Abstract
The integrity of the genome is continuously challenged by both endogenous and exogenous DNA damaging agents. Neurons, due to their post-mitotic state, high metabolism, and longevity are particularly prone to the accumulation of DNA lesions. Indeed, DNA damage has been suggested as a major contributor to both age-associated neurodegenerative diseases and acute neurological injury. The DNA damage response is a key factor in maintaining genome integrity. It relies on highly dynamic posttranslational modifications of the chromatin and DNA repair proteins to allow signaling, access, and repair of the lesion. Drugs that modulate the activity of the enzymes responsible for these modifications have emerged as attractive therapeutic compounds to treat neurodegeneration. In this review, we discuss the role of DNA double-strand breaks and abnormal chromatin modification patterns in a range of neurodegenerative conditions, and the chromatin modifiers that might ameliorate them. Finally, we suggest that understanding the epigenetic modifications specific to neuronal DNA repair is crucial for the development of efficient neurotherapeutic strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0210-9) contains supplementary material, which is available to authorized users.
Keywords: Neurodegeneration, DNA damage, DNA repair, Chromatin modification, Epigenetics
Introduction
One of the most critical processes fundamental to cellular function, integrity, and indeed, survival, is the appropriate response to and repair of DNA damage. DNA damage arises from normal aspects of DNA metabolism such as replication, transcription, or V(D)J recombination in lymphocytes [1, 2], endogenous sources such as reactive oxygen species, or exogenous genotoxic agents such as mutagens and ionizing radiation [3]. Double-strand breaks (DSBs) are the most genotoxic DNA lesions. Unrepaired DSBs can lead to cell death, whereas misrepaired DSBs increase the likelihood of chromosome rearrangement, mutagenesis and loss of crucial genetic information. In replicating cells, such instability can result in apoptosis or cellular transformation. Indeed, increasing evidence suggests that DNA damage in neural stem cells, which are critical to the maintenance of the neural tissue throughout life, underlies the development of brain tumors [4]. In the case of neurons residing in the adult brain, this is even more critical, given that they are post-mitotic and terminally differentiated, and cannot be readily replaced after trauma or disease. Thus, accumulation of genomic (and mitochondrial) DNA damage is an important feature of both acute and chronic neurodegeneration. Neurons exhibit a slower rate of DNA repair as compared to dividing cells, suggesting that DNA damage is more likely to accumulate [5]. In fact, it seems that all differentiated cells display reduced DNA repair capacities [6].
Emerging evidence suggests an active role of chromatin in the DNA damage response (DDR) [7, 8]. The chromatin structure has the potential to impact multiple steps in the DNA repair process, including the detection of DSBs, the elaboration of accessible templates for DNA repair proteins, the activation of DNA damage checkpoint signals, and the restoration of proper chromatin structure following the completion of the repair process. The basic subunit of chromatin is the nucleosome, which consists of approximately 146 bp of DNA wound around an octameric histone core, formed by a dimer of heterotetrameric histones H2A, H2B, H3, and H4. The N- or C-terminal tails of histones can be acetylated, methylated, ubiquitinated, phosphorylated and polyADP-ribosylated [9]. These posttranslational modifications (PTMs) contribute to the regulation of the chromatin structure, and recruit specific binding proteins which recognize modified histones via specific bromo-, chromo- and PHD domains [10, 11]. The combination of histone PTMs has been proposed to constitute a “histone code”, in which distinct histone modification patterns organize the recruitment of specific factors required for particular processes [12].
Aberrant histone modifications are hypothesized to significantly contribute to disease such as cancer and neurodegeneration due to transcriptional dysregulation and defective repair of DNA lesions. Indeed, chromatin-modifying drugs have emerged as attractive therapeutic compounds for neurodegeneration in the last decade. One limitation in understanding the molecular targets of chromatin modifiers in neurons, is that most of what is known comes from studies performed in cycling cells or tumors, and might be therefore irrelevant to post-mitotic cells. In this review, we discuss the role of DNA damage and abnormal histone PTM patterns in a range of neurodegenerative conditions, and the chromatin modifiers that might ameliorate them. Finally, we suggest that understanding the chromatin modifications specific to neuronal DNA repair is crucial for the development of efficient neurotherapeutic strategies.
The DNA Damage Response (DDR)
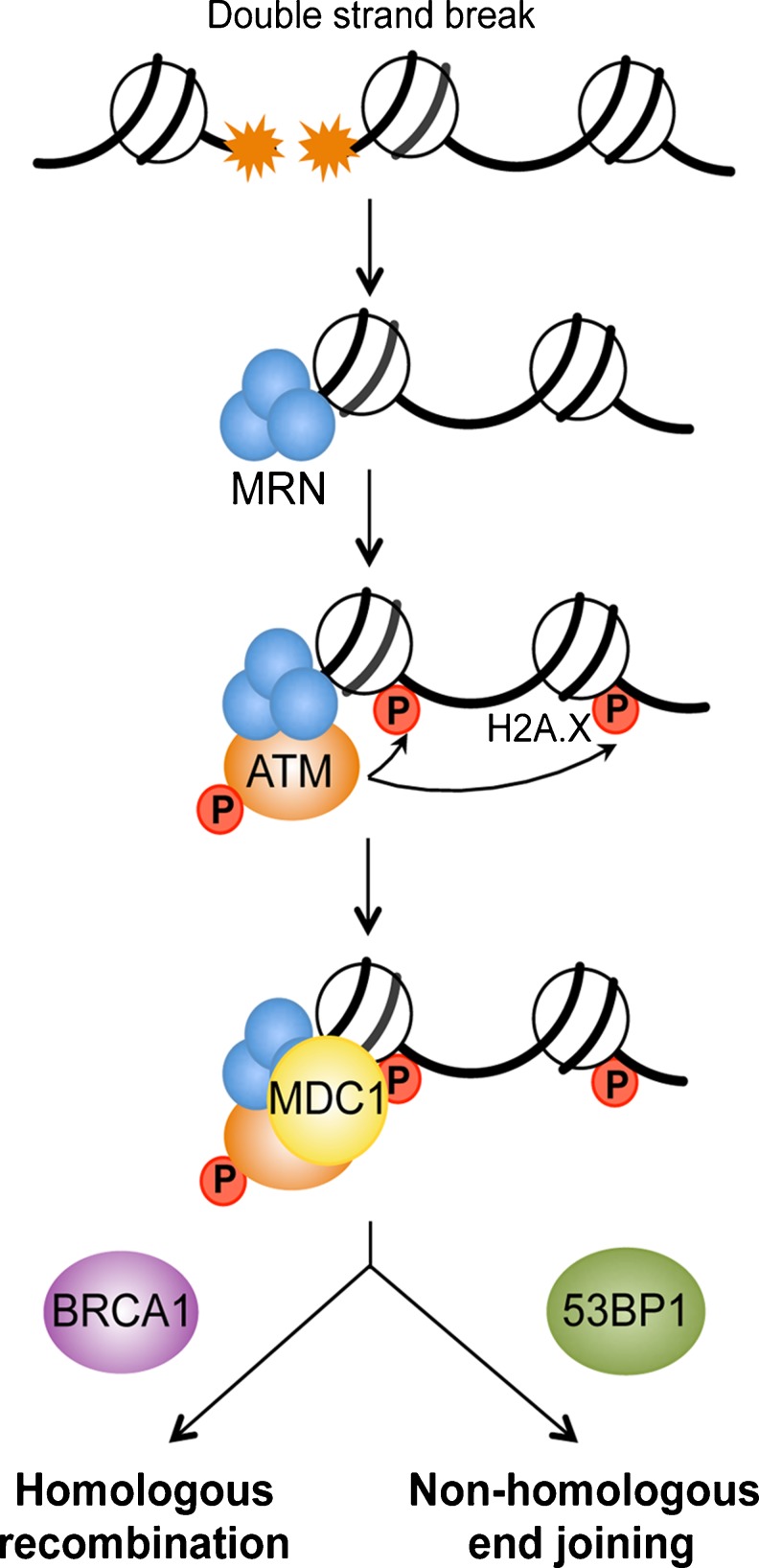
Mammalian cells respond to DSBs by activating control mechanisms, or checkpoints, that delay cell-cycle progression in order to allow cells to repair their DNA, and guarantee the unaltered transmission of genetic information. The DDR is a multi-tiered process that begins with rapid recruitment of early protein sensors to the break site (Fig. 1). The free ends of DNA are recognized by Mre11-Rad50-Nbs1 (MRN) complex, which processes and tethers DNA, and mediates the recruitment and activation of the upstream kinase Ataxia telangectasia mutated (ATM) [13, 14]. Activation of ATM leads to the rapid phosphorylation of the histone variant H2A.X over a large adjacent chromatin region (extending up to 2 Mb in mammalian cells), which facilitates the accumulation of DNA repair and checkpoint proteins to the break site [15]. In addition, other mediator proteins are recruited to the break site via direct interaction with MRN, such as Breast Cancer 1, early onset (BRCA1) [16], and p53-binding protein 1 (53BP1) [17]. One function of these proteins is to facilitate the accumulation and activation of DDR transducers in damaged regions, thus promoting a more efficient checkpoint activation and DNA repair [18]. Furthermore, the balance between BRCA1 and 53BP1 loading at DNA break sites seems to be a determinant in DSB repair mechanism choice [19]. Indeed, DSBs are repaired by two major pathways that compete with each other, namely, homologous recombination (HR), which is mediated by BRCA1, and non-homologous end joining (NHEJ), which involves 53BP1 (Fig. 2). Though both of these systems have been highly conserved throughout evolution, HR appears to be the predominant mechanism of DNA DSB repair throughout embryogenesis, whereas NHEJ is a major pathway for repair in post-natal life [20].
Fig. 1.
Early steps of the DNA damage response. DNA double-strand break is sensed by the MRE11–RAD50–NBS1 (MRN) complex, which leads to activation and recruitment of ataxia telangiectasia mutated (ATM) kinase, and phosphorylation of the histone variant H2A.X. Subsequently, a number of DNA damage sensing proteins are recruited to the break site, such as mediator of DNA damage checkpoint 1 (MDC1), and proteins involved in homologous recombination (Breast Cancer 1 early onset; BRCA1) and non-homologous end joining (p53-binding protein 1; 53BP1).
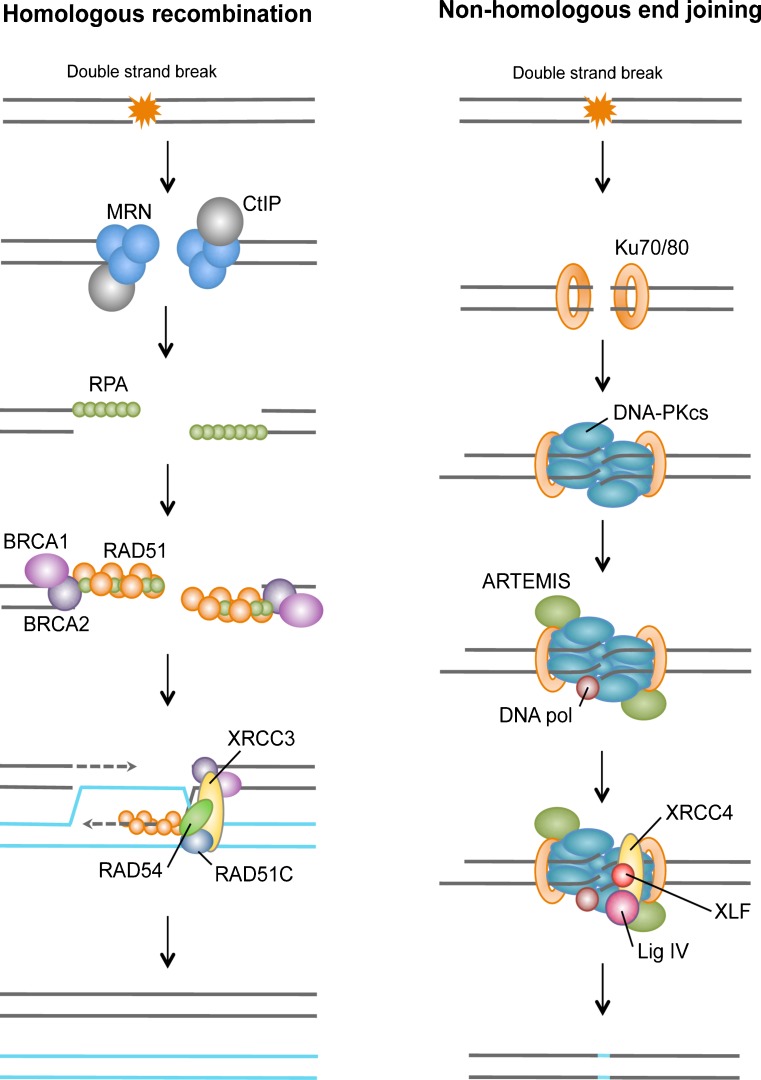
Fig. 2.
Double-strand breaks repair pathways. During homologous recombination, the repair of DSB is initiated by the resection of the DNA ends through the combined action of the MRN complex and CtIP to generate single-stranded DNA. The single-stranded ends are bound by replication protein A (RPA), BRCA1, BRCA2, RAD51 and its homologs, and can subsequently invade the homologous template. In subsequent steps, a Holliday junction is generated to prime DNA synthesis and restore genetic information that was disrupted by the DSB. During non-homologous end joining, the DSB is sensed by the Ku80–Ku70 heterodimer, which in turn recruits the DNA-dependent protein kinase catalytic subunit DNAPKcs, resulting in assembly of the DNAPK complex and activation of its kinase activity (see the figure; left panel). DNAPK increases the recruitment of Artemis, XRCC4, DNA ligase IV, and XLF, which carry out the final rejoining reaction. In many cases, NHEJ may also require the actions of a DNA polymerase(s).
HR uses a homologous copy of the sequence, for example, a sister chromatid, as a template to achieve precise, error-free repair. HR repair relies on extensive DNA processing at the break site via MRN complex and CtIP, in order to generate 3’ single stranded DNA where Rad51 can assemble into a nucleoprotein filament and promote homology search. Rad52, Rad54, and multiple Rad51 paralogs (Xrcc2, Xrcc3, Rad51L2, Rad51L3) are also recruited to the lesion to facilitate strand invasion and subsequent recombination [21]. By contrast, the more error-prone NHEJ pathway, which does not require an undamaged partner molecule, is used to perform the ligation of the DNA ends back together. In mammalian cells, NHEJ is mediated by Ku70/Ku80 heterodimer, a preformed ring that encircles DNA [22]. Ku70/Ku80 then allows the recruitment of the DNA-PK catalytic subunit, DNA-PKcs. If the DNA ends are not compatible for direct religation, nucleases such as Artemis or Exo1 will process damaged or mismatched nucleotides, and the DNA polymerases Polλ and Polμ will fill the gap [23]. Finally, after DNA ends are made compatible, DNA ligase IV and cofactor Xrcc4 mediate their ligation [24]. Despite the different machineries mediating repair, both HR and NHEJ pathways require the relaxation of chromatin to enable access of the repair machinery.
Although DSBs are the most toxic DNA lesions, other types of damage, such as single strand lesions or small chemical alterations of the bases have been linked to neurodegeneration [25, 26]. These lesions, which affect only one of the strands of the double helix, are the target of other repair pathways, nucleotide excision repair (NER) and base excision repair (BER). Both of these pathways are active in neurons, and recent evidence suggests their regulation by chromatin modifiers [27, 28].
Epigenetics of Repair: Histone Modifications
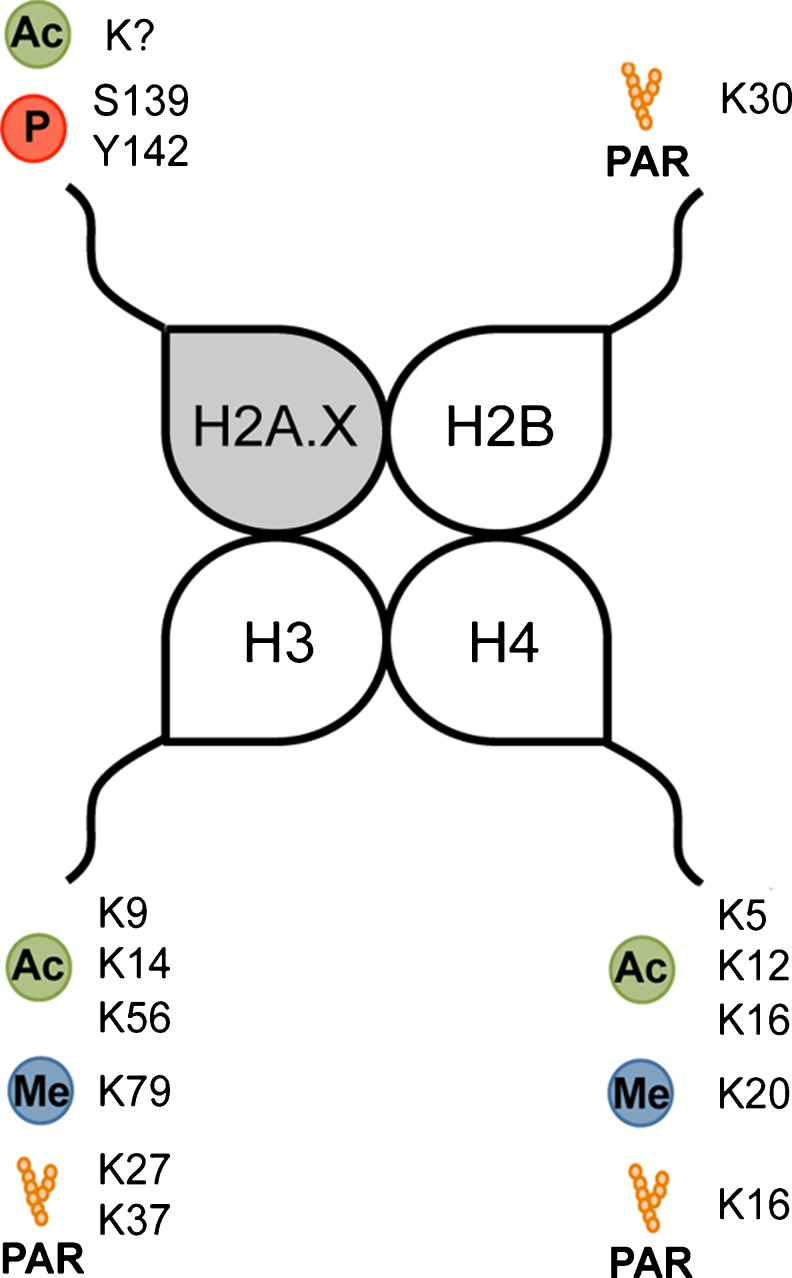
Efficient DNA repair requires a dynamic switch of the chromatin, from condensed to relaxed, in order to allow access to the damaged region by the multiple protein complexes involved in repair. This necessary chromatin remodeling is achieved in part through the reversible acetylation, phosphorylation, methylation, or ADP-ribosylation of histone tails that extend to the accessible surface of the nucleosome core (Fig. 3).
Fig. 3.
Histone modifications influencing the DNA damage response. Ac: acetylation; Me: methylation; P: phosphorylation; PAR: poly-(ADP) ribosylation. The question mark indicates that the specific lysine(s) targeted by acetylation has (have) not been identified.
Acetylation
Histones
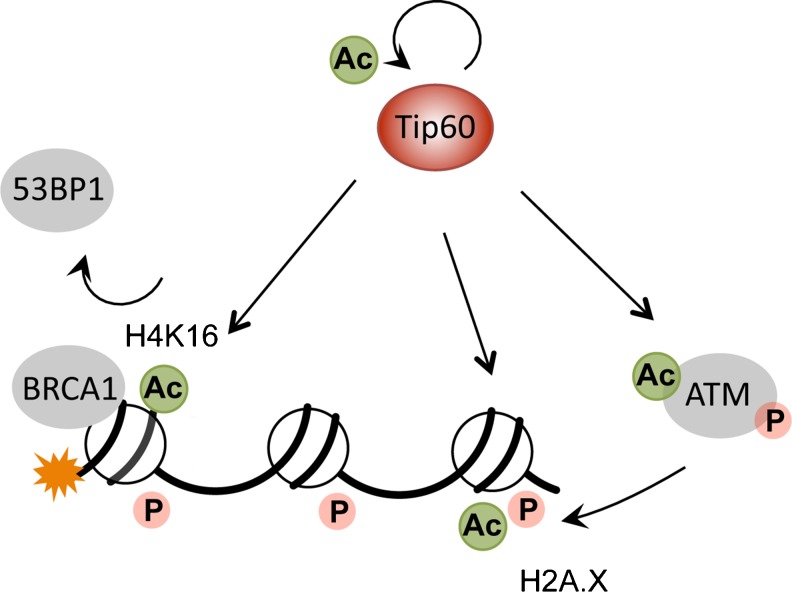
A significant body of evidence suggests that histone acetylation, which is regulated by the concerted actions of histone acetyltransferases (HATs) and histone deacetylases (HDACs), plays a central role in the chromatin remodeling that occurs in response to DNA damage. The mammalian Tat-interacting protein 60 kDa (Tip60), which is part of the chromatin-remodeling NuA4 complex, can acetylate multiple histone marks involved in the DNA damage repair process (Fig. 4). Following DNA damage, Tip60 binds to the chromatin surrounding DSBs and acetylates H2A.X. This process, which is independent of H2A.X phosphorylation, seems to be a prerequisite for H2A.X ubiquitination and eviction from the damaged DNA, which facilitates chromatin remodeling and stimulates the DDR [29]. Additionally, Tip60 induces histone H4 acetylation at lysine 16 and chromatin relaxation, which promotes the accumulation of repair molecules at sites of DSBs [30]. Importantly, Tip60-mediated H4K16 acetylation has been recently shown to play a central role in determining the balance of BRCA1 and 53BP1 loading at DSBs, thereby regulating DNA repair mechanism choice [19].
Fig. 4.
The multiple targets of Tip60 in DSB repair. Following induction of DNA damage, Tip60 autoacetylates, which increases its activity. Furthermore, Tip60 has been shown to acetylate and activate ATM in response to DNA damage. Finally, Tip60 mediates H2A.X and H4 acetylation. H4 acetylation at lysine 16 has important consequences in response to DSBs, as it decreases 53BP1 binding and promotes the recruitment of BRCA1 and homologous recombination repair. Ac: acetylation; P: phosphorylation.
Another acetyltransferase, human ortholog of Drosophila males absent on the first (hMOF), has been shown to regulate histone H4K16 acetylation following DNA damage. hMOF-mediated acetylation of H4K16 is partly responsible for ATM activation and γH2A.X foci formation, notably by modulating the interactions of H4 with H2A.X [31]. Depletion of hMOF delays the formation of irradiation-induced γH2A.X foci and impairs the recruitment of MDC1, 53BP1 and RAD51, which alters DNA repair by both NEHJ and HR pathways [32].
Non-histone
Although this review focuses mainly on the regulation of histone modifications in the context of DNA damage and neurological injury, chromatin modifiers such as HATs and HDACs regulate the expression and activity of many other proteins, and are likely to influence the DNA damage response through acetylation of multiple checkpoint and DNA repair proteins [33]. For instance, Tip60 has been shown to rapidly acetylate ATM after DNA damage, which is required for ATM kinase activity and transduction of the DDR [34]. Additionally, Tip60 autoacetylates in response to DNA damage, which is critical for its activation [35]. Acetylation of Ku70 has been shown to impair its ability to bind DNA extremities, and increases the DNA damage sensitivity of various cancer cells [36, 37]. In addition to its role in DNA repair, Ku70 is present in the cytosol in a complex with the pro-apoptotic protein Bax. Upon acetylation of Ku70, Bax is released from the complex and translocates to mitochondria, where it triggers cytochrome c release and caspase-dependent death [38].
Due to their role as chromatin modifiers, HDAC inhibitors have been extensively studied in their ability to modulate gene expression. Multiple studies have highlighted the modulation of expression of numerous genes involved in cell cycle, DNA replication and DNA repair, by HDAC inhibition [39–41]. Most of the transcriptional reprogramming following treatment with HDAC inhibitors seems to be related to the ATM pathway, as they fail to modulate the expression of DNA repair genes in cells lacking ATM [42, 43]. Regulation of most genes involved in the DDR depends on a common transcription factor, E2F1. Interestingly, the effects of HDAC inhibition on E2F1 activity and on the expression of its target genes, appears to be cell type-specific. In cancer cells, HDAC inhibition decreases the recruitment of E2F1 to DNA repair genes such as Rad51, Brca1 or Chk1, and represses their expression [39, 41, 44, 45]. On the contrary, in non-cancerous cells, E2F1 transcriptional targets are activated in response to HDAC inhibition [42]; for example, HDAC inhibition by trichostatin A or sodium butyrate increases E2F1 expression in neurons [40]. Recent findings suggest that acetylation of E2F1 contributes to the selective activation of its target genes in response to DNA damage, and modulates E2F1 anti-proliferative and pro-apoptotic functions [46, 47]. In fact, several transcription factors playing a central role in the DDR can be acetylated, which appears to be a critical modulator of cell fate decision. One key component of the cellular checkpoint following DNA damage, and a major acetylation target, is the tumor suppressor and transcription factor p53. We have recently shown that specific acetylation sites within the p53 C-terminal domain regulated its transcriptional activity in neurons, thereby promoting or abrogating transcription of cell-cycle arrest, survival, or apoptotic gene programs [48].
Role of HDACs
HATs and HDACs are often found in complexes with transcription factors, DNA repair proteins and chromatin modifiers, where they regulate the balance between gene expression and DNA repair. In yeast, the HDAC complex Sin3p/Rpd3p is recruited to the DNA lesion and deacetylates H4K16, which is required for efficacious repair by NHEJ [49]. Similar regulation appears to be conserved in human cells, where HDAC1 and HDAC2 are rapidly recruited to DNA damage sites to deacetylate histone marks H4K16 and H3K56 [50]. Indeed, deficiency in HDAC1 and HDAC2 activity leads to defective DSB repair by NHEJ, and to a much lesser extent, by HR. Both HDACs seem to be involved in the disassembly of NHEJ factors from the break site since, upon their depletion, Ku70/Ku80, Xrcc4, and Artemis persist at microirradiation-induced DSBs. Consistent with this, Greenberg and collaborators have recently shown that acetylation of H4K16 at the break site has an inhibitory effect on the NHEJ repair pathway by reducing the DNA binding affinity of 53BP1, which in turn promotes BRCA1 loading and repair by HR [19].
Conditional knockout of HDAC3 in mouse embryonic fibroblasts leads to a delay in cell-cycle progression, increased sensitivity to DNA damaging agents and apoptosis [51, 52]. Moreover, deletion of HDAC3 impairs DNA repair by both HR and NHEJ pathways, most likely due to the persistence of H3K9, H3K14, H4K5 and H4K12 acetylation throughout the S phase, and concomitant downregulation of H3K9 tri-methylation. These histone modifications are critical to the recruitment of various DNA repair proteins to the sites of damage, and to chromatin remodeling by Tip60 [52].
HDAC4 has been directly involved in multiple steps of the DDR. In response to DSBs, HDAC4 is rapidly recruited to the break site, where it sits in a complex with 53BP1 [53]. HDAC4 foci formation occurs in both normal and cancer cells, and is independent of the DDR genes NBS1, ATM, or DNA-PK. However, resolution of HDAC4 foci requires a fully functional DDR, and appears to be correlated to specific radio-sensitivities of different cell types, with highly sensitive cells resolving foci less efficiently [53]. Therefore, HDAC4 appears as a mediator of the DDR, which together with 53BP1 accumulates at unrepaired lesions and may signal a cell cycle checkpoint stall where DNA repair can be completed. In addition to its role at the break site, HDAC4 activity as a transcription regulator contributes to the DNA damage-induced block in G2 through the repression of Cyclin B2 and cdc25c promoters [54].
Recently, HDAC9 and 10 activities have also been involved in DNA repair. In HeLa cells, depletion of HDAC9 and 10 by RNA interference leads to large defects in homologous recombination following mitomycin C exposure [55]. However, no clear mechanism of action has been described, and HDAC9 and 10 could be targeting multiple levels of regulation, from chromatin remodeling to the expression of DNA repair genes.
Phosphorylation
Apart from canonical core histone proteins, all eukaryote genomes encode a number of histone variants, some of which have been shown to have specific functions. Of the core histones, histone H2A has the largest number of variants, of which histone H2A.X is a relatively minor variant (comprising of ~10 % of total H2A in the cell). Despite this, it has been extensively studied because of its role in DDR. The phosphorylated form of histone H2A.X (γH2A.X) is an early, sensitive, and selective marker of DSBs. Following damage, activation of ATM leads to the phosphorylation of the histone variant H2A.X at serine 139, which facilitates the accumulation of DNA repair and checkpoint proteins to the break site [15].
Several groups have shown that, in addition to transient phosphorylation at S139, histone H2A.X was constitutively phosphorylated at tyrosine 142 (H2A.X Y142) by the WICH complex [56]. In response to DNA damage, H2A.X Y142 is dephosphorylated by tyrosine phosphatase EYA1/3, which seems required for γH2A.X accumulation and efficient DNA repair. Indeed, it is likely that H2A.X Y142 phosphorylation status determines the relative recruitment of either DNA repair or pro-apoptotic factors to the tail of γH2A.X, and allows it to act as a determinant of cell fate in response to DNA damage [57]. The definition of the specific role of H2A.X Y142 phosphorylation may be a valuable tool to develop strategies to influence the cell’s choice of DNA repair or apoptosis.
Methylation
Several lysine residues on the tails of histone H3 and H4 can be methylated. Methylation at theses sites has been linked to transcriptional activation (H3K4, H3K36 and H3K79) and repression (H3K9 and H3K27), as well as DNA damage response (H3K79 and H4K20), demonstrating a widespread role for histone methylation in various aspects of chromatin function. One (mono-), two (di-), or three (tri-) methyl groups can be added to or removed from lysine residues, and different degrees of methylation on the same residue have been suggested to differentially affect chromatin structure and transcription [58]. Histone methyltransferases (HMTs) catalyze the transfer of methyl groups from S-adenosylmethionine (SAM) to histones. The removal of methyl groups is catalyzed by histone demethylases (HDMs).
A number of studies have demonstrated a relaxation of chromatin in the vicinity of DNA damage sites [59, 60], which may partly be explained by local histone acetylation [30]. Decondensation of the chromatin, which is generally associated with actively transcribed regions, is thought to facilitate the access of repair proteins to the sites of DNA damage [30]. Interestingly, recent evidence suggests the establishment of transcriptionally repressive chromatin patterns in the vicinity of DNA damage sites, a process most likely set up to avoid interference between transcription and DNA repair machineries. Several key proteins involved in initiating and maintaining transcriptional repression are recruited to DSBs, and colocalize with silencing histone modifications, such as tri-methylation of histone H3 at lysine 27 (H3K27me3; [61]). Consistent with this, tri-methylation of histone H3 at lysine 4 (H3K4me3), which labels transcriptionally active regions, is underrepresented at DNA damage sites [62].
Histone H4 methylation has been described as a major participant in the DNA damage response. H4 can be mono-, di- or trimethylated, which results in distinct biological outcomes, i.e., mono- and di-methylated H4K20 (H4K20me1 and H4K20me2) are involved in DNA replication and DNA damage repair, whereas tri-methylated H4K20 (H4K20me3) is a hallmark of silenced heterochromatic regions. These different methylation states are established through specific HMTs. Methylated H4K20 is linked with the DDR in both yeast [63] and mammalian cells [64]. Upon induction of DSBs, the HMT MMSET is responsible for the local increase of H4K20me1/2 at DNA damage sites, which acts as a loading platform for 53BP1, and is required for efficient NHEJ repair [63, 64].
PolyADP-ribosylation
ADP-ribosylation comprises the transfer of the ADP-ribose moiety from the co-substrate nicotinamide adenine dinucleotide (NAD+) onto specific amino acid side chains of acceptor proteins or to pre-existing protein-linked ADP-ribose units by ADP-ribosyltransferases (ARTs). PolyADP-ribosylation (PARylation) is involved in important biological processes, such as maintenance of genomic stability, chromatin modification, cell death and transcriptional regulation. Histone tails are PARylated by Poly [ADP-ribose] polymerases (PARPs) at specific amino acid residues, in particular lysines. Mass spectrometry and electron-transfer dissociation identified H2AK13, H2BK30, H3K27, H3K37 and H4K16 as targets of PARylation by the most abundant PARP family member, PARP-1 [65].
While PARP-1 has been firmly implicated in base excision and SSB repair, it also plays a role in DSBs repair. After DNA damage, the rapid PARylation of chromatin promotes the transient recruitment of chromatin-remodeling enzymes and heterochromatin factors to the DSBs and is critical for efficient DNA repair [66]. PAR functions as a docking polymer for a variety of chromatin and DNA repair proteins [67]. For example, PARylation of the chromatin by PARP-1 is necessary for the recruitment of NuRD, a repressive complex with HDAC activity that maintains higher-order chromatin structure and is required for loading of BRCA1 [68]. Similarly, ALC1, a remodeling ATPase that functions to reposition nucleosomes, is also rapidly recruited to DSBs through direct interaction with PAR chains on the chromatin [69].
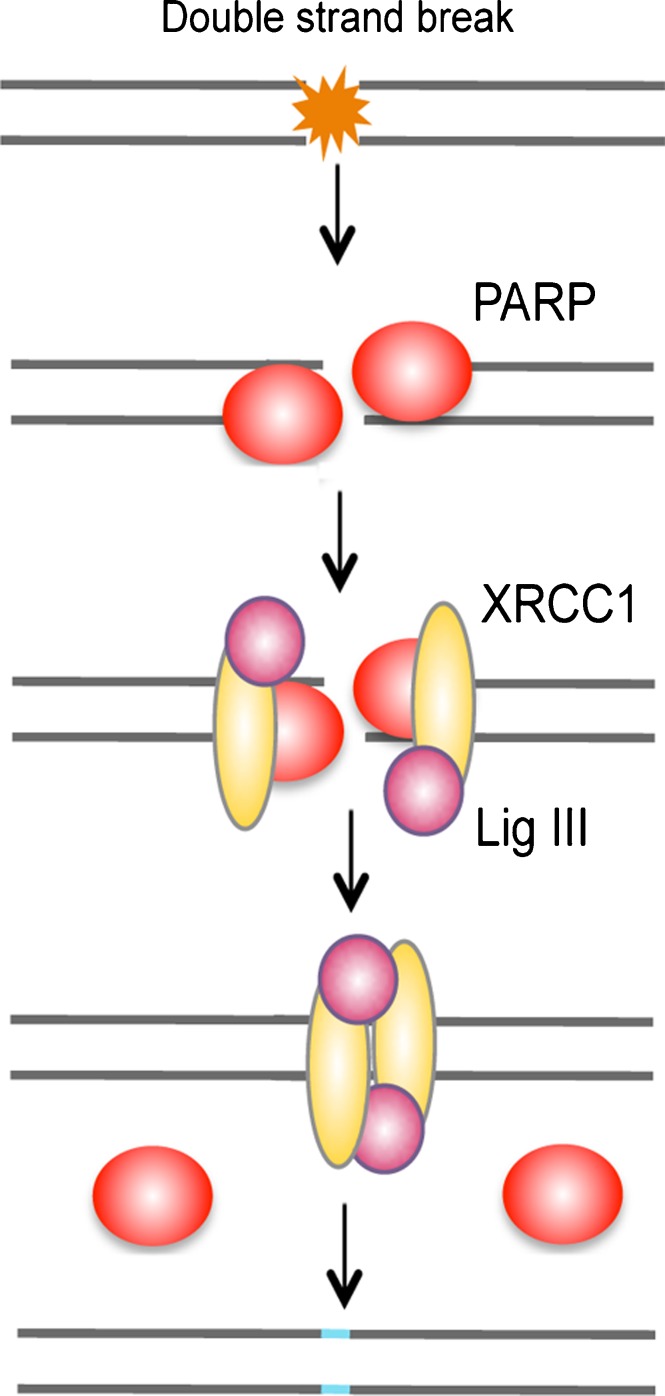
Two types of protein domains that specifically interact with PAR have been identified: macrodomains and PAR-binding zinc finger (PBZ) domains (for review, see [70]). The macrodomain histone variant macroH2A1.1, which is detected in postmitotic and senescent cells, binds PARP-1 in vitro [71]. Upon DNA damage, macroH2A1.1 is rapidly recruited to PARP-1 activation sites, transiently compacts chromatin, reduces the recruitment of Ku70/Ku80, and alters γH2A.X patterns [71]. Consistent with this, earlier studies have suggested that PARP-1 binds to DNA ends in direct competition with Ku70/Ku80 and operates in an alternative pathway of NHEJ, which functions as backup to the classical pathway (Fig. 5) [72].
Fig. 5.
Backup pathway of non-homologous end joining. PARP-1 binds to DNA ends in direct competition with Ku70/Ku80 and operates in an alternative pathway of NHEJ, which functions as backup to the classical pathway.
DNA Damage in Neurodegeneration
Aging
Multiple studies have demonstrated that oxidative stress is a major contributor to the pathophysiological processes of degeneration in both acute and chronic neurodegenerative disorders. The intense metabolism of neurons, associated with high rates of transcription, generates large amounts of reactive oxygen species and increases the potential for genomic DNA damage. This has given rise to the hypothesis that the slow accumulation of neuronal DNA lesions throughout life fosters the transcription of defective proteins in the aging brain, which plays a fundamental role in neurodegeneration. Furthermore, DNA damage-prone sequences, found in selectively vulnerable gene promoters, may reduce the expression of genes involved in learning, memory and neuronal survival after age 40 [73]. Interestingly, different areas of the brain display different rates of DNA damage accumulation during aging, with most DNA damage found in the hippocampus and cortex, consistent with the cognitive decline and memory loss exhibited in neurodegenerative diseases [74]. Compelling support for this also comes from the studies in progeroid syndromes, such as Ataxia telangectasia, Xeroderma pigmentosum, Werner’s and Cockayne’s syndromes. These congenital conditions, which are due to the mutation of genes involved in DNA repair, are all associated with post-developmental neuronal death.
A number of epigenetic marks associated with gene transcription and DNA repair are altered in the aging brain. For instance, dysregulation of H4K16 acetylation in the mouse brain has been linked to aging phenotypes, including memory decline and DNA repair deficiency [75, 76]. The methylation status of histone H3 has also been involved in the epigenetic regulation of aging and lifespan. Direct evidence comes from studies in Caenorhabditis elegans, whose lifespan is regulated by methylation of histone H3 at lysine 4 (H3K4me2/3; associated with transcriptional activation) and lysine 27 (H3K27me2/3; associated with transcriptional repression) [77]. Furthermore, study of the genomic distribution of H3K4me3 in neurons of the human prefrontal cortex has identified age-correlated epigenomic reorganization, with a slight increase of H3K4me3 at specific promoters in the aging brain [78]. Similarly, H3K4me2 levels at gene promoters and enhancers increase during postnatal development and aging, and reflect progressively more open and transcriptionally active chromatin structures associated with metabolic and environmental stresses that induce DNA damage and inflammation. In fact, such changes may form an epigenetic memory of stress and damage experienced by the organism [79]. Finally, SMCX (also known as JARID1C), an H3K4me3-specific demethylase, is one of the most frequently mutated genes to be associated with X-linked mental retardation [80].
Interestingly, supporting the idea of a connection between aging, altered chromatin remodeling and DNA damage accumulation in the CNS, H4K20me1 levels are significantly decreased in the brain of 12-month old senescence-accelerated prone mouse 8, a murine model for premature aging characterized by age-related learning and memory deficits [81].
Stroke
The large majority of strokes are ischemic, caused by the occlusion of a cerebral blood vessel. Stroke is characterized by a rapid excitatory death in the ischemic core, and a more delayed, apoptotic death in the surrounding regions. A significant body of evidence suggests that oxidative stress and DNA damage are important contributors to the neurodegeneration that takes place during cerebral ischemia. Several groups have demonstrated that damage to nuclear DNA is an early event after injury induced by a range of ischemia models, including transient middle cerebral occlusion (MCAO), permanent MCAO and transient global ischemia. In a transient MCAO model, DNA single-strand breaks (SSBs) can be detected in neurons as early as 1 min after reperfusion, whereas double-strand breaks (DSBs) are first seen after 1 h [82]. Between 16 and 72 h of reperfusion, many SSB- and DSB-containing cells, including both neurons and astrocytes, show morphological changes consistent with apoptosis. Similarly, after transient global ischemia, SSBs are detected prior to DSBs in most regions of the rat brain, with the exception of the hippocampal CA1 where they appear simultaneously and in a delayed-fashion [83]. This distinct kinetic pattern suggests that the mechanisms underlying neuronal death in CA1 following transient global ischemia may be different from that of other brain regions. Furthermore, studies in a permanent MCAO model found that oxidative DNA damage, SSBs, and DSBs can be detected in both the ischemic core and the peri-infarct regions, but that their numbers increase over time exclusively in areas outside the core. These data suggest that oxidative injury to nuclear DNA may be a significant cause of secondary damage following ischemia [84].
Spinal Cord Injury
Spinal cord injury (SCI) results from the initial mechanical disruption of structures in the spinal cord (primary injury) and the generation of secondary events that collectively damage the neighboring tissue (secondary damage). A number of studies show that accumulation of DNA strand breaks is a major triggering event for neuronal and non-neuronal apoptosis in spinal cord injury. Rat injured spinal cords show early accumulation of DNA breaks one day after injury, activation of p53, and bax-dependent apoptosis. Furthermore, the expression levels of genes involved in the DNA damage response, such as ATM, Xrcc1, PARP-1 and p53, are increased up to 21 days following injury [85]. Consistent with this, earlier studies in a unilateral sciatic nerve avulsion model, which is used as an in vivo model for apoptosis of spinal motor neurons, have shown that SSBs accumulate early after injury and lead to p53 and bax-dependent apoptosis [86].
Alzheimer’s Disease
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by progressive memory loss and cognitive impairment. The typical hallmarks of the disease are extracellular neuritic plaques of insoluble beta-amyloid peptide, generated from beta-amyloid precursor protein (APP) processing, intracellular neurofibrillary tangles formed by aggregation of hyperphosphorylated tau protein, and neuronal loss in the hippocampus and cerebral cortex. Multiple risk factors, including aging, are associated with AD. Several studies indicate that post-mitotic neurons have a reduced capacity for some types of DNA repair, which is further compromised by aging. DNA SSBs and DSBs are typically detected in AD brains, but the origin and time course of the damage are not fully understood [87, 88]. Although the DNA damage detected in AD brains may be both a consequence and a trigger of neurodegeneration, a number of studies suggest that the repair of DNA DSBs by the NHEJ pathway is deficient in AD [89, 90]. Importantly, APP and its binding partner Fe65 have been directly involved in DNA repair, as either Fe65 or APP depletion impairs the recruitment of Tip60 to the lesion site, and decreases histone H4 acetylation and repair efficiency [90]. Therefore, the role of Fe65-APP complex in the response of neurons to DNA damage should be taken into account as a possible mechanism contributing to neuronal dysfunction observed in AD pathology.
Huntington’s Disease
Huntington's disease (HD) is a neurodegenerative disorder that follows an autosomal-dominant inheritance pattern. The pathogenesis of the disease depends on the degree of expansion of triplet (CAG) repeats located in the first exon on the gene. An expanded polyglutamine tract within the protein huntingtin (Htt) enables a gain-of-function phenotype that is often exhibited by a dysfunctional oligomerization process and the formation of protein aggregates. How this process is connected to neurodegeneration remains undefined. Recent studies have reported that expression of mutant Htt is sufficient to induce DNA damage and activate the DDR in a cell-base model of HD [91]. Interestingly, DNA damage and γH2A.X are present in HD R6/2 transgenic mice before the formation of mutant Htt aggregates and HD pathogenesis [91]. Elaborating on this observation, Ryu and collaborators recently described the abnormal nuclear distribution of γH2A.X and imbalanced levels of phosphorylated BRCA1 in striatal neurons of HD transgenic R6/2 mice [92].
Parkinson’s Disease
Parkinson’s disease (PD) is mainly sporadic, and is characterized by a profound loss of dopaminergic neurons in the substantia nigra. Although the mechanisms of cell death in PD are still poorly understood, substantial evidence implies that DNA damage contributes to the pathogenesis. Midbrain dopaminergic neurons are particularly vulnerable to oxidative stress, partly because the metabolism of dopamine generates cytotoxic hydroxyl radicals [93]. The study of post-mortem brain tissue has shown that the structural integrity and topology of genomic DNA is altered predominantly in the substantia nigra of PD patients, but also in other regions of the brain, such as the caudate putamen, hippocampus, and thalamus [94]. The changes included DNA damage in the form of SSBs and DSBs, and DNA instability.
Intriguingly, mice deficient for the DNA repair protein ATM exhibit locomotor abnormalities similar to a Parkinsonian syndrome, associated with severe degeneration of tyrosine hydroxylase-positive neurons, dopaminergic nigro-striatal neurons, and their terminals in the striatum and the ventral tegmental area [95]. Consistent with a possible involvement of ATM activity in PD pathogenesis, another group has shown that iron chelation therapy had a beneficial effect upon dopaminergic neurons, which was attributed to ATM activation and subsequent ATM-mediated increase in DNA repair and antioxidant defense pathways [96].
Amyotrophic Lateral Sclerosis
The precise mechanisms of motor neuron death in amyotrophic lateral sclerosis (ALS) remain to be identified. However, a mutation in the superoxide dismutase 1 (SOD1) gene is linked to familial forms of ALS, which suggests that oxidative stress has an important role in the etiology of the disease [97]. In transgenic mice expressing human mutant SOD1, the severe degeneration of motor neurons is preceded by accumulation of SSBs [98]. By contrast, DSBs accumulate later in motor neurons, when degenerative structural changes are already prominent [99]. Post-mortem studies of ALS motor cortex reveal the increase of oxidative DNA damage repair activity, supporting the hypothesis that DNA damage is an upstream mechanism for neurodegeneration in this pathology [100].
Neurotherapeutics for DNA Damage
Many neuroprotective strategies are aimed at preventing oxidative stress in neurons, and the consequent oxidative stress-mediated DNA damage. However, the first DNA breaks occur rapidly after injury (for example, within minutes after reperfusion in transient MCAO), which provides a very limited time window to prevent this damage. Therefore, the development of neurotherapeutic strategies that not only limit the extent of DNA damage, but also promote DNA repair, appears critical. To date, HDACs and PARPs are the most studied histone-modifying enzymes as potential therapeutic targets in neurodegenerative diseases. The clinical potential of drugs that can regulate histone methylation is also a promising and expanding field.
HDAC Inhibition
Disruption of the balance between HATs and HDACs activities leads to a disequilibrium in acetylation, an established feature of neurodegeneration [101]. In agreement with this, treatment with various HDAC inhibitors (HDACis) has emerged as an attractive therapeutic approach for neurodegeneration in the last decade (reviewed by Holson and collaborators in this issue). In addition to neuroprotection, there is growing evidence that HDACis can also enhance learning and memory [102] and promote axonal regeneration [103]. Most of the beneficial aspects of HDAC inhibition described in the literature have been attributed to their effect on gene expression [104, 105]. Nevertheless, more studies are needed to determine the effect of HDACis on DNA damage repair in neurodegeneration. For instance, given the recent connection between DNA damage and abnormal BRCA1 recruitment in HD [92], it is possible that this deficiency could be compensated by HDAC inhibition. Indeed, increasing H4K16 acetylation with HDACis disrupts 53BP1 recruitment to the damage site and promotes BRCA1-mediated DNA repair [19]. Although the specific H4K16 deacetylase in neurons remains to be identified, two studies have reported that HDAC1 and HDAC2 depletion was sufficient to increase H4K16 acetylation levels in human cell lines [19, 50]. Yet, loss of HDAC1 and 2 activity has been shown to increase the accumulation rate of γH2A.X at the break site and leads to deficient NHEJ repair [50]. In neurons, deregulation of HDAC1 activity is correlated with aberrant cell cycle activity and accumulation of DSBs. Indeed, pharmacological inhibition or loss of HDAC1 is sufficient to increase the number of unrepaired DNA lesions, which ultimately leads to neuronal apoptosis [106]. In contrast to HDAC1, HDAC2 is an attractive therapeutic target, as its inhibition does not seem to compromise the DNA damage response in neurons. On the contrary, loss of HDAC2 function enhances memory formation in mice, increases the number of synapses, and activates CREB-CBP-dependent transcription [107].
Although HDAC3 inactivation impairs DNA repair and induces apoptosis in MEFs [52], depletion of HDAC3 expression is not toxic in neurons. Rather, HDAC3 deficiency can protect neuronal cells against low potassium-induced death and oxidative stress, whereas its forced expression induces degeneration of otherwise healthy neurons [108]. Interestingly, forced expression of HDAC3 also promotes the death of hippocampus-derived HT22 cells, but has no effect on the viability of primary kidney fibroblasts or the HEK293 and HeLa cell lines. Whereas this cell-selective toxicity is related to a cell type-specific activity of HDAC3 in DNA repair remains to be determined.
A significant amount of data highlights the cell-specific aspect of HDAC inhibition, which seems to impair DNA repair in cancerous, but not normal cells [109], and displays strong protective properties in a broad range of neuropathologies. Eukaryotic cells repair DSBs primarily by two mechanisms—NHEJ and HR—the balance of which differs widely among cell types and during different cell cycle phases [110]. This differential use of repair pathways could account for the specific sensitivities of different cell types to HDAC inhibitors, which is supported by the study of mammalian cells mutated for NHEJ (Ku80 or Lig4) or for HR (Brca2, or Bloom's syndrome Blm). Mouse embryonic fibroblasts ablated for NHEJ by deletion of either Ku80 or Lig4 display hypersensitivity to HDAC inhibition by trichostatin A. However, cells impaired for HR by deletion of Brca2, or derepressed for HR by mutation of HR-influencing gene Bml, exhibit the same level of resistance to trichostatin A as control cells [111]. Therefore, non-cycling cells like neurons, which are less likely than other cell types to use HR repair due to the lack of a readily accessible homologous template, would be less sensitive to HDAC inhibition than cycling cells.
It appears critical to understand the specific contributions of different HDACs to the DNA damage response in neurons to identify safe therapeutic targets. HDAC inhibitors have been extensively described as toxic compounds, compromising genomic stability in highly dividing cells, which partly underlies their death-inducing properties in tumors. This is a specific concern for neural stem cells, in which HDAC inhibition-induced genomic instability could lead to the development of brain tumors. We have shown that the specific outcomes of HDAC inhibitors, i.e. protection in neurons versus apoptosis in cancer cells, were in part attributable to their antagonistic effects on p53 activity [48]. However, very little is known about the effect of HDAC inhibition on genomic stability in neurons. Given the fact that HDAC inhibitors show long-term protection in various neuronal injury paradigms, and stimulate functional recovery, it is likely that their effect on the neuronal genome is not that of cancer cells, although one cannot exclude the possibility that neurons have higher tolerance for DNA damage due to their post-mitotic state.
PARP Inhibition
PARP-1 is activated by DNA strand breaks, and facilitates the DNA repair process. Its activity has been implicated in different pathologies such as cancer, inflammation and neurodegenerative disorders [70]. Indeed, over-activation of PARP may initiate a number of downstream events that may contribute to cell death. PARPs are nicotinamide adenine dinucleotide (NAD+) consuming enzymes and their over-activation may lead to cellular NAD + exhaustion, mitochondrial dysfunction, energy depletion and eventually, death. Furthermore, PARP-1 activation in the context of DNA damage may result in the translocation of apoptosis-inducing factor (AIF) to the nucleus, which initiates a caspase independent cell death termed PARthanatos [112]. Accordingly, mice knocked-out for PARP-1 have a reduced infarct volume following ischemia, which correlates with higher levels of NAD + [113]. Consistent with this, pharmacological inhibition of PARP was shown to increase cellular viability in a number of experimental systems, particularly in the context of neurodegenerative diseases [114]. However, it is not known whether PARP inhibition compromises the DNA damage response in the context of neuronal injury.
HMT Inhibition
The collection of human HMTs is a large and diverse group of nearly 50 enzymes, many of which have been associated with human disease [115]. Indeed, like HDAC inhibitors, a subset of histone methyltransferase inhibitors are in clinical trials for cancer treatment and are likely to be explored in the context of neurological disease in the near future. However, given the reported role of various HMTs in memory formation and consolidation [116–119], more work will be necessary to determine the specificities of histone methylation in neurons, and whether the development of drugs that inhibit HDMTs rather than HMTs would not have more therapeutic potential to treat neurodegeneration.
Conclusion
In mammals, DNA damage occurs naturally and is aggravated following metabolic and oxidative stress. Accordingly, DNA repair processes have evolved to maintain genomic integrity and cellular viability. As the severity of DNA damage escalates from single base damage to DSBs, differing mechanisms are employed to carry out repair. As discussed in this review, posttranslational modification of chromatin and DNA repair enzymes play fundamental roles in orchestrating, regulating, and completing efficient DNA damage repair. However, much of what is known has been identified in proliferating, and in many instances transformed, cells. The unique nature of neurons, which are post-mitotic, terminally differentiated, long-lived, highly metabolic cells, requires a robust response to DNA damage, adapted to their special cellular features. Therefore, more studies are required to determine the types of DNA damage occurring in neurons in healthy and injured brains, and the propensity for damage accumulation with aging. Another important priority is to increase our understanding of the specific roles of DNA repair enzymes and pathways in neurons, and how they are affected by disease, injury, and aging. Furthermore, a major challenge in advancing epigenetic therapy to treat neurodegeneration will be to determine the role of chromatin modifiers and specific histone PTMs in neuronal DNA repair. Besides acetylation, PARylation and methylation, histones can be modified by phosphorylation, ubiquitination or sumoylation, which may represent new directions for therapeutic intervention, and suggests the suitability of combinatorial approaches to target epigenetic modulators. Together these studies will lay the foundation for more precise molecular mechanism-based therapies for progressive neurodegeneration, neuronal injury, and aging.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS071056), the New York State Spinal Cord Injury Research Program (CO19772), the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, and the Burke Medical Research Foundation.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Dudley DD, Chaudhuri J, Bassing CH, Alt FW. Mechanism and control of V(D)J recombination versus class switch recombination: similarities and differences. Adv Immunol. 2005;86:43–112. doi: 10.1016/S0065-2776(04)86002-4. [DOI] [PubMed] [Google Scholar]
- 2.Keeney S, Neale MJ. Initiation of meiotic recombination by formation of DNA double-strand breaks: mechanism and regulation. Biochem Soc Trans. 2006;34:523–525. doi: 10.1042/BST0340523. [DOI] [PubMed] [Google Scholar]
- 3.Aziz K, Nowsheen S, Pantelias G, Iliakis G, Gorgoulis VG, Georgakilas AG. Targeting DNA damage and repair: embracing the pharmacological era for successful cancer therapy. Pharmacol Therapeut. 2012;133:334–350. doi: 10.1016/j.pharmthera.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kenyon J, Gerson SL. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res. 2007;35:7557–7565. doi: 10.1093/nar/gkm1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nouspikel T, Hanawalt PC. Terminally differentiated human neurons repair transcribed genes but display attenuated global DNA repair and modulation of repair gene expression. Mol Cell Biol. 2000;20:1562–1570. doi: 10.1128/mcb.20.5.1562-1570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nouspikel T. DNA repair in differentiated cells: some new answers to old questions. Neuroscience. 2007;145:1213–1221. doi: 10.1016/j.neuroscience.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Luijsterburg MS, van Attikum H. Chromatin and the DNA damage response: the cancer connection. Molec Oncol. 2011;5:349–367. doi: 10.1016/j.molonc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller KM, Jackson SP. Histone marks: repairing DNA breaks within the context of chromatin. Biochem Soc Trans. 2012;40:370–6. doi: 10.1042/BST20110747. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Grunstein M. 25 years after the nucleosome model: chromatin modifications. Trends Biochem Sci. 2000;25:619–23. doi: 10.1016/s0968-0004(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 10.Wysocka J, Swigut T, Milne TA, Dou Y, Zhang X, Burlingame AL, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L, Zhou MM. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513:124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 12.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 13.Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature. 2005;434(7033):605–611. doi: 10.1038/nature03442. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26(56):7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 15.Kinner A, Wu W, Staudt C, Iliakis G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36(17):5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun MH, Hiom K. Understanding the functions of BRCA1 in the DNA-damage response. Biochem Soc Trans. 2009;37(3):597–604. doi: 10.1042/BST0370597. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Goodarzi AA, Jeggo PA, Paull TT. 53BP1 promotes ATM activity through direct interactions with the MRN complex. EMBO J. 2010;29(3):574–585. doi: 10.1038/emboj.2009.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D, et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003;421(6926):952–956. doi: 10.1038/nature01445. [DOI] [PubMed] [Google Scholar]
- 19.Tang J, Cho NW, Cui G, Manion EM, Shanbhag NM, Botuyan MV, et al. Acetylation limits 53BP1 association with damaged chromatin to promote homologous recombination. Nature Struct Molec Biol. 2013;20(3):317–325. doi: 10.1038/nsmb.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nature Rev Molec Cell Biol. 2003;4(9):712–720. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 21.Wyman C, Ristic D, Kanaar R. Homologous recombination-mediated double-strand break repair. DNA Repair. 2004;3(8–9):827–833. doi: 10.1016/j.dnarep.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Doherty AJ, Jackson SP. DNA repair: how Ku makes ends meet. Current Biol: CB. 2001;11(22):R920–924. doi: 10.1016/s0960-9822(01)00555-3. [DOI] [PubMed] [Google Scholar]
- 23.Daley JM, Laan RL, Suresh A, Wilson TE. DNA joint dependence of pol X family polymerase action in nonhomologous end joining. J Biological Chem. 2005;280(32):29030–7. doi: 10.1074/jbc.M505277200. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TE, Grawunder U, Lieber MR. Yeast DNA ligase IV mediates non-homologous DNA end joining. Nature. 1997;388(6641):495–458. doi: 10.1038/41365. [DOI] [PubMed] [Google Scholar]
- 25.Sykora P, Wilson DM, 3rd, Bohr VA. Base excision repair in the mammalian brain: Implication for age related neurodegeneration. Mech Ageing Devel. 2013 doi: 10.1016/j.mad.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canugovi C, Misiak M, Ferrarelli LK, Croteau DL, Bohr VA. The role of DNA repair in brain related disease pathology. DNA Repair. 2013;12(8):578–587. doi: 10.1016/j.dnarep.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamori T, DeRicco J, Naqvi A, Hoffman TA, Mattagajasingh I, Kasuno K, et al. SIRT1 deacetylates APE1 and regulates cellular base excision repair. Nucleic Acids Res. 2010;38(3):832–845. doi: 10.1093/nar/gkp1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan W, Luo J. SIRT1 regulates UV-induced DNA repair through deacetylating XPA. Molecular Cell. 2010;39(2):247–258. doi: 10.1016/j.molcel.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Ikura T, Tashiro S, Kakino A, Shima H, Jacob N, Amunugama R, et al. DNA damage-dependent acetylation and ubiquitination of H2AX enhances chromatin dynamics. Molec Cell Biol. 2007;27(20):7028–7040. doi: 10.1128/MCB.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nature Cell Biol. 2006;8(1):91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, et al. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Molec Cell Biol. 2010;30(22):5335–5347. doi: 10.1128/MCB.00350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma GG, So S, Gupta A, Kumar R, Cayrou C, Avvakumov N, et al. MOF and histone H4 acetylation at lysine 16 are critical for DNA damage response and double-strand break repair. Molec Cell Biol. 2010;30(14):3582–3595. doi: 10.1128/MCB.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 34.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(37):13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Chen J. SIRT1 regulates autoacetylation and histone acetyltransferase activity of TIP60. J Biol Chem. 2010;285(15):11458–11464. doi: 10.1074/jbc.M109.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CS, Wang YC, Yang HC, Huang PH, Kulp SK, Yang CC, et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 2007;67(11):5318–5327. doi: 10.1158/0008-5472.CAN-06-3996. [DOI] [PubMed] [Google Scholar]
- 37.Kerr E, Holohan C, McLaughlin KM, Majkut J, Dolan S, Redmond K, et al. Identification of an acetylation-dependant Ku70/FLIP complex that regulates FLIP expression and HDAC inhibitor-induced apoptosis. Cell Death Differ. 2012;19(8):1317–1327. doi: 10.1038/cdd.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Subramanian C, Jarzembowski JA, Opipari AW, Jr, Castle VP, Kwok RP. HDAC6 deacetylates Ku70 and regulates Ku70-Bax binding in neuroblastoma. Neoplasia. 2011;13(8):726–734. doi: 10.1593/neo.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kachhap SK, Rosmus N, Collis SJ, Kortenhorst MS, Wissing MD, Hedayati M et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PloS One 5(6):e11208. [DOI] [PMC free article] [PubMed]
- 40.Boutillier AL, Trinh E, Loeffler JP. Selective E2F-dependent gene transcription is controlled by histone deacetylase activity during neuronal apoptosis. J Neurochem. 2003;84(4):814–828. doi: 10.1046/j.1471-4159.2003.01581.x. [DOI] [PubMed] [Google Scholar]
- 41.Kachhap SK, Rosmus N, Collis SJ, Kortenhorst MS, Wissing MD, Hedayati M, et al. Downregulation of homologous recombination DNA repair genes by HDAC inhibition in prostate cancer is mediated through the E2F1 transcription factor. PloS One. 2010;5(6):e11208. doi: 10.1371/journal.pone.0011208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang ER, Choi JD, Park MA, Jeong G, Cho H, Lee JS. ATM modulates transcription in response to histone deacetylase inhibition as part of its DNA damage response. Exp Molec Med. 2010;42(3):195–204. doi: 10.3858/emm.2010.42.3.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee JS. Functional link between DNA damage responses and transcriptional regulation by ATM in response to a histone deacetylase inhibitor TSA. Cancer Res Treat. 2007;39(3):116–124. doi: 10.4143/crt.2007.39.3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19482–19487. doi: 10.1073/pnas.0707828104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G, et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res. 2009;15(10):3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 46.Ozaki T, Okoshi R, Sang M, Kubo N, Nakagawara A. Acetylation status of E2F-1 has an important role in the regulation of E2F-1-mediated transactivation of tumor suppressor p73. Biochem Biophys Res Commun. 2009;386(1):207–211. doi: 10.1016/j.bbrc.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 47.Pediconi N, Ianari A, Costanzo A, Belloni L, Gallo R, Cimino L, et al. Differential regulation of E2F1 apoptotic target genes in response to DNA damage. Nature Cell Biol. 2003;5(6):552–558. doi: 10.1038/ncb998. [DOI] [PubMed] [Google Scholar]
- 48.Brochier C, Dennis G, Rivieccio MA, McLaughlin K, Coppola G, Ratan RR, et al. Specific acetylation of p53 by HDAC inhibition prevents DNA damage-induced apoptosis in neurons. J Neurosci. 2013;33(20):8621–8532. doi: 10.1523/JNEUROSCI.5214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jazayeri A, McAinsh AD, Jackson SP. Saccharomyces cerevisiae Sin3p facilitates DNA double-strand break repair. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(6):1644–1649. doi: 10.1073/pnas.0304797101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nature Structural Molec Biol. 2010;17(9):1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhaskara S, Chyla BJ, Amann JM, Knutson SK, Cortez D, Sun ZW, et al. Deletion of histone deacetylase 3 reveals critical roles in S phase progression and DNA damage control. Molecular Cell. 2008;30(1):61–72. doi: 10.1016/j.molcel.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhaskara S, Knutson SK, Jiang G, Chandrasekharan MB, Wilson AJ, Zheng S, et al. Hdac3 is essential for the maintenance of chromatin structure and genome stability. Cancer Cell. 2010;18(5):436–447. doi: 10.1016/j.ccr.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kao GD, McKenna WG, Guenther MG, Muschel RJ, Lazar MA, Yen TJ. Histone deacetylase 4 interacts with 53BP1 to mediate the DNA damage response. J Cell Biol. 2003;160(7):1017–1027. doi: 10.1083/jcb.200209065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basile V, Mantovani R, Imbriano C. DNA damage promotes histone deacetylase 4 nuclear localization and repression of G2/M promoters, via p53 C-terminal lysines. J Biol Chem. 2006;281(4):2347–2357. doi: 10.1074/jbc.M507712200. [DOI] [PubMed] [Google Scholar]
- 55.Kotian S, Liyanarachchi S, Zelent A, Parvin JD. Histone deacetylases 9 and 10 are required for homologous recombination. J Biol Chem. 2011;286(10):7722–7726. doi: 10.1074/jbc.C110.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stucki M. Histone H2A.X Tyr142 phosphorylation: a novel sWItCH for apoptosis? DNA Repair. 2009;8(7):873–876. doi: 10.1016/j.dnarep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458(7238):591–596. doi: 10.1038/nature07849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pattaroni C, Jacob C. Histone methylation in the nervous system: functions and dysfunctions. Molec Neurobiol. 2013;47(2):740–756. doi: 10.1007/s12035-012-8376-4. [DOI] [PubMed] [Google Scholar]
- 59.Kruhlak MJ, Celeste A, Nussenzweig A. Spatio-temporal dynamics of chromatin containing DNA breaks. Cell Cycle. 2006;5(17):1910–1912. doi: 10.4161/cc.5.17.3169. [DOI] [PubMed] [Google Scholar]
- 60.Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, et al. Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nature Cell Biol. 2006;8(8):870–876. doi: 10.1038/ncb1446. [DOI] [PubMed] [Google Scholar]
- 61.O'Hagan HM, Mohammad HP, Baylin SB. Double strand breaks can initiate gene silencing and SIRT1-dependent onset of DNA methylation in an exogenous promoter CpG island. PLoS Genetics. 2008;4(8):e1000155. doi: 10.1371/journal.pgen.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seiler DM, Rouquette J, Schmid VJ, Strickfaden H, Ottmann C, Drexler GA, et al. Double-strand break-induced transcriptional silencing is associated with loss of tri-methylation at H3K4. Chromosome Res. 2011;19(7):883–899. doi: 10.1007/s10577-011-9244-1. [DOI] [PubMed] [Google Scholar]
- 63.Sanders SL, Portoso M, Mata J, Bahler J, Allshire RC, Kouzarides T. Methylation of histone H4 lysine 20 controls recruitment of Crb2 to sites of DNA damage. Cell. 2004;119(5):603–614. doi: 10.1016/j.cell.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 64.Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127(7):1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Messner S, Altmeyer M, Zhao H, Pozivil A, Roschitzki B, Gehrig P, et al. PARP1 ADP-ribosylates lysine residues of the core histone tails. Nucleic Acids Res. 2010;38(19):6350–6362. doi: 10.1093/nar/gkq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monks TJ, Xie R, Tikoo K, Lau SS. Ros-induced histone modifications and their role in cell survival and cell death. Drug Metab Rev. 2006;38(4):755–767. doi: 10.1080/03602530600959649. [DOI] [PubMed] [Google Scholar]
- 67.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Bio. 2006;7(7):517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 68.Chou DM, Adamson B, Dephoure NE, Tan X, Nottke AC, Hurov KE, et al. A chromatin localization screen reveals poly (ADP ribose)-regulated recruitment of the repressive polycomb and NuRD complexes to sites of DNA damage. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18475–18480. doi: 10.1073/pnas.1012946107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahel D, Horejsi Z, Wiechens N, Polo SE, Garcia-Wilson E, Ahel I, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325(5945):1240–1243. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hottiger MO, Boothby M, Koch-Nolte F, Luscher B, Martin NM, Plummer R, et al. Progress in the function and regulation of ADP-Ribosylation. Science Signal. 2011;4(174):mr5. doi: 10.1126/scisignal.2001645. [DOI] [PubMed] [Google Scholar]
- 71.Timinszky G, Till S, Hassa PO, Hothorn M, Kustatscher G, Nijmeijer B, et al. A macrodomain-containing histone rearranges chromatin upon sensing PARP1 activation. Nat Struct Molec Biol. 2009;16(9):923–929. doi: 10.1038/nsmb.1664. [DOI] [PubMed] [Google Scholar]
- 72.Wang M, Wu W, Wu W, Rosidi B, Zhang L, Wang H, et al. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34(21):6170–6182. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 74.Barzilai A, Biton S, Shiloh Y. The role of the DNA damage response in neuronal development, organization and maintenance. DNA Repair. 2008;7(7):1010–1027. doi: 10.1016/j.dnarep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 76.Krishnan V, Chow MZ, Wang Z, Zhang L, Liu B, Liu X, et al. Histone H4 lysine 16 hypoacetylation is associated with defective DNA repair and premature senescence in Zmpste24-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(30):12325–12330. doi: 10.1073/pnas.1102789108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, et al. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479(7373):365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheung I, Shulha HP, Jiang Y, Matevossian A, Wang J, Weng Z, et al. Developmental regulation and individual differences of neuronal H3K4me3 epigenomes in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8824–8829. doi: 10.1073/pnas.1001702107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han Y, Han D, Yan Z, Boyd-Kirkup JD, Green CD, Khaitovich P, et al. Stress-associated H3K4 methylation accumulates during postnatal development and aging of rhesus macaque brain. Aging Cell. 2012;11(6):1055–1064. doi: 10.1111/acel.12007. [DOI] [PubMed] [Google Scholar]
- 80.Jensen LR, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, et al. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X-linked mental retardation. Am J Hum Genet. 2005;76(2):227–236. doi: 10.1086/427563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang CM, Tsai SN, Yew TW, Kwan YW, Ngai SM. Identification of histone methylation multiplicities patterns in the brain of senescence-accelerated prone mouse 8. Biogerontology. 2010;11(1):87–102. doi: 10.1007/s10522-009-9231-5. [DOI] [PubMed] [Google Scholar]
- 82.Ji X, Luo Y, Ling F, Stetler RA, Lan J, Cao G, et al. Mild hypothermia diminishes oxidative DNA damage and pro-death signaling events after cerebral ischemia: a mechanism for neuroprotection. Front Biosci. 2007;12:1737–1747. doi: 10.2741/2185. [DOI] [PubMed] [Google Scholar]
- 83.Jin K, Chen J, Nagayama T, Chen M, Sinclair J, Graham SH, et al. In situ detection of neuronal DNA strand breaks using the Klenow fragment of DNA polymerase I reveals different mechanisms of neuron death after global cerebral ischemia. J Neurochem. 1999;72(3):1204–1214. doi: 10.1046/j.1471-4159.1999.0721204.x. [DOI] [PubMed] [Google Scholar]
- 84.Nagayama T, Lan J, Henshall DC, Chen D, O'Horo C, Simon RP, et al. Induction of oxidative DNA damage in the peri-infarct region after permanent focal cerebral ischemia. J Neurochem. 2000;75(4):1716–1728. doi: 10.1046/j.1471-4159.2000.0751716.x. [DOI] [PubMed] [Google Scholar]
- 85.Kotipatruni RR, Dasari VR, Veeravalli KK, Dinh DH, Fassett D, Rao JS. p53- and Bax-mediated apoptosis in injured rat spinal cord. Neurochem Res. 2011;36(11):2063–2074. doi: 10.1007/s11064-011-0530-2. [DOI] [PubMed] [Google Scholar]
- 86.Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J Neurobiol. 2002;50(3):181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- 87.Mullaart E, Boerrigter ME, Ravid R, Swaab DF, Vijg J. Increased levels of DNA breaks in cerebral cortex of Alzheimer's disease patients. Neurobiol Aging. 1990;11(3):169–173. doi: 10.1016/0197-4580(90)90542-8. [DOI] [PubMed] [Google Scholar]
- 88.Lyras L, Cairns NJ, Jenner A, Jenner P, Halliwell B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer's disease. J Neurochem. 1997;68(5):2061–2069. doi: 10.1046/j.1471-4159.1997.68052061.x. [DOI] [PubMed] [Google Scholar]
- 89.Shackelford DA. DNA end joining activity is reduced in Alzheimer's disease. Neurobiol Aging. 2006;27(4):596–605. doi: 10.1016/j.neurobiolaging.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 90.Minopoli G, Stante M, Napolitano F, Telese F, Aloia L, De Felice M, et al. Essential roles for Fe65, Alzheimer amyloid precursor-binding protein, in the cellular response to DNA damage. J Biol Chem. 2007;282(2):831–835. doi: 10.1074/jbc.C600276200. [DOI] [PubMed] [Google Scholar]
- 91.Illuzzi J, Yerkes S, Parekh-Olmedo H, Kmiec EB. DNA breakage and induction of DNA damage response proteins precede the appearance of visible mutant huntingtin aggregates. J Neurosci Res. 2009;87(3):733–747. doi: 10.1002/jnr.21881. [DOI] [PubMed] [Google Scholar]
- 92.Jeon GS, Kim KY, Hwang YJ, Jung MK, An S, Ouchi M, et al. Deregulation of BRCA1 leads to impaired spatiotemporal dynamics of gamma-H2AX and DNA damage responses in Huntington's disease. Molec Neurobiol. 2012;45(3):550–563. doi: 10.1007/s12035-012-8274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–942. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- 94.Hegde ML, Gupta VB, Anitha M, Harikrishna T, Shankar SK, Muthane U, et al. Studies on genomic DNA topology and stability in brain regions of Parkinson's disease. Arch Biochem Biophys. 2006;449(1–2):143–156. doi: 10.1016/j.abb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 95.Eilam R, Peter Y, Elson A, Rotman G, Shiloh Y, Groner Y, et al. Selective loss of dopaminergic nigro-striatal neurons in brains of Atm-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12653–12656. doi: 10.1073/pnas.95.21.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shackelford RE, Fu Y, Manuszak RP, Brooks TC, Sequeira AP, Wang S, et al. Iron chelators reduce chromosomal breaks in ataxia-telangiectasia cells. DNA Repair. 2006;5(11):1327–1336. doi: 10.1016/j.dnarep.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 97.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 98.Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25(27):6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, et al. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500(1):20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 100.Shaikh AY, Martin LJ. DNA base-excision repair enzyme apurinic/apyrimidinic endonuclease/redox factor-1 is increased and competent in the brain and spinal cord of individuals with amyotrophic lateral sclerosis. Neuromolec Med. 2002;2(1):47–60. doi: 10.1007/s12017-002-0038-7. [DOI] [PubMed] [Google Scholar]
- 101.Rouaux C, Loeffler JP, Boutillier AL. Targeting CREB-binding protein (CBP) loss of function as a therapeutic strategy in neurological disorders. Biochem Pharmacol. 2004;68(6):1157–1164. doi: 10.1016/j.bcp.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 102.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 103.Rivieccio MA, Brochier C, Willis DE, Walker BA, D'Annibale MA, McLaughlin K, et al. HDAC6 is a target for protection and regeneration following injury in the nervous system. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(46):19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kazantsev AG, Thompson LM. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Disc. 2008;7(10):854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- 105.Langley B, Brochier C, Rivieccio MA. Targeting histone deacetylases as a multifaceted approach to treat the diverse outcomes of stroke. Stroke. 2009;40(8):2899–2905. doi: 10.1161/STROKEAHA.108.540229. [DOI] [PubMed] [Google Scholar]
- 106.Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60(5):803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graff J, Rei D, Guan JS, Wang WY, Seo J, Hennig KM, et al. An epigenetic blockade of cognitive functions in the neurodegenerating brain. Nature. 2012;483(7388):222–226. doi: 10.1038/nature10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bardai FH, D'Mello SR. Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta. J Neurosci. 2011;31(5):1746–1751. doi: 10.1523/JNEUROSCI.5704-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11(13):4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 110.Shrivastav M, De Haro LP, Nickoloff JA. Regulation of DNA double-strand break repair pathway choice. Cell Res. 2008;18(1):134–147. doi: 10.1038/cr.2007.111. [DOI] [PubMed] [Google Scholar]
- 111.Yaneva M, Li H, Marple T, Hasty P. Non-homologous end joining, but not homologous recombination, enables survival for cells exposed to a histone deacetylase inhibitor. Nucleic Acids Res. 2005;33(16):5320–5330. doi: 10.1093/nar/gki821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y, Dawson VL, Dawson TM. Poly(ADP-ribose) signals to mitochondrial AIF: a key event in parthanatos. Exp Neurol. 2009;218(2):193–202. doi: 10.1016/j.expneurol.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA. Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cerebr Blood F Met. 1997;17(11):1143–1151. doi: 10.1097/00004647-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 114.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4(5):421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 115.Copeland RA, Olhava EJ, Scott MP. Targeting epigenetic enzymes for drug discovery. Curr Opin Chem Biol. 2010;14(4):505–610. doi: 10.1016/j.cbpa.2010.06.174. [DOI] [PubMed] [Google Scholar]
- 116.Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito-Garagorri E, et al. Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice. J Neurosci. 2013;33(8):3452–3464. doi: 10.1523/JNEUROSCI.3356-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balemans MC, Kasri NN, Kopanitsa MV, Afinowi NO, Ramakers G, Peters TA, et al. Hippocampal dysfunction in the Euchromatin histone methyltransferase 1 heterozygous knockout mouse model for Kleefstra syndrome. Hum Mol Genet. 2013;22(5):852–866. doi: 10.1093/hmg/dds490. [DOI] [PubMed] [Google Scholar]
- 118.Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, et al. G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation. J Neurosci. 2012;32(16):5440–5453. doi: 10.1523/JNEUROSCI.0147-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gupta S, Kim SY, Artis S, Molfese DL, Schumacher A, Sweatt JD, et al. Histone methylation regulates memory formation. J Neurosci. 2010;30(10):3589–3599. doi: 10.1523/JNEUROSCI.3732-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)