Abstract
Niemann–Pick type C disease (NPC) is a devastating, recessive, inherited disorder that causes accumulation of cholesterol and other lipids in late endosomes and lysosomes. Mutations in 2 genes, NPC1 and NPC2, are responsible for the disease, which affects about 1 in 120,000 live births. About 95 % of patients have mutations in NPC1, a large polytopic membrane protein that is normally found in late endosomes. More than 200 missense mutations in NPC1 have been found in NPC patients. The disease is progressive, typically leading to death before the age of 20 years, although some affected individuals live well into adulthood. The disease affects peripheral organs, including the liver, spleen, and lungs, but the most severe symptoms are associated with neurological disease. There are some palliative treatments that slow progression of NPC disease. Recently, it was found that histone deacetylase (HDAC) inhibitors that are effective against HDACs 1, 2, and 3 can reduce the cholesterol accumulation in fibroblasts derived from NPC patients with mutations in NPC1. One example is vorinostat. As vorinostat is a Food and Drug Administration–approved drug for treatment of cutaneous T-cell lymphoma, this opens up the possibility that HDAC inhibitors could be repurposed for treatment of this rare disease. The mechanism of action of the HDAC inhibitors requires further study, but these drugs increase the level of the NPC1 protein. This may be due to post-translational stabilization of the NPC1 protein, allowing it to be transported out of the endoplasmic reticulum.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0217-2) contains supplementary material, which is available to authorized users.
Keywords: Histone deacetylase inhibitors, Cholesterol, Lysosomes, Lipids, Proteostasis
Niemann–Pick Type C Disease
Incidence and Pathology
Niemann–Pick type C disease (NPC) is an autosomal recessive, inherited lysosomal storage disorder that leads to abnormal accumulation of cholesterol and other lipids in late endosomes and lysosomes (LE/Ly) [1]. The lipid accumulation can be seen in tissues throughout the body, but the major disease-associated pathologies are due to progressive neurological deterioration [1]. NPC is a typical example of a large family of lysosomal storage disorders. Other members of this class of diseases include the closely related Niemann–Pick types A and B, as well as Wolman, Gaucher, Fabry, and Tay–Sachs diseases [2]. First described in the early twentieth century [3, 4] and more thoroughly characterized in the 1950s [5], the molecular processes involved were uncovered beginning with the work of Pentchev and coworkers, starting in the 1980s [6–8].
The incidence of NPC disease has been estimated to be approximately 1:120,000 live births [1]. However, patients are frequently misdiagnosed or undiagnosed for several years after the onset of symptoms, and it seems likely that the incidence is actually substantially higher than this [9–11]. The age of onset can be from infancy to adulthood, but the majority of patients develop symptoms in early childhood, with death frequently occurring before the age of 20 years. The rate of progression of the disease is somewhat variable [9], although some reports indicate that once a threshold of disability is reached there is a fairly uniform rate of progression that is apparently independent of age of onset [12]. A slowly progressing form of the disease has been reported with a very small number of patients as old as 68 years [13].
The health of NPC patients is negatively affected by progressive neurodegeneration and inflammatory events in the brain and visceral organs, with effects on the liver, spleen, and lung being most pronounced. Typical neurological manifestations include vertical supranuclear gaze palsy [14], saccadic eye movement abnormalities, cerebellar ataxia, dystonia, dysmetria, dysphagia, and dysarthria [1]. The central nervous system (CNS) deficits are associated with the presence of meganeurites, ectopic dendrites, and axonal spheroids [15]. There is also extensive neurodegeneration, neuroinflammation, and a patterned loss of cerebellar Purkinje cells [15]. Oropharyngeal dysphagia can be particularly problematic as it can often lead to food or fluid aspiration and subsequent pneumonia.
Molecular and Cellular Characterization of NPC Disease
Two genes (NPC1 and NPC2) have been linked to NPC disease in humans [1]. Approximately 95 % of cases are associated with mutations in NPC1. The NPC1 protein is a large membrane protein that is localized in the LE/Ly and is predicted to have 13 transmembrane domains. Most of the NPC1 mutations are missense point mutations, and more than 200 different NPC1 mutations have been found in patients [1]. The NPC2 protein is a small globular protein that is targeted to LE/Ly by a mannose-6-phosphate modification that causes it to bind to mannose-6-phosphate receptors, which direct it to late endosomes [16].
Both NPC1 and NPC2 have been shown to bind cholesterol [16, 17]. In NPC1 there is a well-characterized cholesterol binding site in the N-terminal domain, which faces the lumen of the LE/Ly. X-ray crystallography shows that cholesterol is bound in a pocket in the NPC1 N-terminal domain with the hydroxyl moiety oriented inward away from the surface of the protein [18, 19]. In contrast, cholesterol binds to NPC2 with the hydroxyl oriented outward toward the surface of the protein [20]. It is relatively difficult to directly load the NPC1 termina N-l domain with cholesterol [17], but the rate of loading is greatly increased when cholesterol is first bound to NPC2 and then transferred to NPC1 [18]. This led to a model in which lipoprotein cholesterol esters are hydrolyzed in LE/Ly, and the cholesterol, which is very insoluble in water, binds to NPC2. The NPC2 would then transfer cholesterol to the NPC1 N-terminal domain in the limiting membrane of the LE/Ly [19]. By a process that is yet to be characterized, cholesterol can then exit the LE/Ly and be delivered to other cell membranes. This model of a cholesterol hand-off is strongly supported by in vitro studies showing that NPC2:cholesterol binds to the first lumenal loop of the NPC1 protein, while apo-NPC2 does not bind [21]. Additionally, some NPC1 disease-associated missense mutations found in the first lumenal loop abrogate the binding of NPC2:cholesterol to NPC1 [21], which prevents the efficient transfer of cholesterol from NPC2 to NPC1. These data support a directional transfer of cholesterol from NPC2 to NPC1 in the lumen of the LE/Ly.
The biochemical studies of NPC1 and NPC2 strongly support the hypothesis that defects in cholesterol transport are the underlying mechanism responsible for NPC disease. However, several other lipids accumulate in the lysosomal storage organelles found in NPC mutant cells, including sphingomyelin and bis(monoacylglycero)phosphate, which is also known as lysobisphosphatidic acid [2, 22]. In the CNS the most prominent stored lipids are glycosphingolipids, but cholesterol accumulation is also seen [2].
It has been proposed that the secondary accumulation of sphingolipids may relate to the biophysical properties of cholesterol and sphingolipids as they associate in lipid bilayers. Cholesterol is stabilized in lipid bilayers by the presence of sphingolipids either because of specific interactions or because the structures of the sphingolipids with their large head groups and saturated acyl chains effectively shield the cholesterol from the aqueous phase [23]. As a consequence of these interactions, accumulation of cholesterol may lead to an enrichment of sphingolipids in the internal membranes of lysosomal storage organelles. Similarly, high levels of sphingolipids would be predicted to stabilize the cholesterol in these membranes. Consistent with this model, augmenting the lysosomal hydrolysis of sphingolipids by increasing acid sphingomyelinase levels in NPC mutant fibroblasts leads to a decrease in the cholesterol storage [24]. It is generally unclear if the cellular pathology associated with NPC disease is caused by the accumulation of the cholesterol itself or by the dysregulation of other lipids, including sphingolipids. Miglustat, a drug that inhibits an early step in glycosphingolipid synthesis and is approved for use in NPC patients in several countries, has been found to provide some benefit to NPC patients [25].
Studies on the NPC1I1061T mutant, the most common missense mutation observed in NPC1 patients that represents 15–20 % of all disease alleles, provided insights into the molecular mechanisms of NPC [26]. In NPC patient-derived fibroblasts, the NPC1I1061T protein is synthesized, but is mislocalized and present at reduced levels. In wild-type fibroblasts, NPC1 protein predominantly distributes to the late endosomal compartment, while in human fibroblasts homozygous for the NPC1I1061T the mutant protein fails to localize to the late endosomes. Even though NPC1 mRNA transcript levels are elevated 1.4–2.4-fold in NPC1I1061Tvs WT fibroblasts, NPC1 protein levels are reduced by 85 % in the NPC1I1061T fibroblasts. Thus, the NPC1I1061T substitution affects steady-state levels of endogenously expressed NPC1 protein, possibly by impairing translation of the NPC1 protein or by rendering the protein unstable. Metabolic labeling studies demonstrate that the wild-type protein has 2 distinct rates of degradation [26]. About half of the protein exhibited a t½ of 9 h, after which the remaining protein exhibits a glycosylation pattern shift from an endoglycosidase H-sensitive to an endoglycosidase H-resistant species, extending the t½ to 42 h. In contrast, NPC1I1061T protein remains almost exclusively endoglycosidase H-sensitive and exhibits a t½ of 6.5 h. These data indicate that about half of the wild-type NPC1 and nearly all of the NPC1I1061T protein is degraded in the endoplasmic reticulum (ER). Overexpression of NPC1I1061T or treatment with general protein stabilizers, such as glycerol, led to partial correction of the NPC phenotype [26].
An interesting observation is that siblings with identical mutations in NPC1 can have very different ages of onset and rates of progression of the disease. This observation suggests that factors outside of the NPC1 protein itself can profoundly affect the disease progression, and therefore changes in other genes or epigenetic changes might alter the susceptibility to mutations in NPC1 or NPC2. It also opens the possibility that drugs might be used to alter the sensitivity to these mutations. As discussed in the next section, histone deacetylase inhibitors (HDACi) appear to be good candidates for therapy of most NPC1 patients. Preliminary findings suggest that this role may be due to an increase in the fraction of mutant NPC1 proteins that leave the ER and are delivered to LE/Ly.
HDACi and Treatment of NPC Disease
Therapies Tested Previously for Treatment of NPC Disease
Over the last few decades, several small molecule agents have been investigated as potential treatments for NPC. These studies have been performed mainly in cell culture and mouse models, and, to a lesser extent, in human patients, as summarized in previous reviews [1, 27]. The choices of many of these agents have been based upon previously known effects of these compounds on sterol absorption, biosynthesis, and metabolism, or, alternatively, upon agents involved in sphingolipid pathways. These studies have largely had limited effect on the NPC disease phenotype. The compounds that have been examined include statin drugs (lovastatin and pravastatin) [28–30], a squalene synthesis inhibitor (CP-340868) [31], cholestyramine [28, 29], nicotinic acid [28], ezetimibe (Zetia) [32], peroxisomal inducers (clofibrate, perfluorooctanoic acid, dehydroepiandrosterone, and diethylhexylphthalate) [33, 34], neurosteroids or their mimics (allopregnanolone, ganaxolone, and T-0901317) [35, 36], oxysterols (25- and 27-hydroxycholesterol, 7-ketocholesterol, and 17b-estradiol) [17, 37–40], synthetic liver X receptor ligands (T-0901317 and bexarotene) [40–43], sphingolipid pathway-targeting agents (miglustat and n-butyldeoxygalactonojirimycin) [44–46], calcium regulators (curcumin, thapsigargin, and myriocin; note that curcumin is also an HDACi) [47–49], apoptosis inhibitors (imatinib, minocycline, and B-cell lymphoma 2 protein) [50, 51], a neurodegeneration inhibitor (Nω-nitro-L-arginine methyl ester) [52], α-tocopherol (vitamin E) [53, 54], tamoxifen [53], nitrovin (difurazone) [55], and several members of 3 heterocycle series consisting of highly substituted pyrrolinones, triazines, and thiadiazoles [56–59]. Low cholesterol diets have been found to be ineffective [60].
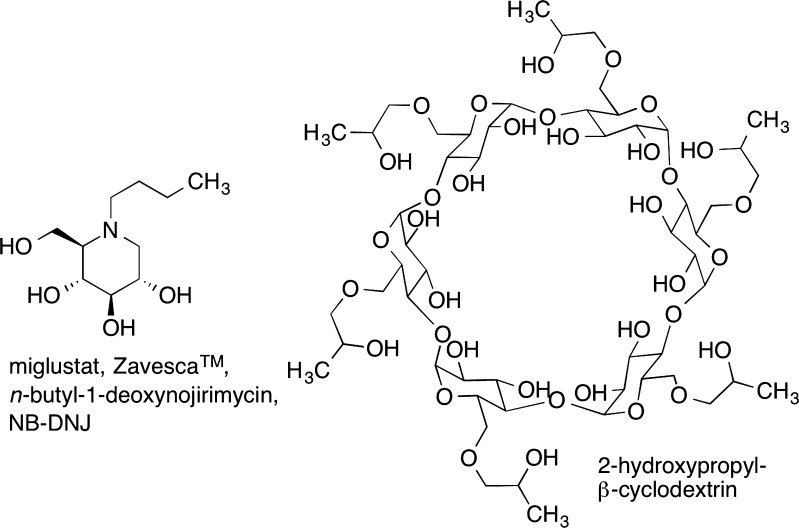
With respect to the current state of available therapies, miglustat (Zavesca; Fig. 1) was approved in 2009 for treatment of NPC in the European Union and, subsequently, in Canada, Brazil, Turkey, Australia, and Japan [44, 61]. Although it has been reviewed by the Food and Drug Administration (FDA), it has not been approved for NPC in the USA. Miglustat functions as a glucosylceramide synthase inhibitor and therefore affects the glycosphingolipid pathway. It had previously been approved by the FDA for treatment of Gaucher disease, which is another lysosomal storage disorder characterized by accumulation of glucosylceramide [62].The past several years of experience with miglustat indicate that it is able to stabilize NPC patients and is likely to extend their life spans [44, 61].
Fig. 1.
Niemann–Pick type C disease therapeutics. Miglustat (left) inhibits the synthesis of glycosphingolipids, which accumulate along with cholesterol in late endosomes and lysosomes of neurons. 2-Hydroxypropyl-β-cyclodextrin (right) binds cholesterol and solubilizes it. This can lead to efflux of stored cholesterol from late endosomes and lysosomes. NB-DNJ = n-butyldeoxygalactonojirimycin
The most effective treatment for NPC disease in animal models is injection of 2-hydroxypropyl-β-cyclodextrin (HPBCD) (Fig. 1), a cholesterol chelating cyclodextrin [63, 64]. Unfortunately, HPBCD is excreted rapidly and crosses the human blood–brain barrier very poorly; therefore, this treatment requires infusion of high-dose HPBCD for several hours a few times per week and intrathecal injections for delivery into the CNS. A clinical trial for HPBCD therapy is currently underway [65]. Significant side effects that have been observed in cat and mouse models of NPC include hearing impairment and lung complications [66].
HDACi and NPC Disease
HDACi were tested as part of a high-throughput screen for compounds that would reduce cholesterol accumulation in LE/Ly of human fibroblasts derived from patients with mutations in NPC1 or NPC2 [67]. A collection of HDACi was specifically included in the screen for several reasons. Cells with the NPC1I1061T mutation could be partially corrected by protein overexpression or by glycerol, which acts as a nonspecific protein chaperone [26]. A basic hypothesis is that up-regulation of mutant, but functional, NPC1 or NPC2 protein or up-regulation of chaperones to overcome protein misfolding may serve to restore normal cholesterol trafficking. By altering chromatin structure, HDACi change the expression of a large number of proteins. HDACi have also been shown to increase production of protein chaperones, which may, in turn, assist transport of the mutant NPC1 protein out of the ER and to avoid protein destruction by ER-associated degradation of misfolded proteins [68]. Preliminary studies of valproic acid supported the hypothesis that HDACi may have a therapeutic effect in NPC disease [69]. Trichostatin A has also been shown to be a repressor of the cholesterol biosynthetic pathway [70], which might provide a secondary benefit. For these reasons, the Maxfield, Wiest, and Helquist laboratories obtained and screened a small set of well-known HDACi representing a range of chemical structural types, potencies, and isoform selectivities. (To avoid duplication, the reader is referred to [71] where the range of HDACi structures, potency, and selectivity are described in greater detail than is the intent here.)
The initial test set consisted of vorinostat (Fig. 2), trichostatin A, panobinostat, PCI-34051, CI-994, and thiophene benzamide (Fig. 3) [67]. The screening was accomplished using filipin staining for fluorescent determination of lysosomal cholesterol in NPC1 mutant GM03123 human fibroblasts carrying heterozygous P237S and I1061T mutations, and GM18443 carrying the homozygous I1061T mutation on both alleles. GM05659 wild-type cells were used as a control. For both of the NPC1 mutants, a dose and time response for lowering of cholesterol levels was observed for 5 of the 6 compounds, with PCI-34051 being the exception.
Fig. 2.
Histone deacetylase (HDAC) inhibitors previously approved by the Food and Drug Administration. The various names used for the compounds are provided. Entries in parentheses indicate the HDAC for which the strongest inhibitory effects are seen.
Fig. 3.
Histone deacetylase (HDAC) inhibitors. Entries in parentheses indicate the HDAC for which the strongest inhibitory effects are seen
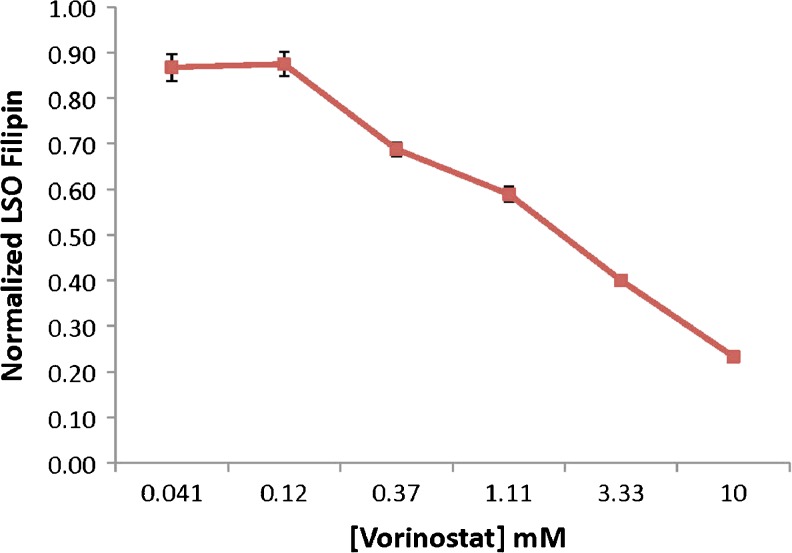
The strongest responses were seen for the most potent HDACi used in this survey, namely panobinostat (LBH-589) and trichostatin A, with EC50 values of <5 nM and ~60 nM, respectively. The dose–response curve for vorinostat is shown in Fig. 4. Essentially, complete correction of the NPC phenotype was seen with 10 μM vorinostat (Fig. 5). Based upon the known isoform selectivities of the HDACi in this survey (see Figs 2 and 3), the relevant target for correction of the NPC1 phenotype is likely to be HDAC1, 2, or 3, whereas HDAC 8 is ruled out by lack of response to PCI-343051. These HDACi were also screened in the NPC2 mutant GM18445 human fibroblast cell line having the homozygous V39M mutation, but there was no significant response. The HDACi examined here did not show cytotoxicity, but they did show cytostatic activity in the NPC1 mutant GM03123 cells.
Fig. 4.
Dose–time response for treatment of GM03123 Niemann–Pick type C disease 1 mutant cells with vorinostat. The vertical axis measures the fluorescence response of filipin staining of cholesterol in lysosomal storage organelles (LSO). Cells were treated for 48 h with varying concentrations of vorinostat. The cells were then fixed and stained with filipin
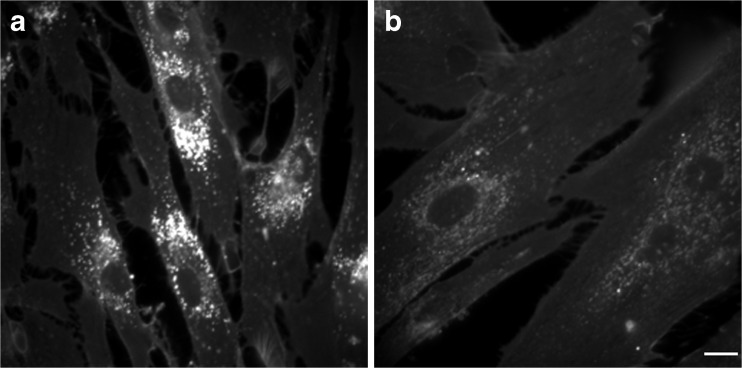
Fig. 5.
Effect of vorinostat on cholesterol storage. Fluorescence microscopy images of filipin-stained GM03123 Niemann–Pick type C disease 1 mutant cells treated with dimethyl sulfoxide vehicle (a) or 10 μM vorinostat (b) for 2 days. Scale bar = 15 μm
With respect to mechanism of action, treatment of the NPC1 mutant cells with the HDACi led to increased production of NPC1 protein in parallel with correction of the NPC phenotype. Also observed was decreased uptake of low-density lipoprotein, reduced proteolytic processing of sterol-responsive element binding protein-2 transcription factor, and increased cholesterol esterification by acyl Co-A:cholesterol acyl transferase—all consistent with restoration of normal cellular cholesterol homeostasis. Studies are in progress to determine if the effect of HDACi treatment is mainly due to protein stabilization in the ER [72]. Ongoing studies in the Maxfield laboratory have shown that many different NPC1 mutations found in several patient-derived fibroblast cell lines respond to HDACi with a dose-dependent reduction in stored cholesterol [72]. Testing is also underway using an engineered cell line expressing many different NPC1 mutations, and preliminary results are encouraging in that a high fraction of NPC1 mutations are responsive (F. Maxfield, unpublished data).
Subsequent studies by Munkacsi et al. [73] confirmed that vorinostat and trichostatin A reduce lysosomal cholesterol levels in human NPC fibroblasts along with lowering sphingolipid accumulation and increasing esterification of free cholesterol, which is deficient in untreated NPC cells. The cholesterol lowering effect was seen to be less pronounced with the more isoform-selective HDACi MC1568 (HDACs 4, 5, 7, 9) and MGCD0103 (HDACs 1, 2, 3, 11) compared with the less selective vorinostat and trichostatin A. Wehrmann et al. [74] have also confirmed the cholesterol lowering effect of vorinostat and panobinostat in human NPC fibroblasts. This effect was enhanced in the presence of HPBCD.
Considerations for Use of HDACi as Therapies for NPC1 Disease
In recent years, a very large number of compounds have been designed and developed specifically as HDACi. The first 2 to receive FDA approval for clinical use were suberoylanilide hydroxamic acid (vorinostat, Zolinza) in 2006 and romidepsin (Istodax, FK-228, FR-901228) in 2009, both for treatment of cutaneous T-cell lymphoma (Fig. 2) [75, 76].
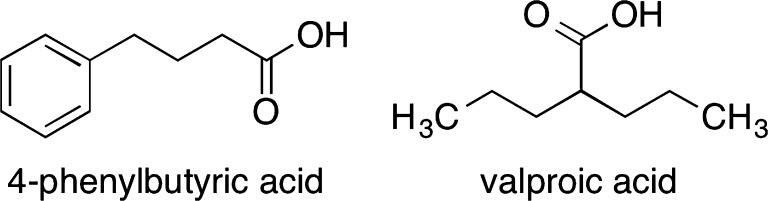
Although HDACi have been developed most prominently for treatment of cancers, their effectiveness in many other diseases has also been investigated [71, 77]. 4-Phenylbutyric acid and valproic acid are among the simplest and also the weakest HDACi (Fig. 6), although their function as HDACi was not recognized until long after early studies of their therapeutic properties. Regardless of its simplicity, the sodium salt of the former was approved by the FDA in 1996 and commercialized as Buphenyl (Ucyclyd Pharma) for the treatment of urea cycle disorders—another group of rare diseases [78, 79]. It was subsequently studied in thalassemia [80], sickle cell anemia [81], and cystic fibrosis [82]. The derivative, glycerol phenylbutyrate (Ravicti), has also been approved for the treatment of urea cycle disorders [83]. The likewise simple valproic acid is used to treat epilepsy, other seizures, and bipolar disorder [84]. Also of relevance in a neurodegenerative disease such as NPC is that HDACi have been studied for treatment of cognitive disorders such as Alzheimer’s, Huntington’s, and Parkinson’s diseases [85]. HDACi are also reported to function as cognitive enhancers [86]. Whereas increased histone acetylation in the brain is associated with improved memory, histone deacetylation correlates with poorer memory [87, 88].
Fig. 6.
Simple, weak histone deacetylase inhibitors with therapeutic applications
For an ultra-rare disease such as NPC, the most reasonable strategy may be to identify a treatment from among previously approved drugs or, at the least, other previously known compounds that have already been through extensive studies, including clinical trials. Developing a new drug de novo would likely encounter enormous costs that could not be recouped from the very small NPC patient population.
Prospective
Although the study of HDACi for treatment of NPC is at a relatively early stage, this therapeutic strategy shows significant promise. In order to accelerate such a treatment into the clinic, the selection of a previously approved drug is a reasonable choice. Of the HDACi screened to date for NPC, vorinostat meets this criterion, whereas romidepsin, another approved HDACi has apparently not been screened yet for this disease. This latter compound is also more chemically complex than vorinostat and may be less accessible.
The advantages of seeking clinical trials of vorinostat are numerous. There has been considerable prior human experience with its use, albeit as a cancer treatment. A search of the MedLine, Web of Science, and Biosis Previews databases shows 81 journal citations beginning with Phase I studies in cancer patients monitoring the pharmacology and pharmacokinetics following intravenous and oral drug administration. The level of acetylated histones in human peripheral blood mononuclear cells (PBMCs) was established as a reliable biomarker of drug action [89, 90]. The acetylation of PBMC histones by vorinostat paralleled its pharmacodynamic effects in tissue. Rapid induction of histone acetylation in PBMCs occurs after intravenous and oral dosing in a time- and concentration-dependent manner [89, 90]. Elevation of acetylated histones was consistently observed 2 h after a single oral dose of vorinostat ranging from 200 to 600 mg. With an increasing vorinostat dose, the duration of the elevated levels of acetylated histones in PBMCs was maintained up to 8 h. In patients who were under study for 6 months or longer, vorinostat was found to continue to stimulate an increase in PBMC acetylated histones, indicative of a sustained pharmacological effect over time [89–91]. The major pathways of vorinostat metabolism involve glucuronidation and hydrolysis followed by α-oxidation [92]. The major metabolites, O-glucuronide and 4-anilino-4-oxobutanoic acids, are both pharmacologically inactive. The clinical pharmacology profile of vorinostat is favorable, exhibiting dose-proportional pharmacokinetics and modest food effect. There appear to be no major differences in the pharmacokinetics of vorinostat in special populations, including varying demographics and hepatic dysfunction. Combination therapy pharmacokinetic data indicate that vorinostat has a low propensity for drug interactions [93].
Until recently, the mouse models for NPC1 deficiency have been NPC1-/-, so they would not be good models for testing a drug that worked by increased expression of a mutant, but somewhat functional, NPC1 protein. Recently, a mouse model with a point mutation in NPC1 (D1005G) has been described [94], and mouse models with other mutations are being developed. These mouse models will provide the capability of testing HDACi therapy in mouse models. In addition, there is a line of cats with a naturally-occurring missense mutation in the feline NPC1 protein [95], and these provide an opportunity for testing in a large mammal. Such animal testing may not be required for regulatory approval of tests of vorinostat in adults, but it would be useful for testing dosing regimens and for testing efficacy in juvenile animals.
In order for vorinostat to be clinically effective for the treatment of NPC, sufficient levels must be achieved in the brain to increase the brain level of acetylated histones leading to pharmacologically correct cholesterol processing and trafficking. The passage of vorinostat across the blood–brain barrier has been observed in laboratory animals using several techniques. An increase of acetylated histones levels in the brain following vorinostat treatment is observed in cancer [96, 97] and noncancer animal models [98, 99]. Following systemic administration, uptake of [14C]vorinostat was significant into normal rodent brain reaching a brain/blood concentration ratio at 30 min that exceeded the brain residual blood volume by 5- to 7-fold [97]. An increase in the level of acetylated histones was measured in biopsies of brain tumor in patients with neuroblastoma treated with vorinostat [100]. Vorinostat is reported to slow neurodegenerative processes in laboratory cellular and animal disease models, indicative of its pharmacological activity in whole animal brain tissue [101].
Inflammation in the brain and visceral organs is also a major pathogenic process in NPC patients that requires therapeutic intervention. The anti-inflammatory activity of vorinostat that has been observed in human cancer patients, and its immunosuppressive properties in multiple laboratory models, may have potential benefits in treated NPC patients. Histone acetylation status has been found to regulate inflammatory gene expression in laboratory models of chronic inflammatory lung diseases and other autoimmune diseases [102].
Since vorinostat was developed as a cancer treatment, most of the safety testing in humans has been in adults, although there have also been trials in pediatric patients. The pediatric studies have, likewise, been for treatment of cancers, including CNS or non-CNS solid tumors, lymphomas, and leukemias [103–106]. Most of the published clinical studies with vorinostat include the tabulation of clinical and laboratory adverse events for patients with recurrent or relapsed carcinoma. For FDA approval of vorinostat to treat cutaneous T-cell lymphoma, the safety and adverse event data came from 73 patients [107]. In a later review of pooled clinical data from 498 patients with solid and hematological malignancies, it was shown that vorinostat was well tolerated as monotherapy or combination therapy [108]. The most commonly reported drug-related adverse events associated with monotherapy were fatigue (61.9 %), nausea (55.7 %), diarrhea (49.3 %), anorexia (48.1 %), and vomiting (32.8 %), and Grade 3/4 drug-related adverse events included fatigue (12.0 %), thrombocytopenia (10.6 %), and dehydration (7.3 %). The clinical use of vorinostat to treat NPC patients will most likely use a cyclic treatment schedule of 3–5 days with intervals of no treatment.
On the whole, the preceding observations are supportive of vorinostat as an epigenetic treatment for NPC, but there are some factors that suggest room for improvement in longer-term drug development efforts. Vorinostat is not nearly as potent as many other HDACi. Its lack of HDAC isoform selectivity likely leads to changes in expression of a large number of genes that have no relevance to NPC. Although there is evidence of some penetration of the blood–brain barrier by vorinostat, other HDACi have measurably superior penetration [109–112].
Much remains to be learned about the function of HDACi in NPC. Which of the several HDAC isoforms is (are) the relevant target for this disease? What is the mechanism by which HDACi correct the NPC phenotype—is it due to up-regulation of NPC protein biosynthesis, a protein chaperone mechanism, or yet additional cellular pathways? Addressing these questions will continue in parallel with medicinal chemists needing to pursue development of a pipeline of other compounds as improved treatments for this disease. Ultimately, the knowledge gained for treatment of NPC will likely translate to other related diseases.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgments
This work was supported by grants from the Ara Parseghian Medical Research Foundation (PH, OW, FRM). FRM also received support from NIH grant R37-DK27083. PH and OW received support from NSF grant Award No. 1058075, NIH grant T32GM075762, Notre Dame Center for Rare and Neglected Diseases, University of Notre Dame, and Circagen. PH received support from the Charles Edison Foundation, Research Council of Sweden, and AstraZeneca. Full conflict of interest disclosures are available in the electronic supplementary material for this article.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis. 2010;5:16. doi: 10.1186/1750-1172-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkley SU, Vanier MT. Secondary lipid accumulation in lysosomal disease. Biochim Biophys Acta. 2009;1793:726–36. doi: 10.1016/j.bbamcr.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niemann A. Ein unbekanntes Krankheitsbild. Jahrbuch für Kinderheilkunde. 1914;79:1–10. [Google Scholar]
- 4.Der PL. Morbus Gaucher und die ihm ähnlichen Krankheiten (die lipoidzellige Splenohepatomegalie Typus Niemann und die diabetische Lipoidzellenhypoplasie der Milz. Ergebnisse der Inneren Medizin und Kinderheilkunde, Berlin. 1926;29:519–627. [Google Scholar]
- 5.Crocker AC, Farber S. Niemann–Pick disease: a review of eighteen patients. Medicine. 1958;37:1–95. doi: 10.1097/00005792-195802000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kruth HS, Comly M, Butler JD, et al. Type C Niemann–Pick disease. Abnormal metabolism of low density lipoprotein in homozygous and heterozygous fibroblasts. J Biol Chem. 1986;261:9290–8. [PubMed] [Google Scholar]
- 7.Pentchev PG, Kruth HS, Comly ME, et al. Type C Niemann–Pick disease. A parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J Biol Chem. 1986;261:16775–80. [PubMed] [Google Scholar]
- 8.Carstea ED, Morris JA, Coleman KG, et al. Niemann–Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–31. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 9.Stampfer M, Theiss S, Amraoui Y, et al. Niemann–Pick disease type C clinical database: cognitive and coordination deficits are early disease indicators. Orphanet J Rare Dis. 2013;8:35. doi: 10.1186/1750-1172-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluenemann H-H, Santosh PJ, Sedel F. Treatable metabolic psychoses that go undetected: What Niemann–Pick type C can teach us. Int J Psych Clin Pract. 2010;16:162–9. doi: 10.3109/13651501.2012.687451. [DOI] [PubMed] [Google Scholar]
- 11.Bauer P, Balding DJ, Klunemann HH, et al. Genetic screening for Niemann–Pick disease type C in adults with neurological and psychiatric symptoms: findings from the ZOOM study. Hum Mol Genet 2013 Jun 26. [DOI] [PMC free article] [PubMed]
- 12.Yanjanin NM, Velez JI, Gropman A, et al. Linear clinical progression, independent of age of onset, in Niemann–Pick disease, type C. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):132–40. doi: 10.1002/ajmg.b.30969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trendelenburg G, Vanier MT, Maza S, et al. Niemann–Pick type C disease in a 68-year-old patient. J Neurol Neurosurg Psychiatry. 2006;77:997–8. doi: 10.1136/jnnp.2005.086785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salsano E, Umeh C, Rufa A, Pareyson D, Zee DS. Vertical supranuclear gaze palsy in Niemann–Pick type C disease. Neur Sci. 2012;33:1225–32. doi: 10.1007/s10072-012-1155-1. [DOI] [PubMed] [Google Scholar]
- 15.Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Liou HL, Dixit SS, Xu S, Tint GS, Stock AM, Lobel P. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem 2006;281:36710–23. [DOI] [PubMed]
- 17.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–63. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 18.Kwon HJ, Abi-Mosleh L, Wang ML, et al. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–24. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ML, Motamed M, Infante RE, et al. Identification of surface residues on Niemann–Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12:166–73. doi: 10.1016/j.cmet.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann–Pick type C2 disease. J Biol Chem. 2007;282:23525–31. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deffieu MS, Pfeffer SR. Niemann–Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc Natl Acad Sci U S A. 2011;108:18932–6. doi: 10.1073/pnas.1110439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Beuchat MH, Lindsay M, et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–8. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 23.Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–45. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devlin C, Pipalia NH, Liao X, Schuchman EH, Maxfield FR, Tabas I. Improvement in lipid and protein trafficking in Niemann–Pick C1 cells by correction of a secondary enzyme defect. Traffic. 2011;11:601–15. doi: 10.1111/j.1600-0854.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wraith JE, Vecchio D, Jacklin E, et al. Miglustat in adult and juvenile patients with Niemann–Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 2010;99:351–7. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Gelsthorpe ME, Baumann N, Millard E, et al. Niemann–Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J Biol Chem. 2008;283:8229–36. doi: 10.1074/jbc.M708735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helquist P, Wiest O. Current status of drug therapy development for Niemann–Pick type C disease. Drugs Future. 2009;34:315–31. [Google Scholar]
- 28.Patterson MC, Di Bisceglie AM, Higgins JJ, et al. The effect of cholesterol-lowering agents on hepatic and plasma cholesterol in Niemann–Pick disease type C. Neurology. 1993;43:61–4. doi: 10.1212/wnl.43.1_part_1.61. [DOI] [PubMed] [Google Scholar]
- 29.Sylvain M, Arnold DL, Scriver CR, Schreiber R, Shevell MI. Magnetic resonance spectroscopy in Niemann–Pick disease type C: correlation with diagnosis and clinical response to cholestyramine and lovastatin. Pediatr Neurol. 1994;10:228–32. doi: 10.1016/0887-8994(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 30.Cheung NS, Koh CH, Bay BH, et al. Chronic exposure to U18666A induces apoptosis in cultured murine cortical neurons. Biochem Biophys Res Commun. 2004;315:408–17. doi: 10.1016/j.bbrc.2004.01.066. [DOI] [PubMed] [Google Scholar]
- 31.Reid PC, Lin S, Vanier MT, et al. Partial blockage of sterol biosynthesis with a squalene synthase inhibitor in early postnatal Niemann–Pick type C npcnih null mice brains reduces neuronal cholesterol accumulation, abrogates astrogliosis, but may inhibit myelin maturation. J Neurosci Methods. 2008;168:15–25. doi: 10.1016/j.jneumeth.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beltroy EP, Liu B, Dietschy JM, Turley SD. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann–Pick type C disease. J Lipid Res. 2007;48:869–81. doi: 10.1194/jlr.M600488-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Schedin S, Pentchev P, Dallner G. Reduced cholesterol accumulation and improved deficient peroxisomal functions in a murine model of Niemann–Pick type C disease upon treatment with peroxisomal proliferators. Biochem Pharmacol. 1998;56:1195–9. doi: 10.1016/s0006-2952(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 34.Beheregaray AP, Souza FT, Coelho JC. Effect of a peroxysomal proliferator agent on intracellular cholesterol accumulation in cultured fibroblasts from Niemann–Pick type C disease patients. Clin Chim Acta. 2003;336:137–42. doi: 10.1016/s0009-8981(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 35.Griffin LD, Gong W, Verot L, Mellon SH. Niemann–Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 36.Langmade SJ, Gale SE, Frolov A, et al. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann–Pick C disease. Proc Natl Acad Sci U S A. 2006;103:13807–12. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lange Y, Ye J, Rigney M, Steck T. Cholesterol movement in Niemann–Pick type C cells and in cells treated with amphiphiles. J Biol Chem. 2000;275:17468–75. doi: 10.1074/jbc.M000875200. [DOI] [PubMed] [Google Scholar]
- 38.Frolov A, Zielinski SE, Crowley JR, Dudley-Rucker N, Schaffer JE, Ory DS. NPC1 and NPC2 regulate cellular cholesterol homeostasis through generation of low density lipoprotein cholesterol-derived oxysterols. J Biol Chem. 2003;278:25517–25. doi: 10.1074/jbc.M302588200. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Li HM, Chen YR, Gu XS, Duan S. Decreased estradiol release from astrocytes contributes to the neurodegeneration in a mouse model of Niemann–Pick disease type C. Glia. 2007;55:1509–18. doi: 10.1002/glia.20563. [DOI] [PubMed] [Google Scholar]
- 40.Ory DS. The Niemann–Pick disease genes; regulators of cellular cholesterol homeostasis. Trends Cardiovasc Med. 2004;14:66–72. doi: 10.1016/j.tcm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Repa JJ, Li H, Frank-Cannon TC, et al. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J Neurosci. 2007;27:14470–80. doi: 10.1523/JNEUROSCI.4823-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boadu E, Choi HY, Lee DW, et al. Correction of apolipoprotein A-I-mediated lipid efflux and high density lipoprotein particle formation in human Niemann–Pick type C disease fibroblasts. J Biol Chem. 2006;281:37081–90. doi: 10.1074/jbc.M606890200. [DOI] [PubMed] [Google Scholar]
- 43.Lalloyer F, Fievet C, Lestavel S, et al. The RXR agonist bexarotene improves cholesterol homeostasis and inhibits atherosclerosis progression in a mouse model of mixed dyslipidemia. Arterioscler Thromb Vasc Biol. 2006;26:2731–7. doi: 10.1161/01.ATV.0000248101.93488.84. [DOI] [PubMed] [Google Scholar]
- 44.Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann–Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–72. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- 45.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann–Pick disease type C. Curr Biol. 2001;11:1283–7. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 46.Patterson MC, Platt F. Therapy of Niemann–Pick disease, type C. Biochim Biophys Acta. 2004;1685:77–82. doi: 10.1016/j.bbalip.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Lloyd-Evans E, Morgan AJ, He X, et al. Niemann–Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med. 2008;14:1247–55. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- 48.Fu S, Kurzrock R. Development of curcumin as an epigenetic agent. Cancer. 2010;116:4670–6. doi: 10.1002/cncr.25414. [DOI] [PubMed] [Google Scholar]
- 49.Borbon IA, Hillman Z, Duran E, Jr, Kiela PR, Frautschy SA, Erickson RP. Lack of efficacy of curcumin on neurodegeneration in the mouse model of Niemann–Pick C1. Pharmacol Biochem Behav. 2012;101:125–31. doi: 10.1016/j.pbb.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alvarez AR, Klein A, Castro J, et al. Imatinib therapy blocks cerebellar apoptosis and improves neurological symptoms in a mouse model of Niemann–Pick type C disease. FASEB J. 2008;22:3617–27. doi: 10.1096/fj.07-102715. [DOI] [PubMed] [Google Scholar]
- 51.Erickson RP, Bernard O. Studies on neuronal death in the mouse model of Niemann–Pick C disease. J Neurosci Res. 2002;68:738–44. doi: 10.1002/jnr.10257. [DOI] [PubMed] [Google Scholar]
- 52.Kim SJ, Lim MS, Kang SK, Lee YS, Kang KS. Impaired functions of neural stem cells by abnormal nitric oxide-mediated signaling in an in vitro model of Niemann–Pick type C disease. Cell Res. 2008;18:686–94. doi: 10.1038/cr.2008.48. [DOI] [PubMed] [Google Scholar]
- 53.Bascunan-Castillo EC, Erickson RP, Howison CM, et al. Tamoxifen and vitamin E treatments delay symptoms in the mouse model of Niemann–Pick C. J Appl Genet. 2004;45:461–7. [PubMed] [Google Scholar]
- 54.Ulatowski L, Parker R, Davidson C, et al. Altered vitamin E status in Niemann–Pick type C disease. J Lipid Res. 2011;52:1400–10. doi: 10.1194/jlr.M015560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liscum L, Arnio E, Anthony M, Howley A, Sturley SL, Agler M. Identification of a pharmaceutical compound that partially corrects the Niemann–Pick C phenotype in cultured cells. J Lipid Res. 2002;43:1708–17. doi: 10.1194/jlr.m200179-jlr200. [DOI] [PubMed] [Google Scholar]
- 56.Pipalia NH, Huang A, Ralph H, Rujoi M, Maxfield FR. Automated microscopy screening for compounds that partially revert cholesterol accumulation in Niemann–Pick C cells. J Lipid Res. 2006;47:284–301. doi: 10.1194/jlr.M500388-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Rujoi M, Pipalia NH, Maxfield FR. Cholesterol pathways affected by small molecules that decrease sterol levels in Niemann–Pick type C mutant cells. PLoS One. 2010;5:e12788. doi: 10.1371/journal.pone.0012788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cosner CC, Markiewicz JT, Bourbon P, et al. Investigation of N-aryl-3-alkylidenepyrrolinones as potential Niemann-Pick type C disease therapeutics. J Med Chem. 2009;52:6494–8. doi: 10.1021/jm900707n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosenbaum AI, Cosner CC, Mariani CJ, Maxfield FR, Wiest O, Helquist P. Thiadiazole carbamates: potent inhibitors of lysosomal acid lipase and potential Niemann–Pick type C disease therapeutics. J Med Chem. 2010;53:5281–9. doi: 10.1021/jm100499s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Somers KL, Brown DE, Fulton R, et al. Effects of dietary cholesterol restriction in a feline model of Niemann–Pick type C disease. J Inherit Metab Dis. 2001;24:427–36. doi: 10.1023/a:1010588112003. [DOI] [PubMed] [Google Scholar]
- 61.Walterfang M, Chien YH, Imrie J, Rushton D, Schubiger D, Patterson MC. Dysphagia as a risk factor for mortality in Niemann–Pick disease type C: systematic literature review and evidence from studies with miglustat. Orphanet J Rare Dis. 2012;7:76. doi: 10.1186/1750-1172-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuter DJ, Mehta A, Hollak CE, et al. Miglustat therapy in type 1 Gaucher disease: clinical and safety outcomes in a multicenter retrospective cohort study. Blood Cells Mol Dis. 2013;51:116–24. doi: 10.1016/j.bcmd.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Ramirez CM, Liu B, Taylor AM, et al. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann–Pick type C1 mouse and markedly prolongs life. Pediatr Res. 2010;68:309–15. doi: 10.1203/PDR.0b013e3181ee4dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fan M, Sidhu R, Fujiwara H, et al. Identification of Niemann–Pick C1 (NPC1) disease biomarkers through sphingolipid profiling. J Lipid Res 2013 Jul 23. [DOI] [PMC free article] [PubMed]
- 65.ClinicalTrials.gov. Hydroxypropyl beta cyclodextrin for Niemann–Pick Type C1 disease. Available at: http://clinicaltrials.gov/ct2/show/NCT01747135. Accessed 10 Sept 2013.
- 66.Crumling MA, Liu L, Thomas PV, et al. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-beta-cyclodextrin. PLoS One. 2012;7:e53280. doi: 10.1371/journal.pone.0053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pipalia NH, Cosner CC, Huang A, et al. Histone deacetylase inhibitor treatment dramatically reduces cholesterol accumulation in Niemann–Pick type C1 mutant human fibroblasts. Proc Natl Acad Sci U S A. 2011;108:5620–5. doi: 10.1073/pnas.1014890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang C, Rahimpour S, Lu J, et al. Histone deacetylase inhibitors increase glucocerebrosidase activity in Gaucher disease by modulation of molecular chaperones. Proc Natl Acad Sci U S A. 2013;110:966–71. doi: 10.1073/pnas.1221046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim SJ, Lee BH, Lee YS, Kang KS. Defective cholesterol traffic and neuronal differentiation in neural stem cells of Niemann–Pick type C disease improved by valproic acid, a histone deacetylase inhibitor. Biochem Biophys Res Commun. 2007;360:593–9. doi: 10.1016/j.bbrc.2007.06.116. [DOI] [PubMed] [Google Scholar]
- 70.Shafaati M, O’Driscoll R, Bjorkhem I, Meaney S. Transcriptional regulation of cholesterol 24-hydroxylase by histone deacetylase inhibitors. Biochem Biophys Res Commun. 2009;378(4):689–94. doi: 10.1016/j.bbrc.2008.11.103. [DOI] [PubMed] [Google Scholar]
- 71.Wagner FF, Weїwer M, Lewis MC, Holson, EB. Small Molecule Inhibitors of Zinc-Dependent Histone Deacetylases (HDACs). Neurotherapeutics 2013. doi:10.1007/s13311-013-0226-1 [DOI] [PMC free article] [PubMed]
- 72.Pipalia NH, Maxfield FR. Investigating the therapeutic potential of HDAC Inhibitors for the treatment of Niemann Pick type C1 Disease. Mol Biol Cell 2011;22:abstract #1642.
- 73.Munkacsi AB, Chen FW, Brinkman MA, et al. An “exacerbate-reverse” strategy in yeast identifies histone deacetylase inhibition as a correction for cholesterol and sphingolipid transport defects in human Niemann–Pick type C disease. J Biol Chem. 2011;286:23842–51. doi: 10.1074/jbc.M111.227645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wehrmann ZT, Hulett TW, Huegel KL, et al. Quantitative comparison of the efficacy of various compounds in lowering intracellular cholesterol levels in Niemann–Pick type C fibroblasts. PLoS One. 2012;7:e48561. doi: 10.1371/journal.pone.0048561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nat Rev Drug Discov. 2007;6(1):21–2. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 76.Tiffon C, Adams J, van der Fits L, et al. The histone deacetylase inhibitors vorinostat and romidepsin downmodulate IL-10 expression in cutaneous T-cell lymphoma cells. Br J Pharmacol. 2011;162:1590–602. doi: 10.1111/j.1476-5381.2010.01188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiech NL, Fisher JF, Helquist P, Wiest O. Inhibition of histone deacetylases: a pharmacological approach to the treatment of non-cancer disorders. Curr Top Med Chem. 2009;9:257–71. doi: 10.2174/156802609788085241. [DOI] [PubMed] [Google Scholar]
- 78.Marini JC, Lanpher BC, Scaglia F, et al. Phenylbutyrate improves nitrogen disposal via an alternative pathway without eliciting an increase in protein breakdown and catabolism in control and ornithine transcarbamylase-deficient patients. Am J Clin Nutr. 2011;93:1248–54. doi: 10.3945/ajcn.110.009043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Batshaw ML, MacArthur RB, Tuchman M. Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr. 2001;138(1 Suppl.):S46–54. doi: 10.1067/mpd.2001.111836. [DOI] [PubMed] [Google Scholar]
- 80.Collins AF, Pearson HA, Giardina P, McDonagh KT, Brusilow SW, Dover GJ. Oral sodium phenylbutyrate therapy in homozygous beta thalassemia: a clinical trial. Blood. 1995;85:43–9. [PubMed] [Google Scholar]
- 81.Dover GJ, Brusilow S, Charache S. Induction of fetal hemoglobin production in subjects with sickle cell anemia by oral sodium phenylbutyrate. Blood. 1994;8:339–43. [PubMed] [Google Scholar]
- 82.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997;100:2457–65. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith W, Diaz GA, Lichter-Konecki U, et al. Ammonia control in children ages 2 months through 5 years with urea cycle disorders: comparison of sodium phenylbutyrate and glycerol phenylbutyrate. J Pediatr. 2013;162:1228–34. doi: 10.1016/j.jpeds.2012.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perucca E. Pharmacological and therapeutic properties of valproate: a summary after 35 years of clinical experience. CNS Drugs. 2002;16:695–714. doi: 10.2165/00023210-200216100-00004. [DOI] [PubMed] [Google Scholar]
- 85.Peedicayil J. Epigenetic drugs in cognitive disorders. Curr Pharm Des 2013 Jul 19. [DOI] [PubMed]
- 86.Graff J, Tsai LH. The potential of HDAC inhibitors as cognitive enhancers. Annu Rev Pharmacol Toxicol. 2013;53:311–30. doi: 10.1146/annurev-pharmtox-011112-140216. [DOI] [PubMed] [Google Scholar]
- 87.Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–59. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- 88.Stilling RM, Fischer A. The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease. Neurobiol Learn Mem. 2011;96:19–26. doi: 10.1016/j.nlm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 89.Kelly WK, O’Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23:3923–31. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kelly WK, Richon VM, O’Connor O, et al. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9:3578–88. [PubMed] [Google Scholar]
- 91.O’Connor OA, Heaney ML, Schwartz L, et al. Clinical experience with intravenous and oral formulations of the novel histone deacetylase inhibitor suberoylanilide hydroxamic acid in patients with advanced hematologic malignancies. J Clin Oncol. 2006;24:166–73. doi: 10.1200/JCO.2005.01.9679. [DOI] [PubMed] [Google Scholar]
- 92.Parise RA, Holleran JL, Beumer JH, Ramalingam S, Egorin MJ. A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), and its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:108–15. doi: 10.1016/j.jchromb.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 93.Iwamoto M, Friedman EJ, Sandhu P, Agrawal NG, Rubin EH, Wagner JA. Clinical pharmacology profile of vorinostat, a histone deacetylase inhibitor. Cancer Chemother Pharmacol. 2013;72:493–508. doi: 10.1007/s00280-013-2220-z. [DOI] [PubMed] [Google Scholar]
- 94.Maue RA, Burgess RW, Wang B, et al. A novel mouse model of Niemann–Pick type C disease carrying a D1005G-Npc1 mutation comparable to commonly observed human mutations. Hum Mol Genet. 2012;21:730–50. doi: 10.1093/hmg/ddr505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bagel JH, Sikora TU, Prociuk M, et al. Electrodiagnostic testing and histopathologic changes confirm peripheral nervous system myelin abnormalities in the feline model of Niemann–Pick disease type C. J Neuropathol Exp Neurol. 2013;72:256–62. doi: 10.1097/NEN.0b013e318286587f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13:1045–52. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 97.Palmieri D, Lockman PR, Thomas FC, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15:6148–57. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100:2041–6. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan JS, Haggarty SJ, Giacometti E, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galanis E, Jaeckle KA, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: a north central cancer treatment group study. J Clin Oncol. 2009;27:2052–8. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov. 2008;7:854–68. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 102.Leoni F, Zaliani A, Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fouladi M, Park JR, Stewart CF, et al. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J Clin Oncol. 2010;28:3623–9. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hummel TR, Wagner L, Ahern C, et al. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: A children’s oncology group phase 1 consortium study. Pediatr Blood Cancer. 2013;60:1452–7. doi: 10.1002/pbc.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muscal JA, Thompson PA, Horton TM, et al. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children’s Oncology Group phase I consortium study (ADVL0916) Pediatr Blood Cancer. 2013;60:390–5. doi: 10.1002/pbc.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Witt O, Milde T, Deubzer HE, et al. Phase I/II intra-patient dose escalation study of vorinostat in children with relapsed solid tumor, lymphoma or leukemia. Klin Padiatr. 2012;224:398–403. doi: 10.1055/s-0032-1323692. [DOI] [PubMed] [Google Scholar]
- 107.Marks PA. Discovery and development of SAHA as an anticancer agent. Oncogene. 2007;26:1351–6. doi: 10.1038/sj.onc.1210204. [DOI] [PubMed] [Google Scholar]
- 108.Siegel D, Hussein M, Belani C, et al. Vorinostat in solid and hematologic malignancies. J Hematol Oncol. 2009;2:31. doi: 10.1186/1756-8722-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Riva L, Blaney SM, Dauser R, et al. Pharmacokinetics and cerebrospinal fluid penetration of CI-994 (N-acetyldinaline) in the nonhuman primate. Clin Cancer Res. 2000;6:994–7. [PubMed] [Google Scholar]
- 110.Fass DM, Reis SA, Ghosh B, et al. Crebinostat: a novel cognitive enhancer that inhibits histone deacetylase activity and modulates chromatin-mediated neuroplasticity. Neuropharmacology. 2013;64:81–96. doi: 10.1016/j.neuropharm.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schroeder FA, Chonde DB, Riley MM, et al. FDG-PET imaging reveals local brain glucose utilization is altered by class I histone deacetylase inhibitors. Neurosci Lett. 2013;550:119–24. doi: 10.1016/j.neulet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Zhang YL, Hennig K, et al. Class I HDAC imaging using [ ( 3) H]CI-994 autoradiography. Epigenetics 2013 Jun 11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)