Abstract
Vaccinia virus, no longer required for immunization against smallpox, now serves as a unique vector for expressing genes within the cytoplasm of mammalian cells. As a research tool, recombinant vaccinia viruses are used to synthesize and analyze the structure-function relationships of proteins, determine the targets of humoral and cell-mediated immunity, and investigate the types of immune response needed for protection against specific infectious diseases and cancer. The vaccine potential of recombinant vaccinia virus has been realized in the form of an effective oral wild-life rabies vaccine, although no product for humans has been licensed. A genetically altered vaccinia virus that is unable to replicate in mammalian cells and produces diminished cytopathic effects retains the capacity for high-level gene expression and immunogenicity while promising exceptional safety for laboratory workers and potential vaccine recipients.
Full text
PDF

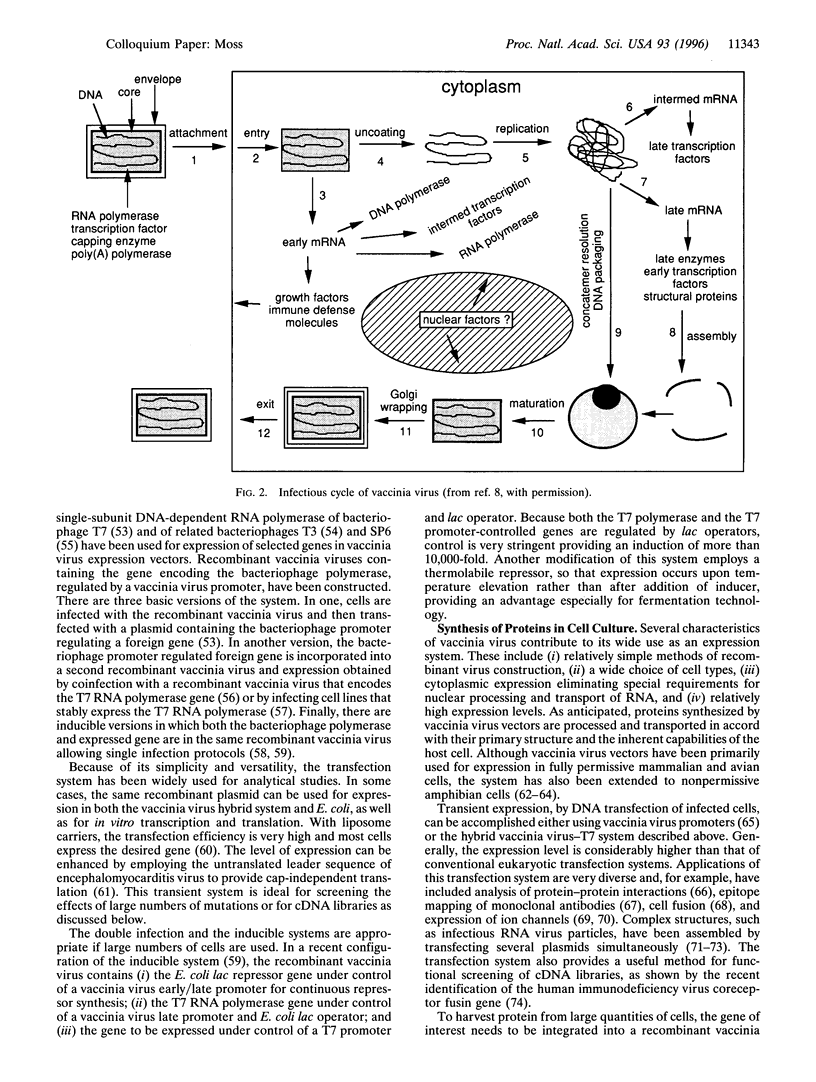





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander W. A., Moss B., Fuerst T. R. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J Virol. 1992 May;66(5):2934–2942. doi: 10.1128/jvi.66.5.2934-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldick C. J., Jr, Keck J. G., Moss B. Mutational analysis of the core, spacer, and initiator regions of vaccinia virus intermediate-class promoters. J Virol. 1992 Aug;66(8):4710–4719. doi: 10.1128/jvi.66.8.4710-4719.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett N., Mitterer A., Mundt W., Eibl J., Eibl M., Gallo R. C., Moss B., Dorner F. Large-scale production and purification of a vaccinia recombinant-derived HIV-1 gp160 and analysis of its immunogenicity. AIDS Res Hum Retroviruses. 1989 Apr;5(2):159–171. doi: 10.1089/aid.1989.5.159. [DOI] [PubMed] [Google Scholar]
- Baxby D. The origins of vaccinia virus. J Infect Dis. 1977 Sep;136(3):453–455. doi: 10.1093/infdis/136.3.453. [DOI] [PubMed] [Google Scholar]
- Bayliss C. D., Peters R. W., Cook J. K., Reece R. L., Howes K., Binns M. M., Boursnell M. E. A recombinant fowlpox virus that expresses the VP2 antigen of infectious bursal disease virus induces protection against mortality caused by the virus. Arch Virol. 1991;120(3-4):193–205. doi: 10.1007/BF01310475. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Destree A., McKenzie S., Gordon E., Weinberg R. A., Panicali D. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6854–6858. doi: 10.1073/pnas.84.19.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancou J., Kieny M. P., Lathe R., Lecocq J. P., Pastoret P. P., Soulebot J. P., Desmettre P. Oral vaccination of the fox against rabies using a live recombinant vaccinia virus. Nature. 1986 Jul 24;322(6077):373–375. doi: 10.1038/322373a0. [DOI] [PubMed] [Google Scholar]
- Blasco R., Moss B. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J Virol. 1992 Jul;66(7):4170–4179. doi: 10.1128/jvi.66.7.4170-4179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco R., Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995 Jun 9;158(2):157–162. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- Boursnell M. E., Green P. F., Campbell J. I., Deuter A., Peters R. W., Tomley F. M., Samson A. C., Chambers P., Emmerson P. T., Binns M. M. Insertion of the fusion gene from Newcastle disease virus into a non-essential region in the terminal repeats of fowlpox virus and demonstration of protective immunity induced by the recombinant. J Gen Virol. 1990 Mar;71(Pt 3):621–628. doi: 10.1099/0022-1317-71-3-621. [DOI] [PubMed] [Google Scholar]
- Boursnell M. E., Green P. F., Samson A. C., Campbell J. I., Deuter A., Peters R. W., Millar N. S., Emmerson P. T., Binns M. M. A recombinant fowlpox virus expressing the hemagglutinin-neuraminidase gene of Newcastle disease virus (NDV) protects chickens against challenge by NDV. Virology. 1990 Sep;178(1):297–300. doi: 10.1016/0042-6822(90)90408-j. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988 May 15;65(1):123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. Construction of recombinant fowlpox viruses as vectors for poultry vaccines. Virus Res. 1988 Jun;10(4):343–356. doi: 10.1016/0168-1702(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Brochier B., Costy F., Pastoret P. P. Elimination of fox rabies from Belgium using a recombinant vaccinia-rabies vaccine: an update. Vet Microbiol. 1995 Sep;46(1-3):269–279. doi: 10.1016/0378-1135(95)00091-n. [DOI] [PubMed] [Google Scholar]
- Brochier B., Kieny M. P., Costy F., Coppens P., Bauduin B., Lecocq J. P., Languet B., Chappuis G., Desmettre P., Afiademanyo K. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature. 1991 Dec 19;354(6354):520–522. doi: 10.1038/354520a0. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Cadoz M., Strady A., Meignier B., Taylor J., Tartaglia J., Paoletti E., Plotkin S. Immunisation with canarypox virus expressing rabies glycoprotein. Lancet. 1992 Jun 13;339(8807):1429–1432. doi: 10.1016/0140-6736(92)92027-d. [DOI] [PubMed] [Google Scholar]
- Carroll M. W., Moss B. E. coli beta-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. Biotechniques. 1995 Sep;19(3):352-4, 356. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain R. S., Carroll M. W., Bronte V., Hwu P., Warren S., Yang J. C., Nishimura M., Moss B., Rosenberg S. A., Restifo N. P. Costimulation enhances the active immunotherapy effect of recombinant anticancer vaccines. Cancer Res. 1996 Jun 15;56(12):2832–2836. [PMC free article] [PubMed] [Google Scholar]
- Chambers T. M., Kawaoka Y., Webster R. G. Protection of chickens from lethal influenza infection by vaccinia-expressed hemagglutinin. Virology. 1988 Dec;167(2):414–421. [PubMed] [Google Scholar]
- Child S. J., Palumbo G. J., Buller R. M., Hruby D. E. Insertional inactivation of the large subunit of ribonucleotide reductase encoded by vaccinia virus is associated with reduced virulence in vivo. Virology. 1990 Feb;174(2):625–629. doi: 10.1016/0042-6822(90)90119-c. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hill M. G., Camargo E., Grosfeld H., Chanock R. M., Murphy B. R. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5' proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11563–11567. doi: 10.1073/pnas.92.25.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., McElrath M. J., Corey L., Hu S. L., Collier A. C., Arditti D., Hoffman M., Coombs R. W., Smith G. E., Greenberg P. D. Enhanced immunity to human immunodeficiency virus (HIV) envelope elicited by a combined vaccine regimen consisting of priming with a vaccinia recombinant expressing HIV envelope and boosting with gp160 protein. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1882–1886. doi: 10.1073/pnas.90.5.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S., Cossart P., Griffiths G., Way M. Actin-based motility of vaccinia virus. Nature. 1995 Dec 7;378(6557):636–638. doi: 10.1038/378636a0. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 1990 Jul 25;18(14):4285–4286. doi: 10.1093/nar/18.14.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus early promoters. J Mol Biol. 1989 Dec 20;210(4):749–769. doi: 10.1016/0022-2836(89)90107-1. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Moss B. Structure of vaccinia virus late promoters. J Mol Biol. 1989 Dec 20;210(4):771–784. doi: 10.1016/0022-2836(89)90108-3. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Hügin A. W., Moss B. Removal of cryptic poxvirus transcription termination signals from the human immunodeficiency virus type 1 envelope gene enhances expression and immunogenicity of a recombinant vaccinia virus. J Virol. 1990 May;64(5):2448–2451. doi: 10.1128/jvi.64.5.2448-2451.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Morrison R. P., Wehrly K., Nishio J., Chesebro B. T-lymphocyte priming and protection against Friend leukemia by vaccinia-retrovirus env gene recombinant. Science. 1986 Nov 7;234(4777):728–731. doi: 10.1126/science.3490689. [DOI] [PubMed] [Google Scholar]
- Earl P. L., Robert-Guroff M., Matthews T. J., Krohn K., London W. T., Moss B. Isolate- and group-specific immune responses to the envelope protein of human immunodeficiency virus induced by a live recombinant vaccinia virus in macaques. AIDS Res Hum Retroviruses. 1989 Feb;5(1):23–32. doi: 10.1089/aid.1989.5.23. [DOI] [PubMed] [Google Scholar]
- Edbauer C., Weinberg R., Taylor J., Rey-Senelonge A., Bouquet J. F., Desmettre P., Paoletti E. Protection of chickens with a recombinant fowlpox virus expressing the Newcastle disease virus hemagglutinin-neuraminidase gene. Virology. 1990 Dec;179(2):901–904. doi: 10.1016/0042-6822(90)90165-n. [DOI] [PubMed] [Google Scholar]
- Edwards K. M., Andrews T. C., Van Savage J., Palmer P. S., Moyer R. W. Poxvirus deletion mutants: virulence and immunogenicity. Microb Pathog. 1988 May;4(5):325–333. doi: 10.1016/0882-4010(88)90060-5. [DOI] [PubMed] [Google Scholar]
- Eisenlohr L. C., Yewdell J. W., Bennink J. R. A transient transfection system for identifying biosynthesized proteins processed and presented to class I MHC restricted T lymphocytes. J Immunol Methods. 1992 Sep 18;154(1):131–138. doi: 10.1016/0022-1759(92)90220-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Prince G. A., Murphy B. R., Venkatesan S., Chanock R. M., Moss B. Resistance to human respiratory syncytial virus (RSV) infection induced by immunization of cotton rats with a recombinant vaccinia virus expressing the RSV G glycoprotein. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1906–1910. doi: 10.1073/pnas.83.6.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Moss B. Cytoplasmic expression system based on constitutive synthesis of bacteriophage T7 RNA polymerase in mammalian cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6743–6747. doi: 10.1073/pnas.87.17.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J., Rovinsky M. Marker rescue of temperature-sensitive mutations of vaccinia virus WR: correlation of genetic and physical maps. J Virol. 1983 Nov;48(2):419–428. doi: 10.1128/jvi.48.2.419-428.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Knight J. C., Shaddock J. H., Novembre F. J., Baer G. M. Successful oral rabies vaccination of raccoons with raccoon poxvirus recombinants expressing rabies virus glycoprotein. Virology. 1988 Jul;165(1):313–316. doi: 10.1016/0042-6822(88)90692-7. [DOI] [PubMed] [Google Scholar]
- Estin C. D., Stevenson U. S., Plowman G. D., Hu S. L., Sridhar P., Hellström I., Brown J. P., Hellström K. E. Recombinant vaccinia virus vaccine against the human melanoma antigen p97 for use in immunotherapy. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1052–1056. doi: 10.1073/pnas.85.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., COMBEN B. M. [Genetic studies with mammalian poxviruses. I. Demonstration of recombination between two strains of vaccina virus]. Virology. 1958 Jun;5(3):530–548. doi: 10.1016/0042-6822(58)90043-6. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J Virol. 1988 Jun;62(6):1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Moss B. Transient dominant selection of recombinant vaccinia viruses. J Virol. 1990 Jun;64(6):3108–3111. doi: 10.1128/jvi.64.6.3108-3111.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Broder C. C., Kennedy P. E., Berger E. A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996 May 10;272(5263):872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Flexner C., Moss B., London W. T., Murphy B. R. Attenuation and immunogenicity in primates of vaccinia virus recombinants expressing human interleukin-2. Vaccine. 1990 Feb;8(1):17–21. doi: 10.1016/0264-410x(90)90171-h. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Rice C. M., Strauss J. H., Hruby D. E. Neomycin resistance as a dominant selectable marker for selection and isolation of vaccinia virus recombinants. Mol Cell Biol. 1985 Aug;5(8):1918–1924. doi: 10.1128/mcb.5.8.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Fernandez M. P., Moss B. Transfer of the inducible lac repressor/operator system from Escherichia coli to a vaccinia virus expression vector. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2549–2553. doi: 10.1073/pnas.86.8.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni L., Jones L., Mebus C., Yilma T. A vaccinia virus double recombinant expressing the F and H genes of rinderpest virus protects cattle against rinderpest and causes no pock lesions. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8011–8015. doi: 10.1073/pnas.88.18.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S. Y., Huang T. M., Ruan L., Miao Y. H., Lu H., Chu C. M., Motz M., Wolf H. First EBV vaccine trial in humans using recombinant vaccinia virus expressing the major membrane antigen. Dev Biol Stand. 1995;84:171–177. [PubMed] [Google Scholar]
- Hany M., Oehen S., Schulz M., Hengartner H., Mackett M., Bishop D. H., Overton H., Zinkernagel R. M. Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein. Eur J Immunol. 1989 Mar;19(3):417–424. doi: 10.1002/eji.1830190302. [DOI] [PubMed] [Google Scholar]
- Hassett D. E., Condit R. C. Targeted construction of temperature-sensitive mutations in vaccinia virus by replacing clustered charged residues with alanine. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4554–4558. doi: 10.1073/pnas.91.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Weber K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J Virol. 1985 Sep;55(3):651–659. doi: 10.1128/jvi.55.3.651-659.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Fuerst T. R., Sutter G., Carroll M. W., Yang L. C., Goldstein S., Piatak M., Jr, Elkins W. R., Alvord W. G., Montefiori D. C. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996 Jun;70(6):3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein-Mintzel V., Hänichen T., Huber H. C., Stickl H. Vaccinia- und variolaprotektive Wirkung des modifizierten Vaccinia-Stammes MVA bei intramuskulärer Immunisierung. Zentralbl Bakteriol Orig A. 1975;230(3):283–297. [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. Reverse guanine phosphoribosyltransferase selection of recombinant vaccinia viruses. Virology. 1990 Oct;178(2):626–630. doi: 10.1016/0042-6822(90)90367-z. [DOI] [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraquemada D., Marti M., Long E. O. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990 Sep 1;172(3):947–954. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. V., Moss B. Mapping of the vaccinia virus DNA polymerase gene by marker rescue and cell-free translation of selected RNA. J Virol. 1984 Jan;49(1):72–77. doi: 10.1128/jvi.49.1.72-77.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., del Val M., Keil G. M., Reddehase M. J., Koszinowski U. H. A nonstructural viral protein expressed by a recombinant vaccinia virus protects against lethal cytomegalovirus infection. J Virol. 1988 May;62(5):1653–1658. doi: 10.1128/jvi.62.5.1653-1658.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanesaki T., Murphy B. R., Collins P. L., Ogra P. L. Effectiveness of enteric immunization in the development of secretory immunoglobulin A response and the outcome of infection with respiratory syncytial virus. J Virol. 1991 Feb;65(2):657–663. doi: 10.1128/jvi.65.2.657-663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor J., Irvine K., Abrams S., Kaufman H., DiPietro J., Schlom J. Antitumor activity and immune responses induced by a recombinant carcinoembryonic antigen-vaccinia virus vaccine. J Natl Cancer Inst. 1992 Jul 15;84(14):1084–1091. doi: 10.1093/jnci/84.14.1084. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Blanden R. V., Ramshaw I. A. Interferon gamma is involved in the recovery of athymic nude mice from recombinant vaccinia virus/interleukin 2 infection. J Exp Med. 1990 Nov 1;172(5):1495–1503. doi: 10.1084/jem.172.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Coupar B. E., Andrew M. E., Boyle D. B., Phillips S. M., Müllbacher A., Blanden R. V., Ramshaw I. A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990 Jan 1;144(1):290–298. [PubMed] [Google Scholar]
- Keil W., Wagner R. R. Epitope mapping by deletion mutants and chimeras of two vesicular stomatitis virus glycoprotein genes expressed by a vaccinia virus vector. Virology. 1989 Jun;170(2):392–407. doi: 10.1016/0042-6822(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish M. R., King N. J., Woodhams C. E., Ramshaw I. A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990 Jan;20(1):157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Konishi E., Pincus S., Paoletti E., Laegreid W. W., Shope R. E., Mason P. W. A highly attenuated host range-restricted vaccinia virus strain, NYVAC, encoding the prM, E, and NS1 genes of Japanese encephalitis virus prevents JEV viremia in swine. Virology. 1992 Sep;190(1):454–458. doi: 10.1016/0042-6822(92)91233-k. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Hügin A. W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989 Aug;171(2):579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. B., Connors M., Firestone C. Y., Morse H. C., 3rd, Murphy B. R. The cytolytic activity of pulmonary CD8+ lymphocytes, induced by infection with a vaccinia virus recombinant expressing the M2 protein of respiratory syncytial virus (RSV), correlates with resistance to RSV infection in mice. J Virol. 1993 Feb;67(2):1044–1049. doi: 10.1128/jvi.67.2.1044-1049.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Morse H. C., 3rd, Bennink J. R., Yewdell J. W., Murphy B. R. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993 Jul;67(7):4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. M., Ruben F. L., Neff J. M., Millar J. D. Complications of smallpox vaccination, 1968. N Engl J Med. 1969 Nov 27;281(22):1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Edwards S. J., Smith G. L., Mitchell G. F., Moss B., Kemp D. J., Anders R. F. Anchoring a secreted plasmodium antigen on the surface of recombinant vaccinia virus-infected cells increases its immunogenicity. Mol Cell Biol. 1986 Sep;6(9):3191–3199. doi: 10.1128/mcb.6.9.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Kieny M. P., Gerlinger P., Clertant P., Guizani I., Cuzin F., Chambon P. Tumour prevention and rejection with recombinant vaccinia. 1987 Apr 30-May 6Nature. 326(6116):878–880. doi: 10.1038/326878a0. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Roos J. M., McGuigan L. C., Smith K. A., Cormier N., Cohen L. K., Roberts B. E., Payne L. G. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol. 1992 May;66(5):2617–2630. doi: 10.1128/jvi.66.5.2617-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. J., Karschin A., Jayashree-Aiyar S., Davidson N., Tanouye M. A., Thomas L., Thomas G., Lester H. A. Expression of Drosophila Shaker potassium channels in mammalian cells infected with recombinant vaccinia virus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7629–7633. doi: 10.1073/pnas.86.19.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier C., Burny A., Meulemans G. Construction of a pigeonpox virus recombinant: expression of the Newcastle disease virus (NDV) fusion glycoprotein and protection of chickens against NDV challenge. Arch Virol. 1991;118(1-2):43–56. doi: 10.1007/BF01311302. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Malvoisin E., Wild F. Contribution of measles virus fusion protein in protective immunity: anti-F monoclonal antibodies neutralize virus infectivity and protect mice against challenge. J Virol. 1990 Oct;64(10):5160–5162. doi: 10.1128/jvi.64.10.5160-5162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Michie C. A., Gotch F. M., Smith G. L., Moss B. Recognition of influenza A virus nucleoprotein by human cytotoxic T lymphocytes. J Gen Virol. 1986 Apr;67(Pt 4):719–726. doi: 10.1099/0022-1317-67-4-719. [DOI] [PubMed] [Google Scholar]
- Meitin C. A., Bender B. S., Small P. A., Jr Enteric immunization of mice against influenza with recombinant vaccinia. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11187–11191. doi: 10.1073/pnas.91.23.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitin C. A., Bender B. S., Small P. A., Jr Influenza immunization: intranasal live vaccinia recombinant contrasted with parenteral inactivated vaccine. Vaccine. 1991 Oct;9(10):751–756. doi: 10.1016/0264-410x(91)90292-e. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M., Moss B. Introduction of foreign DNA into the vaccinia virus genome by in vitro ligation: recombination-independent selectable cloning vectors. Virology. 1992 Sep;190(1):522–526. doi: 10.1016/0042-6822(92)91246-q. [DOI] [PubMed] [Google Scholar]
- Meyer H., Sutter G., Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991 May;72(Pt 5):1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- Miyazawa M., Nishio J., Chesebro B. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J Virol. 1992 Jul;66(7):4497–4507. doi: 10.1128/jvi.66.7.4497-4507.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami T., Fuerst T. R., Berger E. A., Moss B. Binding region for human immunodeficiency virus (HIV) and epitopes for HIV-blocking monoclonal antibodies of the CD4 molecule defined by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9273–9277. doi: 10.1073/pnas.85.23.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. B., Smith G. L. Steroid hormone synthesis by a vaccinia enzyme: a new type of virus virulence factor. EMBO J. 1992 May;11(5):1973–1980. doi: 10.1002/j.1460-2075.1992.tb05251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Grzelecki A., Huang C. Vaccinia virus vectors utilizing the beta-galactosidase assay for rapid selection of recombinant viruses and measurement of gene expression. Gene. 1986;47(2-3):193–199. doi: 10.1016/0378-1119(86)90063-6. [DOI] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. D., Ray C. A., Drucker R. P., Pickup D. J. A poxvirus-derived vector that directs high levels of expression of cloned genes in mammalian cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9431–9435. doi: 10.1073/pnas.85.24.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Perkus M. E., Limbach K., Paoletti E. Cloning and expression of foreign genes in vaccinia virus, using a host range selection system. J Virol. 1989 Sep;63(9):3829–3836. doi: 10.1128/jvi.63.9.3829-3836.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit D. L., Koothan T., Liao D., Malinow R. Vaccinia virus transfection of hippocampal slice neurons. Neuron. 1995 Apr;14(4):685–688. doi: 10.1016/0896-6273(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Pfleiderer M., Falkner F. G., Dorner F. A novel vaccinia virus expression system allowing construction of recombinants without the need for selection markers, plasmids and bacterial hosts. J Gen Virol. 1995 Dec;76(Pt 12):2957–2962. doi: 10.1099/0022-1317-76-12-2957. [DOI] [PubMed] [Google Scholar]
- Pialoux G., Excler J. L., Rivière Y., Gonzalez-Canali G., Feuillie V., Coulaud P., Gluckman J. C., Matthews T. J., Meignier B., Kieny M. P. A prime-boost approach to HIV preventive vaccine using a recombinant canarypox virus expressing glycoprotein 160 (MN) followed by a recombinant glycoprotein 160 (MN/LAI). The AGIS Group, and l'Agence Nationale de Recherche sur le SIDA. AIDS Res Hum Retroviruses. 1995 Mar;11(3):373–381. doi: 10.1089/aid.1995.11.373. [DOI] [PubMed] [Google Scholar]
- Piccini A., Perkus M. E., Paoletti E. Vaccinia virus as an expression vector. Methods Enzymol. 1987;153:545–563. doi: 10.1016/0076-6879(87)53077-4. [DOI] [PubMed] [Google Scholar]
- Polydefkis M., Koenig S., Flexner C., Obah E., Gebo K., Chakrabarti S., Earl P. L., Moss B., Siliciano R. F. Anchor sequence-dependent endogenous processing of human immunodeficiency virus 1 envelope glycoprotein gp160 for CD4+ T cell recognition. J Exp Med. 1990 Mar 1;171(3):875–887. doi: 10.1084/jem.171.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay A. J., Husband A. J., Ramshaw I. A., Bao S., Matthaei K. I., Koehler G., Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994 Apr 22;264(5158):561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- Ramsay A. J., Kohonen-Corish M. Interleukin-5 expressed by a recombinant virus vector enhances specific mucosal IgA responses in vivo. Eur J Immunol. 1993 Dec;23(12):3141–3145. doi: 10.1002/eji.1830231215. [DOI] [PubMed] [Google Scholar]
- Ramsey-Ewing A., Moss B. Restriction of vaccinia virus replication in CHO cells occurs at the stage of viral intermediate protein synthesis. Virology. 1995 Feb 1;206(2):984–993. doi: 10.1006/viro.1995.1021. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Rao J. B., Chamberlain R. S., Bronte V., Carroll M. W., Irvine K. R., Moss B., Rosenberg S. A., Restifo N. P. IL-12 is an effective adjuvant to recombinant vaccinia virus-based tumor vaccines: enhancement by simultaneous B7-1 expression. J Immunol. 1996 May 1;156(9):3357–3365. [PMC free article] [PubMed] [Google Scholar]
- Redfield R. R., Wright D. C., James W. D., Jones T. S., Brown C., Burke D. S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987 Mar 12;316(11):673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Renegar K. B., Small P. A., Jr Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991 Apr;65(4):2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Zhou Y. W., Rodriguez J. R., Durbin R. K., Jimenez V., McAllister W. T., Esteban M. Regulated expression of nuclear genes by T3 RNA polymerase and lac repressor, using recombinant vaccinia virus vectors. J Virol. 1990 Oct;64(10):4851–4857. doi: 10.1128/jvi.64.10.4851-4857.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Esteban M. Plaque size phenotype as a selectable marker to generate vaccinia virus recombinants. J Virol. 1989 Feb;63(2):997–1001. doi: 10.1128/jvi.63.2.997-1001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Smith G. L. Inducible gene expression from vaccinia virus vectors. Virology. 1990 Jul;177(1):239–250. doi: 10.1016/0042-6822(90)90477-9. [DOI] [PubMed] [Google Scholar]
- Romero C. H., Barrett T., Chamberlain R. W., Kitching R. P., Fleming M., Black D. N. Recombinant capripoxvirus expressing the hemagglutinin protein gene of rinderpest virus: protection of cattle against rinderpest and lumpy skin disease viruses. Virology. 1994 Oct;204(1):425–429. doi: 10.1006/viro.1994.1548. [DOI] [PubMed] [Google Scholar]
- Romero C. H., Barrett T., Evans S. A., Kitching R. P., Gershon P. D., Bostock C., Black D. N. Single capripoxvirus recombinant vaccine for the protection of cattle against rinderpest and lumpy skin disease. Vaccine. 1993;11(7):737–742. doi: 10.1016/0264-410x(93)90258-y. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Roth J., Dittmer D., Rea D., Tartaglia J., Paoletti E., Levine A. J. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4781–4786. doi: 10.1073/pnas.93.10.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby J., Brinkman C., Jones S., Ramshaw I. Response of monkeys to vaccination with recombinant vaccinia virus which coexpress HIV gp160 and human interleukin-2. Immunol Cell Biol. 1990 Apr;68(Pt 2):113–117. doi: 10.1038/icb.1990.16. [DOI] [PubMed] [Google Scholar]
- Rupprecht C. E., Wiktor T. J., Johnston D. H., Hamir A. N., Dietzschold B., Wunner W. H., Glickman L. T., Koprowski H. Oral immunization and protection of raccoons (Procyon lotor) with a vaccinia-rabies glycoprotein recombinant virus vaccine. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7947–7950. doi: 10.1073/pnas.83.20.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiflinger F., Dorner F., Falkner F. G. Construction of chimeric vaccinia viruses by molecular cloning and packaging. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9977–9981. doi: 10.1073/pnas.89.21.9977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M., Sodeik B., Ericsson M., Wolffe E. J., Shida H., Hiller G., Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994 Jan;68(1):130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M. J., Mebatsion T., Conzelmann K. K. Infectious rabies viruses from cloned cDNA. EMBO J. 1994 Sep 15;13(18):4195–4203. doi: 10.1002/j.1460-2075.1994.tb06739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983 Nov;25(1):21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Stokes G. V. High-voltage electron microscope study of the release of vaccinia virus from whole cells. J Virol. 1976 May;18(2):636–643. doi: 10.1128/jvi.18.2.636-643.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G., Moss B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10847–10851. doi: 10.1073/pnas.89.22.10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter G., Ohlmann M., Erfle V. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 1995 Aug 28;371(1):9–12. doi: 10.1016/0014-5793(95)00843-x. [DOI] [PubMed] [Google Scholar]
- Sutter G., Wyatt L. S., Foley P. L., Bennink J. R., Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994 Aug;12(11):1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Perkus M. E., Taylor J., Norton E. K., Audonnet J. C., Cox W. I., Davis S. W., van der Hoeven J., Meignier B., Riviere M. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Taylor J., Edbauer C., Rey-Senelonge A., Bouquet J. F., Norton E., Goebel S., Desmettre P., Paoletti E. Newcastle disease virus fusion protein expressed in a fowlpox virus recombinant confers protection in chickens. J Virol. 1990 Apr;64(4):1441–1450. doi: 10.1128/jvi.64.4.1441-1450.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J., Trimarchi C., Weinberg R., Languet B., Guillemin F., Desmettre P., Paoletti E. Efficacy studies on a canarypox-rabies recombinant virus. Vaccine. 1991 Mar;9(3):190–193. doi: 10.1016/0264-410x(91)90152-v. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Kawaoka Y., Webster R. G., Paoletti E. Protective immunity against avian influenza induced by a fowlpox virus recombinant. Vaccine. 1988 Dec;6(6):504–508. doi: 10.1016/0264-410x(88)90101-6. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Languet B., Desmettre P., Paoletti E. Recombinant fowlpox virus inducing protective immunity in non-avian species. Vaccine. 1988 Dec;6(6):497–503. doi: 10.1016/0264-410x(88)90100-4. [DOI] [PubMed] [Google Scholar]
- Taylor J., Weinberg R., Tartaglia J., Richardson C., Alkhatib G., Briedis D., Appel M., Norton E., Paoletti E. Nonreplicating viral vectors as potential vaccines: recombinant canarypox virus expressing measles virus fusion (F) and hemagglutinin (HA) glycoproteins. Virology. 1992 Mar;187(1):321–328. doi: 10.1016/0042-6822(92)90321-f. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bastin J., Gould K., Brownlee G., Andrew M., Coupar B., Boyle D., Chan S., Smith G. Defective presentation to class I-restricted cytotoxic T lymphocytes in vaccinia-infected cells is overcome by enhanced degradation of antigen. J Exp Med. 1988 Oct 1;168(4):1211–1224. doi: 10.1084/jem.168.4.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin T. B., Brownstein M. J., Moss B., Isaacs S. N. SP6 RNA polymerase containing vaccinia virus for rapid expression of cloned genes in tissue culture. Biotechniques. 1993 Feb;14(2):222–224. [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Ward G. A., Stover C. K., Moss B., Fuerst T. R. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6773–6777. doi: 10.1073/pnas.92.15.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Kawaoka Y., Taylor J., Weinberg R., Paoletti E. Efficacy of nucleoprotein and haemagglutinin antigens expressed in fowlpox virus as vaccine for influenza in chickens. Vaccine. 1991 May;9(5):303–308. doi: 10.1016/0264-410x(91)90055-b. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S. P., Ball L. A., Barr J. N., Wertz G. T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Zou D. J., Koothan T., Cline H. T. Infection of frog neurons with vaccinia virus permits in vivo expression of foreign proteins. Neuron. 1995 Apr;14(4):681–684. doi: 10.1016/0896-6273(95)90211-2. [DOI] [PubMed] [Google Scholar]
- Wyatt L. S., Moss B., Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995 Jun 20;210(1):202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Karschin A., Labarca C., Elroy-Stein O., Moss B., Davidson N., Lester H. A. Expression of ion channels and receptors in Xenopus oocytes using vaccinia virus. FASEB J. 1991 May;5(8):2209–2216. doi: 10.1096/fasebj.5.8.1708738. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilma T., Hsu D., Jones L., Owens S., Grubman M., Mebus C., Yamanaka M., Dale B. Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science. 1988 Nov 18;242(4881):1058–1061. doi: 10.1126/science.3194758. [DOI] [PubMed] [Google Scholar]
- Yilma T., Ristow S. S., Moss B., Jones L. A novel approach for the production of monoclonal antibodies using infectious vaccinia virus recombinants. Hybridoma. 1987 Aug;6(4):329–335. doi: 10.1089/hyb.1987.6.329. [DOI] [PubMed] [Google Scholar]
- Yuen L., Moss B. Oligonucleotide sequence signaling transcriptional termination of vaccinia virus early genes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6417–6421. doi: 10.1073/pnas.84.18.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Lasky L. A., Moss B. Herpes simplex virus (HSV)-specific human T-cell clones recognize HSV glycoprotein D expressed by a recombinant vaccinia virus. J Virol. 1986 Aug;59(2):506–509. doi: 10.1128/jvi.59.2.506-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Leek M. L., Feller J. A., Sorensen G., Isaacson W., Adams C. L., Borde D. J., Pfeiffer N., Tran T., Moyer R. W., Gibbs E. P. Evaluation of swinepox virus as a vaccine vector in pigs using an Aujeszky's disease (pseudorabies) virus gene insert coding for glycoproteins gp50 and gp63. Vet Rec. 1994 Jan 1;134(1):13–18. doi: 10.1136/vr.134.1.13. [DOI] [PubMed] [Google Scholar]





