Abstract
Objective
The aim of this study was to evaluate the learning curve and perioperative outcomes of robot-assisted laparoscopic procedure for cervical cancer.
Methods
A series of 65 cases of robot-assisted laparoscopic radical hysterectomies with bilateral pelvic lymph node dissection for early stage cervical cancer were included. Demographic data and various perioperative parameters including docking time, console time, and total operative time were reviewed from the prospectively collected database. Console time was set as a surrogate marker for surgical competency, in addition to surgical outcomes. The learning curve was evaluated using cumulative summation method.
Results
The mean operative time was 190 minutes (range, 117 to 350 minutes). Two unique phases of the learning curve were derived using cumulative summation analysis; phase 1 (the initial learning curve of 28 cases), and phase 2 (the improvement phase of subsequent cases in which more challenging cases were managed). Docking and console times were significantly decreased after the first 28 cases compared with the latter cases (5 minutes vs. 4 minutes for docking time, 160 minutes vs. 134 minutes for console time; p<0.001 and p<0.001, respectively). There was a significant reduction in blood loss during operation (225 mL vs. 100 mL, p<0.001) and early postoperative complication rates (28% vs. 8.1%, p=0.003) in phase 2. No conversion to laparotomy occurred.
Conclusion
Improvement of surgical performance in robot-assisted surgery for cervical cancer can be achieved after 28 cases. The two phases identified by cumulative summation analysis showed significant reduction in operative time, blood loss, and complication rates in the latter phase of learning curve.
Keywords: Cervical neoplasms, Laparoscopic surgery, Learning curve, Robotics
INTRODUCTION
Since Food and Drug Administration (FDA) approval for use of the da Vinci Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) in gynecologic procedures in 2005, many studies have demonstrated the advantages of robotic assistance in overcoming the drawbacks of traditional laparoscopy [1-4]. These advantages include improved surgeon ergonomics, a magnified three-dimensional view, wider range of motion with wristed instruments, and filtration of hand tremor allowing for increased surgical precision [5,6]. Therefore, theoretically, the incorporation of robotic technology is expected to help accomplish tasks such as intracorporeal suturing and knot tying, lymphadenectomy, ureterolysis and pelvic adhesiolysis with ease compared to conventional straight stick laparoscopy. Also, application of robotics in the treatment of complex gynecologic oncology procedures such as radical hysterectomy and lymph node dissection seem feasible and safe [7-11]. However, the initial learning curve to acquire competency may be steep, and extensive assessment is important when adopting a novel surgical technique.
Cumulative summation (CUSUM) analysis is a statistical tool originally used in the industrial sector for quality assurance and detection of areas for improvement. In the medical field, it has been used since the 1970s to analyze the learning curve for surgical procedures [12,13]. With this methodology, raw data can be transformed into running total data deviations from the group mean, enabling surgeons to visualize data for trends and to determine when a level of proficiency has been attained [14]. Analysis of learning curves related to robotic surgery in non-gynecologic field is well documented [15-19]. Meanwhile, the majority of data published on robotic surgery in gynecology primarily focused on surgical data and patient outcomes, and aspects of the learning curve have not been well described. Moreover, data on the learning experiences of a skilled conventional laparoscopic surgeon are scarce and no previous study has been reported on the learning curve for robot-assisted radical hysterectomy using the CUSUM analysis. Therefore, the purpose of this study was to determine the learning curve as well as differences in surgical outcomes before and after reaching proficiency, in robot-assisted laparoscopy for cervical cancer using CUSUM methodology.
MATERIALS AND METHODS
A total of 164 robot-assisted cases were performed between May 2006 to May 2011 in our institution. After excluding hysterectomy for benign conditions and endometrial cancer staging operation, we evaluated 65 consecutive cases of robot-assisted laparoscopic radical hysterectomy with pelvic lymphadenectomy for early stage cervical cancer. Inclusion criteria were women with newly diagnosed invasive cervical cancer without treatment, International Federation of Gynecology and Obstetrics (FIGO) stage IB2 or less disease, Gynecologic Oncology Group (GOG) performance ≤1, and financial capability to pay the surgical cost ($10,000). Exclusion criteria for performing robotic surgery were patients with uterine size greater than 16 gestational weeks by pelvic examination and those with previous history of 3 or more open abdominal surgeries. All procedures were performed by a single surgeon already experienced and proficient in advanced laparoscopic gynecologic procedures. Prior to performing robot-assisted laparoscopic procedures for gynecologic cancer, the surgeon had accomplished computer-based training, observed cases, and also had completed 8 cases of robotic laparoscopic hysterectomy for benign conditions. The surgical team consisted of a fellow as bedside assistant and a resident as second assistant to manipulate the uterus.
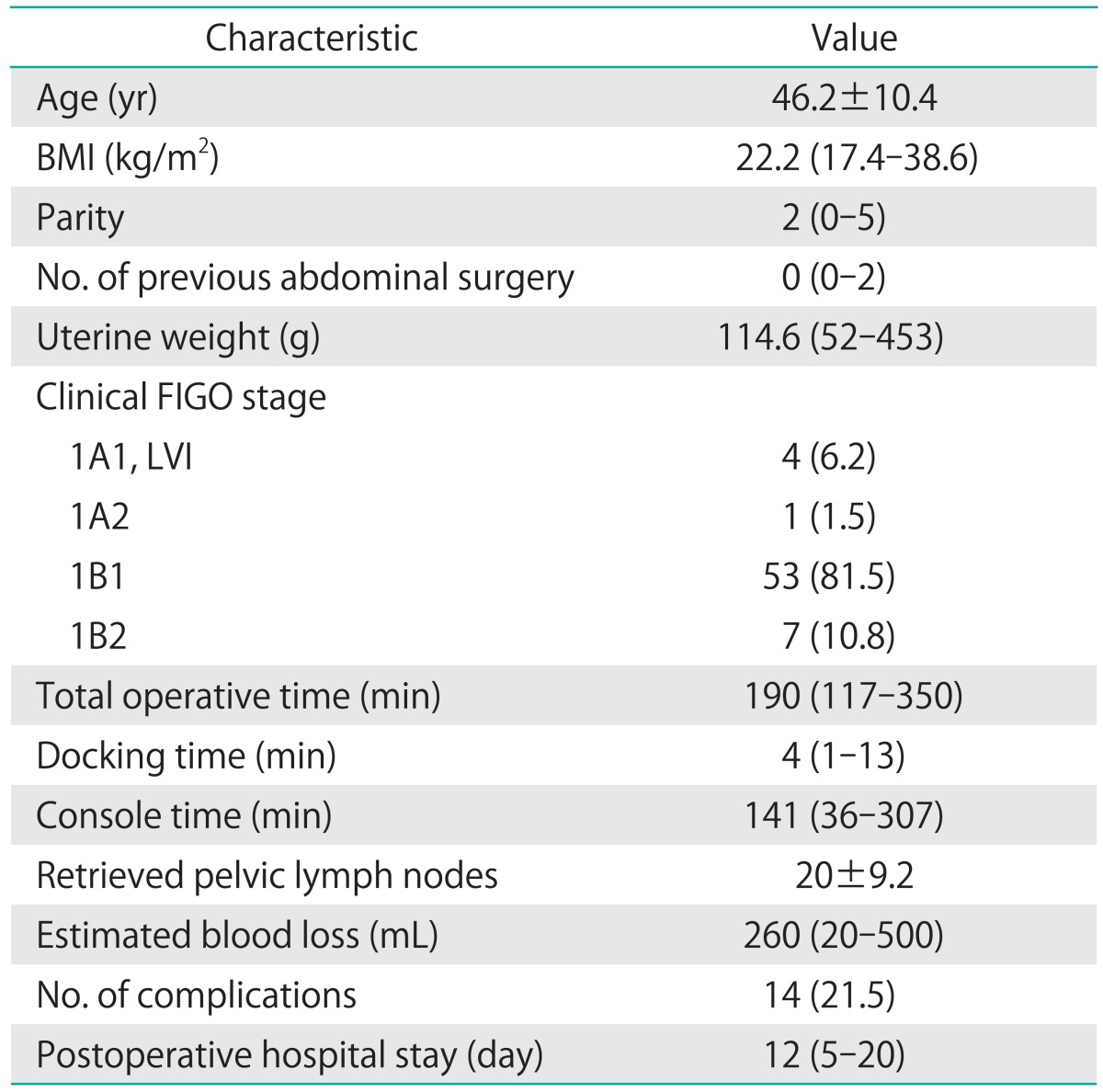
Data pertaining to patient characteristics (age, parity, body mass index, and general health status) and perioperative parameters including docking time (DT), console time (CT), total operative time (OT), estimated blood loss, number of retrieved lymph nodes, length of hospital stay, and perioperative complications were retrospectively reviewed from the prospectively entered database. DT was defined as time to position the robot and install the robotic arms securely to the port sites. CT was defined as the time the surgeon spent at the robotic console for performing the main procedural steps of radical hysterectomy and pelvic lymph node dissection. The total OT was the time from the first skin incision to the last port site skin closure. Lymph node retrieval was the number of pelvic lymph nodes at pathologic analysis. Complications were categorized as intraoperative and postoperative (early/late) events.
1. Surgical techniques
All surgeries were performed using da Vinci Robotic Surgical with Maryland Bipolar and Permanent Cautery Spatula or needle holder on each robotic arms. After general anesthesia, the patient was placed in a low dorsal lithotomy and steep Trendelenburg position. A nelaton catheter was inserted to drain the bladder. We routinely placed a RUMI uterine manipulator with a Koh colpotomy ring and vaginal balloon pneumo-occluder (Cooper Surgical Inc., Trumball, CT, USA) for adequate pelvic exposure. After creating pneumoperitoneum by Veress needle insertion at the umbilicus, four trocars were placed: a 12-mm trocar at the umbilicus for the camera, two 8-mm lateral trocars at each lower quadrant of the abdomen 2 to 3 cm below the umbilical level, and a fourth assistant port (either 5 or 10 mm) at mid-distance between the umbilicus and the left robotic arm. The surgical management of cervical cancer included radical hysterectomy with removal of bilateral pelvic lymph nodes as described in our previous report [20]. In brief, the procedure consisted of eight component parts: 1) right and left pelvic lymphadenectomy, 2) development of the paravesical and pararectal spaces, 3) ureteral dissection, 4) ligation and dissection of the uterine artery, 5) development of the vesicouterine and rectovaginal spaces, 6) resection of the parametria, 7) resection of the upper vagina, and 8) vaginal cuff closure. Adequacy of the component parts of this procedure was routinely determined and documented on video. The vaginal cuff closure was performed intracorporeally using interrupted sutures of 1-0 Vicryl (Ethicon, Piscataway, NJ, USA) and extracorporeally using a Clarke-Reich knot pusher. The surgeon would usually place 1 or 2 intracorporeal sutures and 2 extracorporeal sutures for the bedside assistant to proceed for training purposes. Upon completion of the procedure, the fascia of the port sites greater than 8 mm in diameter were closed with interrupted suture using 2-0 Vicryl (Ethicon).
2. Statistical analysis
CUSUM analysis was used to quantitatively assess the learning curve of docking (CUSUMDT) and console time (CUSUMCT). This technique provides a graphical information of the trend in the outcome of consecutive procedures performed over time, since it is a plot of cumulative total of differences between each data point and the mean of all data points [21]. Therefore, CUSUMDT was calculated as the difference between the DT for the first case and the mean DT for all 65 cases mounted chronologically. The CUSUMDT for the second case would be the previous case's CUSUMDT added to the difference between the DT for the second case and the mean DT for all the cases. The same calculation applied for CUSUMCT. When this process is continued until the last case, a recursive curve can be achieved as shown in previous studies [18,22].
Statistical analysis was performed with SPSS ver. 18 (SPSS Inc., Chicago, IL, USA) and SAS ver. 9.2 (SAS Inc., Cary, NC, USA). Kolmogorov Smirnov test was used to verify standard normal distributional assumptions. Student's t-test and Mann-Whitney U-test were used for parametric and non-parametric variables, respectively. Differences between proportions were compared using Fisher's test or χ2 test. A p-value of less than 0.05 was regarded as statistically significant.
RESULTS
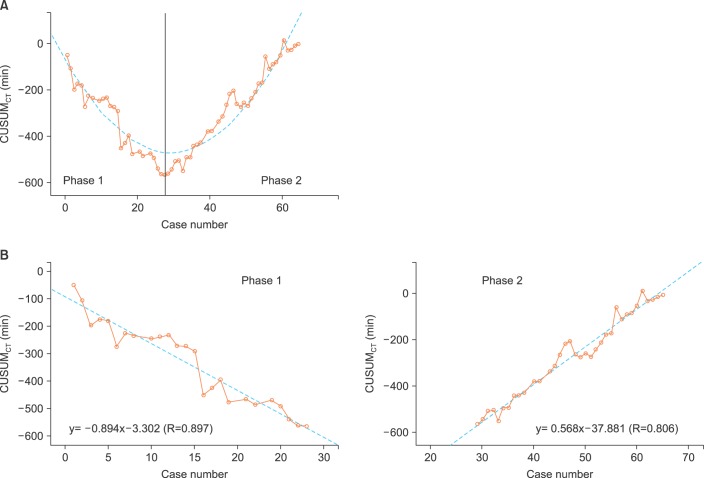
Sixty-five consecutive robot-assisted laparoscopic radical hysterectomy procedures were performed during the study period. Patient demographics, operative characteristics, and postoperative outcomes are shown in Table 1. Most of the patients (53/65, 81.5%) had clinical FIGO stage 1B1 disease. All cases were completed robotically without conversion to conventional straight stick laparoscopy or laparotomy. Fig. 1 shows the raw surgeon CT plotted chronologically against the case number (Fig. 1A). Using CUSUMCT, the learning curve was drawn by plotting the cumulative sequential differences between each CT data point and the process average over time. This curve was best modeled as a second-order parabola with the equation: CUSUM in minutes equals 0.46×case number2-27.43×case number-63.04, with a high R-value of 0.89 (Fig. 1B). The CUSUM value of 28 divided the learning curve into two distinct phases: phase 1, a negative slope representing the initial 28 cases with an average lower than the process average, and phase 2, a positive slope indicating the next 37 cases with an average higher than the process average (Fig. 2A). From this data, increased competence of surgeon could be seen after the first 28 cases.
Table 1.
Overall patient characteristics (n=65)
Values are presented as mean±SD, median (range), or number (%).
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular space infiltration.
Fig. 1.
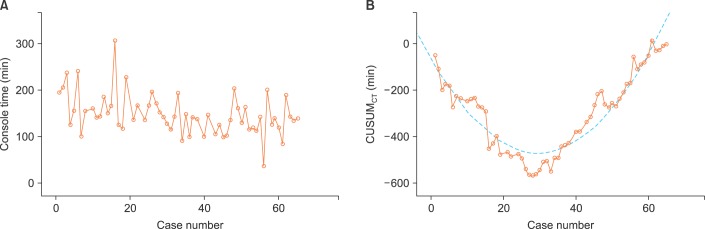
Console time (CT) plots. (A) The raw CT plotted against chronological case number. (B) Cumulative sum (CUSUM) of CT plotted against case number (solid line). CUSUM curve of best modeled fit for the plot (dashed line).
Fig. 2.
Two phases and the lines of best fit for each phase of the cumulative sum (CUSUM) learning curve. (A) The CUSUM value 28 divides the learning curve of the console time (CT) into two phases. (B) Lines best fit for each phase. Phase 1 represents the initial learning curve. Phase 2 represents increasing competence of surgeon after the initial 28 case.
Patient characteristics and perioperative outcomes were compared between the two phases identified by CUSUMCT analysis (Table 2). Age, body mass index, previous surgical history, and clinical stage did not differ significantly between the two groups. The median uterine weight was slightly higher in phase 2 compared to the first phase (140 g vs. 105.5 g; p=0.0243). A significant reduction in total OT was observed during phase 2 compared with phase 1 (167 minutes and 206.5 minutes, respectively; p<0.001). The median DT for the first 28 cases (phase 1: 5 minutes; range, 2 to 13 minutes) was slightly longer than that for the last 37 cases (phase 2: 4 minutes; range, 1 to 11 minutes; p<0.001). The median console time of phase 2 was also significantly shorter than that of phase 1 (134 minutes [range, 36 to 203 minutes] vs. 160 minutes [range, 100 to 307 minutes], respectively, p<0.001). The shortest CT in the last 37 cases was 36 minutes. This particular case with the shortest CT was a 42-year-old patient with clinical stage 1A1 squamous cell carcinoma. Modified radical hysterectomy was performed with bilateral pelvic lymph node dissection with total yield of 11 nodes (with no lymph node extension on final pathology). The absence of pelvic adhesion, normal sized uterus, and no intraoperative enlarged or bulky pelvic lymph node resulted in the shortest CT for this case which was performed during phase 2. There was no significant difference in the acquired number of pelvic lymph nodes and the length of postoperative hospital stay between the two phases. Intraoperative and postoperative complications (including all minor complications such as transient febrile event to major complications including bowel injury) occurred in 10 patients (35.7%) during the first phase of 28 cases, and in 4 patients (10.8%) during phase 2. Early postoperative complications (≤6 weeks after surgery) were significantly lower in the later phase of the learning curve (28.6% vs. 8.1%, respectively; p=0.033).There was one case of intraoperative blood transfusion to a patient with known anemia because of an ongoing estimated surgical blood loss of about 500 mL. No postoperative severe leg edema, ileus, or wound dehiscence developed during the later phase. Most of the early and late postoperative complications were spontaneously resolved by conservative management, except one case of panperitonitis due to bowel perforation, which developed postoperatively in the 59th patient (phase 2). The patient had developed fever and abdominal pain from postoperative day 5, and diagnostic laparoscopy revealed extensive stool soilage in the abdominal cavity leaking from an approximately 1-cm sized laceration site on the anterior wall of the upper rectum. Low anterior resection with diverting ileostomy was proceeded via a laparotomy. The same patient developed vesicovaginal fistula with ureteral stricture about 2 months after the bowel surgery. However, it healed spontaneously without surgical intervention after prolonged bladder catheterization. Ileostomy was successfully repaired 3 months after low anterior resection and she is currently being followed up without further complication or cancer recurrence.
Table 2.
Interphase comparisons of patient characteristics and perioperative outcomes
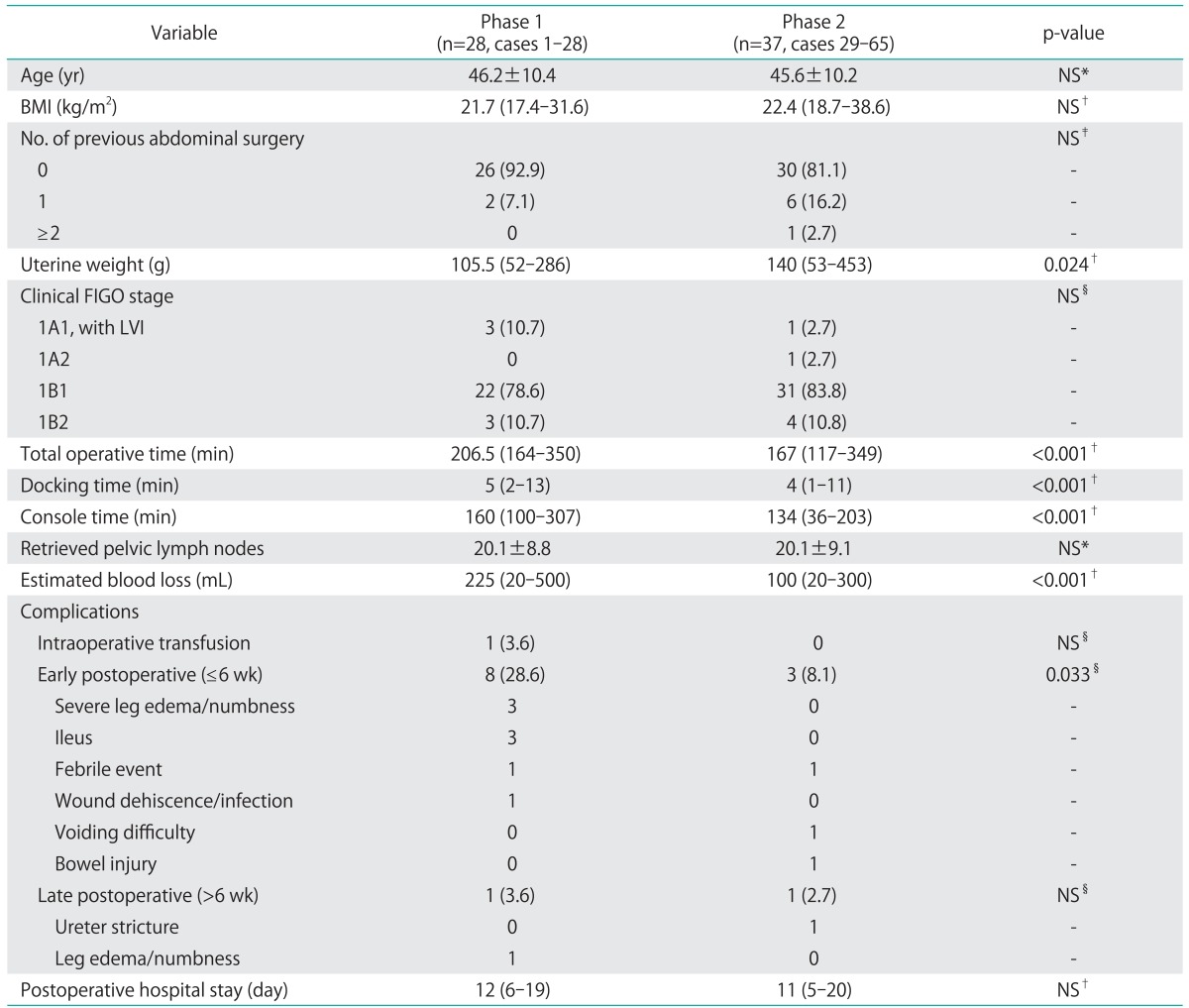
Values are presented as mean±SD, median (range), or number (%).
BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LVI, lymphovascular space infiltration; NS, not significant.
*Student's t-test, †Mann-Whitney U-test, ‡Fisher's exact test, §chi-square test
DISCUSSION
The results of this study showed that improvements in the surgical performance of robot-assisted radical hysterectomy with pelvic lymph node dissection in the treatment of cervical cancer can be achieved after 28 cases in terms of procedural time, blood loss, and complication rates. We used CUSUM analysis to identify two phases of learning in regards to surgeon CT as a surrogate marker for surgical competency.
To date, most publications investigating the learning curve for robot-assisted laparoscopy for gynecologic conditions split chronological cases into predetermined phases and used simple comparisons such as univariate and/or regression analysis [23-31]. It may not be easy to identify a significant trend in surgical performance when the raw data are simply plotted against the chronological case number, such as that shown in Fig. 1A. CUSUM analysis is a technique which plots the cumulative sequential differences between each data point and the process average over time [13,14]. Therefore, a shift in the learning average can be observed more easily by a graph than in a standard control chart format, enabling investigators to visualize the data for trends not discernable with other approaches. As shown in Fig. 2A, the CUSUMCT graph showed variance from the mean for each case, yielding a parabolic curve with two distinct phases from which the learning curve could be assessed. The negative slope of the graph indicates longer CT with greater variance from the overall average, and represents the initial learning curve (Fig. 2B). A positive slope of the graph indicates an average higher than the overall performance average, and represents a proficiency phase with a reduction in CT. The high R values for the line of best fit in each phase (0.897 and 0.806 for phase 1 and 2, respectively) indicate the unique components of the surgeon's learning curve. Likewise, the CUSUM method can be used to demonstrate the level of competence with a surgical technique, since it is better at detecting small shifts in the process mean.
We compared various parameters between the two distinct phases of learning identified by the CUSUM analysis. There was a significant decrease in total OT during phase 2, attributed mainly to a decrease in CT (Table 2). Of particular note, the longest CT of 307 minutes occurred during phase 1, while the shortest (36 minutes) was performed during phase 2 (case number 33). This decrease in OT, despite an increase in median uterine weight and no decrease in lymph node yields in later cases, may indicate the performance and selection of more challenging cases taken on with increased surgeon competence. As for DT, only a subtle decrease in phase 2 may be explained by rotating bed side assistants every 4 months (usually a fellow) who assisted or performed most of the docking procedure under the supervision of the operator. Therefore, DT may not entirely reflect the learning curve of a single surgeon in this study. Still, the overall total OT decreased in phase 2, and the performance of the first assistant is speculated to have improved at the same time, since robotic surgery is a procedure that requires well orchestrated team work. The number of retrieved pelvic lymph nodes remained consistent during the study period with less blood loss in the later phase. No difference in hospital stay was documented since we routinely start bladder catheter training from postoperative day 6 and discharge patients after resuming self-voiding function. Therefore, the length of postoperative hospital stay was longer than 10 days in both groups.
Our data appears to be compatible with other studies that have addressed learning curves in robot-assisted gynecologic surgery. Published series with specific suggestions on the number of cases needed for proficiency or that analyzed surgical performance in robotic gynecologic surgery are summarized in Table 3. Robot-assisted urogynecologic and infertility procedures, and studies addressing less than 20 cases were excluded from review. Many publications have set OT as the standard to assess proficiency, and the number of cases needed to achieve competency ranged from 9 to as many as 50 cases. Learning curve analyses for benign procedures demonstrated proficiency upon completion of approximately 20 cases with improvement over time [25,31]. However, in a prospective evaluation of 113 patients with benign gynecologic conditions by Lenihan et al. [29], robotic CT and total OT plateaued after approximately 50 cases. This is probably due to many changes in the surgeons' techniques and instrumentation during the initial cases. Also, performance assessment was not stratified by the type of procedure or surgeon, because two surgeons unequally operated or assisted on various cases.
Table 3.
Published series on learning curve of robot-assisted for gynecologic surgery
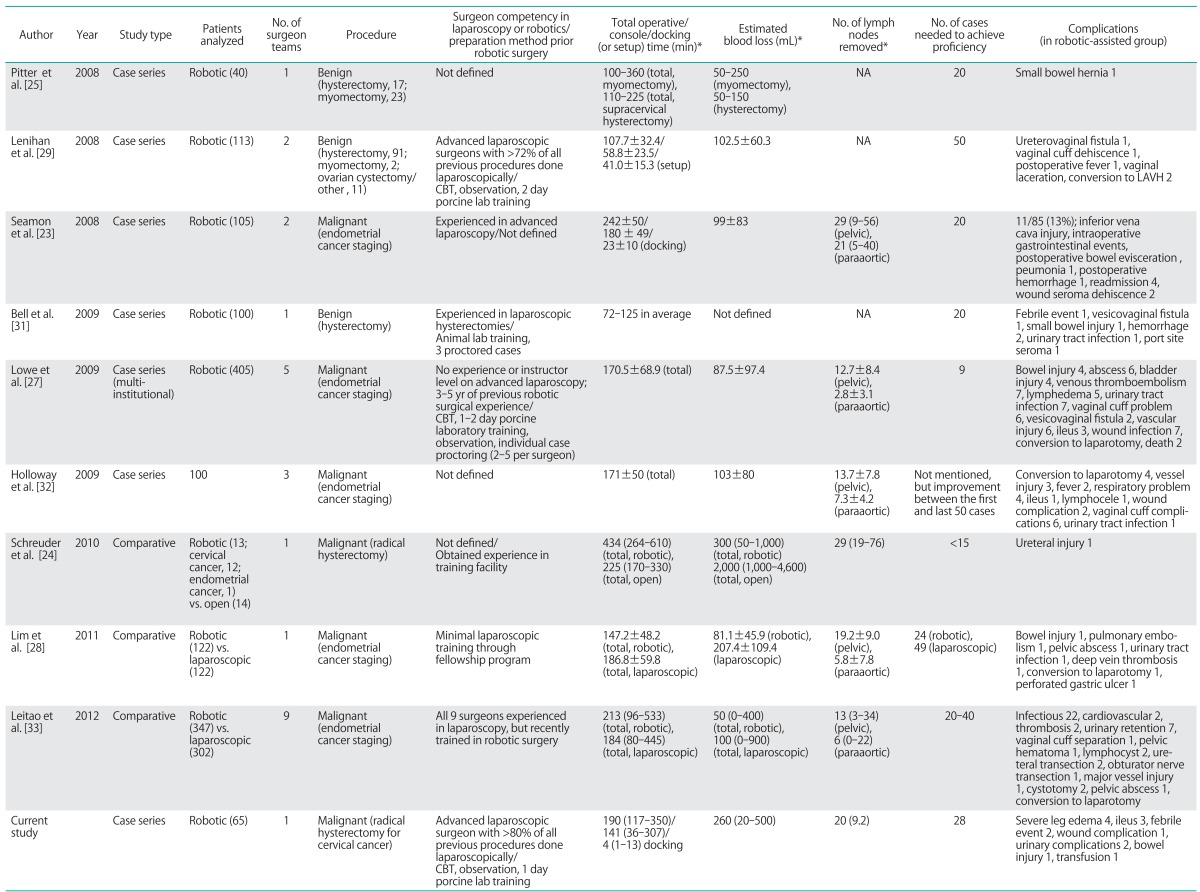
CBT, computer-based training; LAVH, laparoscopic assisted vaginal hysterectomy; NA, not applicable.
*Mean±SD or median (range).
Several contributing factors may affect learning curve outcomes for robotic surgery. First is the surgeon's previous experience in robotics or advanced laparoscopy. Whether prior proficiency in advanced laparoscopy contributes to faster learning curve in robotic surgery is still under discussion. Moreover, previous surgical experience and training prior to adopting robotic surgery has varied between studies or has not been well described. In this study, an advanced laparoscopic surgeon who had been performing more than 80% of all procedures laparoscopically, received computer-based training, case observation, porcine lab training, and had operated on benign gynecologic cases prior to commencing robot-assisted radical hysterectomy with lymph node dissection. However, the number of cases needed to achieve proficiency in our study was not significantly less than that of other studies performed by inexperienced or under trained surgeons [24,27,28].
Second, differences in the metrics used to assess surgical proficiency may contribute to the discrepancy among studies. Most published articles have used total OT in addition to other factors such as estimated blood loss and complication rates to evaluate surgical proficiency. However, the use of certain standard may not accurately reflect competency over time [23,24,29,32]. For example, as a surgeon becomes more comfortable and competent with the procedure, he or she may operate on more challenging patients such as those expected to have adhesions from prior surgeries, obese patients, advanced stages of cancer, or increase the extent of certain procedures such as lymphadenectomy. Holloway et al. [32] compared the first 50 and the last 50 cases in a cohort of 100 patients undergoing robotic surgical staging for endometrial cancer. Three attending surgeons and two fellows-in-training performed all or portions of each procedure, and the results revealed a shorter OT, higher number of retrieved lymph nodes, and a lower rate of operative complications in the later cases. Not surprisingly, the OT for one of the surgeons increased from 120 minutes during the first-half to 176 minutes in the second-half, with an almost two-fold increase in the lymph node yield. This finding obviously indicates evolving operative limits set by the surgeon as he or she gains more experience.
Third, the specific characteristics of robotic surgery, i.e., the various surgical steps of the procedure, may alter learning curve outcomes. Total OT, for example, may be subdivided into specific components such as initial robotic system setup, trocar insertion, docking, and console time according to the steps of procedures. A recent analysis by Lim et al. [28] revealed that each specific robotic procedure has its own unique learning curve. The efficiency for docking and trocar placement (10th case), hysterectomy (8th case), cuff closure (21st case), pelvic lymph node dissection (55th case), and paraaortic lymph node dissection (17th case) stabilized after a varying number of cases. Also, the set-up including draping and docking of the robotic system may be time consuming at first and affect data when mastered rapidly by a designated and well-trained team [34].
The major limitation of this study is that this cohort represents a small window of learning curve data of a single surgeon that may not be generalized to the entire gynecologic oncology public. However, data available in the literature on robotic radical hysterectomy is limited, and single surgeon experience is important to consider, especially when taking in account that our institution is one of the early adopters of robotic surgery and a leading national/international teaching center in Asia. Therefore, the authors believe that the current data could be applied to surgeons of similar settings (single or small surgeon practice, prior instructor-level laparoscopic experience considering robotic surgery, extensive background with open procedures, and dedicated robotic surgical team). In addition, we stress that the number of cases need to achieve competency may be vastly different according to previous experience and individual skills of surgeon. Another limitation is that our robotic cohort was not compared with a conventional laparoscopic cohort. Nonetheless, the fact that cases were performed by a single surgeon without any fluctuation in the surgical procedure is one of the major strengths of this study. Studies including different surgeons at different time points in their learning curve may cause variability in the outcome.
The method of learning curve analysis in this study is distinct from previous studies because the unique phases of the learning curve were not derived from a predetermined grouping of cases. In the field of obstetrics and gynecology, studies using the CUSUM method have been limited to assessing proficiency in procedures such as embryo transfer for in vitro fertilization [35], certain diagnostic ultrasound procedures [36,37], and fetoscopic laser ablation [38]. To our knowledge, this is the first report to evaluate the learning curve of robot-assisted surgery in gynecology using the CUSUM method. Graphical representation of learning experience by CUSUM analysis can be a useful tool for quality assessment of surgical novices. Furthermore, the quantitative assessment of individual performance by CUSUM analysis can help prevent wasting of resources and time needed to train surgeons who are already proficient [14].
In conclusion, a minimum of 28 cases of robot-assisted laparoscopic radical hysterectomies is suggested in the treatment of early cervical cancer for an experienced laparoscopic surgeon to achieve surgical improvement. With exponential growth and rapid adaptation of robotic surgery in many institutions, there may not be enough time for surgeons to thoroughly assess their learning curves or develop structured training programs. However, we believe that defining the learning curve is important to reduce the risks associated with incompletely trained surgeons performing complex procedures. The results of this study may serve as a reference for learning assessment in similar clinical settings.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084120).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Mendivil A, Holloway RW, Boggess JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol. 2009;114(2 Suppl):S24–S31. doi: 10.1016/j.ygyno.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Lin PS, Wakabayashi MT, Han ES. Role of robotic surgery in endometrial cancer. Curr Treat Options Oncol. 2009;10:33–43. doi: 10.1007/s11864-009-0086-4. [DOI] [PubMed] [Google Scholar]
- 3.Jung YW, Kim SW, Kim YT. Recent advances of robotic surgery and single port laparoscopy in gynecologic oncology. J Gynecol Oncol. 2009;20:137–144. doi: 10.3802/jgo.2009.20.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Advincula AP, Wang K. Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol. 2009;16:291–301. doi: 10.1016/j.jmig.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynecology: scientific dream or reality. Fertil Steril. 2009;91:2620–2622. doi: 10.1016/j.fertnstert.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 6.Chen CC, Falcone T. Robotic gynecologic surgery: past, present, and future. Clin Obstet Gynecol. 2009;52:335–343. doi: 10.1097/GRF.0b013e3181b08adf. [DOI] [PubMed] [Google Scholar]
- 7.Yim GW, Kim SW, Nam EJ, Kim YT. Role of robot-assisted surgery in cervical cancer. Int J Gynecol Cancer. 2011;21:173–181. doi: 10.1097/IGC.0b013e318200f7a7. [DOI] [PubMed] [Google Scholar]
- 8.Soliman PT, Frumovitz M, Sun CC, Dos Reis R, Schmeler KM, Nick AM, et al. Radical hysterectomy: a comparison of surgical approaches after adoption of robotic surgery in gynecologic oncology. Gynecol Oncol. 2011;123:333–336. doi: 10.1016/j.ygyno.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer. 2010;20:438–442. doi: 10.1111/IGC.0b013e3181cf5c2c. [DOI] [PubMed] [Google Scholar]
- 10.Magrina JF, Kho R, Magtibay PM. Robotic radical hysterectomy: technical aspects. Gynecol Oncol. 2009;113:28–31. doi: 10.1016/j.ygyno.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez PT, Soliman PT, Schmeler KM, dos Reis R, Frumovitz M. Laparoscopic and robotic techniques for radical hysterectomy in patients with early-stage cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S21–S24. doi: 10.1016/j.ygyno.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Wohl H. The cusum plot: its utility in the analysis of clinical data. N Engl J Med. 1977;296:1044–1045. doi: 10.1056/NEJM197705052961806. [DOI] [PubMed] [Google Scholar]
- 13.Williams AK, Chalasani V, Martinez CH, Osbourne E, Stitt L, Izawa JI, et al. Cumulative summation graphs are a useful tool for monitoring positive surgical margin rates in robot-assisted radical prostatectomy. BJU Int. 2011;107:1648–1652. doi: 10.1111/j.1464-410X.2010.09634.x. [DOI] [PubMed] [Google Scholar]
- 14.Biau DJ, Williams SM, Schlup MM, Nizard RS, Porcher R. Quantitative and individualized assessment of the learning curve using LC-CUSUM. Br J Surg. 2008;95:925–929. doi: 10.1002/bjs.6056. [DOI] [PubMed] [Google Scholar]
- 15.Secin FP. The learning curve of robotic assisted laparoscopic radical prostatectomy: what is the evidence? Arch Esp Urol. 2011;64:830–838. [PubMed] [Google Scholar]
- 16.Pierorazio PM, Patel HD, Feng T, Yohannan J, Hyams ES, Allaf ME. Robotic-assisted versus traditional laparoscopic partial nephrectomy: comparison of outcomes and evaluation of learning curve. Urology. 2011;78:813–819. doi: 10.1016/j.urology.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Yun JH, Nam KH, Soh EY, Chung WY. The learning curve for robotic thyroidectomy: a multicenter study. Ann Surg Oncol. 2011;18:226–232. doi: 10.1245/s10434-010-1220-z. [DOI] [PubMed] [Google Scholar]
- 18.Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25:855–860. doi: 10.1007/s00464-010-1281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu SC, Clapp BL, Lee MJ, Albrecht WC, Scarborough TK, Wilson EB. Robotic assistance provides excellent outcomes during the learning curve for laparoscopic Roux-en-Y gastric bypass: results from 100 robotic-assisted gastric bypasses. Am J Surg. 2006;192:746–749. doi: 10.1016/j.amjsurg.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol. 2008;108:312–316. doi: 10.1016/j.ygyno.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins DM, Olwell DH. Cumulative sum charts and charting for quality improvement. New York: Springer; 1998. [Google Scholar]
- 22.Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, et al. The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg. 2010;251:249–253. doi: 10.1097/SLA.0b013e3181b7fdb0. [DOI] [PubMed] [Google Scholar]
- 23.Seamon LG, Cohn DE, Richardson DL, Valmadre S, Carlson MJ, Phillips GS, et al. Robotic hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Obstet Gynecol. 2008;112:1207–1213. doi: 10.1097/AOG.0b013e31818e4416. [DOI] [PubMed] [Google Scholar]
- 24.Schreuder HW, Zweemer RP, van Baal WM, van de Lande J, Dijkstra JC, Verheijen RH. From open radical hysterectomy to robot-assisted laparoscopic radical hysterectomy for early stage cervical cancer: aspects of a single institution learning curve. Gynecol Surg. 2010;7:253–258. doi: 10.1007/s10397-010-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitter MC, Anderson P, Blissett A, Pemberton N. Robotic-assisted gynaecological surgery-establishing training criteria; minimizing operative time and blood loss. Int J Med Robot. 2008;4:114–120. doi: 10.1002/rcs.183. [DOI] [PubMed] [Google Scholar]
- 26.Payne TN, Dauterive FR. A comparison of total laparoscopic hysterectomy to robotically assisted hysterectomy: surgical outcomes in a community practice. J Minim Invasive Gynecol. 2008;15:286–291. doi: 10.1016/j.jmig.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Lowe MP, Johnson PR, Kamelle SA, Kumar S, Chamberlain DH, Tillmanns TD. A multiinstitutional experience with roboticassisted hysterectomy with staging for endometrial cancer. Obstet Gynecol. 2009;114(2 Pt 1):236–243. doi: 10.1097/AOG.0b013e3181af2a74. [DOI] [PubMed] [Google Scholar]
- 28.Lim PC, Kang E, Park DH. A comparative detail analysis of the learning curve and surgical outcome for robotic hysterectomy with lymphadenectomy versus laparoscopic hysterectomy with lymphadenectomy in treatment of endometrial cancer: a case-matched controlled study of the first one hundred twenty two patients. Gynecol Oncol. 2011;120:413–418. doi: 10.1016/j.ygyno.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 29.Lenihan JP, Jr, Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008;15:589–594. doi: 10.1016/j.jmig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Fanning J, Hojat R, Johnson J, Fenton B. Robotic radical hysterectomy. Minerva Ginecol. 2009;61:53–55. [PubMed] [Google Scholar]
- 31.Bell MC, Torgerson JL, Kreaden U. The first 100 da Vinci hysterectomies: an analysis of the learning curve for a single surgeon. S D Med. 2009;62:91, 93–95. [PubMed] [Google Scholar]
- 32.Holloway RW, Ahmad S, DeNardis SA, Peterson LB, Sultana N, Bigsby GE, 4th, et al. Robotic-assisted laparoscopic hysterectomy and lymphadenectomy for endometrial cancer: analysis of surgical performance. Gynecol Oncol. 2009;115:447–452. doi: 10.1016/j.ygyno.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Leitao MM, Jr, Briscoe G, Santos K, Winder A, Jewell EL, Hoskins WJ, et al. Introduction of a computer-based surgical platform in the surgical care of patients with newly diagnosed uterine cancer: outcomes and impact on approach. Gynecol Oncol. 2012;125:394–399. doi: 10.1016/j.ygyno.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 34.Iranmanesh P, Morel P, Wagner OJ, Inan I, Pugin F, Hagen ME. Set-up and docking of the da Vinci surgical system: prospective analysis of initial experience. Int J Med Robot. 2010;6:57–60. doi: 10.1002/rcs.288. [DOI] [PubMed] [Google Scholar]
- 35.Dessolle L, Freour T, Barriere P, Jean M, Ravel C, Darai E, et al. How soon can I be proficient in embryo transfer? Lessons from the cumulative summation test for learning curve (LC-CUSUM) Hum Reprod. 2010;25:380–386. doi: 10.1093/humrep/dep391. [DOI] [PubMed] [Google Scholar]
- 36.Bazot M, Darai E, Biau DJ, Ballester M, Dessolle L. Learning curve of transvaginal ultrasound for the diagnosis of endometriomas assessed by the cumulative summation test (LC-CUSUM) Fertil Steril. 2011;95:301–303. doi: 10.1016/j.fertnstert.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Balsyte D, Schaffer L, Burkhardt T, Wisser J, Zimmermann R, Kurmanavicius J. Continuous independent quality control for fetal ultrasound biometry provided by the cumulative summation technique. Ultrasound Obstet Gynecol. 2010;35:449–455. doi: 10.1002/uog.7545. [DOI] [PubMed] [Google Scholar]
- 38.Papanna R, Biau DJ, Mann LK, Johnson A, Moise KJ., Jr Use of the Learning Curve-Cumulative Summation test for quantitative and individualized assessment of competency of a surgical procedure in obstetrics and gynecology: fetoscopic laser ablation as a model. Am J Obstet Gynecol. 2011;204:218.e1–218.e9. doi: 10.1016/j.ajog.2010.10.910. [DOI] [PubMed] [Google Scholar]