoids derive mostly from in vitro studies in heterologous systems, we aimed to study the effect of increases in circulating glucocorticoids within the physiological range on the interaction of the GR with the proximal CRH promoter as well as the activation of transcription factors essential for transcriptional activation. The major findings of this study are that association of GR with the proximal CRH promoter does not appear to be a component of the mechanism for glucocorticoid repression of CRH gene transcription in vivo and further that glucocorticoids do not affect either nuclear accumulation of the CRH transcriptional regulators pCREB and TORC2 or the recruitment of pCREB to the CRH promoter.
Although the proximal CRH promoter does not contain classic paired and palindromic consensus GRE sites, it is generally accepted that GR interacts with the CRH promoter, acting as a transcriptional repressor. GRE half sites have been identified in the CRH promoter and DNase I protection assays in promoter fragments incubated with the recombinant GR DNA-binding domain have revealed several potential GR binding sites (19). Of these putative sites, Malkoski et al (20) identified a highly conserved region between −278 and −249 bp of the CRH promoter, which was shown to be essential for glucocorticoid-dependent repression. Deletion of this negative GRE region decreased both cAMP stimulation and glucocorticoid repression of CRH promoter activity in AtT-20 cells (22). The same region of the CRH promoter in Xenopus laevis was shown to bind GR in gel shift assays (24). However, the role of these putative GREs in transcriptional repression of the CRH gene is unclear. For example, mutagenesis of none of the sites identified by GR DNA-binding domain DNase I protection assays was found to mediate negative glucocorticoid regulation of CRH reporter gene expression in AtT-20 cells (19). Similarly, deletion or mutation of the negative GRE did not affect GR-dependent glucocorticoid suppression of CRH promoter activity in the human neuronal cell line BE(2)C (21).
The effects of glucocorticoids on CRH promoter activity using the wild-type promoter are variable, depending on the cell line and experimental conditions used. In this regard, the inhibitory effect of glucocorticoids on CRH promoter activity in the hypothalamic cell line 4B in the present experiments, using a reporter gene containing the putative negative GRE, and in previous reports using a larger promoter fragment (31) is much smaller than the consistent inhibition reported in the pituitary cell line AtT-20 (19, 20, 32). Because the aim of the present work was to understand the physiological effects of glucocorticoids on endogenous CRH transcription, AtT-20 cells, which do not express CRH, were not used in this study. Although reporter gene assays and in vitro DNA binding assays are useful tools for characterizing the promoter region of a gene, conclusions concerning physiological regulation can be questionable due to the lack of context of the reporter gene with the chromatin landscape. On the other hand, the use of intronic real-time qPCR to measure changes in the primary transcript in primary cultures of hypothalamic neurons provides a useful tool to evaluate the effects of glucocorticoids on endogenous hypothalamic CRH transcription. Nevertheless, similar to the results in 4B cells, corticosterone was a weak inhibitor of cAMP-stimulated endogenous CRH transcription in primary cultures of hypothalamic neurons. This minor effect (statistically significant only after log transformation of the data) was evident solely after a short-term (30-minute) exposure to corticosterone. Previous studies showing decreased expression of CRH mRNA after exposure of hypothalamic neuronal cultures to micromolar concentrations of the synthetic corticosteroid dexamethasone (33) or long-term (24-hour) hypothalamic organotypic cultures to dexamethasone or corticosterone (34) have suggested that glucocorticoids have a direct repressor effect on CRH transcription. However, these long-term effects of glucocorticoids on CRH mRNA levels could be influenced by mRNA degradation and do not necessarily reflect changes in transcription. The lack of significant inhibition of CRH primary transcript production in hypothalamic neurons by physiological concentrations of the natural glucocorticoid, corticosterone, in the present experiments, is against the view that glucocorticoids directly repress CRH gene transcription. Although not assessed in primary neuronal cultures, the concentrations of corticosterone used were highly effective in causing marked GR translocation to the nucleus both at 30 minutes and 18 hours of exposure in 4B cells.
An additional approach to address this problem was to examine the interaction of the GR with the endogenous CRH promoter after in vivo changes in circulating glucocorticoids. In contrast to the DNA-GR interactions observed in gel shift and DNase I protection assays on CRH promoter fragments, in the present study using chromatin immunoprecipitation, ex vivo, there was no increase in recruitment of GR by the endogenous proximal CRH promoter after increases in circulating corticosterone in any of the experimental conditions used (adrenalectomized and intact rats and physiological and supraphysiological doses of glucocorticoids). These ChIP experiments were performed at time points showing that the marked elevations in circulating corticosterone and CRH hnRNA observed during stress had already declined. Because it can be assumed that occupancy of the CRH negative GRE by the GR precedes transcriptional repression, it is not likely that the examination of additional time points beyond those in the current study would have shown association of the GR with the CRH promoter. In addition, genome-wide studies show that GR interaction with chromatin is very rapid with sites being occupied by 1 hour (35). The lack of increased CRH promoter binding was consistently observed despite use of a cocktail of GR antibodies designed to recognize different epitopes of the GR. The immunoprecipitation of chromatin was clearly effective as seen by marked increases in the Per1 promoter, a recognized GR-regulated gene (36), after stress in intact rats or glucocorticoid administration in intact or adrenalectomized rats. Consistent with a previous report in prefrontal cortex chromatin (37), immunoprecipitation with the GR antibody yielded detectable CRH promoter compared with that for the negative controls. However, the significance of this in vivo association of GR to the CRH promoter in the absence of data showing changes after increased circulating glucocorticoids is unclear. Recent in vitro reports show increases in GR recruitment by the endogenous or transiently transfected CRH promoter in 4B cells after administration of the synthetic corticosteroid dexamethasone (38, 39). However, current passages of these cells in our laboratory do not exhibit regulated endogenous CRH expression (26, 40). Although the effects of dexamethasone on GR binding to the CRH promoter in 4B cells may reflect a mechanism for regulation of CRH expression in some physiological or pathological conditions, examination of the interaction of the GR with the endogenous CRH promoter in vivo was a primary goal of the current study, and, therefore, we did not assess the effects of corticosterone on GR recruitment by the CRH promoter in this cell line. The 112-bp PCR product amplified by the CRH promoter primers used in the present study included both the CRE and negative GRE, and thus the lack of an increase of this fragment after GR immunoprecipitation suggests that direct interaction of the GR with the chromatin at this site does not mediate repression of CRH transcription by glucocorticoids. Because chromatin fragmentation before immunoprecipitation was programmed to yield 500- to 1000-bp fragments and our primer sets covered up to −1800, it is not likely that there are active in vivo GR binding sites within 2000 bp upstream of the transcription start site.
On the other hand, based on the observation that an 18-bp fragment containing the CRE was sufficient for regulation by both cAMP and glucocorticoids and the inability of mutagenesis of the GRE-like sites in the proximal CRH promoter to alter glucocorticoid repression, Guardiola-Diaz et al (19) suggested that transcriptional repression by glucocorticoids involves protein-protein interactions of the GR with a transcriptional complex binding the CRE. This possibility cannot be ruled out in the present immunoprecipitation experiments, because a short-arm cross-linker, formaldehyde, was used to stabilize GR-DNA interactions before sonication and immunoprecipitation. It is also possible that the GR interacts with sites upstream of the 2000 bp scanned in the present experiments, resulting in changes in chromatin configuration and interaction with the CRE region in the proximal promoter. This kind of interaction of the GR with distant elements has been described for a number of genes, including 54-kDa progesterone receptor-associated immunophilin (FKBP5), Lipocalin2 (Lcn2), and others (41–43).
In addition, it is likely that indirect mechanisms play a role in glucocorticoid-induced repression of CRH expression observed in vivo. This could involve alteration of signaling pathways and/or the activation of transcription factors essential for CRH transcription. Consistent with previous reports and the recognized importance of CREB in the activation of CRH transcription, activation of neural circuitry by restraint stress induced CREB recruitment by the CRH promoter irrespective of the presence of glucocorticoids (intact and adrenalectomized rats). Although it has been reported that glucocorticoid administration abolishes stress-induced increases in pCREB immunoreactivity in the PVN (44), this effect could reflect inhibition of afferent pathways to the PVN, rather than direct effects on the CRH neuron. The present demonstration that glucocorticoids do not inhibit nuclear accumulation of pCREB is consistent with previous studies (31, 33) and suggests that glucocorticoids do not interfere with CREB phosphorylation. Most importantly, the similar basal and stress-stimulated pCREB recruitment by the CRH promoter observed in intact and adrenalectomized rats shown in this study indicates that glucocorticoids do not impede CREB access to the CRE in the CRH promoter.
We have previously shown that CREB is essential but not sufficient for activation of CRH transcription, which also requires the coactivator TORC, also known as CREB-regulated transcription coactivator (CRTC) (29, 45). There are 3 TORC isoforms (46), all of which are present in CRH neurons (47), but TORC2 appears to be the most important (29). Glucocorticoids up-regulate mRNA levels of salt-inducible kinase 1 (SIK1), the enzyme responsible for TORC inactivation via phosphorylation of Ser171 (48, 49). Therefore, it is conceivable that glucocorticoids prevent TORC activation and nuclear translocation by increasing SIK1 activity. However, in this study incubation of hypothalamic cells with corticosterone had no effect on TORC2 translocation to the nucleus, suggesting that glucocorticoids do not affect TORC2 activation directly in the cell. On the other hand, mice with GR-targeted deletion in the PVN have decreased cytoplasmic phosphorylated TORC in PVN CRH neurons (33). The same study shows a reduction in nuclear TORC2 6 hours after administration of high doses of dexamethasone (10 mg/kg) in wild-type mice but not in PVN GR knockouts. A number of possibilities, including lack of specificity of the Sim1-Cre mouse for CRH neurons, long-term GR knockdown, the use of supraphysiological doses of a synthetic glucocorticoid, and species differences could explain these disparate results. Additional factors are known to positively regulate CRH expression in the PVN, including biogenic amines, cytokines, and the protein kinase A and C pathways (for review, see Ref. 50), and these factors may be targets for negative regulation by glucocorticoids. Protein kinase A (PKA) has been implicated as a target for glucocorticoid regulation of CRH based on known protein-protein interactions between GR and PKA (51) and the inability of dexamethasone to inhibit CRH expression induced by overexpression of the PKA catalytic subunit (21). Kageyama and Suda (31), on the other hand, have shown that dexamethasone increased rather than decreased cAMP production and PKA activity in 4B cells. Based on the present experiments in vivo, it is not likely that activated GR significantly suppressed the cAMP/PKA pathway, because we observed no effects on the recruitment of pCREB to the CRH promoter.
An additional mechanism for glucocorticoid inhibition of CRH transcription could involve afferent pathways to the PVN. Against this possibility are reports showing a failure of glucocorticoids to block stress-induced c-fos activation in the PVN (52, 53), although others have shown opposite findings (54), and there is evidence supporting the modulatory effects of glucocorticoids at sites distal from the CRH neuron. For example, microdialysis studies have shown that glucocorticoids inhibit stress-induced norepinephrine release from the PVN (55). More recently, toxin-induced lesions of catecholaminergic innervation of the PVN were shown to abolish the inhibitory effect of corticosterone on CRH expression (56). Modulation of catecholaminergic receptors could also contribute to glucocorticoid inhibition of CRH neuron function, as suggested by the converse effects of adrenalectomy and glucocorticoid administration, with respective increases and decreases of α-adrenergic receptor content in the PVN (57). In addition, in vitro studies have shown switching in adrenergic receptor α1-α2 in hypothalamic sections after incubation with glucocorticoids (58). Additional evidence for extra-PVN sites for feedback is provided by experiments in mice bearing deletion of GR in the limbic forebrain but preserved GR in the PVN (59). No data on the effect of glucocorticoids on hypothalamic CRH transcription are available in these mice, but they have delayed inhibition of HPA axis responses to psychogenic stressors and deficient suppression of the increases in circulating corticosterone by dexamethasone (60).
The current study indicates that negative glucocorticoid feedback on CRH gene expression is mediated neither via direct interaction between GR and DNA response elements in the CRH proximal promoter nor by interfering with the activation of transcriptional proteins essential for CRH transcription, such as pCREB and TORC2. Although GR interaction at distal promoter sites or with other transcriptional proteins may play a role in the minor inhibition of CRH transcription observed in vitro, the data suggest that the repressor effects of glucocorticoids on CRH expression in vivo are predominantly indirect, through modulation of pathways regulating CRH neuron function.
Acknowledgments
This work was supported by the Intramural Research Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Current address for A.N.E.: University of Southern Mississippi, Gulf Coast Research Laboratory, 703 East Beach Drive, Ocean Springs, Mississippi 39564.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- chromatin immunoprecipitation
- CRE
- cAMP responsive element
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid response element
- HBC
- 2-hydroxypropyl-β-cyclodextrin
- HDAC1
- histone deacetylase 1
- hn
- heteronuclear
- HPA
- hypothalamic pituitary adrenal
- pCREB
- phospho-CREB
- Per1
- period 1
- PKA
- protein kinase A
- PVN
- periventricular nucleus
- q
- quantitative
- TORC2
- transducer of regulated CREB activity 2.
References
- 1. Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33:67–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7:351–378 [DOI] [PubMed] [Google Scholar]
- 3. Claes S. Corticotropin-releasing hormone (CRH) in psychiatry: from stress to psychopathology. Ann Med (Helsinki). 2004;36:50–61 [DOI] [PubMed] [Google Scholar]
- 4. McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179 [DOI] [PubMed] [Google Scholar]
- 5. Munck A, Náray-Fejes-Tóth A. Glucocorticoids and stress: permissive and suppressive actions. Ann NY Acad Sci. 1994;746:115–130; discussion 131–133 [DOI] [PubMed] [Google Scholar]
- 6. Dallman MF. Fast glucocorticoid actions on brain: back to the future. Front Neuroendocrinol. 2005;26:103–108 [DOI] [PubMed] [Google Scholar]
- 7. Myers B, McKlveen J, Herman J. Neural regulation of the stress response: the many faces of feedback. Cell Mol Neurobiol. 2012;32:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dallman MF, Akana SF, Levin N, et al. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann NY Acad Sci. 1994;746:22–28; discussion 32–32, 64–67 [DOI] [PubMed] [Google Scholar]
- 9. Lightman SL, Young WS., 3rd Corticotrophin-releasing factor, vasopressin and pro-opiomelanocortin mRNA responses to stress and opiates in the rat. J Physiol. 1988;403:511–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma XM, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140:5642–5650 [DOI] [PubMed] [Google Scholar]
- 11. Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309 [DOI] [PubMed] [Google Scholar]
- 12. Aguilera G, Kiss A, Liu Y, Kamitakahara A. Negative regulation of corticotropin releasing factor expression and limitation of stress response. Stress. 2007;10:153–161 [DOI] [PubMed] [Google Scholar]
- 13. Harbuz MS, Lightman SL. Glucocorticoid inhibition of stress-induced changes in hypothalamic corticotrophin-releasing factor messenger RNA and proenkephalin A messenger RNA. Neuropeptides. 1989;14:17–20 [DOI] [PubMed] [Google Scholar]
- 14. Kovács KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–210 [DOI] [PubMed] [Google Scholar]
- 15. Sawchenko PE. Evidence for a local site of action for glucocorticoids in inhibiting CRF and vasopressin expression in the paraventricular nucleus. Brain Res. 1987;403:213–223 [DOI] [PubMed] [Google Scholar]
- 16. Kretz O, Reichardt HM, Schütz G, Bock R. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999;818:488–491 [DOI] [PubMed] [Google Scholar]
- 17. Fenoglio KA, Brunson KL, Avishai-Eliner S, Chen Y, Baram TZ. Region-specific onset of handling-induced changes in corticotropin-releasing factor and glucocorticoid receptor expression. Endocrinology. 2004;145:2702–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liposits Z, Uht RM, Harrison RW, Gibbs FP, Paull WK, Bohn MC. Ultrastructural localization of glucocorticoid receptor (GR) in hypothalamic paraventricular neurons synthesizing corticotropin releasing factor (CRF). Histochemistry. 1987;87:407–412 [DOI] [PubMed] [Google Scholar]
- 19. Guardiola-Diaz HM, Kolinske JS, Gates LH, Seasholtz AF. Negative glucorticoid regulation of cyclic adenosine 3′, 5′-monophosphate-stimulated corticotropin-releasing hormone-reporter expression in AtT-20 cells. Mol Endocrinol. 1996;10:317–329 [DOI] [PubMed] [Google Scholar]
- 20. Malkoski SP, Handanos CM, Dorin RI. Localization of a negative glucocorticoid response element of the human corticotropin releasing hormone gene. Mol Cell Endocrinol. 1997;127:189–199 [DOI] [PubMed] [Google Scholar]
- 21. Yamamori E, Iwasaki Y, Taguchi T, et al. Molecular mechanisms for corticotropin-releasing hormone gene repression by glucocorticoid in BE(2)C neuronal cell line. Mol Cell Endocrinol. 2007;264:142–148 [DOI] [PubMed] [Google Scholar]
- 22. Malkoski SP, Dorin RI. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol Endocrinol. 1999;13:1629–1644 [DOI] [PubMed] [Google Scholar]
- 23. Van LP, Spengler DH, Holsboer F. Glucocorticoid repression of 3′,5′-cyclic-adenosine monophosphate-dependent human corticotropin-releasing-hormone gene promoter activity in a transfected mouse anterior pituitary cell line. Endocrinology. 1990;127:1412–1418 [DOI] [PubMed] [Google Scholar]
- 24. Yao M, Schulkin J, Denver RJ. Evolutionarily conserved glucocorticoid regulation of corticotropin-releasing factor expression. Endocrinology. 2008;149:2352–2360 [DOI] [PubMed] [Google Scholar]
- 25. Nicholson RC, King BR, Smith R. Complex regulatory interactions control CRH gene expression. Front Biosci. 2004;9:32–39 [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Kamitakahara A, Kim AJ, Aguilera G. Cyclic adenosine 3′,5′-monophosphate responsive element binding protein phosphorylation is required but not sufficient for activation of corticotropin-releasing hormone transcription. Endocrinology. 2008;149:3512–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kageyama K, Suda T. Transcriptional regulation of hypothalamic corticotropin-releasing factor gene. Vitam Horm. 2010;82:301–317 [DOI] [PubMed] [Google Scholar]
- 28. Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G. Cyclic adenosine 3′-5′-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 Cells and in a transformed hypothalamic cell line. Endocrinology. 2003;144:1292–1300 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y, Coello AG, Grinevich V, Aguilera G. Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription. Endocrinology. 2010;151:1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130 [DOI] [PubMed] [Google Scholar]
- 31. Kageyama K, Akimoto K, Suda T. Corticotrophin-releasing factor gene transcription is directly activated after deprivation of glucocorticoids in hypothalamic cells. J Neuroendocrinol. 2010;22:971–978 [DOI] [PubMed] [Google Scholar]
- 32. Adler GK, Smas CM, Majzoub JA. Expression and dexamethasone regulation of the human corticotropin-releasing hormone gene in a mouse anterior pituitary cell line. J Biol Chem. 1988;263:5846–5852 [PubMed] [Google Scholar]
- 33. Jeanneteau FD, Lambert WM, Ismaili N, et al. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA. 2012;109:1305–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bali B, Ferenczi S, Kovács KJ. Direct inhibitory effect of glucocorticoids on corticotrophin-releasing hormone gene expression in neurones of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neuroendocrinol. 2008;20:1045–1051 [DOI] [PubMed] [Google Scholar]
- 35. Grøntved L, John S, Baek S, et al. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J. 2013;32:1568–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol. 2010;22:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meng QY, Chen XN, Tong DL, Zhou JN. Stress and glucocorticoids regulated corticotropin releasing factor in rat prefrontal cortex. Mol Cell Endocrinol. 2011;342:54–63 [DOI] [PubMed] [Google Scholar]
- 38. Przybycien-Szymanska MM, Mott NN, Pak TR. Alcohol dysregulates corticotropin-releasing-hormone (CRH) promoter activity by interfering with the negative glucocorticoid response element (nGRE). PLoS One. 2011;6:e26647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sharma D, Bhave S, Gregg E, Uht R. Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol Endocrinol. 2013;27:1142–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y, Kalintchenko N, Sassone-Corsi P, Aguilera G. Inhibition of corticotrophin-releasing hormone transcription by inducible cAMP-early repressor in the hypothalamic cell line, 4B. J Neuroendocrinol. 2006;18:42–49 [DOI] [PubMed] [Google Scholar]
- 41. Hakim O, John S, Ling JQ, Biddie SC, Hoffman AR, Hager GL. Glucocorticoid receptor activation of the Ciz1-Lcn2 locus by long range interactions. J Biol Chem. 2009;284:6048–6052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paakinaho V, Makkonen H, Jääskeläinen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol Endocrinol. 2010;24:511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. So A, Chaivorapol C, Bolton E, Li H, Yamamoto K. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLOS Genet. 2007;3:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Légárdi G, Holzer D, Kapcala LP, Lechan RM. Glucocorticoids inhibit stress-induced phosphorylation of CREB in corticotropin-releasing hormone neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1997;66:86–97 [DOI] [PubMed] [Google Scholar]
- 45. Liu Y, Knobloch HS, Grinevich V, Aguilera G. Stress induces parallel changes in corticotrophin-releasing hormone (CRH) transcription and nuclear translocation of transducer of regulated cAMP response element-binding activity 2 in hypothalamic CRH neurones. J Neuroendocrinol. 2011;23:216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Conkright MD, Canettieri G, Screaton R, et al. TORCs: transducers of regulated cREB activity. Mol Cell. 2003;12:413–423 [DOI] [PubMed] [Google Scholar]
- 47. Watts AG, Sanchez-Watts G, Liu Y, Aguilera G. The distribution of messenger RNAs encoding the three isoforms of the transducer of regulated cAMP responsive element binding protein activity in the rat forebrain. J Neuroendocrinol. 2011;23:754–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu Y, Poon V, Sanchez-Watts G, Watts AG, Takemori H, Aguilera G. Salt-inducible kinase is involved in the regulation of corticotropin-releasing hormone transcription in hypothalamic neurons in rats. Endocrinology. 2012;153:223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takemori H, Okamoto M. Regulation of CREB-mediated gene expression by salt inducible kinase. J Steroid Biochem Mol Biol. 2008;108:287–291 [DOI] [PubMed] [Google Scholar]
- 50. Kasckow J, Mulchahey JJ, Aguilera G, et al. Corticotropin-releasing hormone (CRH) expression and protein kinase A mediated CRH receptor signalling in an immortalized hypothalamic cell line. J Neuroendocrinol. 2003;15:521–529 [DOI] [PubMed] [Google Scholar]
- 51. Doucas V, Shi Y, Miyamoto S, West A, Verma I, Evans RM. Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-κB and the glucocorticoid receptor. Proc Natl Acad Sci USA. 2000;97:11893–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andrews MH, Wood SA, Windle RJ, Lightman SL, Ingram CD. Acute glucocorticoid administration rapidly suppresses basal and stress-induced hypothalamo-pituitary-adrenal axis activity. Endocrinology. 2012;153:200–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15:1075–1083 [DOI] [PubMed] [Google Scholar]
- 54. Imaki T, Xiao-Quan W, Shibasaki T, et al. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pacak K, Palkovits M, Kvetnansky R, et al. Catecholaminergic inhibition by hypercortisolemia in the paraventricular nucleus of conscious rats. Endocrinology. 1995;136:4814–4819 [DOI] [PubMed] [Google Scholar]
- 56. Kaminski KL, Watts AG. Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J Neuroendocrinol. 2012;24:1517–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Day H, Campeau S, Watson SJ, JR, Akil H. Expression of α1b adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci. 1999;19:10098–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Feuvrier E, Aubert M, Mausset AL, et al. Glucocorticoids provoke a shift from alpha2- to alpha1-adrenoreceptor activities in cultured hypothalamic slices leading to opposite noradrenaline effect on corticotropin-releasing hormone release. J Neurochem. 1998;70:1199–1209 [DOI] [PubMed] [Google Scholar]
- 59. Boyle MP, Brewer JA, Funatsu M, et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA. 2005;102:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain- and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol. 2011;336:2–5 [DOI] [PMC free article] [PubMed] [Google Scholar]