Abstract
Objective
To investigate the utility of serum squamous cell carcinoma antigen (SCC-Ag) levels upon the diagnosis of recurrent cervical cancer for decision making in patient management.
Methods
Clinical records from 167 cervical cancer patients who developed recurrence between April 1996 and September 2010 were reviewed. A Cox proportional hazards regression model was used to investigate the prognostic significance of serum SCC-Ag levels at the time of recurrence. The effects of various salvage treatments on survival outcomes of recurrent cervical cancer were examined with respect to serum SCC-Ag levels.
Results
Serum SCC-Ag levels were elevated (>2.0 ng/mL) in 125 patients (75%) when recurrence was diagnosed. These patients exhibited significantly shorter postrecurrence survival than those with normal SCC-Ag levels (log-rank; p=0.033). Multivariate analyses revealed that an elevated serum SCC-Ag level was an independent prognostic factor for poor postrecurrence survival. In patients with SCC-Ag levels <14.0 ng/mL, radiotherapy or surgery resulted in improved survival compared with chemotherapy or supportive care. In contrast, in patients with SCC-Ag levels of ≥14.0 ng/mL, salvage treatment with radiotherapy had only a minimal impact on postrecurrence survival.
Conclusion
The serum SCC-Ag level measured when cervical cancer recurrence is diagnosed can be useful for deciding upon the appropriate salvage treatment.
Keywords: Decision-making, Recurrent cervical cancer, Squamous cell carcinoma antigen, Survival
INTRODUCTION
Cervical cancer is the third most common cancer worldwide, with an annual incidence of 530,000 new cases. Although largely preventable, it still results in approximately 250,000 deaths globally each year [1]. In Japan, 6,000-7,000 new cases of the disease are reported annually [2]. Cervical cancer patients that suffer recurrence still have a very poor prognosis [3], despite recent advances in surgery [4], the introduction of new radiotherapy techniques such as intensity-modulated radiotherapy and image-guided radiotherapy [5,6], and the development of cisplatin-based combination chemotherapy [7].
Because of the short life expectancy of recurrent cervical cancer patients, it is very important to identify factors that predict the outcome of salvage treatments, enabling physicians and patients to choose the optimal treatment. The prognostic factors for recurrent cervical cancer patients have been investigated in several studies [8-11]. Recurrence within the previously irradiated area, young age, a poor performance status, an elevated leukocyte count, and a short time to progression from the initial diagnosis have been reported as significant predictors of shorter survival [8-11].
Squamous cell carcinoma antigen (SCC-Ag) is used as a serological tumor marker in women with SCC of the uterine cervix. It is 1 of 14 subtypes of the tumor antigen TA-4, a 48-kDa glycoprotein that was first isolated by Kato and Torigoe [12] in 1977. Although it is found in normal cervical epithelium, its expression is increased in cervical neoplasms, particularly in well- and moderately differentiated SCC of the uterine cervix [13]. The serum SCC-Ag level is reported to be elevated in 28%-88% of patients with SCC of the uterine cervix at the time of the initial diagnosis [14,15]. Previous studies demonstrated that elevated serum SCC-Ag levels before the start of treatment were associated with advanced stage disease [16]; pelvic lymph node metastasis [17]; shorter survival [18-20]; and poor responses to radiotherapy [19], chemoradiotherapy [21], or chemotherapy [16]. Moreover, measuring the SCC-Ag level has been shown to aid the early detection of disease recurrence [20,22].
Several previous studies have reported that recurrent cervical cancer patients with elevated serum SCC-Ag levels survived for a shorter time than those with normal serum SCC-Ag levels [20]. However, the clinical value of using serum SCC-Ag levels to decide the optimal salvage treatment for recurrent cervical cancer has not yet been determined.
In the present study, we investigated the utility of the serum SCC-Ag level at the time of recurrence in predicting the outcome of salvage treatment. In addition, we established a prognostic model for estimating survival outcomes in patients with recurrent cervical cancer based on factors including the serum SCC-Ag level.
MATERIALS AND METHODS
1. Patients
Permission to proceed with data acquisition and analysis was obtained from Osaka University Hospital's Institutional Review Board. A list of patients who had been diagnosed with recurrent cervical cancer at Osaka University Hospital from April 1996 to September 2010 was generated from our institutional tumor registry. Patients with SCC histology were then identified through a chart review. Patients with non-SCC histology were excluded. Records for 167 recurrent cervical cancer patients were analyzed in this study.
2. Follow-up
The follow-up examinations after the initial treatment were conducted by gynecological oncologists with or without radiation oncologists in an outpatient clinic, as reported previously [10,23]. Briefly, follow-up visits occurred every month in the first year, every 2 months in the second year, every 3 months in the third year, every 4 months in the fourth year, every 6 months in the fifth year, and annually thereafter until 10 years after treatment. The standard follow-up surveillance program consisted of clinical history taking, a physical examination, and blood analyses including an SCC-Ag assay and a complete blood analysis. Chest radiography and computed tomography (CT) of the pelvis and abdomen were performed every 6 months in the first year and annually thereafter. In patients who displayed suspicious histological or physical findings or who had persistently elevated serum SCC-Ag levels during follow-up, further evaluations were performed including chest radiography, CT of the pelvis and abdomen, and magnetic resonance imaging (MRI). All lesions that were suspected recurrent tumors were confirmed as such by histological or cytological diagnosis whenever possible. Recurrence was defined as the presence of disease after a 3-month or longer treatment-free interval after the end of the primary treatment. The disease-free interval (DFI) was defined as the time from the end of the primary treatment to the detection of recurrence.
3. Measurement of serum SCC-Ag levels
Serum SCC-Ag levels were measured using an immunoradiometric assay (Abbott Diagnostics, Chicago, IL, USA). The intra-assay coefficient of variation (CV) ranged from 1.3% to 3.4%, and the inter-assay CV ranged from 3.8% to 4.6%. A concentration of 2.0 ng/mL, which represented the 98th percentile in a random sample of 616 healthy Japanese women, was defined as the upper limit of normal. Serum SCC-Ag levels were measured at every follow-up visit after the initial treatment. Serum SCC-Ag levels at the time of diagnosis of recurrence were evaluated in the current study.
4. Salvage treatments
Recurrent disease was treated in accordance with the institutional treatment guidelines. Single distant recurrent tumors were treated with salvage external beam radiotherapy or salvage surgery. Patients who had central recurrence were treated with salvage surgery or, when possible, with interstitial brachytherapy. Patients with pelvic sidewall disease or multiple recurrences were treated with platinum-based chemotherapy [24]. Patients with a single distant tumor that could not be salvaged by surgery or radiotherapy were also treated with platinum-based chemotherapy. Patients who refused salvage chemotherapy or radiotherapy were given only supportive care. Postrecurrence survival was defined as the time from the diagnosis of recurrence to death or the last follow-up visit.
5. Statistical analysis
Continuous parameters such as age and DFI were analyzed using the Student's t-test or Kolmogorov-Smirnov test. Categorical data such as disease stage were compared between the groups using the chi-square test or Fisher's exact test. The logistic likelihood ratio test was used to determine the best cut-off value of SCC-Ag levels for our patient population. Using this approach, we found that a SCC-Ag level of 14.0 ng/mL was associated with the greatest difference in postrecurrence survival between the patient groups.
We performed a Kaplan-Meier survival analysis using a log-rank test. Cox proportional hazards regression analysis was performed to identify significant independent prognostic factors for postrecurrence survival. All p-values were two-sided, and p<0.05 was considered statistically significant. All statistical analyses were performed with SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Patients
The clinical and pathological characteristics of the patients included in the current study are summarized in Table 1. The mean age of the patients was 58 years. The clinical stage at the initial diagnosis was stage I-II in 88 patients and stage III-IV in 79 patients. Radiotherapy was administered to 160 patients (95.8%) as part of their initial treatment. Diagnosis of recurrent disease occurred within 1 year of the primary treatment for 87 patients (52.1%). The median DFI was 11 months. Single recurrences occurred in 83 patients (49.7%), while 84 patients (50.3%) suffered multiple recurrences. Recurrent disease was treated with supportive care alone in 46 cases, platinum-based chemotherapy in 61 cases, radiotherapy in 50 cases, and surgery in 10 cases. The median survival period after recurrence was 17 months.
Table 1.
Patient characteristics (n=167)
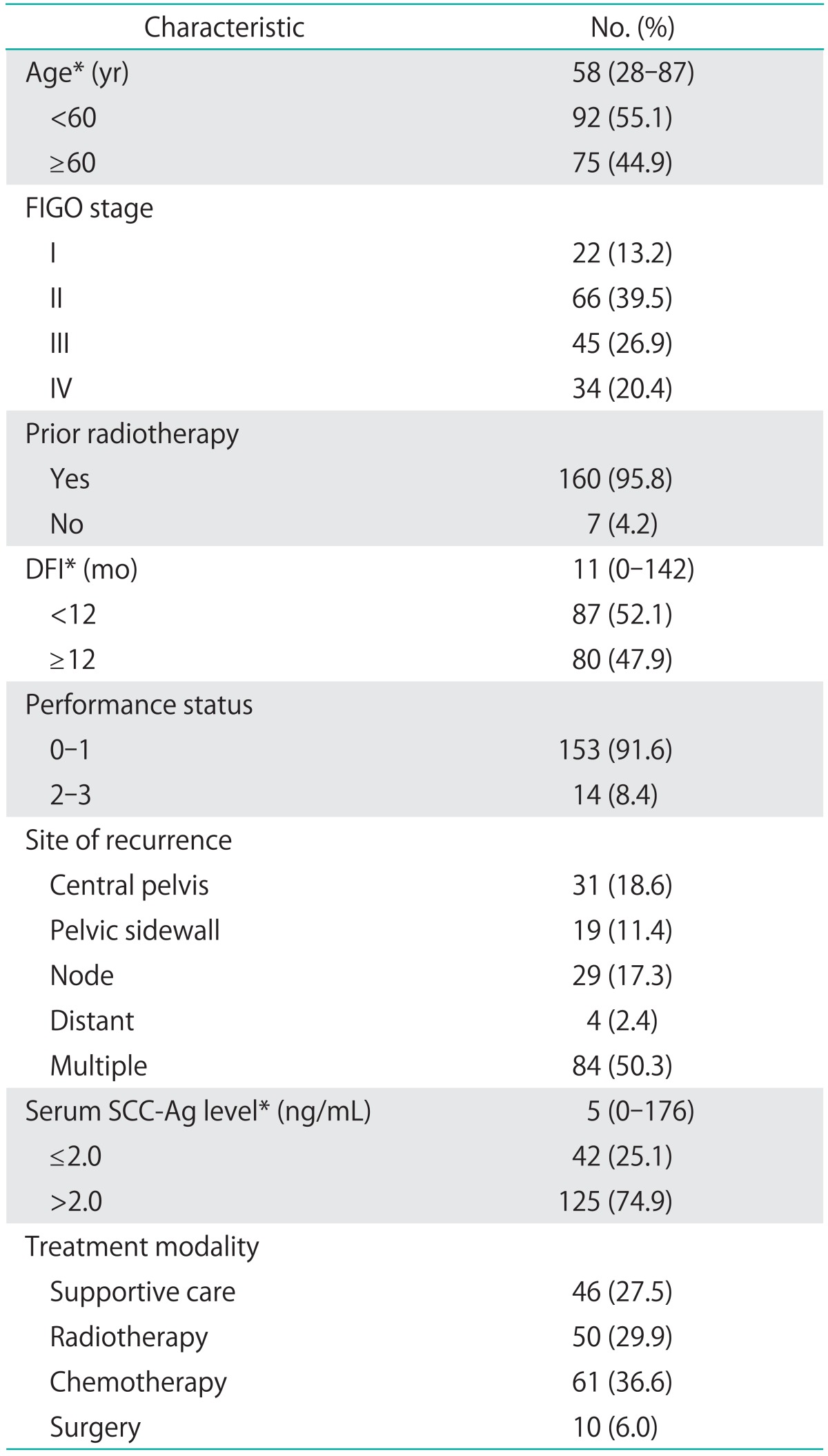
DFI, disease free interval; FIGO, International Federation of Gynecology and Obstetrics; SCC-Ag, squamous cell carcinoma antigen.
*Median (range).
2. The prognostic significance of the SCC-Ag level at the time of recurrence diagnosis
As shown in Table 1, of the 167 patients, 125 had elevated serum SCC-Ag levels (SCC-Ag>2.0 ng/mL) at the time that recurrence was diagnosed. The mean and median SCC-Ag level of these patients was 13.9 ng/mL and 5.0 ng/mL (range, 0 to 176 ng/mL), respectively. Patients with SCC-Ag levels>2.0 ng/mL exhibited significantly shorter postrecurrence survival than those with SCC-Ag levels ≤2.0 ng/mL (median postrecurrence survival: 16 months vs. 23 months, log-rank; p<0.033) (Fig. 1A). We next determined the optimum cutoff serum SCC-Ag level for our study population, as described above in the Materials and Methods section, and found that a SCC-Ag level of 14.0 ng/mL resulted in the greatest difference in postrecurrence survival between the patient groups (Fig. 1B) (median postrecurrence survival: 12 months vs. 25 months, log-rank; p<0.001).
Fig. 1.
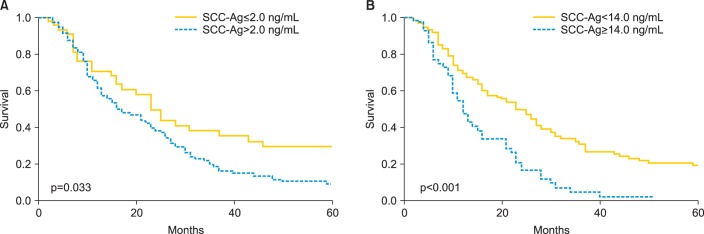
Kaplan-Meier estimates of postrecurrence survival according to the serum squamous cell carcinoma antigen (SCC-Ag) level. (A) Patients with serum SCC-Ag levels >2.0 ng/mL had a significantly shorter postrecurrence survival than those with serum SCC-Ag levels ≤2.0 ng/mL (p=0.033). (B) Patients with serum SCC-Ag levels ≥14.0 ng/mL also had a significantly shorter postrecurrence survival than those with serum SCC-Ag levels <14.0 ng/mL (p<0.001).
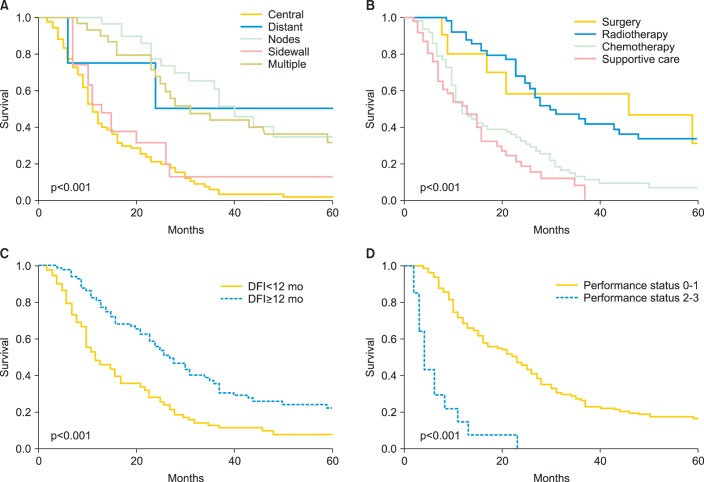
We further investigated the prognostic significance of serum SCC-Ag levels using multivariate analysis (Table 2). In the separate univariate analyses, patients with pelvic central, single distant, or lymph node recurrences achieved longer postrecurrence survival than did those with pelvic sidewall or multiple recurrences (Fig. 2A). Moreover, the patients who were treated with surgery or radiotherapy survived significantly longer after recurrence than those who were treated with chemotherapy or supportive care, indicating that the role of chemotherapy in this subset of patients is still palliative (Fig. 2B). Thus, in the subsequent multivariate analysis, the following parameters were combined to produce single prognostic variables: salvage treatment with surgery or radiotherapy; chemotherapy or supportive care; pelvic central, single distant, or lymph node recurrence; and pelvic sidewall recurrence or multiple recurrences. The Cox multivariate analyses showed that DFI, performance status, recurrence site, and the serum SCC-Ag level were independent prognostic factors for postrecurrence survival (Table 2, Figs. 1B, 2).
Table 2.
Univariate and multivariate analysis for postrecurrence survival
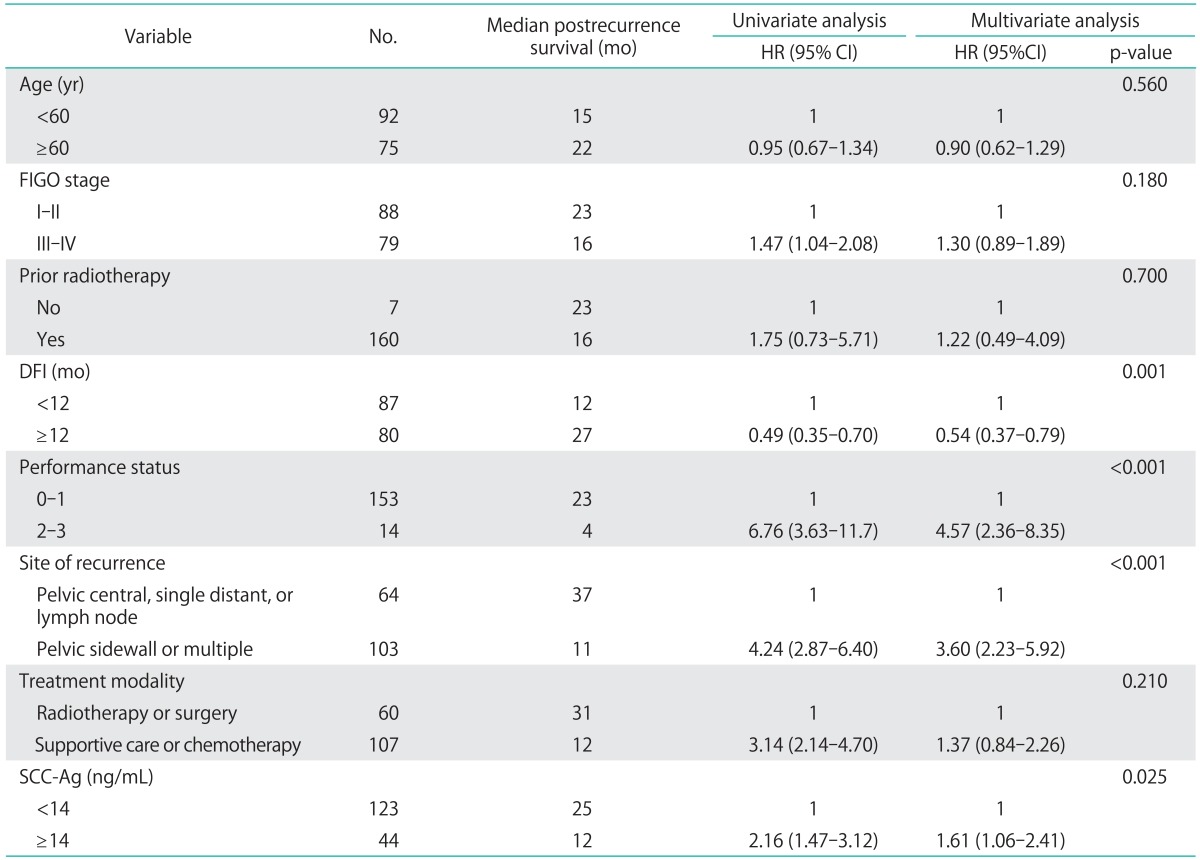
CI, confidence interval; DFI, disease free interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; SCC-Ag, squamous cell carcinoma antigen.
Fig. 2.
Postrecurrence survival according to (A) the site of recurrence, (B) salvage treatment, (C) disease-free interval (DFI), and (D) performance status.
3. Usefulness of serum SCC-Ag levels in deciding upon optimal the treatment for recurrent cervical cancer
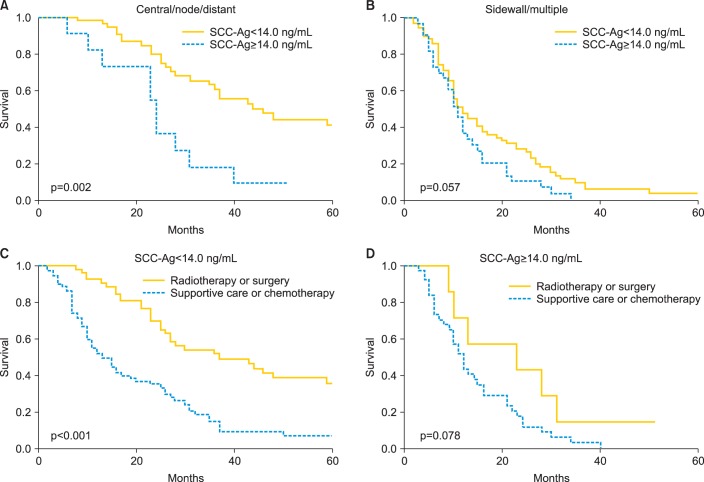
As shown in Fig. 3A, in patients with pelvic central, single distant, or lymph node recurrences, elevated SCC-Ag levels (SCC-Ag ≥14.0 ng/mL) were associated with significantly shorter postrecurrence survival (p<0.002). In contrast, although patients with pelvic sidewall or multiple recurrences (Fig. 3B) as well as those with SCC-Ag levels ≥14.0 ng/mL tended to have shorter postrecurrence survival, this difference was not statistically significant (p=0.057). These results indicate that the prognostic significance of SCC-Ag levels is more evident in patients with pelvic central, single distant, or lymph node recurrences that can be salvaged with curative treatments including radiotherapy or surgery, than in those with pelvic sidewall or multiple recurrences that are usually treated with chemotherapy of supportive care alone.
Fig. 3.
Kaplan-Meier estimates of postrecurrence survival according to the serum squamous cell carcinoma antigen (SCC-Ag) level in patients with (A) pelvic central, single distant, or lymph node recurrences and (B) pelvic sidewall or multiple recurrences. Kaplan-Meier estimates of postrecurrence survival according to salvage treatment in patients with (C) serum SCC-Ag levels<14.0 ng/mL and (D) serum SCC-Ag levels ≥ 14.0 ng/mL.
To investigate the utility of SCC-Ag levels for deciding upon the optimal treatment of recurrent cervical cancer, we examined the effects of various salvage treatments on post-recurrence survival with respect to the SCC-Ag level. Patients with SCC-Ag levels <14.0 ng/mL who were treated with surgery or radiotherapy survived significantly longer after recurrence than those treated with chemotherapy or supportive care (p<0.001). In contrast, in patients with SCC-Ag levels ≥14.0 ng/mL, salvage treatment with surgery or radiotherapy was not associated with improved postrecurrence survival (p=0.078) (Fig. 3D).
To establish a predictive model in this population, we assessed postrecurrence survival with respect to the number of poor prognostic factors, with the exception of serum SCC-Ag levels. Patients were analyzed according to whether they had 0, 1, or ≥2 poor prognostic factors. Amongst patients with serum SCC-Ag levels<14.0 ng/mL, postrecurrence survival was inversely associated with the number of poor prognostic factors (p<0.001) (Fig. 4A). The median postrecurrence survival times of the patients with 0, 1, and ≥2 poor prognostic factors other than elevated SCC-Ag levels were 46, 23, and 12 months, respectively. In contrast, among the patients with serum SCC-Ag levels ≥ 14.0 ng/mL, all groups displayed similar survival outcomes regardless of whether they had 0, 1, or ≥2 poor prognostic factors (p=0.387) (Fig. 4B).
Fig. 4.
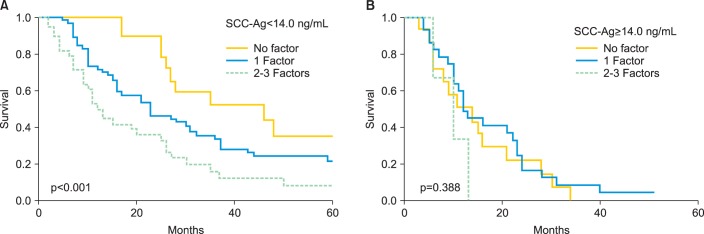
Kaplan-Meier estimates of postrecurrence survival according to the number of poor prognostic factors. (A) Patients with serum squamous cell carcinoma antigen (SCC-Ag) levels <14.0 ng/mL. (B) Patients with serum SCC-Ag levels ≥14.0 ng/mL.
DISCUSSION
The measurement of serum SCC-Ag levels has been used successfully in the posttreatment surveillance of cervical cancer patients for predicting recurrence. In the current study, the serum SCC-Ag level was greater than 2.0 ng/mL at the time when recurrence was diagnosed in 125 patients (75%), which was consistent with the rate found in a previous study [20].
The concept of follow-up surveillance of cervical cancer patients is based on the premise that the early detection of recurrence would allow effective salvage treatment, resulting in decreased morbidity and mortality rates [25]. However, whether or not early detection of recurrent cervical cancer via the assessment of serum SCC-Ag levels could improve survival remains controversial. Some authors have suggested that follow-up measurement of serum SCC-Ag levels could allow the early detection of cervical cancer recurrence and improve survival [22,26]. However, other studies have shown that while the routine assessment of serum SCC-Ag levels during follow-up resulted in the earlier detection of recurrence, it did not contribute to prolonged survival [18,27]. In the current study, the postrecurrence survival of patients with elevated serum SCC-Ag levels (>2.0 ng/mL) was significantly shorter than that of patients with normal SCC-Ag levels (log-rank; p=0.033) (Fig. 1). Further studies are needed to investigate the value of routine surveillance of serum SCC-Ag levels for improving postrecurrence survival.
Although the serum SCC-Ag level has been widely used to predict recurrence, it has not been used to aid decision-making regarding salvage treatment for recurrent disease. We found that in patients with serum SCC-Ag levels <14.0 ng/mL, salvage treatment with surgery or radiotherapy was associated with improved survival compared with chemotherapy or supportive care. In contrast, patients with SCC-Ag levels >14.0 ng/mL had poor postrecurrence survival irrespective of whether salvage treatment was used (Fig. 3D), indicating that it is not effective in this population. Traditionally, the treatment of choice for recurrent cervical cancer depends on the site and extent of the recurrent disease; however, our results indicate that the serum SCC-Ag level might be a useful marker in deciding whether to use salvage treatment, and that serum SCC-Ag levels could predict the survival benefits of different salvage treatments.
We did not include 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) in our routine follow-up program. It has recently been demonstrated that FDG-PET is a reliable modality for detecting the asymptomatic recurrence of uterine cervical cancer [28]. The use of FDG-PET in asymptomatic patients with elevated SCC-Ag levels was shown to allow the early diagnosis of recurrence, which resulted in improved survival in patients with recurrent cervical cancer [29]. Moreover, it was reported that FDG-PET could be used to predict the clinical outcomes of patients with recurrent cervical carcinoma [30]. Using a combination of FDG-PET and serum SCC-Ag level measurement might enable accurate prediction of the prognosis of recurrent cervical cancer patients, and the clinical value of this combination should be investigated in future studies.
One limitation of the current study is the relatively small sample size. In addition, given the retrospective nature of this study, we cannot exclude the potential sources of biases such as lead time bias and length time bias. Moreover, selection bias might have played a role in the physicians' choice of salvage treatments, and the educational level and/or the socio-economic status of the patient might also have influenced the choice of salvage treatment. Further prospective studies are necessary to eliminate these potential biases.
In conclusion, the current study demonstrates that the serum SCC-Ag level at the time of diagnosis of recurrent cervical cancer aids decision-making regarding salvage treatment and could allow more accurate, individualized pretreatment assessments of the benefits that patients will derive from particular salvage treatments. By considering the serum SCC-Ag level, physicians might be able to identify high-risk patients who would not benefit from conventional salvage treatments, which would in turn give them the opportunity to offer patients other types of treatment such as best supportive care for those with a poor performance status and entry into a novel clinical trial for patients with a good performance status. Moreover, our prognostic model, composed of 4 prognostic variables, allows us to estimate postrecurrence survival more precisely in this population. The utility of the SCC-Ag level at the time of diagnosis of recurrence in assessing prognosis and influencing decision-making regarding salvage treatment should be validated in future clinical studies.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ajiki W, Tsukuma H, Oshima A Research Group for Population-based Cancer Registration in Japan. Cancer incidence and incidence rates in Japan in 1999: estimates based on data from 11 population-based cancer registries. Jpn J Clin Oncol. 2004;34:352–356. doi: 10.1093/jjco/hyh056. [DOI] [PubMed] [Google Scholar]
- 3.Long HJ., 3rd Management of metastatic cervical cancer: review of the literature. J Clin Oncol. 2007;25:2966–2974. doi: 10.1200/JCO.2006.09.3781. [DOI] [PubMed] [Google Scholar]
- 4.Hockel M, Dornhofer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol. 2006;7:837–847. doi: 10.1016/S1470-2045(06)70903-2. [DOI] [PubMed] [Google Scholar]
- 5.Fokdal L, Tanderup K, Nielsen SK, Christensen HK, Rohl L, Pedersen EM, et al. Image and laparoscopic guided interstitial brachytherapy for locally advanced primary or recurrent gynaecological cancer using the adaptive GEC ESTRO target concept. Radiother Oncol. 2011;100:473–479. doi: 10.1016/j.radonc.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Powell ME. Modern radiotherapy and cervical cancer. Int J Gynecol Cancer. 2010;20(11 Suppl 2):S49–S51. doi: 10.1111/igc.0b013e3181f7b241. [DOI] [PubMed] [Google Scholar]
- 7.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke-Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–171. doi: 10.1200/JCO.1997.15.1.165. [DOI] [PubMed] [Google Scholar]
- 9.Vermorken JB, Zanetta G, De Oliveira CF, van der Burg ME, Lacave AJ, Teodorovic I, et al. Randomized phase III trial of bleomycin, vindesine, mitomycin-C, and cisplatin (BEMP) versus cisplatin (P) in disseminated squamous-cell carcinoma of the uterine cervix: an EORTC Gynecological Cancer Cooperative Group study. Ann Oncol. 2001;12:967–974. doi: 10.1023/a:1011165115426. [DOI] [PubMed] [Google Scholar]
- 10.Mabuchi S, Isohashi F, Yoshioka Y, Temma K, Takeda T, Yamamoto T, et al. Prognostic factors for survival in patients with recurrent cervical cancer previously treated with radiotherapy. Int J Gynecol Cancer. 2010;20:834–840. doi: 10.1111/IGC.0b013e3181dcadd1. [DOI] [PubMed] [Google Scholar]
- 11.Mabuchi S, Matsumoto Y, Hamasaki T, Kawano M, Hisamatsu T, Mutch DG, et al. Elevated white blood cell count at the time of recurrence diagnosis is an indicator of short survival in patients with recurrent cervical cancer. Int J Gynecol Cancer. 2012;22:1545–1551. doi: 10.1097/IGC.0b013e31826ea0eb. [DOI] [PubMed] [Google Scholar]
- 12.Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer. 1977;40:1621–1628. doi: 10.1002/1097-0142(197710)40:4<1621::aid-cncr2820400435>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 13.Maruo T, Shibata K, Kimura A, Hoshina M, Mochizuki M. Tumor-associated antigen, TA-4, in the monitoring of the effects of therapy for squamous cell carcinoma of the uterine cervix: serial determinations and tissue localization. Cancer. 1985;56:302–308. doi: 10.1002/1097-0142(19850715)56:2<302::aid-cncr2820560217>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Bolli JA, Doering DL, Bosscher JR, Day TG, Jr, Rao CV, Owens K, et al. Squamous cell carcinoma antigen: clinical utility in squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 1994;55:169–173. doi: 10.1006/gyno.1994.1272. [DOI] [PubMed] [Google Scholar]
- 15.Duk JM, de Bruijn HW, Groenier KH, Hollema H, ten Hoor KA, Krans M, et al. Cancer of the uterine cervix: sensitivity and specificity of serum squamous cell carcinoma antigen determinations. Gynecol Oncol. 1990;39:186–194. doi: 10.1016/0090-8258(90)90430-s. [DOI] [PubMed] [Google Scholar]
- 16.Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol. 1994;12:2309–2316. doi: 10.1200/JCO.1994.12.11.2309. [DOI] [PubMed] [Google Scholar]
- 17.Takeda M, Sakuragi N, Okamoto K, Todo Y, Minobe S, Nomura E, et al. Preoperative serum SCC, CA125, and CA19-9 levels and lymph node status in squamous cell carcinoma of the uterine cervix. Acta Obstet Gynecol Scand. 2002;81:451–457. doi: 10.1034/j.1600-0412.2002.810513.x. [DOI] [PubMed] [Google Scholar]
- 18.Pras E, Willemse PH, Canrinus AA, de Bruijn HW, Sluiter WJ, ten Hoor KA, et al. Serum squamous cell carcinoma antigen and CYFRA 21-1 in cervical cancer treatment. Int J Radiat Oncol Biol Phys. 2002;52:23–32. doi: 10.1016/s0360-3016(01)01805-3. [DOI] [PubMed] [Google Scholar]
- 19.Ogino I, Nakayama H, Okamoto N, Kitamura T, Inoue T. The role of pretreatment squamous cell carcinoma antigen level in locally advanced squamous cell carcinoma of the uterine cervix treated by radiotherapy. Int J Gynecol Cancer. 2006;16:1094–1100. doi: 10.1111/j.1525-1438.2006.00449.x. [DOI] [PubMed] [Google Scholar]
- 20.Esajas MD, Duk JM, de Bruijn HW, Aalders JG, Willemse PH, Sluiter W, et al. Clinical value of routine serum squamous cell carcinoma antigen in follow-up of patients with early-stage cervical cancer. J Clin Oncol. 2001;19:3960–3966. doi: 10.1200/JCO.2001.19.19.3960. [DOI] [PubMed] [Google Scholar]
- 21.Yoon SM, Shin KH, Kim JY, Seo SS, Park SY, Kang S, et al. The clinical values of squamous cell carcinoma antigen and carcinoembryonic antigen in patients with cervical cancer treated with concurrent chemoradiotherapy. Int J Gynecol Cancer. 2007;17:872–878. doi: 10.1111/j.1525-1438.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 22.Forni F, Ferrandina G, Deodato F, Macchia G, Morganti AG, Smaniotto D, et al. Squamous cell carcinoma antigen in follow-up of cervical cancer treated with radiotherapy: evaluation of cost-effectiveness. Int J Radiat Oncol Biol Phys. 2007;69:1145–1149. doi: 10.1016/j.ijrobp.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 23.Mabuchi S, Okazawa M, Isohashi F, Matsuo K, Ohta Y, Suzuki O, et al. Radical hysterectomy with adjuvant radiotherapy versus definitive radiotherapy alone for FIGO stage IIB cervical cancer. Gynecol Oncol. 2011;123:241–247. doi: 10.1016/j.ygyno.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Hisamatsu T, Mabuchi S, Yoshino K, Fujita M, Enomoto T, Hamasaki T, et al. Prediction of progression-free survival and response to paclitaxel plus carboplatin in patients with recurrent or advanced cervical cancer. Int J Gynecol Cancer. 2012;22:623–629. doi: 10.1097/IGC.0b013e3182473277. [DOI] [PubMed] [Google Scholar]
- 25.Kew FM, Roberts AP, Cruickshank DJ. The role of routine follow-up after gynecological malignancy. Int J Gynecol Cancer. 2005;15:413–419. doi: 10.1111/j.1525-1438.2005.15302.x. [DOI] [PubMed] [Google Scholar]
- 26.Chou HH, Wang CC, Lai CH, Hong JH, Ng KK, Chang TC, et al. Isolated paraaortic lymph node recurrence after definitive irradiation for cervical carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:442–448. doi: 10.1016/s0360-3016(01)01628-5. [DOI] [PubMed] [Google Scholar]
- 27.Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002;84:7–11. doi: 10.1006/gyno.2001.6497. [DOI] [PubMed] [Google Scholar]
- 28.Brooks RA, Rader JS, Dehdashti F, Mutch DG, Powell MA, Thaker PH, et al. Surveillance FDG-PET detection of asymptomatic recurrences in patients with cervical cancer. Gynecol Oncol. 2009;112:104–109. doi: 10.1016/j.ygyno.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang TC, Law KS, Hong JH, Lai CH, Ng KK, Hsueh S, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004;101:164–171. doi: 10.1002/cncr.20349. [DOI] [PubMed] [Google Scholar]
- 30.Sharma DN, Rath GK, Kumar R, Malhotra A, Kumar S, Pandjatcharam J, et al. Positron emission tomography scan for predicting clinical outcome of patients with recurrent cervical carcinoma following radiation therapy. J Cancer Res Ther. 2012;8:23–27. doi: 10.4103/0973-1482.95169. [DOI] [PubMed] [Google Scholar]