Abstract
Parkinson’s disease (PD) is a complex multifactorial disorder marked by extensive system-wide pathology, including a substantial loss of nigrostriatal dopaminergic neurons. The etiology of PD remains elusive, but there is considerable evidence that, in addition to well-defined genetic mechanisms environmental factors play a crucial role in disease pathogenesis. How the environment might influence the genetic factors and contribute to disease development and progression remains unclear. In recent years, epigenetic mechanisms such as DNA methylation, chromatin remodeling and alterations in gene expression via non-coding RNAs have begun to be revealed as potential factors in PD pathogenesis. Epigenetic modulation exists throughout life, beginning in prenatal stages, is dependent on the lifestyle, environmental exposure and genetic makeup of an individual and may serve as a missing link between PD risk factors and development of the disease. This chapter sheds light on the emerging role of epigenetics in disease pathogenesis and on prospective interventional strategies for the therapeutic modulation of PD.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0211-8) contains supplementary material, which is available to authorized users.
Keywords: Parkinson’s disease, Epigenetics, DNA methylation, Histones, MicroRNA
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease and affects millions worldwide. It is estimated that within the world’s ten most populated countries alone, more than 4 million individuals are affected by PD [1]. Within the next 20 years, the number of PD patients is projected to reach 10 million and is a matter of significant concern. Clinically, the disease presents with classic motor symptoms, such as resting tremor, rigidity, bradykinesia, postural instability, and slowness of movements that develops over time and becomes debilitating. These motor symptoms are often accompanied by non-motor symptoms, including cognitive impairment, sleep disturbance, olfactory dysfunction, obsessive compulsive behavior, depression, constipation, and urinary difficulty. The pathological hallmark of PD that gives rise to the constellation of motor dysfunctions is the loss of dopamine-producing neurons within the substantia nigra pars compacta (SNpc). The loss of SNpc dopaminergic neurons results in a concomitant depletion of dopamine (DA) in the striatum, impairing the nigrostriatal system such that the execution of coordinated movements is prevented. The neuropathology of PD is widespread in the brain, with the involvement of SNpc beginning only in the middle stages of the disease. This disease stage is also accompanied by the formation of fibrillary cytoplasmic inclusions, known as Lewy bodies, which contain ubiquitin and α-synuclein [2]. The etiology of the disease remains poorly understood despite being recognized and intensively studied for nearly two centuries. Some symptomatic therapies exist, but none can cure or prevent PD. Epidemiological studies reveal that less than 10 % of PD cases have a strict familial etiology, while the majority of cases are sporadic and are thought to arise from the combination of environmental factors and susceptibility genes by mechanisms that are not fully understood. Thus, it is necessary to understand how the environment can affect the function of specific genes to fully understand their role in disease development and design strategies to alter these genes or the pathways that they modulate for therapeutic interventions. In this chapter, we provide an overview of how epigenetic analysis technology over recent years has revolutionized the depth of our understanding of both environmental factors and the function of familial PD-linked genes. This knowledge is essential in deciphering the molecular events leading to PD and in identifying prospective epigenetics-based neuroprotective therapies to prevent this disabling neurological illness.
What is Epigenetics?
An exact copy of the genome is replicated in every cell in our body. Despite this unanimity, cells differ functionally, each maintaining a dynamic yet highly regulated unique expression pattern constantly interacting with the environment. This constant repertoire of expression maintained throughout the lifespan above the basic regulation provided by the DNA sequence information constitutes the epigenome. Conrad Waddington in 1942 coined the term epigenetics and provided a basic definition: “the branch of biology which studies the causal interactions between genes and their products, which bring the phenotype into being” [3]. The recent interpretation of epigenetics involves all potentially heritable variations of gene expression without a change in DNA sequence [4]. Bird [5] put forth a more inclusive definition: “the structural adaptation of chromosomal regions so as to register, signal or perpetuate altered activity states.”
At the molecular level, epigenetic processes manipulate protein expression via post-translational modifications of histones (e.g., acetylation, methylation, phosphorylation, ubiquitination), the methylation of cytosine bases and positioning of nucleosome, and by employing microRNAs (miRNAs), long non-coding RNAs and circular RNAs. These processes control cell fate through regulating gene and miRNA expression, as well as through parental imprinting, X chromosome inactivation, suppressing of transposons, and regulating developmental processes [4, 6]. The epigenome provides the flexibility to address a changing environment above the rather rigid architecture of DNA sequence information and thus greatly affects the development and formation of a phenotype with no alteration in the genotype. Epigenetic modifications provide phenotypic plasticity for cells to react with the environment and are simultaneously essential for normal differentiation and development. In particular, environmental exposure to nutritional, chemical, physical, as well as intellectual or social factors can alter gene expression and affect an individual’s phenotype via epigenetic modifications at labile genomic regions. The three distinct mechanisms of epigenetic regulation that are complex and interrelated are DNA methylation, histone modification, and RNA-based mechanisms.
DNA Methylation
This is a covalent modification process that refers to the addition of a methyl group to the 5-position of those cytosines, which are followed by guanines (forming CpG dinucleotides referred to as the “fifth base of DNA”). DNA methylation plays a key role in regulating chromatin structure and remodeling, X chromosome inactivation and genomic imprinting. Methylation of the amino acid cytosine is the most widely studied epigenetic modification. 5-methyl-cytosine (5MeC) represents 2–5 % of all cytosines in mammalian genomes and is found on CpG dinucleotides which tend to cluster in regions called CpG islands [7]. DNA methylation is heritable in somatic cells and is carried along to the next generation. The methylation process is specific not only to CpG islands but also on CpG island shores, i.e., regions of low CpG density located near (~ 2 kb) to CpG islands. The hypermethylation of CpG sequences might induce a conformational modification in chromatin, thereby inhibiting the access of transcriptional machinery to the gene promoter regions and decreasing expression of the gene [8]. Conversely, the hypomethylation of a non-coding region causes chromosome instability [9]. Often, methylation occurring at CpG islands and shores located at promoter regions recruits methyl-CpG-binding domain (MBD) proteins, which in turn recruits epigenetic factors to remodel chromatin. Alternatively, CpG methylation within the gene body is sometimes associated with transcriptional activation [4]. The epigenetic regulators of DNA methylation are DNA methylation enzymes known as DNA methyltransferases (DNMT). In mammals, there are five members of the DNMT family; however, only three of them—DNMT1, DNMT3a, and DNMT3b—have methyltransferase activity [10]. They catalyze the transfer of a methyl group from S-adenosyl-L-methionine (SAM) to cytosine. All methylation reactions generate S-adenosylhomocysteine (SAH) as a by-product, which rapidly becomes metabolized to homocysteine. Because SAH has been shown to inhibit methylation-dependent reactions, the ratio of SAM to SAH is often used as an index of methylation status. DNA methylation serves as a principal epigenetic signal that participates in cell-specific gene expression and plays a quintessential role in multiple physiological processes to regulate gene expression, cellular differentiation, and development.
Histone Modification
The fundamental repeating unit of chromatin is the nucleosome, which is comprised of an octamer of core histone proteins. Posttranslational modifications of the amino (N)-terminal tails of histone proteins and the density of these proteins per unit length of DNA can significantly affect chromatin structure and constitute a putative “histone code” (defined as histone modifications). Histones are key players in epigenetics. The core histones subunits H2A, H2B, H3 and H4 along with the linker histone H1, form the nucleosome, which is wrapped around by 147 base pairs of DNA in 1.65 turns. Neighboring nucleosomes are separated by an average of ~50 bp of free DNA [11]. Each of the histone proteins has an N-terminal tail that protrudes from the DNA, making these linear amino-acid chains accessible for post-translational modifications, including acetylation, methylation, phosphorylation, and ubiquitination. The dynamic pattern of these chemical modifications on histones is essential for chromatin remodeling and, ultimately, for the regulation of gene expression. Histone acetylation by histone acetyl transferases (HATs) is primarily associated with transcriptional activation machinery, whereas deacetylation catalyzed by the enzymes known as histone deacetylases (HDACs), is associated with transcriptional repression. HATs such as p300/CREB binding protein (CBP) catalyze the transfer of an acetyl group from acetyl coenzyme A to a lysines of a histone tail [12]. There are 18 different HDACs known in humans. They are generally classified into four groups: Class I (HDACs 1,2,3 and 8); class II (HDACs 4,5,6,7,9 and 10); Class III (Sirtuins [SIRT] 1,2,3,4,5,6 and 7); and Class IV (HDAC11) [13]. Class III SIRTs require nicotine adenine dinucleotide (NAD+) for their active site, while all other HDACs are zinc-dependent. Cellular localization and tissue-specific expression of HDACs varies. HDAC2 is predominantly neuronal, whereas HDAC1 is strongly expressed in glia [14, 15]. Histone methylation occurs at either lysine or arginine side chains on H3 or H4. Methylation can cause either the activation or repression of transcription based on the occurrence or degree of methylation either at lysine or arginine residues on the histone tail. On H3, methylation at K4, K36, K79 and R2, R17, and R26 as well as H4R3 promote transcription. Alternatively, methylation of K9, K27 and R8 on H3, as well as H4K20 and H4R3 are repressive. Further degree of methylation and symmetry adds to the complexity of transcriptional regulation [16]. Histone lysine methyltransferase and protein arginine methyltransferases (PRMT) protein family enzymes are known to methylate histones, whereas two families of histone demethylases remove the methyl groups from histones. Phosphorylation and ubiquitination regulate gene expression by activating or repressing transcription, while sumoylation is only known to cause repressive modification of genes [16]. Post-translational modifications of histones regulate important physiological functions such as gene expression, chromatin remodeling, cell survival and death.
RNA-Based Mechanisms
These are regulated primarily by miRNAs. Once neglected as ‘junk’, the greater than over 98 % of the human genome that does not contain any protein coding sequence has been found to harbor new classes of regulatory elements. miRNAs are single-stranded RNAs approximately 21–23 nucleotides in length that are transcribed from DNA but not translated into proteins (and therefore referred to as small non-coding RNAs). They are well known as post-transcriptional regulators of gene expression with relevant roles in physiological and pathological aspects of the central nervous system [17]. miRNAs conduct targeted mRNA degradation or translational inhibition through sequence complementarity to the open reading frames and 3′-untranslated regions (3′UTRs) of mRNAs. One single species of miRNA is known to regulate the expression of several hundred different target genes, and a single gene can be regulated by hundreds of different miRNAs. In addition to their roles in pathophysiology and neuroprotection miRNAs are also known to play a major role in diverse biological processes, including cell proliferation, differentiation, and development.
Epigenetic Modulation in Familial Parkinson’s Disease
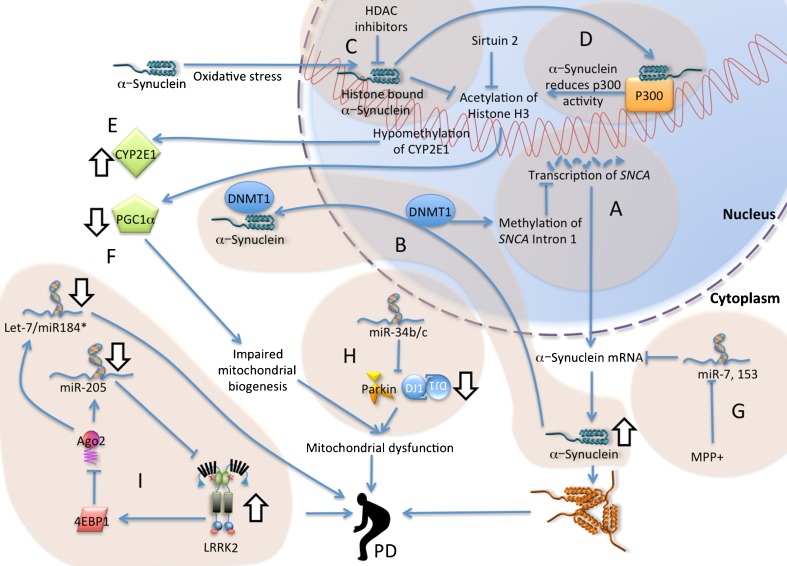
For the most part, PD is considered a sporadic disorder with onset later in life, and its etiology is incompletely understood. For most of the 20th century, genetic involvement was thought to play a negligible role in the disease, but over the past 15 years, the identification of distinct genetic loci responsible for (both the dominant and recessive) inherited forms of PD has provided us with tremendous insights into its molecular causes. To date, approximately 17 PD-related genetic loci have been described [18, 19]. Among these genes, five have been studied extensively: α-synuclein (also known as SNCA), parkin, PINK1, DJ-1, and leucine-rich repeat kinase 2 (LRRK2). Recent genome-wide association studies (GWAS) have revealed variations in two of the familial PD genes (SNCA and LRRK2) as important risk factors for sporadic PD [20]. In addition, other studies have uncovered variability in novel alleles associated with increased risk for PD [18]. The in silico meta-analyses of the GWAS data have allowed for more systematic and detailed investigations of the role of gene–gene and gene–environments interactions in PD. The emerging view from the existing knowledge is that the etiology of PD is multifactorial and presumably involves a complex interplay between a myriad of gene networks, including epigenetics, and the environment. However, the contribution of epigenetic mechanisms to the onset and development of both sporadic and genetic forms of PD is still emerging. The existing evidence based on the most studied familial genes regarding epigenetic alterations in PD are highlighted here (Fig. 1).
Fig. 1.
Epigenetic processes in familial PD. a Transcription at the SNCA locus is decreased following methylation of its intron 1. α-synuclein enters the nucleus following oxidative stress and (b) sequesters DNA methyl transferase 1 (DNMT1) into the cytoplasm, (c) binds to histones to reduce histone H3 acetylation and (d) binds to p300 to reduce histone acetyl transferase activity. e Hypomethylation of cytochrome P450 2E1 (CYP2E1) increases the transcription of this detoxifying gene. f Histone deacetylation contributes to a decrease in the expression of PGC1-α which controls mitochondrial biogenesis. HDAC inhibition rescues from nuclear α-synuclein toxicity. g miRs-7 and miR-153 targets the 3’UTR of SNCA (miR-7 is in turn suppressed by MPP+). h miR-34b/c downregulates Parkin and DJ-1 and in turn affects mitochondrial activity. i LRRK2 phosphorylate and activate 4EBP1, which through Argonaute-2 (Ago2) of RISC complex downregulates miRs let-7, -184* and −205. Familial PD genes Parkin, DJ-1, LRRK2 and SNCA, as well as mitochondrial dysfunctions are risk factors for PD. Abbreviations: 4EBP1 eukaryotic translation initiation factor 4E-binding protein 1, Ago2 Argonaute RISC Catalytic component 2, CYP2E1 cytochrome P450 2E1, DNMT1 DNA (cytosine-5)-methyltransferase 1, HDAC histone deacetylase, LRRK2 leucine-rich repeat kinase 2, miR miRNA, MPP + 1-methyl-4-phenyl-pyridinium ion, PD Parkinson’s disease, PGC1α- peroxisome proliferator-receptor gamma coactivator-1, SNCA α-synuclein
Evidence for the direct epigenetic regulation of familial PD-linked genes is emerging, and the most studied of these genes via epigenetics is SNCA. SNCA, which encodes the presynaptic protein α-synuclein, was the first causative familial PD gene identified. SNCA gene duplication and triplication as well as missense point mutations- A53T, A30P and E46K are known to cause autosomal dominant forms of PD and Lewy body (LB) pathology [19]. Moreover, single nucleotide polymorphisms within the SNCA promoter region or 3’UTR have been found in PD patients [21]. Irrespective of the differences between missense mutations and gene multiplications, both variations of SNCA are known to cause the accumulation of the α-synuclein protein into cytoplasmic aggregates, which serve as the structural component of LBs and are signature pathological hallmarks of sporadic PD. The toxic effects of α-synuclein are also exerted in the neuronal nuclei, where it has been hypothesized that α-synuclein can perturb the distribution and organization of DNA and the epigenetic modifications of histone [22]. Although the role of DNA methylation and its link to PD pathogenesis is currently unclear, methylation of the SNCA gene may be involved in pathology via structural changes or the overexpression of the protein, leading to protein aggregation, or via impaired gene expression. Methylation of SNCA intron 1 has been demonstrated to be associated with decreased SNCA transcription, whereas reduced methylation at this site was found to be decreased in several brain regions, including the substantia nigra, of sporadic PD patients, causing the increased expression of the SNCA gene [23, 24]. These results raise the possibility that the increased α-synuclein production that is associated with PD may result from increased SNCA expression, as a consequence of a decreased methylation state of the SNCA gene. Furthermore, it has been demonstrated that α-synuclein could sequester DNA methyltransferase 1 (which maintains DNA methylation) in the cytoplasm, leading to global DNA hypomethylation in PD and dementia with Lewy body (DLB) patients, as well as in transgenic human α-synuclein mouse models [25]. Conversely, the overexpression of DNMT1, in both cellular and transgenic mouse models, restored the nuclear level of the protein, underscoring the significance of epigenetic dysregulation in PD and Lewy body disorders. A differential methylation pattern of additional genes apart from SNCA, such as ARK16, GPNMB, and STX1B, has been reported in PD [26], although their mechanistic link to PD has yet to be established. Interestingly, studies in anorexia patients and alcoholics have also reported hypermethylation of the SNCA locus [27, 28]. However, epidemiological evidence linking such obsessive behaviors to PD pathogenesis is still lacking. Contrary to the study by Jowaed et al. [23], a high-resolution methylome study detected no difference in overall methylation at intron 1 of the SNCA gene compared to controls [29]. However, significant differences in hyper- and hypomethylation patterns existed in single CpG analysis at different points with little functional consequence of the formation of 5-methylcytosine emerging from the study. The epigenetic regulation of SNCA has also been reported in an A53T-linked familial case of PD. Lymphoblastoid cells and blood cells of a patient heterozygous for A53T SNCA exhibited monoallelic expression, and the epigenetic silencing of the mutated allele involved histone modification but not DNA methylation. The steady state mRNA levels derived from the normal SNCA allele in this patient exceeded those of the two normal SNCA alleles combined in matched control individuals [30].
The regulation of SNCA by epigenetic histone modifications is yet to be studied in human PD subjects. However, studies performed in pharmacologically and genetically manipulated animal models and cell lines that indicate that SNCA expression can be altered by histone modifications. Indeed, in Drosophila models, nuclear-targeted α-synuclein has been shown to bind to histones and reduce histone H3 acetylation through its association with the HDAC SIRT2 [22]. Consistent with these findings, the targeted downregulation of SIRT2 has been shown to rescue α-synuclein toxicity and dopaminergic cell loss in flies and in primary mesencephalic culture [31]. Moreover, toxicity associated with nuclear-targeted α-synuclein in both flies and SH-SY5Y cells can be rescued by employing HDAC inhibitors (HDACIs) [22, 31, 32]. In addition, oxidative stress is known to cause nuclear translocation of α-synuclein, which then binds to the promoter element (peroxisome proliferator-receptor gamma coactivator-1) PGC1-αto to cause histone deacetylation and lowered PGC1-α levels [33]. Significantly lower PGC1-α levels are reported in post-mortem PD SNpc neurons, and these lower levels are associated with a loss of mitochondrial function due to a reduction in mitochondrial biogenesis [34]. A recent study identified a region of the HAT p300 that is highly disordered and displays similarities to prion-like domains [35]. This region encoded as an alternative splicing variant independent of the acetyltransferase domain provides an interaction interface for various misfolded proteins, thereby promoting their aggregation. The HAT p300 enhanced the aggregation of misfolded proteins in cell models and in LBs of PD patients containing α-synuclein [35]. It is noteworthy that the expression of human wild-type α-synuclein at physiological levels in dopaminergic neuronal cells resulted in an isoform-dependent transcriptional suppression of PKCδ, a key oxidative stress-sensitive kinase [36].
The diminished PKCδ transactivation was mediated via the suppression of constitutive NFkB activity, resulting in decreased NFkB-mediated p65 acetylation and reduced α-synuclein induced p300 levels and its HAT activity to protect dopaminergic neuronal cells from apoptosis. These data point towards potentially disease-modifying effects of epigenetic histone modification in the regulation of α-synuclein function in the context of various PD-related neuropathologies.
Several miRNAs and their corresponding targets have been identified as regulating SNCA function, thus providing new mechanistic insights regarding the possibility of using RNA interference (RNAi) as a therapeutic approach. A 3’UTR polymorphism (rs1270208) in fibroblast growth factor 20 (FGF20) is linked to an increased risk of developing PD [37]. This single nucleotide polymorphism disrupted a binding site for miR-433 to increase the translation of FGF20 both in vitro and in vivo. This study also demonstrated that increased α-synuclein expression, both in cellular models and in postmortem PD brains, correlated with the increased translation of FGF20. Importantly, miR-433b overexpression in midbrain dopaminergic neuronal cultures was demonstrated to prevent dopaminergic differentiation, while miR-433b inhibition increased the numbers of tyrosine hydroxylase (TH)-positive neurons [37]. miR-7 and miR-153 were found to possess target sites in the 3’UTR of SNCA [38, 39]. miR-7 which is mainly expressed in neurons, has been demonstrated to repress α-synuclein protein levels via the 3’UTR of α-synuclein mRNA. Cellular toxicity induced by mutant forms of SNCA was attenuated by miR-7, suggesting that miR-7 plays a role in protection against neuronal death [38]. miR-153 is another miRNA known to repress α-synuclein expression at both the mRNA and protein levels [39]. Additionally, there are reports of the altered expression of miRNAs in in vivo α-synuclein models. In transgenic mice overexpressing human A30P α-synuclein, the levels of miR-10a, -10b, -212, -132 and −495 were found to follow an altered expression pattern compared to non-transgenic littermates [40]. In yet another study that analyzed the differential expression of miRNAs in human α-synuclein transgenic Caenorhabditis elegans, the expression of 12 miRNAs was altered, including miR-64, and miR-65 and those of the let-7 family [41]. However, human orthologs of these microRNAs are not yet known. Although the functional significance of these altered miRNA levels in these model systems have yet to be established, as stated above, miRNAs could be explored as therapeutic targets to block α-synuclein-induced abnormalities in PD.
The LRRK2 gene, responsible for the most common familial form of PD with autosomal dominant inheritance [19], is also regulated by epigenetics. In sporadic PD patients with enhanced LRRK2 expression, miR-205 was found to be downregulated [42]. miR-205 has a target sequence at the 3’UTR of LRRK2, and when introduced to neurons expressing the PD-causing LRRK2 mutation R1441G, miR-205 ameliorated neurite outgrowth defects. In a Drosophila model, expressing pathogenic mutants of LRRK2- I1915T or G2019S, antagonized the miRNA-mediated downregulation of transcription factors E2F1 and DP, which are implicated in cell cycle regulation and survival [43]. Mutant LRRK2 dysregulates the translation of these two factors by antagonizing miRNAs let-7 and miR-184*. Various genetic manipulations that interfere with these two microRNAs and lead to the increased expression of E2F1 and/or DP have toxic effects similar to those observed with mutant LRRK2 resulting in reduced locomotor activity and dopamine neuron count in flies. These manipulations include the deletion of let-7, antagomir-mediated inhibition of let-7 and miR-184* actions, and transgenic expression of a target protector that specifically interferes with the interaction between let-7 and its target site in the 3′-UTR of DP mRNA. Conversely, increasing the level of let-7 or miR-184* attenuates the pathogenic effects of LRRK2 effects. In all cases studied, the over-expression of E2F1 was sufficient to cause dopaminergic neuron degeneration and impaired locomotor activity [43].
The ability of LRRK2 to antagonize miRNAs is dependent on its kinase activity, a finding that further substantiates their relevance to PD. The common PD-causing LRRK2 mutants induce increased kinase activity, which is an important factor in neurodegeneration [44]. LRRK2-mediated miRNA regulation is exacerbated by these mutations, whereas a kinase-dead LRRK2 mutant does not affect gene repression [43]. The LRRK2 substrate 4E-BP associates with Argonaute 2, a component of the RNA-induced silencing complex (RISC), and this interaction is promoted by I1915T and G2019S mutants of LRRK2, presumably due to increased phosphorylation of 4E-BP1 [45]. Thus, compared to wild type, the phosphomimetic 4E-BP(TE) is more toxic to dopamine neurons and is more effective than wild-type 4E-BP in attenuating let-7 activity in vivo. In addition, in the aged fly brain, Drosophila Argonaute 1 protein levels are negatively regulated by LRRK2 [43]. LRRK2 may also affect gene expression and neuronal function by influencing Dicer. The deficits in dopaminegic neurons and climbing ability of the fly due to the inhibition of Dicer via RNAi are partially attenuated by knocking down LRRK2 [43]. These complex mechanisms by which mutant LRRK2 affects the miRNA machinery and causes neuronal death in the Drosophila must be replicated in a mammalian system, and their applicability to the human disease needs to be investigated.
In addition to SNCA and LRRK2, there are scarce reports of epigenetic modification of other familial PD-linked genes. A loss of function mutations in parkin is associated with an autosomal juvenile form of PD. A loss of heterozygosity of parkin has been observed in several types of malignant tumors, and abnormal methylation of the parkin promoter has been found in chronic myelogenous leukemia and acute lymphoblastic leukemia [46]. However, studies in human PD cases did not detect any difference in methylation of the parkin promoter, downplaying its significance in PD [47]. Similarly, the promoter of the deubiquitinating enzyme ubiquitin carboxy-terminal hydrolase L1 (UCHL1), another recessive PD-linked gene, is hypermethylated in cancer, but not differentially methylated in brain samples from PD patients [48]. In the same study, the methylation profiles of the MAPT promoter in the frontal cortex and hippocampus of subjects with Alzheimer’s disease, PD or tauopathies or synucleinopathies contained no differences compared to controls. Mutations of the ATP13A2 gene at the PARK9 locus cause an autosomal recessive juvenile form of PD called Kufor-Rakeb syndrome [19]. Analysis of the ATP13A2 promoter regions did not provide any tangible correlation between disease progression and DNA methylation. However, a large collaborative study between the International Parkinson's Disease Genomics Consortium (IPDGC) and the Wellcome Trust Case Control Consortium 2 identified PD risk variants of three loci, PARK16/1q32, GPNMB/7p15, and STX1B/16p11, that were associated with methylation and expression changes, suggesting that additional genes could be subject to epigenetic modification in PD brains [26].
The number of methylated sites in DNA increases with aging [49]. These changes may aid the very process of aging, which is a major risk factor for PD, or may arise as a response to increased stress during aging. A GWAS on methylation of candidate genes identified changes in methylation status of proximal DNA CpG sites of RAB7L1, GPNMB and STX1B [26]. Results from the GWAS have already provided many novel and significant insights for mechanisms of development of complex diseases like PD. However, it is plausible that interpreting specific epigenetic modifications could be greatly aided by knowledge of genetic susceptibility loci determined from GWAS. This would be particularly useful in loci that meet intermediate p-values in GWAS studies. Therefore a major avenue that has opened recently involves mating the advances in GWAS study with epigenetics [50]. Developing technologies that allow detection of fluctuations in epigenome in a genome wide scale could one day reveal perturbed regulatory networks that link multiple seemingly disparate genome loci and ultimately aid towards better diagnosis and treatment for PD. Overall, these studies support the notion that epigenetic modifications do influence the functioning of familial PD linked genes and could regulate the pathophysiological mechanisms associated with PD. However, more extensive investigation will be needed in this area to tease out the links of epigenetic modifications with various disease mechanisms and for subsequent therapeutic interventions.
Epigenetic Modifications in Idiopathic PD and due to Environmental Toxins
Approximately 90 % of PD cases are idiopathic and are characterized by late onset Parkinsonism. The causes of idiopathic PD are unknown but are considered to result from a combination of environmental factors and genetic predisposition. For example, living in a rural environment appears to confer an increased risk of PD, perhaps due to increased exposure to pesticides and wood preservatives. In addition, lifestyle choices such as consuming coffee and smoking cigarettes are inversely associated with risk of developing PD [51]. For sporadic PD, a definitive role of epigenetic modification in neurodegeneration has not yet been clearly established. Evidence for a role of DNA methylation in sporadic PD is based on homocysteine cycle dysregulation. Increased levels of plasma homocysteine have been reported in PD patients, which will increase SAH and decrease SAM, leading to an overall increase in methylation potential (the SAM/SAH ratio) [52]. A correlation between increased SAM/SAH ratios and better cognitive function in PD patients has been suggested, implicating an association of methylation with the disease process [53]. Evidence for fewer short telomeres with constant subtelomeric methylation status was demonstrated in peripheral leukocytes of Japanese PD patients compared to healthy controls. Subtelomeric hypomethylation is associated with the increased accessibility of DNA-binding proteins for suppressing the “telomeric position effect”, a mechanism known to silence genes neighboring a telomere. The telomeric and subtelomeric regions impaired by oxidative stress progress to become hypomethylated and are available for easy access by free radicals [54]. The most important findings linking epigenetic regulation to sporadic PD are related to abnormal miRNA profiles. Analysis of miRNA levels from blood samples of non-treated PD patients identified miR-1, miR-22*, and miR-29 as differently expressed compared to healthy subjects. miR-16-2*, miR-26a2*, and miR-30a were differentially expressed between treated and untreated PD patients [55]. Similarly, miRNA-profiling of PD brains revealed the early downregulation of miR-34b/c, which is known to modulate mitochondrial function. In particular, the downregulation of miR-34b/c was detected in premotor stages (stages 1–3) of the disease, such that these subjects did not receive any PD-related treatment during life. Additionally, miR-34b/c downregulation was coupled to a decrease in the expression of mitochondria-associated and familial PD-linked proteins DJ-1 and parkin [56]. These findings provide an intriguing connection between mitochondrial dysfunction and the genetic mechanisms of epigenetic regulation in PD pathogenesis.
On the environmental toxin front, the most concrete evidence of environmental exposure as a cause of PD was the accidental exposure of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), leading to PD-like symptoms in humans, which was subsequently validated in animal models [57, 58]. In addition to MPTP, other environmental toxins, such as pesticides and industrial agents [59, 60], have been associated with increased risk for developing PD, although a definitive causal role continues to be debated [61]. Emerging evidence indicates that chronic environmental exposure can alter gene expression via epigenetic mechanisms and can be a key risk factor in the pathogenesis of late-onset neurodegenerative diseases. For instance, in a significant number of genes, DNA methylation causes allelic skewing, such that one allele can be transcribed or expressed at a higher level than the other allele based on the maternal or paternal origin of the allele. This skewing may determine how an individual’s genotype can alter the effect, an environmental factor has on the risk of developing a neurodegenerative disorder [62]. Among the various epigenetic mechanisms, histone modification appears to be the most prominent epigenetic change observed due to environmental toxins such as pesticides (Table 1). Paraquat, a widely used herbicide, as well as the organochlorine insecticide dieldrin, are among the environmental chemicals potentially linked with PD [59]. Histone acetylation may represent a key epigenetic modification in dopaminergic neuronal cells due to neurotoxic insults. The exposure of paraquat to N27 dopaminergic cells induced histone H3 acetylation and decreased the total HDAC activity [63, 64]. Similarly, dieldrin induced a time-dependent increase in the acetylation of core histones H3 and H4 [63]. This hyperacetylation was attributed to dieldrin-induced proteasomal dysfunction, resulting in the accumulation of the critical HAT cAMP response element-binding protein (CBP). In mouse models, exposure to dieldrin induced histone acetylation in the nigrostriatal system. Anacardic acid, a HAT inhibitor, significantly attenuated dieldrin-induced histone acetylation and dieldrin-associated apoptosis, which was independent of its antioxidant effect [63]. In addition, administration of MPTP in murine and non-human primate models has been associated with decreased striatal presence of H3 histone K4 trimethylation, and chronic administration of the DA precursor L-DOPA has been demonstrated to reverse these changes [65]. Alterations in miRNA profiles are also reported following exposures with environmental toxins. Decreased miR-7 expression levels were reported in animal and cell culture models following exposure to MPP + (1-methyl-4-phenyl-pyridinium ion, a toxic metabolite of MPTP), thereby contributing to increased α-synuclein expression [38]. This is an interesting observation because DA neurodegeneration in PD is directly associated with expression levels of α-synuclein [66], suggesting that miR-7 could be a potential target for therapeutics. Collectively, the studies reviewed above suggest that the effects on the epigenome caused by chronic exposure of environmental toxins may lead to alterations in gene regulation, which could explain the noxious effects that these chemicals have on PD pathogenesis.
Table 1.
Epigenetic modulation due to environmental toxins and therapeutic drugs in Parkinson’s disease
| Chemical | Epigenetic modification | References |
|---|---|---|
| Toxins | ||
| MPTP | Demethylation | 65 |
| Paraquat | H3 Hyperacetylation | 63, 64 |
| Rotenone | Unknown mechanisms/HDAC inhibitor protect toxicity | 32 |
| Dieldrin | H3, H4 hyperacetylation | 63 |
| Chronic drug use | ||
| Levodopa | H4K5, K8, K12, K16 deacetylation | 65 |
| Histone acetyltransferase inhibitor | ||
| Anacardic acid | Deacetylation | 63 |
| HDAC (histone deacetylases) inhibitors | ||
| Valproic acid | H3 Hyperacetylation/ Promoter activation /Di, trimethylation | 70-73, 75 |
| Trichostatin A | Hyperacetylation/Promoter activation /Di, trimethylation | 74–76 |
| Suberoyl anilide hydroxamic acid | Hyperacetylation/Promoter activation /Di, trimethylation | 73–76 |
| Sodium butyrate | Hyperacetylation/Promoter activation | 73–78 |
| AGK2 | Hyper acetylation | 31 |
| MS-275 | Promoter activation /Di, trimethylation | 73, 77, 78 |
| Apicidin | Promoter activation /Di, trimethylation | 73, 77, 78 |
| Phenyl butyrate | Promoter activation | 79, 80 |
| Urocortin | Hyper acetylation | 81 |
MPTP 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, AGK2 a SIRT2 inhibitor, MS-275 entinostat
Therapeutic Approaches and Epigenetic Remodeling in PD
In recent years, there has been considerable progress in the development of epigenetic-based drugs for the treatment of neurodegenerative disorders. Several drugs that target epigenetic alterations are known and well studied (Table 1). Such inhibitors of HDACs and DNMTs are currently approved and available for clinical investigation [67]. One effective epigenetic pharmacological strategy for the selective reduction of genes could involve methylating cytosine bases in the promoter regions of their DNA by increasing the endogenous levels SAM, the methyl donor used in DNA methylation reactions, via exogenous delivery of methionine, choline, folates, and vitamin B12 [67]. The selectivity of the latter epigenetic therapeutic strategy depends on increasing DNA methylation, thereby interfering with the binding of transcription factors to the promoter regions that contain a high guanine-cytosine content of CpG-enriched dinucleotides. Increased levels of SAM could also lead to the binding of proteins that recognize methylated cytosine bases in enriched CpG islands in promoters and recruit enzymes with HDAC activity, leading to chromatin compaction as an alternative mechanism of gene repression [68]. DNMT inhibitors, also referred to as demethylating agents have been under preclinical and clinical investigation for a long period of time. The most widely studied inhibitors are the cytidine nucleoside analogs, which incorporate into DNA after activation by a triphosphate moiety [67]. After the formation of an irreversible complex with DNMT1, enzyme degradation occurs, which prevents the methylation of daughter DNA in CpG islands during DNA replication. In addition, the acetylation of histones is also a promising therapeutic target. Increased acetylation of histone proteins via HDAC inhibitors shifts the balance toward chromatin relaxation, thereby activating gene expression.
In human PD patients, the current pharmacological agents used for therapy are those that provide symptomatic relief because drugs that stop or delay the progression of the disease are still lacking. Among the drugs used for symptomatic relief is the psychoactive drug and DA precursor levodopa (L-DOPA). Unfortunately, the chronic use of L-DOPA is associated with the development of complications, such as wearing-off and L-DOPA induced dyskinesia (LDID). In animal models of LDID, dopamine depletion was associated with a marked reduction in histone H3 trimethylation at Lys 4, while chronic L-DOPA therapy leading to hyperkinesia was marked by the deacetylation of histone H4 at K5, K8, K12, and K16 in the striatum [65]. This study highlighted the presence of histone modifications and supported the hypothesis that chromatin remodeling could contribute to the development and persistence of LDID in PD. Moreover, one study of the dopamine D1 receptor (shown to be associated with L-DOPA-induced dyskinesia) demonstrated that H3 phosphoacetylation was blocked by D1 receptor inactivation, suggesting that H3 acetylation and/or phosphorylation inhibitors could be useful in preventing or reversing dyskinesia [69].
Enhancement of histone acetylation has been studied for its potent neuroprotective effects in several Parkinsonism models. Therefore HDAC inhibitors are the most recent emerging therapeutic targets in the treatment of PD. HDAC inhibitors can be classified into four main chemical families: the short chain fatty acids (e.g., sodium butyrate [SB], phenylbutyrate [4PBA], and valproic acid [VPA]; the hydroxamic acids (e.g. trichostatin A [TSA] and suberoylanilide hydroxamic acid [SAHA]); the epoxyketones (e.g. trapoxin); and the benzamides. One important study demonstrated that the neurotoxicity of α-synuclein in the nucleus could be rescued by the administration of HDAC inhibitors in both cellular models and transgenic fly models [22]. α-synuclein was found to bind directly to histones, reducing the levels of acetylated histone H3 in cultured cells and inhibiting acetylation in HAT assays [22]. Similarly, the pharmacological inhibition of SIRT2 by AGK2 rescued α-synuclein toxicity and modified inclusion morphology in cellular and fly models of PD [31]. Other HDAC inhibitors such as VPA, which has been extensively studied and have been shown to protect against MPTP, rotenone, lipopolysaccharide, and α-synuclein-mediated toxicity by enhancing H3 histone acetylation, accompanied by a decrease in inflammatory mediators and increased apoptosis in microglial cells [70–72]. VPA treatment enhanced the expression of brain-derived neurotrophic factor (BDNF) and glial cell-derived neurotrophic factor (GDNF) in in vitro primary astrocyte cell culture. Both GDNF and BDNF are known to play critical roles in the growth, survival, and synaptic plasticity of neurons. The inhibitory effect of VPA on HDACs indirectly induces histone H3 acetylation within the GDNF promoter, resulting in increased GDNF mRNA levels. VPA treatment appeared to markedly up-regulated the Hsp70 protein levels, and this upregulation was accompanied by promoter hyperacetylation and increased levels of histone 3 lysine 4 dimethylation and trimethylation (H3K4Me2 and H3K4Me3). These processes were linked to the recruitment of the HAT p300 and to the transcriptional activation of Hsp70 in rat cortical neurons and astrocytes [73]. Similar to VPA, TSA, SAHA and SB enhanced GDNF promoter activity and promoter-associated histone H3 acetylation in astrocyte cell culture indicating that HDAC inhibitors upregulate GDNF and BDNF expression in astrocytes and protect DA neurons [74–76]. TSA and other HDAC inhibitors including SB, and Class I HDAC-specific inhibitors, such as MS-275 and apicidin, all mimicked the ability of VPA to induce Hsp70 [73, 77, 78]. HDAC inhibitors are also known to modulate important cytoprotective pathways independent of their actions on histones, providing additional beneficial effects to therapy. For instance, 4PBA has been demonstrated to protect DA neurons, possibly via increased DJ-1 expression and the activation of the tyrosine hydroxylase promoter in the substantia nigra [79]. In another study, 4PBA was found to alter gene transcription in a number of genes associated with antioxidant enzyme chaperones, including those that are critical for cell survival [80]. Consistent with these mechanisms, treatment with 4PBA rescued MPTP-induced dopaminergic neurotoxicity in mice [80]. Several HDAC inhibitors including urocortin and VPA inhibit the activity of glycogen synthase kinase 3 β (Gsk3β), which is negatively correlated with neuronal viability [81]. Together, these data suggest that HDAC inhibitors can render disease modifying therapeutic benefits in various model systems related to PD, and these pharmacological agents could also activate additional genes or pathways to render additive neuroprotective effects. These pharmacological studies further reinforce the mechanistic basis of epigenetic regulation in the pathogenesis of PD.
Concluding Remarks
It is evident that epigenetic mechanisms are responsible for orchestrating complex biological processes. However, we are just beginning to understand the role played by epigenetic regulation in the pathophysiology of complex multifactorial diseases such as PD. Epigenetics may represent a missing link between genetic and environmental factors and their potential interactions in PD pathophysiology. Research has revealed that DNA methylation histone modification, and alterations in miRNA profiles often precede disease pathology and that these might prove useful as early disease biomarkers. Additionally, they might be targeted with epigenetic drugs, such as DNMT inhibitors, HDAC inhibitors or drugs that target histone demethylases, histone methyltransferases or SIRTs. However, future studies are needed to analyze epigenetic pathways, elaborate epigenetic profiles and to correlate these molecular signatures with genome-wide association studies, and with clinical features and outcomes in PD. In particular, the Human Epigenome Project has begun cataloguing and interpreting epigenetic profiles and their association with various disease states [82]. These epigenomic data should ultimately integrate genomic and phenomic (e.g., transcriptomic, proteomic, metabolomics) profiles in the context of both familial and sporadic PD to generate a comprehensive understanding of the disease. We anticipate that new and evolving technologies to better understand the epigenetic landscape of PD will give rise to better molecular tools to improve the prognosis, diagnosis and ultimately, therapeutic intervention.
Electronic Supplementary Material
(PDF 498 kb)
Acknowledgments
N.A.K is a Parkinson’s Disease Foundation fellow. This work was supported in part by grants from the National Institutes of Health (grant #NS060885), the Michael J Fox Foundation for Parkinson’s disease, and STARTUP funds from the Medical College of Georgia to B.T.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Navneet Ammal Kaidery and Shaista Tarannum contributed equally to this work.
Contributor Information
Navneet Ammal Kaidery, Phone: +1-706-7216356, FAX: +1-706-7212347.
Shaista Tarannum, Phone: +1-706-7216356, FAX: +1-706-7212347.
Bobby Thomas, Email: bthomas1@gru.edu.
References
- 1.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55(3):259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann NY Acad Sci. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- 4.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 5.Bird A. Perceptions of epigenetics. Nature. 2007;447(7143):396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 6.Jiang C, Pugh BF. Nucleosome positioning and gene regulation: advances through genomics. Nat Rev Genet. 2009;10(3):161–172. doi: 10.1038/nrg2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat Struct Mol Biol. 2013;20(3):274–281. doi: 10.1038/nsmb.2518. [DOI] [PubMed] [Google Scholar]
- 8.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108(4):439–451. doi: 10.1016/S0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe Y, Maekawa M. Methylation of DNA in cancer. Adv Clin Chem. 2010;52:145–167. doi: 10.1016/S0065-2423(10)52006-7. [DOI] [PubMed] [Google Scholar]
- 10.Kinney SR, Pradhan S. Regulation of expression and activity of DNA (cytosine-5) methyltransferases in mammalian cells. Prog Mol Biol Transl Sci. 2011;101:311–333. doi: 10.1016/B978-0-12-387685-0.00009-3. [DOI] [PubMed] [Google Scholar]
- 11.Luger K. Structure and dynamic behavior of nucleosomes. Curr Opin Genet Dev. 2003;13(2):127–135. doi: 10.1016/S0959-437X(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 12.Marmorstein R, Roth SY. Histone acetyltransferases: function, structure, and catalysis. Curr Opin Genet Dev. 2001;11(2):155–161. doi: 10.1016/S0959-437X(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 13.Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res. 2007;5(10):981–989. doi: 10.1158/1541-7786.MCR-07-0324. [DOI] [PubMed] [Google Scholar]
- 14.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MacDonald JL, Roskams AJ. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev Dyn. 2008;237(8):2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- 16.Habibi E, Masoudi-Nejad A, Abdolmaleky HM, Haggarty SJ. Emerging roles of epigenetic mechanisms in Parkinson’s disease. Funct Integr Genomics. 2011;11(4):523–537. doi: 10.1007/s10142-011-0246-z. [DOI] [PubMed] [Google Scholar]
- 17.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 18.Houlden H, Singleton AB. The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol. 2012;124(3):325–338. doi: 10.1007/s00401-012-1013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas B, Beal MF. Molecular insights into Parkinson’s disease. F1000 Med Rep. 2011;3:7. [DOI] [PMC free article] [PubMed]
- 20.Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet. 2009;41(12):1303–1307. doi: 10.1038/ng.485. [DOI] [PubMed] [Google Scholar]
- 21.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. [DOI] [PMC free article] [PubMed]
- 22.Kontopoulos E, Parvin JD, Feany MB. Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Human molecular genetics. 2006;15(20):3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- 23.Jowaed A, Schmitt I, Kaut O, Wullner U. Methylation regulates alpha-synuclein expression and is decreased in Parkinson’s disease patients’ brains. J Neurosci. 2010;30(18):6355–6359. doi: 10.1523/JNEUROSCI.6119-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto L, Takuma H, Tamaoka A, Kurisaki H, Date H, Tsuji S, et al. CpG demethylation enhances alpha-synuclein expression and affects the pathogenesis of Parkinson’s disease. PLoS One. 2010;5(11):e15522. doi: 10.1371/journal.pone.0015522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desplats P, Spencer B, Coffee E, Patel P, Michael S, Patrick C, et al. Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem. 2011;286(11):9031–9037. doi: 10.1074/jbc.C110.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A two-stage meta-analysis identifies several new loci for Parkinson’s disease. PLoS genetics. 2011;7(6):e1002142. [DOI] [PMC free article] [PubMed]
- 27.Bonsch D, Lenz B, Kornhuber J, Bleich S. DNA hypermethylation of the alpha synuclein promoter in patients with alcoholism. Neuroreport. 2005;16(2):167–170. doi: 10.1097/00001756-200502080-00020. [DOI] [PubMed] [Google Scholar]
- 28.Frieling H, Gozner A, Romer KD, Lenz B, Bonsch D, Wilhelm J, et al. Global DNA hypomethylation and DNA hypermethylation of the alpha synuclein promoter in females with anorexia nervosa. Mol Psychiatry. 2007;12(3):229–230. doi: 10.1038/sj.mp.4001931. [DOI] [PubMed] [Google Scholar]
- 29.de Boni L, Tierling S, Roeber S, Walter J, Giese A, Kretzschmar HA. Next-generation sequencing reveals regional differences of the alpha-synuclein methylation state independent of Lewy body disease. Neuromolecular Med. 2011;13(4):310–320. doi: 10.1007/s12017-011-8163-9. [DOI] [PubMed] [Google Scholar]
- 30.Voutsinas GE, Stavrou EF, Karousos G, Dasoula A, Papachatzopoulou A, Syrrou M, et al. Allelic imbalance of expression and epigenetic regulation within the alpha-synuclein wild-type and p.Ala53Thr alleles in Parkinson disease. Hum Mutat. 2010;31(6):685–691. doi: 10.1002/humu.21248. [DOI] [PubMed] [Google Scholar]
- 31.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317(5837):516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 32.St Laurent R, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, et al. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free Radical Biol Med. 2012;53(4):993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci TransMed. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirilyuk A, Shimoji M, Catania J, Sahu G, Pattabiraman N, Giordano A, et al. An intrinsically disordered region of the acetyltransferase p300 with similarity to prion-like domains plays a role in aggregation. PLoS One. 2012;7(11):e48243. doi: 10.1371/journal.pone.0048243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin H, Kanthasamy A, Ghosh A, Yang Y, Anantharam V, Kanthasamy AG. alpha-Synuclein negatively regulates protein kinase Cdelta expression to suppress apoptosis in dopaminergic neurons by reducing p300 histone acetyltransferase activity. J Neurosci. 2011;31(6):2035–2051. doi: 10.1523/JNEUROSCI.5634-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, et al. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82(2):283–289. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junn E, Lee KW, Jeong BS, Chan TW, Im JY, Mouradian MM. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc Natl Acad Sci USA. 2009;106(31):13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doxakis E. Post-transcriptional regulation of alpha-synuclein expression by mir-7 and mir-153. J Biol Chem. 2010;285(17):12726–12734. doi: 10.1074/jbc.M109.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillardon F, Mack M, Rist W, Schnack C, Lenter M, Hildebrandt T, et al. MicroRNA and proteome expression profiling in early-symptomatic alpha-synuclein(A30P)-transgenic mice. Proteomics Clin Appl. 2008;2(5):697–705. doi: 10.1002/prca.200780025. [DOI] [PubMed] [Google Scholar]
- 41.Asikainen S, Rudgalvyte M, Heikkinen L, Louhiranta K, Lakso M, Wong G, et al. Global microRNA expression profiling of Caenorhabditis elegans Parkinson’s disease models. J Mol Neurosci. 2010;41(1):210–218. doi: 10.1007/s12031-009-9325-1. [DOI] [PubMed] [Google Scholar]
- 42.Cho HJ, Liu G, Jin SM, Parisiadou L, Xie C, Yu J, et al. MicroRNA-205 regulates the expression of Parkinson’s disease-related leucine-rich repeat kinase 2 protein. Human molecular genetics. 2013;22(3):608–620. doi: 10.1093/hmg/dds470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gehrke S, Imai Y, Sokol N, Lu B. Pathogenic LRRK2 negatively regulates microRNA-mediated translational repression. Nature. 2010;466(7306):637–641. doi: 10.1038/nature09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9(10):1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 45.Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, et al. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27(18):2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agirre X, Roman-Gomez J, Vazquez I, Jimenez-Velasco A, Garate L, Montiel-Duarte C, et al. Abnormal methylation of the common PARK2 and PACRG promoter is associated with downregulation of gene expression in acute lymphoblastic leukemia and chronic myeloid leukemia. Int J Cancer. 2006;118(8):1945–1953. doi: 10.1002/ijc.21584. [DOI] [PubMed] [Google Scholar]
- 47.Cai M, Tian J, Zhao GH, Luo W, Zhang BR. Study of methylation levels of parkin gene promoter in Parkinson’s disease patients. Int J Neurosci. 2011;121(9):497–502. doi: 10.3109/00207454.2011.580866. [DOI] [PubMed] [Google Scholar]
- 48.Barrachina M, Ferrer I. DNA methylation of Alzheimer disease and tauopathy-related genes in postmortem brain. J Neuropathol Exp Neurol. 2009;68(8):880–891. doi: 10.1097/NEN.0b013e3181af2e46. [DOI] [PubMed] [Google Scholar]
- 49.Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, van der Brug M, Chong S, et al. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Human Molec Genet. 2011;20(6):1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardison RC. Genome-wide epigenetic data facilitate understanding of disease susceptibility association studies. J Biol Chem. 2012;287(37):30932–30940. doi: 10.1074/jbc.R112.352427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 52.Blandini F, Fancellu R, Martignoni E, Mangiagalli A, Pacchetti C, Samuele A, et al. Plasma homocysteine and l-dopa metabolism in patients with Parkinson disease. Clin Chem. 2001;47(6):1102–1104. [PubMed] [Google Scholar]
- 53.Obeid R, Schadt A, Dillmann U, Kostopoulos P, Fassbender K, Herrmann W. Methylation status and neurodegenerative markers in Parkinson disease. Clin Chem. 2009;55(10):1852–1860. doi: 10.1373/clinchem.2009.125021. [DOI] [PubMed] [Google Scholar]
- 54.Maeda T, Guan JZ, Oyama J, Higuchi Y, Makino N. Aging-associated alteration of subtelomeric methylation in Parkinson’s disease. J Gerontol A Biol Sci Med Sci. 2009;64(9):949–955. doi: 10.1093/gerona/glp070. [DOI] [PubMed] [Google Scholar]
- 55.Margis R, Margis R, Rieder CR. Identification of blood microRNAs associated to Parkinsonis disease. J Biotechnol. 2011;152(3):96–101. doi: 10.1016/j.jbiotec.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 56.Minones-Moyano E, Porta S, Escaramis G, Rabionet R, Iraola S, Kagerbauer B, et al. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Human Molec Genet. 2011;20(15):3067–3078. doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 57.Kopin IJ. Toxins and Parkinson’s disease: MPTP parkinsonism in humans and animals. Adv Neurol. 1987;45:137–144. [PubMed] [Google Scholar]
- 58.Fukuda T. Neurotoxicity of MPTP. Neuropathology. 2001;21(4):323–332. doi: 10.1046/j.1440-1789.2001.00402.x. [DOI] [PubMed] [Google Scholar]
- 59.Van Maele-Fabry G, Hoet P, Vilain F, Lison D. Occupational exposure to pesticides and Parkinson’s disease: a systematic review and meta-analysis of cohort studies. Environ Int. 2012;46:30–43. doi: 10.1016/j.envint.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Zaheer F, Slevin JT. Trichloroethylene and Parkinson disease. Neurol Clin. 2011;29(3):657–665. doi: 10.1016/j.ncl.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Franco R, Li S, Rodriguez-Rocha H, Burns M, Panayiotidis MI. Molecular mechanisms of pesticide-induced neurotoxicity: Relevance to Parkinson’s disease. Chem Biol Interact. 2010;188(2):289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanthasamy A, Jin H, Anantharam V, Sondarva G, Rangasamy V, Rana A, et al. Emerging neurotoxic mechanisms in environmental factors-induced neurodegeneration. Neurotoxicology. 2012;33(4):833–837. doi: 10.1016/j.neuro.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song C, Kanthasamy A, Anantharam V, Sun F, Kanthasamy AG. Environmental neurotoxic pesticide increases histone acetylation to promote apoptosis in dopaminergic neuronal cells: relevance to epigenetic mechanisms of neurodegeneration. Mol Pharmacol. 2010;77(4):621–632. doi: 10.1124/mol.109.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song C, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG. Paraquat induces epigenetic changes by promoting histone acetylation in cell culture models of dopaminergic degeneration. Neurotoxicology. 2011;32(5):586–595. doi: 10.1016/j.neuro.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicholas AP, Lubin FD, Hallett PJ, Vattem P, Ravenscroft P, Bezard E, et al. Striatal histone modifications in models of levodopa-induced dyskinesia. J Neurochem. 2008;106(1):486–494. doi: 10.1111/j.1471-4159.2008.05417.x. [DOI] [PubMed] [Google Scholar]
- 66.Thomas B, Mandir AS, West N, Liu Y, Andrabi SA, Stirling W, et al. Resistance to MPTP-neurotoxicity in alpha-synuclein knockout mice is complemented by human alpha-synuclein and associated with increased beta-synuclein and Akt activation. PLoS One. 2011;6(1):e16706. doi: 10.1371/journal.pone.0016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Z, Li H, Jin P. Epigenetics-based therapeutics for neurodegenerative disorders. Curr Transl Geriatr Exp Gerontol Rep. 2012;1(4):229–236. doi: 10.1007/s13670-012-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Human Molec Genet. 2008;17(23):3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66(6):603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 70.Kidd SK, Schneider JS. Protective effects of valproic acid on the nigrostriatal dopamine system in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2011;194:189–194. doi: 10.1016/j.neuroscience.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, et al. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience. 2007;149(1):203–212. doi: 10.1016/j.neuroscience.2007.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peng GS, Li G, Tzeng NS, Chen PS, Chuang DM, Hsu YD, et al. Valproate pretreatment protects dopaminergic neurons from LPS-induced neurotoxicity in rat primary midbrain cultures: role of microglia. Brain Res Mol Brain Res. 2005;134(1):162–169. doi: 10.1016/j.molbrainres.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 73.Marinova Z, Ren M, Wendland JR, Leng Y, Liang MH, Yasuda S, et al. Valproic acid induces functional heat-shock protein 70 via class I histone deacetylase inhibition in cortical neurons: a potential role of Sp1 acetylation. J Neurochem. 2009;111(4):976–987. doi: 10.1111/j.1471-4159.2009.06385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP + −mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen H, Dzitoyeva S, Manev H. Effect of valproic acid on mitochondrial epigenetics. Eur J Pharmacol. 2012;690(1–3):51–59. doi: 10.1016/j.ejphar.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11(8):1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marinova Z, Leng Y, Leeds P, Chuang DM. Histone deacetylase inhibition alters histone methylation associated with heat shock protein 70 promoter modifications in astrocytes and neurons. Neuropharmacology. 2011;60(7–8):1109–1115. doi: 10.1016/j.neuropharm.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leng Y, Marinova Z, Reis-Fernandes MA, Nau H, Chuang DM. Potent neuroprotective effects of novel structural derivatives of valproic acid: potential roles of HDAC inhibition and HSP70 induction. Neurosci Lett. 2010;476(3):127–132. doi: 10.1016/j.neulet.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou W, Bercury K, Cummiskey J, Luong N, Lebin J, Freed CR. Phenylbutyrate up-regulates the DJ-1 protein and protects neurons in cell culture and in animal models of Parkinson disease. J Biol Chem. 2011;286(17):14941–14951. doi: 10.1074/jbc.M110.211029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy A, Ghosh A, Jana A, Liu X, Brahmachari S, Gendelman HE, et al. Sodium phenylbutyrate controls neuroinflammatory and antioxidant activities and protects dopaminergic neurons in mouse models of Parkinson’s disease. PLoS One. 2012;7(6):e38113. doi: 10.1371/journal.pone.0038113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang HY, Lin SZ, Chen WF, Li KW, Kuo JS, Wang MJ. Urocortin modulates dopaminergic neuronal survival via inhibition of glycogen synthase kinase-3beta and histone deacetylase. Neurobiol Aging. 2011;32(9):1662–1677. doi: 10.1016/j.neurobiolaging.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 82.Moving AHEAD with an international human epigenome project. Nature. 2008;454(7205):711–5. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 498 kb)