Abstract
Objective
To investigate the changes of incidence and prognosis of epithelial ovarian cancer in thirty years in Taiwan.
Methods
The databases of women with epithelial ovarian cancer during the period from 1979 to 2008 were retrieved from the National Cancer Registration System of Taiwan. The incidence and prognosis of these patients were analyzed.
Results
Totally 9,491 patients were included in the study. The age-adjusted incidences of epithelial ovarian cancer were 1.01, 1.37, 2.37, 3.24, 4.18, and 6.33 per 100,000 person-years, respectively, in every 5-year period from 1979 to 2008. The age-specific incidence rates increased especially in serous, endometrioid and clear cell carcinoma, and the age of diagnosis decreased from sixty to fifty years old in the three decades. Patients with mucinous, endometrioid, or clear cell carcinoma had better long-term survival than patients with serous carcinoma (log rank test, p<0.001). Patients with undifferentiated carcinoma or carcinosarcoma had poorer survival than those with serous carcinoma (log rank test, p<0.001). The mortality risk of age at diagnosis of 30-39 was significantly higher than that of age of 70 years or more (test for trend, p<0.001). The mortality risk decreased from the period of 1996-1999 (hazard ratio [HR], 0.90; p=0.054) to the period after 2000 (HR, 0.74; p<0.001) as compared with that from the period of 1991-1995.
Conclusion
An increasing incidence and decreasing age of diagnosis in epithelial ovarian cancer patients were noted. Histological type, age of diagnosis, and treatment period were important prognostic factors for epithelial ovarian carcinoma.
Keywords: Epithelial ovarian carcinoma, Histological type, Population-based study, Prognosis
INTRODUCTION
Ovarian cancer, especially epithelial ovarian carcinoma, has the highest mortality rate among gynecologic malignancies and is an important disease in the field [1]. Early diagnosis is difficult due to the lack of obvious initial symptoms, therefore ovarian cancer patients are usually diagnosed at advanced stage. Current treatments include debulking surgery and adjuvant chemotherapy with the regimen of platinum and paclitaxel which have a response rate of 80 for all patients, but these patients usually relapse after initial response and ultimately die of recurrence [2]. However, the current managements are the same for all histological types of which the underlying carcinogenesis are different [3]. It is important to understand any difference in the prognosis among these types of epithelial ovarian carcinoma.
According to World Health Organization (WHO) database-CI5plus: cancer incidence in five continents-the age-standardized rate of ovarian cancer in 2002 was around 9-11 per 100,000 person-years in Western countries, such as USA, Canada, UK, France, and Italy, but it was around 5-7 per 100,000 person-years in Asia, including China, Japan, and India [4]. Also, the mortality of epithelial ovarian cancer declined gradually around the world in recent two decades, and it might in part result from the improvements in managements [5-9]. Therefore, we conducted the nationwide database analysis through Taiwan cancer registry system to evaluate the pattern, incidence and prognosis of epithelial ovarian carcinomas in the last three decades.
MATERIALS AND METHODS
1. Data sources from the National Cancer Registry System
A population-based cancer registry collected basic information by the short-form system on newly diagnosed cancer patients from hospitals with more than 50-bed capacity in Taiwan since 1979 [10]. From 2002, a long-form system was used to collect more detailed information in hospitals with more than 500 new cancer cases annually. The National Health Insurance program was launched in 1995, and more than 99 of all Taiwan residents participate in the program, which provides health care of acceptable quality, comprehensive benefits and convenient access to treatment, especially for cancer patients [11]. The National Public Health Association had been contracted to operate the registry and organized an advisory board to standardize definitions of terminology, coding, and procedures of the registry's reporting system. The data were collected and stored in computers, and quality controls were run periodically. The information in the system was processed according to the standard guides of International Agency for Research on Cancer. Therefore the population-based cancer registry not only provides cancer incidence data at the national level but also is the fundamental support in cancer prevention.
The study protocol was approved by the Institutional Review Board of National Taiwan University Hospital (The reference number, 201103107RC).
2. Ascertainment of incidence and cases of death
The database was accurate and complete as gradual improving the quality of registration. Percentage of death certificate only cases (DCO) is an indicator of quality registration, and it is generally considered as satisfactory when the value of DCO less than 10. In our registration system, DCO was less than 10 since 1998, less than 5 since 2000, less than 3 since 2003 and 1.24 in 2008. Percentage of morphologically verified cases (MV) is another indicator, and it is generally considered as accuracy in diagnosis of registered cases when the value of MV is higher. MV of ovarian carcinoma was more than 85 since 1990, more than 90 since 1995 and 94.41 in 2008.
3. Classification of histological types of ovarian cancers
The women diagnosed with epithelial ovarian cancer during the period from January 1, 1979 to December 31, 2008, were retrieved from the database of the National Cancer Registration System. The disease codes are based on the International Classification of Diseases for Oncology, 3rd ed. (ICD-O-3). Women diagnosed with a primary cancer of the ovary (ICD-O-3 C56) between 1989 and 2008 were eligible for this study. The histological types were according to the World Health Organization Classification of Tumors [12]. The included histological types were serous (8441/3, 8460/3, 8461/3), mucinous (8470/2, 8470/3, 8471/3, 8480/3, 8482/3), endometrioid (8380/3, 8382/3, 8383/3), clear cell (8310/3, 8313/3), malignant Brenner (9000/3), undifferentiated (8020/3, 8021/3), and carcinosarcoma (8950/3, 8980/3, 8981/3). Non-epithelial ovarian cancer including ovarian germ cell tumors and ovarian sex-cord stromal tumors were excluded. Mixed cell adenocarcinoma generally representing a heterogeneous group of different histological subtypes was excluded in the current study. Patients with undefined histological types of ovarian cancer or other primary tumors were also excluded. The pathological diagnostic criteria of mucinous ovarian carcinoma had been changed recently [13-17], and the diagnosis of mucinous ovarian carcinoma in our registry system also followed the change in pathological criteria. Registered data, including date of birth, date of diagnosis, histological diagnosis, and date of death, were retrieved for investigation.
4. Statistical analysis
The survival of each patient (in person-months) was defined as the period from the date of diagnosis to the date of death related to ovarian cancer, or last date available from the National Cancer Registration System as described in our previous study [10]. Survival curves were generated using the Kaplan-Meier method and differences in survival curves were calculated using the log rank test. Cox's regression model was used to evaluate prognostic factors for survival. The 95% confidence intervals (CIs) for the hazard ratios (HRs) also were calculated. Statistical significance levels were determined by two-tailed test, and a p<0.05 was considered statistically significant. Statistical analysis was performed with SAS ver. 9.1 (SAS Inc., Cary, NC, USA).
RESULTS
1. Basic characteristics of women with epithelial ovarian cancer
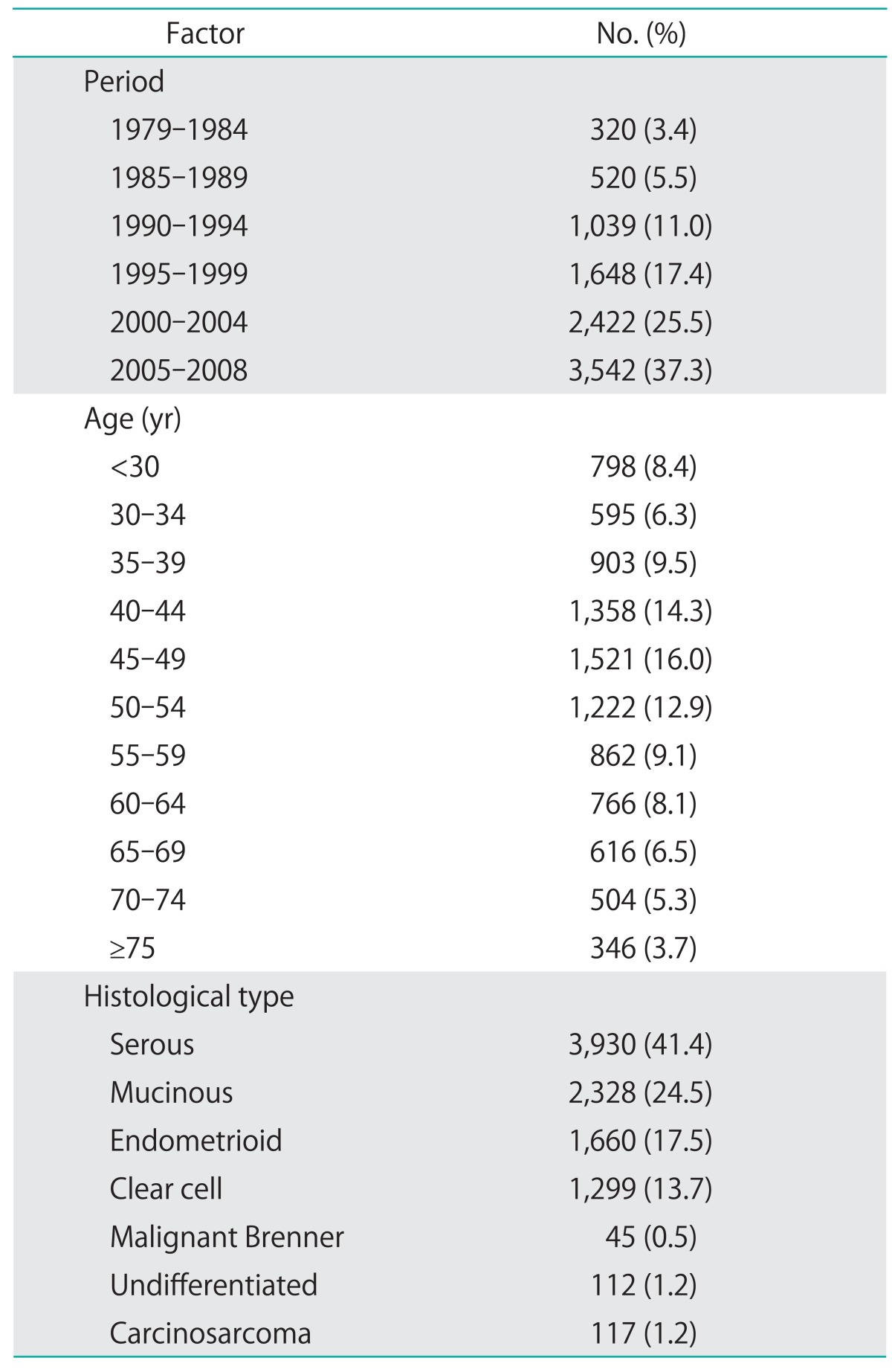
Initially 13,380 patients were obtained from the registry database, but 3,889 patients were excluded for the undefined histological types, which consisted of a heterogenous group of carcinoma and might have biased effects on the analysis of the study. Totally 9,491 women who met the study criteria were included and the basic information was shown in Table 1. There were 3,930 patients with serous carcinoma, 2,328 patients with mucinous carcinoma, 1,660 patients with endometrioid carcinoma, 1,299 patients with clear cell carcinoma, 45 patients with malignant Brenner tumor, 112 patients with undifferentiated carcinoma, and 117 patients with carcinosarcoma.
Table 1.
Basic information on epithelial ovarian cancer in Taiwan, 1979-2008 (n=9,491)
2. Comparison of the incidence rates between epithelial ovarian cancer and female gastric cancer
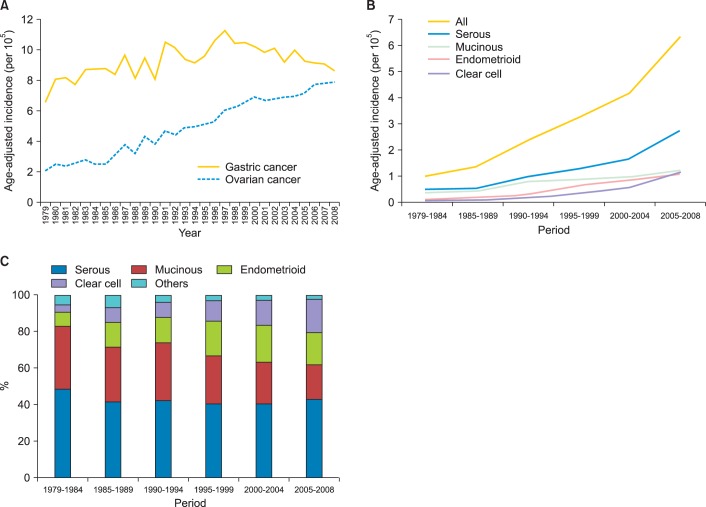
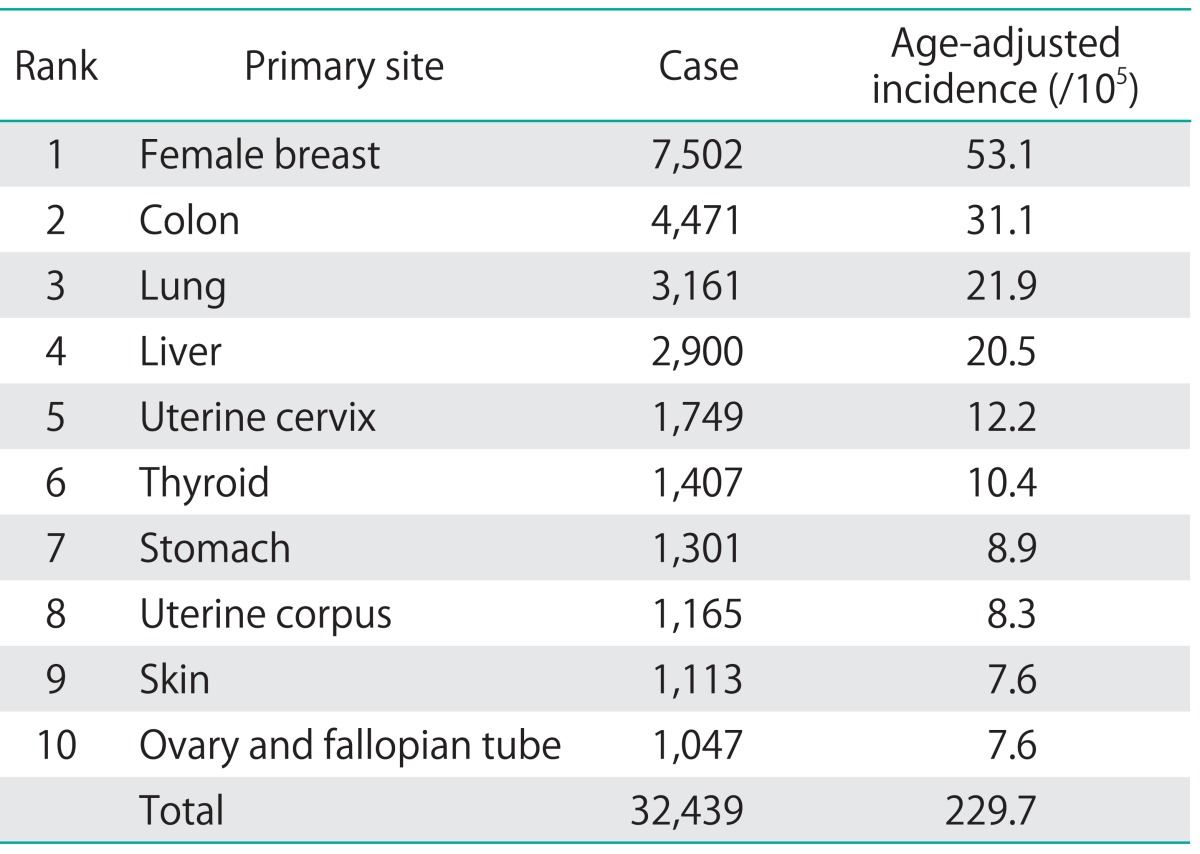
To avoid or reduce potential bias due to incomplete cancer registry data at the beginning of implementing the registry, we compared the age-adjusted incidence rates of ovarian cancer with female gastric cancer over the same period of time. As shown in Fig. 1A and Table 2, from 1979 to 2008, the age-adjusted incidence rate of ovarian cancer increased gradually (2.08 per 100,000 women per year in 1979 and 7.87 per 100,000 women per year in 2008). In contrast, the incidence of female gastric cancer did not show a similarly increasing trend during the observation period (6.60 per 100,000 women per year in 1979 and 8.63 per 100,000 women per year in 2008), by using the same registration system. Our results indicated that the incidence of ovarian cancer really increased in the thirty years not due to the bias of registry.
Fig. 1.
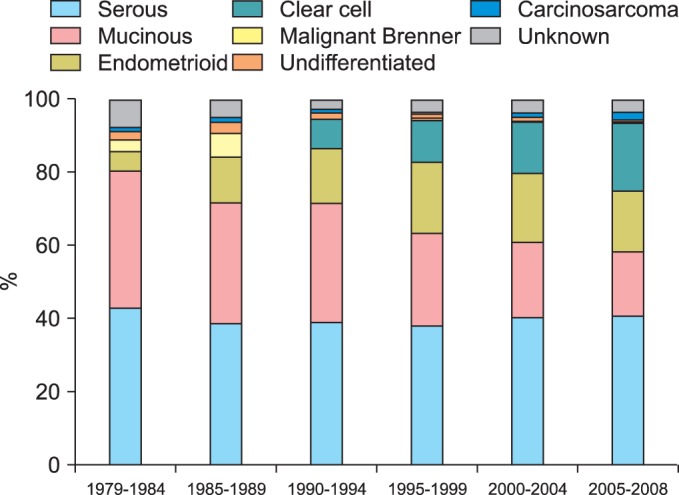
(A) Secular trend of age-adjusted incidence rates of ovarian cancer and female gastric carcinoma, 1979-2008. (B) Secular trend of age-adjusted incidence of epithelial ovarian cancer in Taiwan, 1979-2008. Especially the incidence of serous and clear cell carcinoma increased over the period. (C) The percentage of histological types of epithelial ovarian cancer in Taiwan, 1979-2008. The percentages decreased in mucinous carcinoma but increased in clear cell carcinoma over these decades. Others include malignant Brenner tumor, undifferentiated carcinoma, and carcinosarcoma.
Table 2.
The ranking of Taiwanese female cancer incidence in 2007
3. Age-specific incidence rates of epithelial ovarian cancer by calendar year
The age-adjusted incidence in a 5-year interval presented an increasing trend in each histological type, especially in serous and clear cell carcinoma (Fig. 1B). The age-adjusted incidences of epithelial ovarian carcinoma were 1.01, 1.37, 2.37, 3.24, 4.18, and 6.33 per 100,000 person-years, respectively, in every 5-year period from 1979 to 2008. It increased from 0.49 to 2.73 for serous carcinoma, from 0.35 to 1.21 for mucinous carcinoma, from 0.08 to 1.11 for endometrioid carcinoma, and from 0.04 to 1.19 for clear cell carcinoma.
The percentages of each histological type over these decades are shown in Fig. 1C. The percentages of the mucinous carcinoma decreased, but the percentages of clear cell carcinoma increased. The percentage remained around 40 in serous carcinoma and 15-20 in endometrioid carcinoma steadily except in the period from 1979 to 1984. The percentage decreased from 37.3 to 19.6 in mucinous carcinoma. It might reflect that the change in pathological diagnostic criteria of mucinous ovarian carcinoma [13-17], and a large proportion of mucinous ovarian tumor were metastatic from gastrointestinal tracts based on current diagnostic criteria. However, the percentages significantly increased from 4.0 to 18.6 in clear cell carcinoma. It remained less than 1 in malignant Brenner tumor and less than 3 in undifferentiated carcinoma and carcinosarcoma.
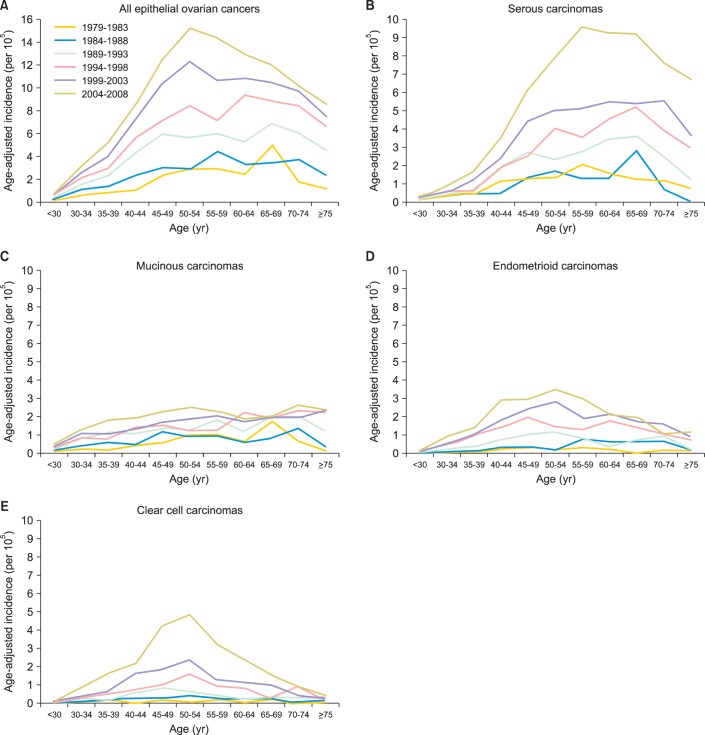
Fig. 2 shows the age-specific incidences in each histological type of epithelial ovarian carcinoma. In addition to relatively steady in mucinous carcinoma, the age-specific incidence increased especially in serous carcinoma and clear cell carcinoma, and the most prevalent age of diagnosis shifted gradually to fifty years old. The age-specific incidences increased gradually in almost all age groups from 1979 to 2008 in a 5-year interval, and the most prevalent age of diagnosis moved from the sixty years old to fifty years old (Fig. 2A). The age-specific incidences of serous carcinoma and clear cell carcinoma increased significantly with the most prevalent age of diagnosis around 55-59 years old in serous carcinoma (Fig. 2B) and 50-54 years old in clear cell carcinoma (Fig. 2E). In mucinous carcinoma, the incidence did not change a lot in the period (Fig. 2C). In endometrioid carcinoma, it also increased gradually with the most prevalent age of diagnosis around 50-54 years old (Fig. 2D).
Fig. 2.
Age-specific incidence of epithelial ovarian cancers in Taiwan, 1979-2008. (A) All epithelial ovarian cancers. The incidence of all age groups increased gradually over the period, and the peak age of diagnosis shifted gradually to 50 years old. (B) Serous carcinomas. The incidence of all age groups increased gradually over the period, and the peak age of diagnosis was around 60 years old. (C) Mucinous carcinoma. The incidence remained steady in all age groups, and the peak age of diagnosis was around 70 years old. (D) Endometrioid carcinomas. The incidence of all age groups increased gradually over the period, and the peak age of diagnosis was around 50 years old. (E) Clear cell carcinomas. The incidence increased especially over latest decade, and the peak age of diagnosis was around 50 years old.
Our results showed that the distribution of different histological types changed with increasing clear cell type, but decreasing mucinous type. Besides, the age-specific incidences increased gradually in almost all age groups and the most prevalent age of diagnosis moved from the sixty years old to fifty years old especially in serous and clear cell types.
4. Analyses of long-term survival of ovarian carcinoma patients
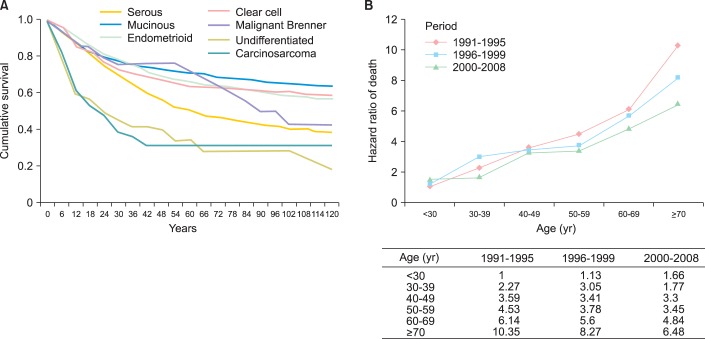
Because of the relative few cases, more than 40 of undefined histology and less precise death registry in the first decade from 1979 to 1990, we analyzed the prognosis of the 8,196 patients diagnosed since 1991. The 10-year survival curves of histological types by Kaplan-Meier analyses were shown in the Fig. 3A. Generally, patients with mucinous, endometrioid or clear cell carcinoma had better long-term outcomes than patients with serous carcinoma (log rank test, p<0.001). In contrast, patients with undifferentiated carcinoma or carcinosarcoma had a poorer survival outcome than serous carcinoma (log rank test, p<0.001). In 2-year follow-up, the survival rate of undifferentiated carcinoma and carcinosarcoma were less than 50, but the other types were more than 75. However, the survival rate of serous carcinoma gradually dropped to 50 in 5-year follow-up. In 10-year follow-up, the survival rate of serous carcinoma was less than 40. However, the survival rates were more than 50 in the mucinous, endometrioid and clear cell carcinoma, but less than 25 in undifferentiated carcinoma and carcinosarcoma.
Fig. 3.
(A) The survival rates of epithelial ovarian cancer by histological types in Taiwan, 1991-2008. The 2-year survival rate of undifferentiated carcinoma and carcinosarcoma were less than 50. The survival rate of serous carcinoma gradually dropped to 50 in 5-year. In 10-year survival rate, mucinous carcinoma, endometrioid carcinoma and clear cell carcinoma were more than 50. (B) Hazard ratios of death for patients with epithelial ovarian cancer in Taiwan, 1991-2008. The risk of death increased with the age of diagnosis in all the period, but the risk decreased when patients diagnosed after 2000 in almost all ages of diagnosis.
The risk of death in these patients related to age of diagnosis and time period of diagnosis is shown in Fig. 3B. The prognosis of patients diagnosed in the period of 1991-1995 and less than 30 years old was set as a referent, the risk of death increased significantly in aged patients in all the time periods. However, the risk of death decreased gradually with the time period of diagnosis, especially those diagnosed after 2000.
The relationship of survival, age of diagnosis, time period of diagnosis and histological types was further evaluated by multivariate analysis (Table 3). The risk for death increased with the age of diagnosis: 1.85 (95% CI, 1.45 to 2.31) for aged 30-39, 2.81 (95% CI, 2.28 to 3.47) for aged 40-49, 3.29 (95% CI, 2.66 to 4.06) for aged 50-59, 4.74 (95% CI, 3.84 to 5.87) for aged 60-69, and 7.50 (95% CI, 6.04 to 9.30) for more than 70 years old (test for trend, p<0.001). The risk of death decreased gradually from the period of 1996-1999 (HR, 0.90; 95% CI, 0.81 to 1.01; p=0.054) to the period after 2000 (HR, 0.74; 95% CI, 0.67 to 0.82; p<0.001) as compared with that from the period of 1991-1995. In compared to serous carcinoma, higher risk of death was noted in undifferentiated carcinoma (HR, 1.98; 95% CI, 1.52 to 2.58; p<0.001) and carcinosarcoma (HR, 1.76; 95% CI, 1.53 to 2.53; p<0.001). The prognosis of mucinous carcinoma (HR, 0.65; 95% CI, 0.59 to 0.72; p<0.001), endometrioid carcinoma (HR, 0.72; 95% CI, 0.65 to 0.79; p<0.001), clear cell carcinoma (HR, 0.8; 95% CI, 0.72 to 0.89; p<0.001) or malignant Brenner tumor (HR, 0.54; 95% CI, 0.33 to 0.89; p=0.016) was better than serous carcinoma.
Table 3.
Multivariate analysis of risk of death for epithelial ovarian cancer in Taiwan, 1991-2008
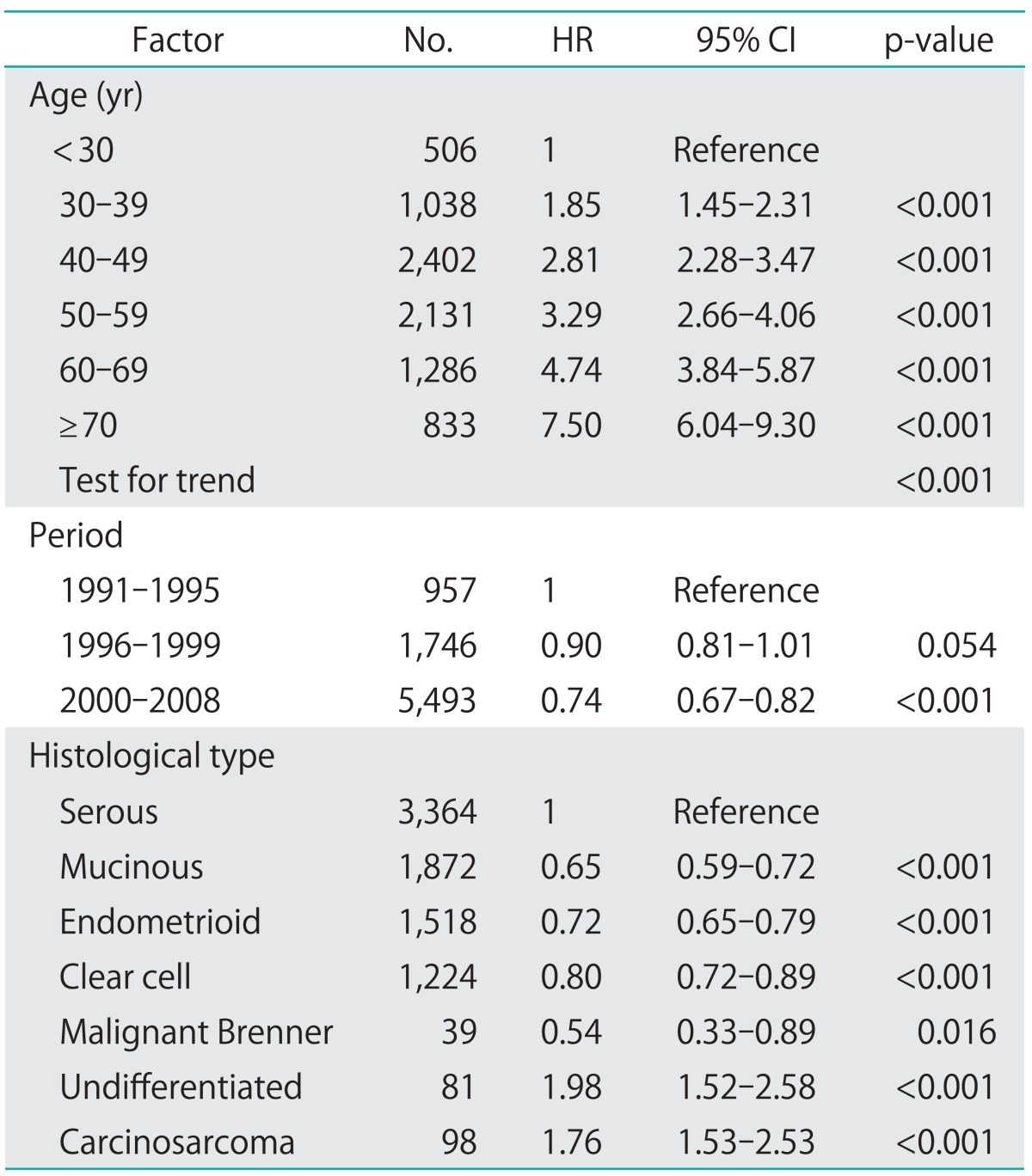
CI, confidence interval; HR, hazard ratio.
Our results indicated that certain histological types such as serous carcinoma, undifferentiated carcinoma, and carcinosarcoma had poor prognosis. However, patients with younger age and those treated in recent years had better prognosis.
DISCUSSION
The incidence of epithelial ovarian carcinoma increased in Taiwan and the peak age of diagnosis dropped from sixty years old to fifty years old. The incidence was comparable to that of other Asian countries, but still lower than that of Western countries. In contrast with Taiwan, the incidence is gradually decreasing and the peak age of diagnosis is around seventy years old in the Europe and Americas according to the WHO database [4]. Completeness of the cancer registry may play a role in the changing incidence, especially in the early period. The ascertainment of registered cases was less complete in the beginning days of the cancer registry system, therefore the cases were fewer and the estimates might be less precise in the first decade. The percentage of unknown histology, which was excluded from the study, was only 6.38 in 1979-1984, 4.84 in 1985-1989, 2.88 in 1990-1994, 3.58 in 1995-1999, 3.85 in 2000-2004, and 3.41 in 2005-2008. More than 90% of the cancer patients were recruited and analyzed in this study in every 5-year period (Table 4, Fig. 4). The satisfactory value of quality indicators of cancer registry system, DCO, and MV were both achieved in mid-1990. As the quality of registration improved over the time, more cases were being correctly registered as epithelial ovarian carcinoma that might have been previously unreported or misclassified. Therefore, the improved quality of cancer registry system partly contributed to increasing incidence of epithelial ovarian carcinoma especially before mid-1990.
Table 4.
Percentages of different histological types from 1979 to 2008
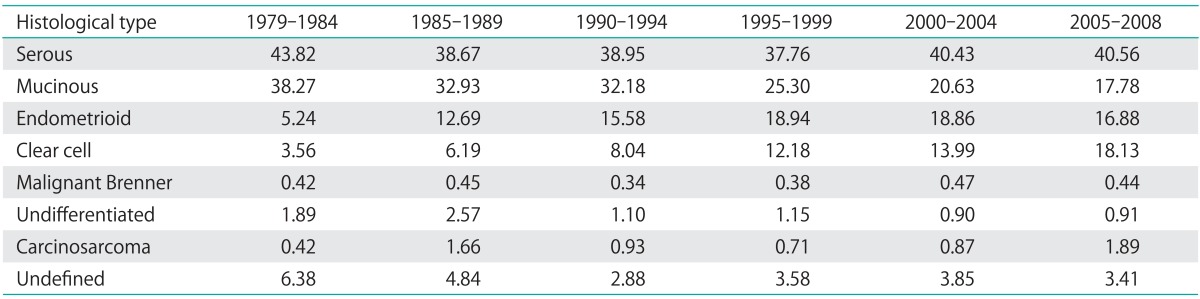
Fig. 4.
Percentages of different histological types from 1979 to 2008.
Increasing incidence of epithelial ovarian cancer is partly due to the effect of screening by image studies and accessible medical services [18]. The national health insurance of Taiwan was established in 1995, and almost all the citizens were covered by the national health insurance in Taiwan. In contrast to the past when fewer patients were able to receive standard cancer treatments, the insurance provided medical service with low costs for all the insured people including epithelial ovarian cancer patients who previously might have no chance to receive medical services because of the high medical cost. The sensitivity and specificity of imaging technique for detecting pelvic masses, such as sonography, computed tomography or magnetic resonance imaging have made progress in recent years, which provided more opportunities to find asymptomatic early-stage ovarian carcinoma confined to pelvis. Both the convenience of medical service and improvement of diagnostic tools contributed to increasing incidence of epithelial ovarian carcinoma.
Changes in lifestyle and reproductive factors, including early menarche, late menopause, delay in marriage, lower fertility rate in life, and changes in infant feeding patterns constitute risk factors of ovarian cancer [19]. Three or more years of oral contraceptive use reduces the risk of epithelial ovarian carcinoma, and the protective effect of oral pills increases with the duration of use [19,20]. However, the prevalence of oral contraceptive use in Taiwan never exceeded 10 of the married women of childbearing age, and the average duration of use was 10.5 months [21]. Therefore the oral contraceptive has little influence on the incidence of epithelial ovarian cancer in Taiwan. Higher parity might have protective effect against ovarian cancer [19]. The total fertility rate (TFR) in Taiwan has declined more rapidly than expected. The TFR kept in 1.75 for decades since 1984, but it declined gradually to 1.3-1.4 in the period from 1998 to 2005 [22]. The very low fertility rate and lower parity of the Taiwanese women in recent decades can partly explain the increasing incidence and younger age of diagnosis of epithelial ovarian cancer in Taiwan.
During the past three decades, urbanization, which has brought with it significant environmental changes, and the adoption of a "westernized" lifestyle, characterized by delayed age at first birth, decreased parity, a diet rich in saturated fats, and a sedentary lifestyle, may have contributed to the progressive rise of ovary cancer incidence [23]. Estrogen-related cancers rapidly emerge in young women in Taiwan [24], our present study observed the increasing incidence and percentages of ovarian endometrioid and clear cell carcinoma. Both endometrioid and clear cell carcinoma are known to be associated with endometriosis which responds to excess estrogen stimulation [25,26]. With rapid industrialization since the 1960s, the Taiwanese women tended to have earlier menarche, obesity, decreased fertility rates and childbearing which are associated with excessive endogenous estrogen [23]. Besides, exogenous estrogen exposure from environmental pollutants had been reported in Taiwan, such as estrogenic steroid pollutants in water, polycyclic aromatic hydrocarbons in the air and water [27,28] and Di-(2-ethylhexyl) phthalate (DEHP) from plastics bags in food handling [29]. Excessive estrogen exposure also partly contributes to the increasing incidence and younger age of diagnosis of epithelial ovarian carcinoma in Taiwan. Besides, different genetic or epigenetic regulating mechanisms underlying each histological subtypes of epithelial ovarian carcinoma might partly contributes to the different percentages of each histological subtypes, incidences and age of diagnosis between Western and Asian patients with ovarian cancer [3].
Histological type is a prognostic factor for patients of epithelial ovarian carcinomas [30,31]. Our data showed the 10-year survival of patients with mucinous, endometrioid or clear cell carcinoma was better than those of serous carcinoma, undifferentiated carcinoma or carcinosarcoma. It provided evidence to the current hypothesis of carcinogenesis of ovarian carcinoma. The type I tumors, including mainly mucinous, endometrioid, clear cell, and Brenner carcinomas, generally have an indolent behavior and favorable prognosis than the type II tumors consisting of high-grade serous carcinoma, undifferentiated carcinoma, and carcinosarcoma. Although the current managements for epithelial ovarian carcinoma were similar regardless different histological types, the modification of the treatments based on the histological types is necessary especially for patients with undifferentiated carcinoma or carcinosarcoma because of their poorer outcome. Interestingly, the survival of serous carcinoma dropped after 2 year follow-up, it might reflect the higher risk of recurrence and disease progression of serous carcinoma within two years after diagnosis.
Age of diagnosis was another prognostic factor for patients of epithelial ovarian carcinomas in this survey. Our finding that the younger patients of epithelial ovarian carcinoma had better survival than older patients was consistent with other large population-based studies [5,6,32,33]. Several possible explanations had been proposed, including performance status [34], earlier stage and low grade at presentation [32], improved response to paclitaxel/platinum-based chemotherapy [35], and more tolerance to intensive chemotherapeutic regimens [35]. Young age itself is indeed an independent prognostic factor after adjusting other well-known clinical-pathological prognostic factors for epithelial carcinoma [32].
Period of treatment was another prognostic factor for patients of epithelial ovarian carcinomas in Taiwan. The risk of death in epithelial ovarian carcinoma in Taiwan declined gradually, especially those patients diagnosed and treated after 2000 in this survey. The trend of prognosis was also comparable to other countries [5,7,8,36,37]. It might result from improvements of treatment strategies for ovarian cancer, including comprehensive service provided by gynecologic oncologists and benefits of paclitaxel-platinum chemotherapy. Surgery plays an important role in the optimal management of epithelial ovarian carcinoma, and surgical cytoreduction of tumor volume is highly correlated with survival [38]. But the ability to successfully perform optimal cytoreduction ranges from 20 to 90 of the patients depending on the experience of the surgeon [38,39]. Managements of gynecologic cancer served by gynecologic oncologists improved the survival of the patients [40-42]. Taiwan Association of Gynecologic Oncologists was established by gynecologic oncologists in 1998 [43], and the members are well trained to manage and promote the quality of care for patients with gynecologic cancers, which might contribute to the improvements of prognosis in the patients with epithelial ovarian carcinoma. Besides, patients undergoing platinum plus paclitaxel adjuvant chemotherapy have been reported to reach better response and outcome [44]. Paclitaxel plus carboplatin is currently the first-line regimen for epithelial ovarian carcinoma [44-49], which was introduced to Taiwan in 2000. Our findings suggested that chemotherapeutic regimens influence the outcomes of ovarian cancer patients.
The limitations of this study were lack of cancer stage and detailed information of treatment courses, such as residual tumor size of cytoreductive surgery and adjuvant chemotherapy regimens. The registry system did not recruit the information of cancer stage, residual tumor volume after surgery, the routes and regimens of chemotherapy until 2008. So it is difficult to evaluate the effects of these factors in the current study. However, further comprehensive analysis to include these above-mentioned factors can be investigated in the future.
ACKNOWLEDGMENTS
We thank the Taiwan Cancer Registry taskforce for their technical assistance in data collection. This study was granted by the Bureau of Health promotion, Department of Health, Executive Yuan, Taipei, Taiwan.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Huang YW, Kuo CT, Stoner K, Huang TH, Wang LS. An overview of epigenetics and chemoprevention. FEBS Lett. 2011;585:2129–2136. doi: 10.1016/j.febslet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer. CI5plus: cancer incidence in five continents annual dataset [Internet] Cedex: International Agency for Research on Cancer; [cited 2013 Aug 20]. Available from: http://ci5.iarc.fr/CI5plus/ci5plus.htm. [Google Scholar]
- 5.Chan JK, Cheung MK, Husain A, Teng NN, West D, Whittemore AS, et al. Patterns and progress in ovarian cancer over 14 years. Obstet Gynecol. 2006;108(3 Pt 1):521–528. doi: 10.1097/01.AOG.0000231680.58221.a7. [DOI] [PubMed] [Google Scholar]
- 6.Cabanes A, Vidal E, Perez-Gomez B, Aragones N, Lopez-Abente G, Pollan M. Age-specific breast, uterine and ovarian cancer mortality trends in Spain: changes from 1980 to 2006. Cancer Epidemiol. 2009;33:169–175. doi: 10.1016/j.canep.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Bray F, Loos AH, Tognazzo S, La Vecchia C. Ovarian cancer in Europe: Cross-sectional trends in incidence and mortality in 28 countries, 1953-2000. Int J Cancer. 2005;113:977–990. doi: 10.1002/ijc.20649. [DOI] [PubMed] [Google Scholar]
- 8.Barnholtz-Sloan JS, Schwartz AG, Qureshi F, Jacques S, Malone J, Munkarah AR. Ovarian cancer: changes in patterns at diagnosis and relative survival over the last three decades. Am J Obstet Gynecol. 2003;189:1120–1127. doi: 10.1067/s0002-9378(03)00579-9. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Diego P, Lopez-Abente G, Pollan M, Ruiz M. Time trends in ovarian cancer mortality in Europe (1955-1993): effect of age, birth cohort and period of death. Eur J Cancer. 2000;36:1816–1824. doi: 10.1016/s0959-8049(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 10.Health Promotion Administration, Ministry of Health and Welfare. National Cancer Registry [Internet] [cited 2013 Aug 20]. Available from: http://www.bhp.doh.gov.tw/BHPNET/Portal/StatisticsShow.aspx?No=200911300001.
- 11.Chiang CJ, Chen YC, Chen CJ, You SL, Lai MS Taiwan Cancer Registry Task Force. Cancer trends in Taiwan. Jpn J Clin Oncol. 2010;40:897–904. doi: 10.1093/jjco/hyq057. [DOI] [PubMed] [Google Scholar]
- 12.Tavassoeli FA. World Health Organization classification of tumours: pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC press; 2003. [Google Scholar]
- 13.Leen SL, Singh N. Pathology of primary and metastatic mucinous ovarian neoplasms. J Clin Pathol. 2012;65:591–595. doi: 10.1136/jclinpath-2011-200162. [DOI] [PubMed] [Google Scholar]
- 14.McCluggage WG. Immunohistochemistry in the distinction between primary and metastatic ovarian mucinous neoplasms. J Clin Pathol. 2012;65:596–600. doi: 10.1136/jcp.2010.085688. [DOI] [PubMed] [Google Scholar]
- 15.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part II. Adv Anat Pathol. 2007;14:149–177. doi: 10.1097/PAP.0b013e3180504abf. [DOI] [PubMed] [Google Scholar]
- 16.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27:985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–25. [PubMed] [Google Scholar]
- 18.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013;129:258–264. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55:3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 20.Collaborative Group on Epidemiological Studies of Ovarian Cancer. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 21.Chow LP, Nair NK. Oral contraceptive use and diseases of the circulatory system in Taiwan: an analysis of mortality statistics. Int J Gynaecol Obstet. 1980;18:420–432. doi: 10.1002/j.1879-3479.1980.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang TM, Liu Y. Re-examination of Taiwan's total fertility rate: an application of the ACF method. J Popul Stud. 2008;36:37–65. [Google Scholar]
- 23.Lin YC, Yen LL, Chen SY, Kao MD, Tzeng MS, Huang PC, et al. Prevalence of overweight and obesity and its associated factors: findings from National Nutrition and Health Survey in Taiwan, 1993-1996. Prev Med. 2003;37:233–241. doi: 10.1016/s0091-7435(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 24.Lin CH, Chen YC, Chiang CJ, Lu YS, Kuo KT, Huang CS, et al. The emerging epidemic of estrogen-related cancers in young women in a developing Asian country. Int J Cancer. 2012;130:2629–2637. doi: 10.1002/ijc.26249. [DOI] [PubMed] [Google Scholar]
- 25.Nagle CM, Olsen CM, Webb PM, Jordan SJ, Whiteman DC, Green AC, et al. Endometrioid and clear cell ovarian cancers: a comparative analysis of risk factors. Eur J Cancer. 2008;44:2477–2484. doi: 10.1016/j.ejca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Vlahos NF, Kalampokas T, Fotiou S. Endometriosis and ovarian cancer: a review. Gynecol Endocrinol. 2010;26:213–219. doi: 10.1080/09513590903184050. [DOI] [PubMed] [Google Scholar]
- 27.Fang GC, Wu YS, Fu PP, Yang IL, Chen MH. Polycyclic aromatic hydrocarbons in the ambient air of suburban and industrial regions of central Taiwan. Chemosphere. 2004;54:443–452. doi: 10.1016/S0045-6535(03)00706-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen CY, Wen TY, Wang GS, Cheng HW, Lin YH, Lien GW. Determining estrogenic steroids in Taipei waters and removal in drinking water treatment using high-flow solid-phase extraction and liquid chromatography/tandem mass spectrometry. Sci Total Environ. 2007;378:352–365. doi: 10.1016/j.scitotenv.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Chen ML, Chen JS, Tang CL, Mao IF. The internal exposure of Taiwanese to phthalate--an evidence of intensive use of plastic materials. Environ Int. 2008;34:79–85. doi: 10.1016/j.envint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 31.Ioka A, Tsukuma H, Ajiki W, Oshima A. Ovarian cancer incidence and survival by histologic type in Osaka, Japan. Cancer Sci. 2003;94:292–296. doi: 10.1111/j.1349-7006.2003.tb01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan JK, Urban R, Cheung MK, Osann K, Shin JY, Husain A, et al. Ovarian cancer in younger vs older women: a population-based analysis. Br J Cancer. 2006;95:1314–1320. doi: 10.1038/sj.bjc.6603457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatta G, Capocaccia R, De Angelis R, Stiller C, Coebergh JW EUROCARE Working Group. Cancer survival in European adolescents and young adults. Eur J Cancer. 2003;39:2600–2610. doi: 10.1016/j.ejca.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Chan JK, Loizzi V, Lin YG, Osann K, Brewster WR, DiSaia PJ. Stages III and IV invasive epithelial ovarian carcinoma in younger versus older women: what prognostic factors are important? Obstet Gynecol. 2003;102:156–161. doi: 10.1016/s0029-7844(03)00399-5. [DOI] [PubMed] [Google Scholar]
- 35.Kosary CL. FIGO stage, histology, histologic grade, age and race as prognostic factors in determining survival for cancers of the female gynecological system: an analysis of 1973-87 SEER cases of cancers of the endometrium, cervix, ovary, vulva, and vagina. Semin Surg Oncol. 1994;10:31–46. doi: 10.1002/ssu.2980100107. [DOI] [PubMed] [Google Scholar]
- 36.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 37.Hirabayashi Y, Marugame T. Comparison of time trends in ovary cancer mortality (1990-2006) in the world, from the WHO Mortality Database. Jpn J Clin Oncol. 2009;39:860–861. doi: 10.1093/jjco/hyp168. [DOI] [PubMed] [Google Scholar]
- 38.Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25:2873–2883. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- 39.Wakabayashi MT, Lin PS, Hakim AA. The role of cytoreductive/debulking surgery in ovarian cancer. J Natl Compr Canc Netw. 2008;6:803–810. doi: 10.6004/jnccn.2008.0060. [DOI] [PubMed] [Google Scholar]
- 40.Tan W, Stehman FB, Carter RL. Mortality rates due to gynecologic cancers in New York state by demographic factors and proximity to a Gynecologic Oncology Group member treatment center: 1979-2001. Gynecol Oncol. 2009;114:346–352. doi: 10.1016/j.ygyno.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 41.Chan JK, Sherman AE, Kapp DS, Zhang R, Osann KE, Maxwell L, et al. Influence of gynecologic oncologists on the survival of patients with endometrial cancer. J Clin Oncol. 2011;29:832–838. doi: 10.1200/JCO.2010.31.2124. [DOI] [PubMed] [Google Scholar]
- 42.Stewart SL, Rim SH, Richards TB. Gynecologic oncologists and ovarian cancer treatment: avenues for improved survival. J Womens Health (Larchmt) 2011;20:1257–1260. doi: 10.1089/jwh.2011.3053. [DOI] [PubMed] [Google Scholar]
- 43.Taiwan Association of Gynecologic Oncologists. TAGO [Internet] Taiwan: TAGO; [cited 2013 Aug 20]. Available from: http://www.tago.org.tw/eng/about.asp. [Google Scholar]
- 44.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 45.Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, Lentz SL, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106–115. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 46.du Bois A, Luck HJ, Meier W, Adams HP, Mobus V, Costa S, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S, Mahdi H, Bryant C, Shah JP, Garg G, Munkarah A. Clinical trials and progress with paclitaxel in ovarian cancer. Int J Womens Health. 2010;2:411–427. doi: 10.2147/IJWH.S7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 49.International Collaborative Ovarian Neoplasm Group. Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505–515. doi: 10.1016/S0140-6736(02)09738-6. [DOI] [PubMed] [Google Scholar]