Abstract
It is well known that most of the ionizing radiation-induced damage is caused by hydroxyl radicals (·OH) follows radiolysis of H2O. Molecular hydrogen (H2) has antioxidant activities by selectively reducing ·OH and peroxynitrite(ONOO-). We firstly hypothesized and demonstrated the radioprotective effect of H2 in vitro and in vivo, which was also repeated on different experimental animal models by different departments. A randomized, placebo-controlled study showed that consumption of hydrogen-rich water reduces the biological reaction to radiation-induced oxidative stress without compromising anti-tumor effects. These encouraging results suggested that H2 represents a potentially novel preventative strategy for radiation-induced oxidative injuries. H2 is explosive. Therefore, administration of hydrogen-rich solution (physiological saline/pure water/other solutions saturated with H2) may be more practical in daily life and more suitable for daily consumption. This review focuses on major scientific and clinical advances of hydrogen-rich solution/H2 as a new class of radioprotective agent.
Keywords: Hydrogen, free radical, radiation, radioprotection.
Introduction
Great benefits for people have been got because of extensive utilization of atom energy in many fields such as medicine, civil industry, agriculture, military etc. In the meantime, how to protect human health and safety from severe nuclear radiation was always a critical problem in the world 1. It is a novel scientific topic for radiologists to look for high-effective radiation protectants with low toxicity. In fact, exploitation the ideal radiation protectants has always been emphasized in the field of radiation 2, 3. However, it is always an obstacle. As we know, damaging effects of radiation are divided into direct effect and indirect effect, which accounts for about most of the damages 4. Since the indirect effect mainly due to the abundant free radicals caused from radiation, so blocking and scavenging of free radicals become our most important protecting strategy. In 2007, Ohsawa et al. 5 found that molecular hydrogen could selectively reduce cytotoxic reactive oxygen species in vitro and exert therapeutic antioxidant activity. From then on, research on hydrogen set off a worldwide upsurge 6-10. Hydrogen could selectively reduce hydroxyl radicals excited our interest, because most of irradiation induced injury was caused by hydroxyl radicals. Therefore, we firstly reasoned and demonstrated that hydrogen might have great radioprotective effects in 201011, 12. Since then, application of hydrogen on radioprotection was well investigated 13-17. It was also then used in clinic to improve the quality of life of patients treated with radiotherapy for liver tumours 18. The objective of this review was to offer an overview of major scientific and clinical advances of hydrogen as a new class of radioprotective agent.
Detrimental biological effects of radiation
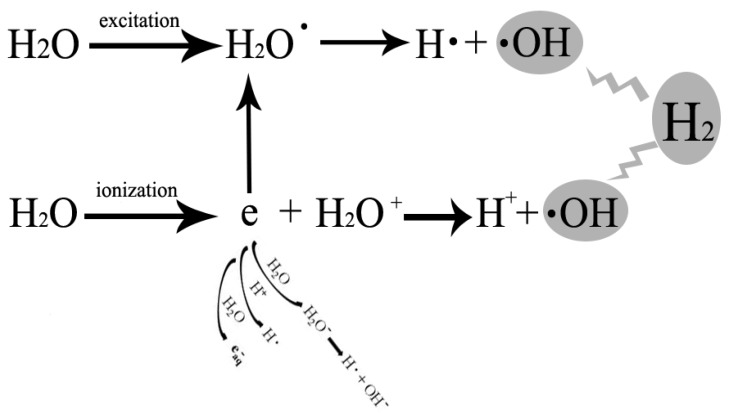
The detrimental biological effects of radiation are divided into direct effect and indirect effect. In physics, radiation is a process in which energetic particles travel through a medium or space. Direct detrimental biological effect of radiation is caused by radiation energy in the exposure pathway, which targets molecules including DNA, proteins and lipids, et al 14. Direct effect could be prevented by radiation shielding materials. However, indirect detrimental biological effect of radiation is caused by free radicals generated from radiolysis of H2O (Figure 1). Among those free radicals generated from radiolysis of H2O, hydroxyl radical is the most reactive product. Detrimental effects of IR on biological tissues are, in major part, mediated via increased production of hydroxyl radicals. It was estimated that 60-70% of the IR-induced cellular damage was caused by hydroxyl radicals 4. Hydroxyl radicals produced during radiolysis of water can trigger oxidation of DNA, lipids, amino acids, saccharides etc leading to formation of various secondary free radicals. These free radicals can produce severe health impairments due to injury and failure to susceptible cells and organs19, 20. DNA is one of the major targets of free radicals and 8-OHdG is formed from deoxyguanosine in DNA by hydroxyl radicals 21, which is also a biomarker of carcinogenesis 22. Membrane lipids are also one of the major targets of free radicals 23. Lipid peroxidation products including malondialdehyde, TBARs, etc are the indices of lipid damage 24, leading to changes in membrane permeability 25, 26. Structure of proteins could also be changed by hydroxyl radical and other free radicals, which induced the change in the function of proteins 27.
Figure 1.
Free radicals generated during radiolysis of water and cytotoxic oxygen radicals that hydrogen could selectively reduce. Initial radiation chemistry of ionization and excitation is to generate H2O+ and e- from the ionization of H2O, and hydrogen atom (H∙) and hydroxyl radical (∙OH) from the excited H2O. ∙OH is the cytotoxic oxygen radical that hydrogen could selectively reduce which caused most of the IR-induced cellular damage.
Hydrogen
Hydrogen is the lightest and most abundant chemical element. Molecular hydrogen is a colorless, odorless, nonmetallic, tasteless, highly flammable diatomic gas which was firstly documented by Philippus Aureolus Paracelsus in 1520 as a kind of flammable gas 28. It was named by Lavoisier in 1783 by a french word” hydrogene” 28. However, hydrogen was extensively used in aeronautical nature such as hydrogen balloon and in chemical field such as fuel processing, fertilizer production ((3H2 + N2 → 2NH3), etc since 1783 while it was seldom used in medicine. Early in 1888, Pilcher JE et al. reported one of the very publications linking hydrogen and medicine. They reported that hydrogen gas was insufflated the gastro-intestinal canal to locate visceral injuries, avoiding unwarranted surgeries 29. This paper is probably the first document linking hydrogen and medicine. Until 1975, Dole et al. 30 reported that hyperbaric hydrogen may be a possible treatment for cancer. They found a marked regression of the skin squamous cell carcinoma by inhalation of a mixture (2.5% O 2 and 97.5% H 2) at a total of 0.8 MPa for 2 weeks in a mouse model. They tried to elucidate the phenomenon with the possibility of H2 as a free radical decay catalyser. In 1988, H2 was demonstrated reducing hydroxyl radicals produced by radiolysis or photolysis of H2O in cell free systems by Buxton et al 31. In 2001, Gharib B et al. reported that H2 attenuated schistosomiasis-associated chronic liver inflammation in mice 32. However, these investigations did not draw attention from researchers. Until 2007, Ohsawa et al. 5 discovered that hydrogen gas has antioxidant and antiapoptotic properties that protect the brain against ischemia-reperfusion injury and stroke by selectively neutralizing hydroxyl and peroxynitrite radicals. This study was well designed and took hydrogen gas into a new era. It has been demonstrated that H2 exerted preventive or therapeutic effects on cerebral, myocardial, hepatic ischemia-reperfusion injuries, intestinal transplantation, lung transplantation, renal transplantation, heart transplantation, acute graft-versus-host disease post allogeneic hematopoietic stem cell transplantation, etc 33, 34. Recent basic and clinical research revealed that hydrogen is an important physiological regulatory factor with antioxidant, anti-inflammatory and anti-apoptotic protective effects on cells and organs 35-40. Hydrogen was also demonstrated has radioprotective effects on cultured cells and mice 11-16, 41 (Figure 2). H2 exert antioxidative effects with few toxic side effects. Saitoh et al. detected mutagenicity, genotoxicity and subchronic oral toxicity of hydrogen in a rat model 42. In the model they observed significant changes of basophil ratio of blood in female rats and decreased aspartate aminotransferase and alanine aminotransferase in male rats. However, these changes were not considered biologically significant. In another study on human beings, Nakao A et al. also observed similar clinical chemistry parameters 9.
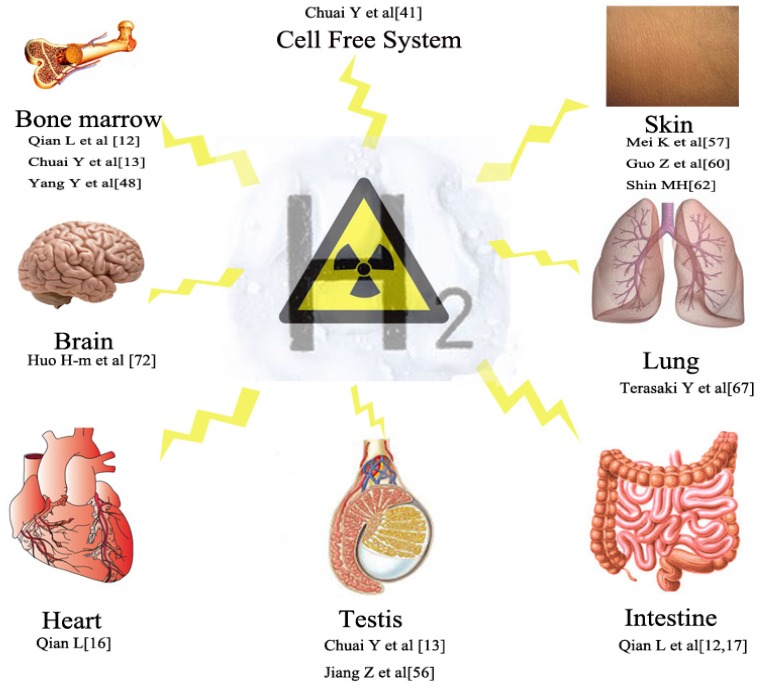
Figure 2.
Radioprotective effects of hydrogen on different systems including cell free system, intestine, haematopoietic system, testis, skin, Lung, cardiovascular, brain, etc.
Radioprotective effects of hydrogen Cell free system
In cell free systems, our group produced ∙OH by radiolysis of H2O and Fenton reaction 41. In the study, we found that 71.2% of ·OH in the Fenton reaction was reduced by H2 dissolved in phosphate buffer (10 mM) solution at 23 ℃ and pH 7.4 under 0.4 MPa for 2 hour, while 88.7% of ·OH was reduced in radiolysis of H2O by 5 Gy 60Co γ -ray. When H2 was replaced with nitrogen (N2), the levels of ·OH did show significant change.
Intestine
The gastrointestinal tract is one of the most susceptible organs to radiation 43. As low as 1 Gy of radiation induces dramatic increase in apoptosis in mouse small intestinal crypt within three to six hours after exposure, predominantly in the stem cell region 44 . To study the radioprotective effects of H2 on intestine, we chose Human intestinal crypt HIEC cells to study which was sensitive to irradiation 12, 17. In the study, cells were treated with or without different concentrations (0.1-0.4 mmol/L) of H2 before exposed to different doses ofγ-radiation (up to 8 Gy). We demonstrated that treating cells with hydrogen before irradiation could significantly inhibit IR-induced cell apoptosis, increase viability of Human intestinal crypt HIEC cells. In 2010, our group also demonstrated that intraperitoneal injection of hydrogen-rich saline before radiation in a mouse model protected the gastrointestinal endothelia from radiation-induced injury, decreased plasma MDA and intestinal 8-hydroxydeoxyguanosine levels and protected plasma levels of endogenous antioxidant enzymes including superoxide dismutase and glutathione-S-transferase 12.
Haematopoietic system
The haematopoietic system has a high level of cells turnover which is also one of the most sensitive system to IR 45. Myelosuppression is a critical issue for individuals exposed to IR which always treated by hematopoietic transplantation but with low efficacy 46, 47.The primary cause of radiation death in sublethal dose range is due to sepsis, resulting from low numbers in bone marrow cells and leukocytes of peripheral blood. To study the radioprotective effects of H2 on haematopoietic system, we chose Human Lymphocyte AHH-1 cells to study which were sensitive to irradiation 12. In the study, cells were treated with or without different concentrations (0.1-0.4 mmol/L) of H2 before exposed to different doses of γ-radiation (up to 8 Gy). We demonstrated that treating cells with hydrogen before irradiation could significantly inhibit IR-induced cell apoptosis, increase viability of Human Lymphocyte AHH-1 cells. Endogenous antioxidants (superoxide, glutathione) were protected by hydrogen, while malondialdehyde and 8-hydroxydeoxyguanosine concentrations of Human Lymphocyte AHH-1 cells were decreased. Those results showed that the protective effects of hydrogen had positive correlation with the concentrations of hydrogen. However, if we treated cells after radiation, the therapeutic effects of H2 was not significant 12.
In a study by Chuai Y et al, hydrogen-rich saline prior to IR increased bone marrow nucleated cells (BMNCs) and leukocyte counts in irradiated mice compared with those without hydrogen. In the study, Hydrogen -rich saline prior to IR also increased the levels of spleen weights, endogenous haematopoietic spleen colony formations (endoCFUs) 13.
In another study by Yang Y et al., they demonstrated H2 reduced ROS level in Human lymphocyte AHH-1 cells as well as in the radiolysis of water induced by 12C6+ heavy ion radiation.48 They also showed H2 attenuated cell apoptosis and inhibited Caspase 3 activation induced by 12C6+ heavy ion radiation-induced.
Testis
Testis is an important organ of the male reproductive system, which is especially sensitive to ionizing radiation (IR) because of the presence of rapidly proliferating cells (spermatogonia)49. Doses as low as 0.1 Gy are known to cause damage to spermatogonia 41, 49.
Biochemical and morphological injury can be caused by irradiation in testicular tissue50-53.Only 2% of men who received total-body IR are able to father children later in life54. In 2012, Chuai Y et al. reported the radioprotective effect of H2 on male germ cells by ameliorating apoptotic cells in testicular tissue, and by preserving viability of stem spermatogonia, daily sperm production and sperm quality. In the study, WR-2721 was used as a reference compound, which is the only radioprotectant approved by FDA 55. Inspiringly, although the radioprotective effects of H2 have been shown to be less than WR-2721, the exceeded protective effect of WR-2721 seems not to be prominent. In accordance with these results, in later papers by Chuai Y et al and by Jiang Z et al, they also demonstrated the radioprotective effects of hydrogen on testis 13, 56 by protecting seminiferous epithelium, preserving testis weight, testis dimensions, sperm count, sperm motility from IR. However, in the paper by Jiang Z et al, it was found that the radioprotective effect of hydrogen on testis is similar to WR-2721 at one time point (after a 4-day initiation of irradiation) which is better than the results by our group.
Skin
Skin is a biological defense barrier of human body. Radiation injuries skin directly by radiation energy or indirectly by free radicals, causing radiodermatitis which occurred in nearly 95% of patients receiving radiation therapy 57. There are generally two types of radiodermatitis: acute radiodermatitis(usually occur within 90 days), chronic radiodermatitis (may occurred over a prolonged period) which often exhibited by the onset of erythema, swelling, blisters, and ulceration, followed by development of chronic inflammation, necrosis, fibrosis, and lymphedema58, 59. In 2012, Guo Z et al.60 firstly reported that hydrogen-rich saline protected against UVB radiation injury, possibly by reducing inflammation and oxidative stress. They demonstrated that hydrogen-rich saline had protective effects by altering the levels of markers including necrosis factor alpha, interleukin (IL)-1β, IL-6, tissue superoxide dismutase, malondialdehyde and nitric oxide activity and relieved morphological skin injury against UVB radiation injury on C57BL/6 rats. In 2013, Mei K et al.57 reported the protective effects of hydrogen on skin in vitro and vitro against γ ray. In the study, our group found hydrogen significantly reduced the severity of dermatitis caused by radiation, accelerated tissue recovery, and reduced the extent of radiation-induced weight loss in rats after a single dose of 15 or 20 Gy radiation. We also found hydrogen protected rats from cumulative doses of 30 Gy delivered in three fractions. In the study, hydrogen also protected Immortalized human keratinocytes (HaCaT cells) from radiation-induced injury. In 2013, Rosa Mistica Ignacio et al. 61 demonstrated the protective effect of hydrogen reduced water (HRW) on UVB-mediated skin injury in hairless mice by balneotherapy. In their study, bathing with HRW significantly reduced the levels of skin damage by decreasing the level of inflammatory cytokines including IL-1β, IL-6, TNF-α and IFN-γ, as well as increased activity of glutathione peroxidase.
In their study, HRW bathing also protect UV-induced corneocytes damage and ultrastructural changes.
Interestingly, in a recent study by Mi Hee Shin et al.62, they observed that H(H2O) (atomic hydrogen surrounded by water molecules) application to human skin prevented UV-induced erythema and DNA damage. In their stuy, H(H2O) significantly prevented UV-induced MMP-1, COX-2, IL-6 and IL-1β mRNA expressions in human skin in vivo. They also found that H(H2O) prevented UV-induced ROS generation and inhibited UV-induced MMP-1, COX-2 and IL-6 expressions, and UV-induced JNK and c-Jun phosphorylation in HaCaT cells.
Lung
The lung is one of the organs most susceptible to radiation injury 63. Radiation pneumonitis is an inflammation of the lungs that occurs when lung or whole body was irradiated. Development of interstitial pneumonitis increases according to radiation dose, especially single-fraction total body irradiation at higher dose rates 64 and higher total lung doses 65, 66. In 2011, our group hypothesized that hydrogen may be a possible prevention strategy of radiation pneumonitis 15. In 2011, Terasaki et al. 67 showed that H2 reduced irradiation-induced ·OH levels in human lung epithelial cell line A549 cells. They demonstrated that pretreatment of H2 could reduce the fluorescence intensity of hydroxyphenyl fluorescein in irradiated A549 cells. They demonstrated pretreatment of H2 reduced the products of oxidative stress including 4-hydroxy-2-nonenal, 8-hydroxydeoxyguanosine, etc. H2 also could significantly reduce levels of apoptosis-associated proteins including Bax and active caspase 3 in irradiated A549 cells after a 24h incubation with H2-rich solution. In vivo, they demonstrated that H2 treatment reduced oxidative stress and apoptosis, measures of acute damage in the lungs of mice within 1 week after whole thorax irradiation by immunohistochemistry and immunoblotting. In their study, H2 treatment reduced lung fibrosis by chest computed tomography, Ashcroft scores, and type III collagen deposition at 5 months after irradiation.
Cardiovascular
Irradiation of the heart can cause chronic impairment of cardiac pump function and cardiac disease 68. The most significant type of radiation-induced heart disease (RIHD) appears to be that of myocardial damage, which may result from loss of alkaline phosphatase activity of capillary endothelial cells 6-10 weeks after irradiation 69, 70. In addition to myocardial degeneration, perivascular and interstitial fibrosis are seen 71. Injury of heart by irradiation has been shown to be caused by the hydroxyl radical. Intervention to protect the heart from the damage of the hydroxyl radical by hydrogen has been proposed, suggested, and performed by our group. We investigated the cardioprotective properties of hydrogen by pretreating mice with hydrogen rich water prior to irradiation. The results were pleasing in the study, 90% of the mice without hydrogen rich water pretreatment died, while 80% of the mice with hydrogen treatment lived after 30 days post-radiation. When focusing on the myocardium, hydrogen pre-treatment proved to have cardioprotective properties by decreasing melanodialdehyde (MDA) and eight-hydroxydeoxyguanosine (8-OHdG) levels as opposed to the non-treatment counterparts, which showed increased levels of those oxidative stress markers 16.
Brain
In a study by Huo H. et al. in Chinese 72, hydrogen was demonstrated alleviating oxidative stress and early-phase radiation-induced brain injury. They found that the brain water content and the contents of 8-OHdG in the hydrogen group were lower than the control group at 7th day and 14th day post irradiation. The contents of SOD were significantly higher in the hydrogen group from 1st to 7th day post irradiation, while the contents of MDA were significantly lower in the hydrogen group from 1st to 14th day post irradiation. They also found the damage degree in the nerve cells of hippocampus was less in the hydrogen group.
Lymphoma
Ionizing radiation (IR) is also a well-known carcinogen for various human tissues and a complete carcinogen that is able to both initiate and promote tumor progression 73-75. A study by Zhao L. et al. found that molecular hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice 76. They found that radiation-induced thymic lymphoma rate in the hydrogen group was significantly lower than in the control group and hydrogen treatment significantly prolonged the latency of lymphoma development after the split dose irradiation by reducing ROS, which has been found to be a factor of inducing cancers.
Radiotherapy
Although a remarkable progress about the application of hydrogen as a radioprotectant has been made in past 6 years, there were only a few clinical data. Ki-Mun Kang et al. performed a randomised, placebo-controlled study on 49 patients receiving radiotherapy for malignant liver tumour. Hydrogen-rich water was produced by metallic magnesium (Mg) [Mg + 2H 2 O = Mg (OH)2+ H2 ], and the final concentration of H2 reached to 0.55-0.65 mM. Results showed that daily consumption of hydrogen-rich water for 6 weeks reduced reactive oxygen metabolites in the blood and maintained blood oxidation potential of these patients. Quality of life (QOL) scored during radiotherapy were improved in patients with hydrogen-rich water compared to those with placebo. This study did not find differences in tumor response to radiotherapy as application of hydrogen-rich water 18.
Future Directions
The application of hydrogen on radioprotection has been widely studied. Some investigators even proposed application hydrogen therapy in space flight 77. Definition of hydrogen as a kind of antioxidant seems cannot explain all radioprotective effects of hydrogen. But people have not found any other mechanism to replace the reduction of ∙ OH by H2 41. The exact mechanism and signaling pathway involved in the protection role of hydrogen in ionizing radiation injury need to be studied in the future. There were only a few researches explain molecular hydrogen exerted its effect not only as an antioxidant. Itoh et al. 78, 79 suggest that H2 may become a gaseous signalling molecule like nitric oxide (NO), carbon monoxide (CO) and hydrogen sulphide (H2S) for the first time. They showed that H2 suppressed FcɛRI-associated signal transduction and prevented degranulation of mast cell, but not through reducing the ∙ OH, and they also demonstrated that H2 inhibited lipopolysaccharide/interferon γ induced NO production through modulation of signal transduction in the macrophage. An assay of DNA microarrays in the livers detected 548 up-regulated and 695 down-regulated genes in rats after 4 weeks of drinking hydrogen-rich water, and those genes for oxidoreduction-related proteins were enriched in the up-regulated groups 80. However, at present there is no direct evidence could confirm hydrogen gas is a new signalling molecule, which may need further investigations.
Conclusion
Researchers have always been hammering at looking for an ideal radioprotectant. The sulfhydryl compound amifostine, named WR-2721, which is the only radioprotectant registered for using in human, has shown good radioprotective effects 55. But it has many side effects limiting its clinical use such as hypertension, nausea, vomiting, and other side effects 81. Some nature antioxidants such as Vitamins, Flavonoids, etc. have fewer toxic effects but also with lower radioprotection. Therefore, an ideal radioprotectant should be effective with few side effects. To date, no radioprotectant can fulfill the criteria. H2 as a new class of radioprotective agent may give us more hope, although there are still lots of future research needs to be done.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (Grant No.81072241).
References
- 1.Damron TA, Spadaro JA, Horton JA, Margulies BS, Strauss JA, Farnum CE. Novel radioprotectant drugs for sparing radiation-induced damage to the physis. Int J Radiat Biol. 2004;80:217–28. doi: 10.1080/09553000410001669524. [DOI] [PubMed] [Google Scholar]
- 2.Vijayalaxmi Reiter RJ, Tan DX Herman TS, Thomas CR Jr. Melatonin as a radioprotective agent: a review. Int J Radiat Oncol Biol Phys. 2004;59:639–53. doi: 10.1016/j.ijrobp.2004.02.006. doi:10.1016/j.ijrobp.2004.02.006S0360301604002469 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Maisin JR. Bacq and Alexander Award lecture--chemical radioprotection: past, present, and future prospects. Int J Radiat Biol. 1998;73:443–50. doi: 10.1080/095530098142284. [DOI] [PubMed] [Google Scholar]
- 4.Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- 5.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94. doi: 10.1038/nm1577. doi:nm1577 [pii]10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 6.Qian L, Mei K, Shen J, Cai J. Administration of hydrogen-rich saline protects mice from lethal acute graft-versus-host disease (aGVHD) Transplantation. 2013;95:658–62. doi: 10.1097/TP.0b013e31827e6b23. doi:10.1097/TP.0b013e31827e6b2300007890-201303150-00003 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Qian L, Shen J. Hydrogen therapy may be an effective and specific novel treatment for Acute Graft-versus-host disease (GVHD) Journal of Cellular and Molecular Medicine. 2013 doi: 10.1111/jcmm.12081. doi: 10.1111/jcmm.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qian L, Wu Z, Shen J. Advances in the treatment of acute graft-versus-host disease. Journal of Cellular and Molecular Medicine. 2013 doi: 10.1111/jcmm.12093. doi: 10.1111/jcmm.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010;46:140–9. doi: 10.3164/jcbn.09-100. doi:10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–82. doi: 10.3109/10715762.2010.500328. doi:10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 11.Qian L, Li B, Cai J, Gao F. The hypothesis of an effective safe and novel radioprotective agent: hydrogen-rich solution. West Indian Med J. 2010;59:122–4. [PubMed] [Google Scholar]
- 12.Qian L, Cao F, Cui J, Huang Y, Zhou X, Liu S. et al. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic Res. 2010;44:275–82. doi: 10.3109/10715760903468758. doi:10.3109/10715760903468758. [DOI] [PubMed] [Google Scholar]
- 13.Chuai Y, Shen J, Qian L, Wang Y, Huang Y, Gao F. et al. Hydrogen-rich saline protects spermatogenesis and hematopoiesis in irradiated BALB/c mice. Med Sci Monit. 2012;18:BR89–94. doi: 10.12659/MSM.882513. doi:882513 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chuai Y, Qian L, Sun X, Cai J. Molecular hydrogen and radiation protection. Free Radic Res. 2012;46:1061–7. doi: 10.3109/10715762.2012.689429. doi:10.3109/10715762.2012.689429. [DOI] [PubMed] [Google Scholar]
- 15.Chuai Y, Zhao L, Ni J, Sun D, Cui J, Li B. et al. A possible prevention strategy of radiation pneumonitis: combine radiotherapy with aerosol inhalation of hydrogen-rich solution. Med Sci Monit. 2011;17:HY1–4. doi: 10.12659/MSM.881698. doi:881698 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L, Cao F, Cui J, Wang Y, Huang Y, Chuai Y. et al. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res. 2010;51:741–7. doi: 10.1269/jrr.10093. doi:JST.JSTAGE/jrr/10093 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Liren Qian, Bailong Li, Fei Cao, Yuecheng Huang, Shulin Liu, Jianming Cai. et al. Hydrogen-rich PBS protects cultured human cells from ionizing radiation-induced cellular damage. Nuclear Technology and Radiation Protection. 2010;25:23–9. [Google Scholar]
- 18.Kang KM, Kang YN, Choi IB, Gu Y, Kawamura T, Toyoda Y. et al. Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors. Med Gas Res. 2011;1:11.. doi: 10.1186/2045-9912-1-11. doi:2045-9912-1-11 [pii]10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. doi:10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 20.Fan X. Ionizing radiation induces formation of malondialdehyde, formaldehyde, and acetaldehyde from carbohydrates and organic acid. J Agric Food Chem. 2003;51:5946–9. doi: 10.1021/jf0344340. doi:10.1021/jf0344340. [DOI] [PubMed] [Google Scholar]
- 21.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–63. doi: 10.1016/s1383-5742(97)00035-5. doi:S1383-5742(97)00035-5 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Floyd RA. The role of 8-hydroxyguanine in carcinogenesis. Carcinogenesis. 1990;11:1447–50. doi: 10.1093/carcin/11.9.1447. [DOI] [PubMed] [Google Scholar]
- 23.BN Pandey KM. Fluorescence and ESR studies on membrane oxidative damage by γ-radiation. Appl Magn Reson. 2000;18:483–92. [Google Scholar]
- 24.Dubner D, Gisone P, Jaitovich I, Perez M. Free radicals production and estimation of oxidative stress related to gamma irradiation. Biol Trace Elem Res. 1995;47:265–70. doi: 10.1007/BF02790126. doi:10.1007/BF02790126. [DOI] [PubMed] [Google Scholar]
- 25.Verma SP, Sonwalkar N. Structural changes in plasma membranes prepared from irradiated Chinese hamster V79 cells as revealed by Raman spectroscopy. Radiat Res. 1991;126:27–35. [PubMed] [Google Scholar]
- 26.Giusti AM, Raimondi M, Ravagnan G, Sapora O, Parasassi T. Human cell membrane oxidative damage induced by single and fractionated doses of ionizing radiation: a fluorescence spectroscopy study. Int J Radiat Biol. 1998;74:595–605. doi: 10.1080/095530098141177. [DOI] [PubMed] [Google Scholar]
- 27.Pohl LR. An immunochemical approach of identifying and characterizing protein targets of toxic reactive metabolites. Chem Res Toxicol. 1993;6:786–93. doi: 10.1021/tx00036a006. [DOI] [PubMed] [Google Scholar]
- 28.Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013;3:10.. doi: 10.1186/2045-9912-3-10. doi:2045-9912-3-10 [pii]10.1186/2045-9912-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilcher JE. Senn on the Diagnosis of Gastro-Intestinal Perforation by the Rectal Insufflation of Hydrogen Gas. Ann Surg. 1888;8:190–204. doi: 10.1097/00000658-188807000-00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–4. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 31.Buxton GV GC, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals in aqueous solution. J Phys Chem. 1988:513– 886. [Google Scholar]
- 32.Gharib B, Hanna S, Abdallahi OM, Lepidi H, Gardette B, De Reggi M. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C R Acad Sci III. 2001;324:719–24. doi: 10.1016/s0764-4469(01)01350-6. doi:S0764446901013506 [pii] [DOI] [PubMed] [Google Scholar]
- 33.Hayashida K, Sano M, Ohsawa I, Shinmura K, Tamaki K, Kimura K. et al. Inhalation of hydrogen gas reduces infarct size in the rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 2008;373:30–5. doi: 10.1016/j.bbrc.2008.05.165. doi:S0006-291X(08)01083-8 [pii]10.1016/j.bbrc.2008.05.165. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz BM, Kaczorowski DJ, Sugimoto R, Yang R, Wang Y, Billiar TR. et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant. 2008;8:2015–24. doi: 10.1111/j.1600-6143.2008.02359.x. doi:AJT2359 [pii]10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 35.Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang JH. et al. Hydrogen-rich saline protects myocardium against ischemia/reperfusion injury in rats. Exp Biol Med (Maywood) 2009;234:1212–9. doi: 10.3181/0812-RM-349. doi:0812-RM-349 [pii]10.3181/0812-RM-349. [DOI] [PubMed] [Google Scholar]
- 36.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–4. doi: 10.1016/j.bbrc.2007.07.088. doi:S0006-291X(07)01552-5 [pii]10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 37.Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH. et al. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–72. doi: 10.1016/j.neulet.2008.05.077. doi:S0304-3940(08)00732-5 [pii]10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- 38.Fu Y, Ito M, Fujita Y, Ichihara M, Masuda A, Suzuki Y. et al. Molecular hydrogen is protective against 6-hydroxydopamine-induced nigrostriatal degeneration in a rat model of Parkinson's disease. Neurosci Lett. 2009;453:81–5. doi: 10.1016/j.neulet.2009.02.016. doi:S0304-3940(09)00183-9 [pii]10.1016/j.neulet.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology. 2009;34:501–8. doi: 10.1038/npp.2008.95. doi:npp200895 [pii]10.1038/npp.2008.95. [DOI] [PubMed] [Google Scholar]
- 40.Mao YF, Zheng XF, Cai JM, You XM, Deng XM, Zhang JH. et al. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2009;381:602–5. doi: 10.1016/j.bbrc.2009.02.105. doi:S0006-291X(09)00376-3 [pii]10.1016/j.bbrc.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 41.Chuai Y, Gao F, Li B, Zhao L, Qian L, Cao F. et al. Hydrogen-rich saline attenuates radiation-induced male germ cell loss in mice through reducing hydroxyl radicals. Biochem J. 2012;442:49–56. doi: 10.1042/BJ20111786. doi:BJ20111786 [pii]10.1042/BJ20111786. [DOI] [PubMed] [Google Scholar]
- 42.Saitoh Y, Harata Y, Mizuhashi F, Nakajima M, Miwa N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health. 2010;26:203–16. doi: 10.1177/0748233710362989. doi:0748233710362989 [pii]10.1177/0748233710362989. [DOI] [PubMed] [Google Scholar]
- 43.Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol. 2000;15:109–20. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 44.Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–21. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- 45.Grande T, Bueren JA. The mobilization of hematopoietic progenitors to peripheral blood is predictive of the hematopoietic syndrome after total or partial body irradiation of mice. Int J Radiat Oncol Biol Phys. 2006;64:612–8. doi: 10.1016/j.ijrobp.2005.09.036. doi:S0360-3016(05)02725-2 [pii]10.1016/j.ijrobp.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 46.Baranov A, Gale RP, Guskova A, Piatkin E, Selidovkin G, Muravyova L. et al. Bone marrow transplantation after the Chernobyl nuclear accident. N Engl J Med. 1989;321:205–12. doi: 10.1056/NEJM198907273210401. doi:10.1056/NEJM198907273210401. [DOI] [PubMed] [Google Scholar]
- 47.Normile D. Nuclear accident. Special treatment set for radiation victim. Science. 1999;286:207–9. doi: 10.1126/science.286.5438.207b. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Gao F, Zhang H, Hunag Y, Zhang P, Liu C. et al. Molecular hydrogen protects human lymphocyte AHH-1 cells against C heavy ion radiation. Int J Radiat Biol. 2013 doi: 10.3109/09553002.2013.817704. doi:10.3109/09553002.2013.817704. [DOI] [PubMed] [Google Scholar]
- 49.Oakberg EF. Sensitivity and time of degeneration of spermatogenic cells irradiated in various stages of maturation in the mouse. Radiat Res. 1955;2:369–91. [PubMed] [Google Scholar]
- 50.Mauduit C, Siah A, Foch M, Chapet O, Clippe S, Gerard JP. et al. Differential expression of growth factors in irradiated mouse testes. Int J Radiat Oncol Biol Phys. 2001;50:203–12. doi: 10.1016/s0360-3016(01)01461-4. doi:S0360-3016(01)01461-4 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Bruheim K, Svartberg J, Carlsen E, Dueland S, Haug E, Skovlund E. et al. Radiotherapy for rectal cancer is associated with reduced serum testosterone and increased FSH and LH. Int J Radiat Oncol Biol Phys. 2008;70:722–7. doi: 10.1016/j.ijrobp.2007.10.043. doi:S0360-3016(07)04462-8 [pii]10.1016/j.ijrobp.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 52.Samarth RM, Samarth M. Protection against radiation-induced testicular damage in Swiss albino mice by Mentha piperita (Linn.) Basic Clin Pharmacol Toxicol. 2009;104:329–34. doi: 10.1111/j.1742-7843.2009.00384.x. doi:PTO384 [pii]10.1111/j.1742-7843.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 53.Meistrich ML, Finch MV, Hunter N, Milas L. Cytotoxic effects of WR-2721 on mouse testicular cells. Int J Radiat Oncol Biol Phys. 1984;10:1551–4. doi: 10.1016/0360-3016(84)90501-7. doi:0360-3016(84)90501-7 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Costabile RA. The effects of cancer and cancer therapy on male reproductive function. J Urol. 1993;149:1327–30. doi: 10.1016/s0022-5347(17)36384-x. [DOI] [PubMed] [Google Scholar]
- 55.Gosselin TK, Mautner B. Amifostine as a radioprotectant. Clin J Oncol Nurs. 2002;6:175–6. doi: 10.1188/02.CJON.175-176. 80. doi:10.1188/02.CJON.175-176. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Z, Xu B, Yang M, Li Z, Zhang Y, Jiang D. Protection by hydrogen against gamma ray-induced testicular damage in rats. Basic Clin Pharmacol Toxicol. 2013;112:186–91. doi: 10.1111/bcpt.12016. doi:10.1111/bcpt.12016. [DOI] [PubMed] [Google Scholar]
- 57.Mei K, Zhao S, Qian L, Li B, Ni J, Cai J. Hydrogen protects rats from dermatitis caused by local radiation. J Dermatolog Treat. 2013 doi: 10.3109/09546634.2012.762639. doi:10.3109/09546634.2012.762639. [DOI] [PubMed] [Google Scholar]
- 58.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19:35–42. doi: 10.1016/j.semradonc.2008.09.007. doi:S1053-4296(08)00062-3 [pii]10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28–46. doi: 10.1016/j.jaad.2005.08.054. doi:S0190-9622(05)02707-6 [pii]10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 60.Guo Z, Zhou B, Li W, Sun X, Luo D. Hydrogen-rich saline protects against ultraviolet B radiation injury in rats. J Biomed Res. 2012;26:365–71. doi: 10.7555/JBR.26.20110037. doi:10.7555/JBR.26.20110037jbr-26-05-365 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ignacio RM, Yoon Y-S, Sajo MEJ, Kim C-S, Kim D-H, Kim S-K. et al. The balneotherapy effect of hydrogen reduced water on UVB-mediated skin injury in hairless mice. Molecular & Cellular Toxicology. 2013;9:15–21. [Google Scholar]
- 62.Shin MH, Park R, Nojima H, Kim HC, Kim YK, Chung JH. Atomic hydrogen surrounded by water molecules, H(H2O)m, modulates basal and UV-induced gene expressions in human skin in vivo. PLoS One. 2013;8:e61696.. doi: 10.1371/journal.pone.0061696. doi:10.1371/journal.pone.0061696PONE-D-12-32948 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Para AE, Bezjak A, Yeung IW, Van Dyk J, Hill RP. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92:500–10. doi: 10.1016/j.radonc.2009.04.005. doi:S0167-8140(09)00162-5 [pii]10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 64.Deeg HJ. Acute and delayed toxicities of total body irradiation. Seattle Marrow Transplant Team. Int J Radiat Oncol Biol Phys. 1983;9:1933–9. doi: 10.1016/0360-3016(83)90365-6. doi:0360-3016(83)90365-6 [pii] [DOI] [PubMed] [Google Scholar]
- 65.Beyzadeoglu M, Oysul K, Dirican B, Arpaci F, Balkan A, Surenkok S. et al. Effect of dose-rate and lung dose in total body irradiation on interstitial pneumonitis after bone marrow transplantation. Tohoku J Exp Med. 2004;202:255–63. doi: 10.1620/tjem.202.255. [DOI] [PubMed] [Google Scholar]
- 66.Sampath S, Schultheiss TE, Wong J. Dose response and factors related to interstitial pneumonitis after bone marrow transplant. Int J Radiat Oncol Biol Phys. 2005;63:876–84. doi: 10.1016/j.ijrobp.2005.02.032. doi:S0360-3016(05)00395-0 [pii]10.1016/j.ijrobp.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 67.Terasaki Y, Ohsawa I, Terasaki M, Takahashi M, Kunugi S, Dedong K. et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011;301:L415–26. doi: 10.1152/ajplung.00008.2011. doi:ajplung.00008.2011 [pii]10.1152/ajplung.00008.2011. [DOI] [PubMed] [Google Scholar]
- 68.Lee CK, Aeppli D, Nierengarten ME. The need for long-term surveillance for patients treated with curative radiotherapy for Hodgkin's disease: University of Minnesota experience. Int J Radiat Oncol Biol Phys. 2000;48:169–79. doi: 10.1016/s0360-3016(00)00647-7. doi:S0360-3016(00)00647-7 [pii] [DOI] [PubMed] [Google Scholar]
- 69.Lauk S. Endothelial alkaline phosphatase activity loss as an early stage in the development of radiation-induced heart disease in rats. Radiat Res. 1987;110:118–28. [PubMed] [Google Scholar]
- 70.Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–8. doi: 10.1016/0360-3016(85)90314-1. doi:0360-3016(85)90314-1 [pii] [DOI] [PubMed] [Google Scholar]
- 71.Stewart JR, Fajardo LF, Gillette SM, Constine LS. Radiation injury to the heart. Int J Radiat Oncol Biol Phys. 1995;31:1205–11. doi: 10.1016/0360-3016(94)00656-6. doi:0360-3016(94)00656-6 [pii]10.1016/0360-3016(94)00656-6. [DOI] [PubMed] [Google Scholar]
- 72.Huo H-m, Yang S, Chen L-s, Lu H-j, Wang A-d, Zhang L-y. Hydrogen-rich saline alleviation on the oxidative stress and early-phase radiation-induced brain injury in rats. Chinese Journal of Radiological Medicine and Protection. 2012;32:485–7. [Google Scholar]
- 73.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 74.Potworowski EF, Gagnon F, Beauchemin C, St Pierre Y. Dendritic cells prevent radiation-induced thymic lymphoma. Leukemia. 1996;10:1639–47. [PubMed] [Google Scholar]
- 75.Shin SC, Kang YM, Kim HS. Life span and thymic lymphoma incidence in high- and low-dose-rate irradiated AKR/J mice and commonly expressed genes. Radiat Res. 2010;174:341–6. doi: 10.1667/RR1946.1. doi:10.1667/RR1946.1. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Zhou C, Zhang J, Gao F, Li B, Chuai Y. et al. Hydrogen protects mice from radiation induced thymic lymphoma in BALB/c mice. Int J Biol Sci. 2011;7:297–300. doi: 10.7150/ijbs.7.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schoenfeld MP, Ansari RR, Zakrajsek JF, Billiar TR, Toyoda Y, Wink DA. et al. Hydrogen therapy may reduce the risks related to radiation-induced oxidative stress in space flight. Med Hypotheses. 2010;76:117–8. doi: 10.1016/j.mehy.2010.08.046. doi:S0306-9877(10)00350-6 [pii]10.1016/j.mehy.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itoh T, Fujita Y, Ito M, Masuda A, Ohno K, Ichihara M. et al. Molecular hydrogen suppresses FcepsilonRI-mediated signal transduction and prevents degranulation of mast cells. Biochem Biophys Res Commun. 2009;389:651–6. doi: 10.1016/j.bbrc.2009.09.047. doi:S0006-291X(09)01849-X [pii]10.1016/j.bbrc.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 79.Itoh T, Hamada N, Terazawa R, Ito M, Ohno K, Ichihara M. et al. Molecular hydrogen inhibits lipopolysaccharide/interferon gamma-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem Biophys Res Commun. 2011;411:143–9. doi: 10.1016/j.bbrc.2011.06.116. doi:S0006-291X(11)01096-5 [pii]10.1016/j.bbrc.2011.06.116. [DOI] [PubMed] [Google Scholar]
- 80.Nakai Y, Sato B, Ushiama S, Okada S, Abe K, Arai S. Hepatic oxidoreduction-related genes are upregulated by administration of hydrogen-saturated drinking water. Biosci Biotechnol Biochem. 2011;75:774–6. doi: 10.1271/bbb.100819. doi:JST.JSTAGE/bbb/100819 [pii] [DOI] [PubMed] [Google Scholar]
- 81.Genvresse I, Lange C, Schanz J, Schweigert M, Harder H, Possinger K. et al. Tolerability of the cytoprotective agent amifostine in elderly patients receiving chemotherapy: a comparative study. Anticancer Drugs. 2001;12:345–9. doi: 10.1097/00001813-200104000-00007. [DOI] [PubMed] [Google Scholar]