Abstract
One of the exciting findings in recent cancer genome studies is the discovery of somatic mutations in several chromatin remodeling genes. These studies not only illuminate the emerging roles of chromatin remodeling in the pathogenesis of human cancer but also provide molecular genetic basis of aberrant epigenomic regulation as one of the key mechanisms driving cancer development. This is because chromatin remodeling influences a variety of DNA activities such as replication, transcription, repair, methylation, and recombination. Among the mutated chromatin remodeling genes reported, ARID1A is frequently mutated in a variety of human cancers, especially in endometrium-related neoplasms including ovarian clear cell carcinoma, ovarian endometrioid carcinomas, and uterine endometrioid carcinomas, all of which arise from endometrial epithelium. This review will summarize the recent advances in studying the roles of ARID1A mutations in gynecologic cancers with special emphasis on how this new knowledge will further extend our understanding of the pathogenesis of endometrium-related carcinomas.
Keywords: ARID1A, BAF250a, Chromatin remodeling, Endometriosis, Ovarian cancer
INTRODUCTION
The AT-rich interactive domain 1A (SWI-like) gene (ARID1A) encodes BAF250A which is a member of the SWI/SNF adenosine triphosphate-dependent chromatin-remodeling complexes [1]. The SWI/SNF complex has been shown to play an essential role in controlling gene expression [2] and is also involved in tissue development and cellular differentiation [3,4]. Inactivation of several components of this complex had been found to be associated with certain type of cancer. For example, INI1 (also called BAF47, SNF5) is frequently deleted in rhabdoid tumor and atypical teratoid/rhabdoid tumor of the central nervous system [5-7]. The ATPase subunit genes including BRG1 and BRM have been found to be lost in about 15%-20% of primary non-small cell lung cancers, and loss of BRG1/BRM are associated with poor prognosis [8,9]. ARID1A, containing a DNA-binding motif (ARID), is located at Ch1p36.11, a region frequently deleted in human cancers [10]. Functional genomics analysis demonstrates homozygous deletion involving the 5' end of ARID1A in a lung adenocarcinoma cell line, suggesting ARID1A has a tumor suppressor role [11]. Deficient expression of ARID1A had been found in carcinomas from several organs, and most frequent in carcinomas of the breast and kidney [12]. Recently, using whole exome sequencing and transcriptome sequencing, mutation of ARID1A was found in 43%-57% of ovarian clear cell carcinomas [13,14] and 30% of ovarian endometrioid carcinomas [14].
MUTATION PROFILES OF ARID1A IN MALIGNANCIES
Following the discovery of inactivating mutations of ARID1A in gynecologic tumors, mutations of ARID1A had been found in many human malignancies based on next generation sequencing. These mutation frequency varies among tumor types: 3.2%-3.5% in breast carcinoma [15,16], 9.1%-15% in esophageal adenocarcinoma [17,18], 8%-27% in gastric carcinoma [16,19,20], 8% in pancreatic carcinoma [16,21], 10%-13% in hepatocellular carcinoma [22,23], 13% in transitional cell carcinoma of the bladder [20], 8% in prostate carcinoma [16], 6% in neuroblastoma [24], 2% in medulloblastoma [16], and 17% in Burkitt lymphoma [25].
Study on the characteristics of gastric carcinomas harboring ARID1A mutations revealed the higher frequency of microsatellite instability (MSI) and Epstein-Barr virus infection and the less likelihood of having mutation of TP53 [20]. Besides, ARID1A-mutated gastric carcinomas with MSI usually have indels involving the mononucleotide repeats of C or G, especially one single G tract located in exon 20. Zang et al. [19] also reported a significant association of PIK3CA mutation and MSI with ARID1A mutation in gastric carcinomas.
BIOLOGY OF ARID1A AS A TUMOR SUPPRESSOR
The majority of ARID1A mutations are frame shift or nonsense mutations, suggesting that ARID1A is a tumor suppressor. It has been reported that restoring the expression of wild-type ARID1A in ovarian cancer cells harboring deleterious ARID1A genes was sufficient to suppress cellular proliferation and tumor growth in mice [26]. Conversely, silencing ARID1A expression in non-transformed epithelial cells enhanced cellular proliferation and tumorigenicity in a mouse tumor xenograft model [26]. Similarly, in an esophageal cancer model, knockdown of ARID1A in cell lines enhanced cellular proliferation, and re-expression of ARID1A in ARID1A-deleted cell lines significantly suppressed proliferation [18]. At the molecular level, ARID1A/BRG1 complex has been shown to directly interact and collaborate with p53 to transcriptionally regulate several downstream effectors including those encoded by CDKN1A (p21) and SMAD3 [26]. Using biochemistry approaches, investigators have found that ARID1A acts as a nucleocytoplasmic protein whose stability depends on its subcellular localization [27]. Nuclear ARID1A is unstable as compared to cytoplasmic ARID1A because the protein is rapidly degraded by the ubiquitin-proteasome system in the nucleus. In-frame deletions that disrupt the consensus nuclear export signal are associated with a reduced steady-state protein level of ARID1A due to its retention in the nuclei and subsequent degradation [27]. These findings delineate the basic biological mechanism regulating ARID1A subcellular distribution and protein stability [27]. In summary, recently published studies provided new evidence that ARID1A functions as a tumor suppressor and may participate in both tumor initiation and progression of in human cancer including endometrium-related neoplasms.
ARID1A EXPRESSION AND MUTATION IN GYNECOLOGIC NEOPLASMS
1. Alteration of ARID1A in ovarian tumors
As previously discussed, among human tumors studied so far, gynecologic tumors have the highest frequency of mutation of ARID1A, in which ovarian clear cell carcinoma is most frequenty mutated (43%-57%) [13,14]. Mutation of ARID1A correlates with loss of protein expression [14,28], since most mutations are frameshift or nonsense mutations, leading to loss of protein expression. Ovarian endometrioid carcinoma has a mutation rate of 30%, while none of the high-grade serous carcinomas and mucinous caricnomas has mutation of ARID1A [14,29]. Ovarian clear cell carcinoma can be devided into cystic and adenofibromatous type based on the growth pattern of the tumor [30]. Cystic type clear cell carcinomas are more frequently associated with endometriosis, present at early stage, and tend to have favorable outcome. Yamamoto et al. [31] compared endometriosis and adenofibroma-associated ovarian clear cell carcinomas, and found that loss of ARID1A was more frequent in endometriosis-associated carcinomas than in adenofibroma-assocaited carcinomas (61% vs. 43%), although the difference was not statistically significant. The endometriotic cyst epithelium of non-atypical endometriosis, atypical endometriosis, benign and borderline clear cell adenofibroma adjacent to carcinomas with deficient ARID1A expression, showed ARID1A loss in 86% to 100% of the cases, reflecting that inactivation of ARID1A occurs in the early stages of tumor development. In contrast to the endometriotic lesions adjacent to carcinomas, endometriosis distant from ARID1A-deficient carcinomas and solitary endometrioses had retained ARID1A expression. Similarly, Ahyan et al. [32] studied the expression of ARID1A in ovarian clear cell and endometrioid carcinomas arising from endometrioma. Concurrent loss of ARID1A was found in carcinomas and the contiguous endometriotic epithelium in all tumors with deficient ARID1A expression, while the cystic epithelium distant from the tumors had retained ARID1A expression. PIK3CA, another gene frequently mutated in ovarian clear cell carcinoma (33%-46%) [33,34], was also found to be frequently mutated in the endometriotic epithelium adjacent to clear cell carcinomas [34]. The same authors further found that mutation of PIK3CA frequently co-exists with loss of ARID1A expression in ovarian clear cell caricnoma [31,35].
In borderline ovarian tumors, loss of ARID1A immunoreactivity was found in 33% of endocervical-type mucinous (seromucinous) borderline tumors, 1 (13%) of borderline endometrioid tumor, and none of the borderline serous tumor and gastrointestinal-type borderline mucinous tumors [36]. Endocerivcal-type mucinous borderline tumors, although also named seromucinous tumors due to endocervical and serous morphology, frequently contain features of endometrioid tumors, including ciliated cells, endometrial-type cells with abundant eosinophilic cytoplasm, and hobnail-shaped cells. In conjunction with the frequent association of endometriosis and mutation and/or loss of ARID1A, they are more closely related to endometrial-type proliferative lesions such as ovarian clear cell carcinoma and endometrioid carcinoma. Collectively, these tumors are called "endometriosis-related ovarian neoplasms" [37]. How endometriosis leads to the development of these neoplasms is unclear. It is postulated that repeated epithelial damage and repair in an endometriotic microenvironment rich in iron-induced free radicals contribute to the neoplastic transformation of the endometriotic epithelial cells [38,39].
2. Alteration of ARID1A in uterine carcinomas
Similar to the ovarian tumors, endometrial endometrioid carcinomas have high frequency of alteration of ARID1A. Mutation was found in 40% of low-grade endometrioid carcinoma and loss of expression was founded in 26%-29% of low-grade endometrioid carcinoma and up to 39% of high-grade endometrioid carcinoma [29,40]. Interesting, ARID1A mutations frequently co-ocurr with mutations of PTEN and PIK3CA, as well as overall PIK3CA pathway aberration. Furthermore, PI3K pathway activity is regulated by ARID1A through phohsphorylation of AKT [41]. In the dualistic pathogenetic pathway of endometrial carcinoma, endometrioid carcinoma belongs to type I carcinoma which shows frequent mutations of KRAS, PTEN, PIK3CA, CTNNB1, and microsatellite instability [42]. Alteration of PI3K pathway was found in >80% of endometrial endometrioid carcinoma [43]. Regulation of PI3K pathway by ARID1A further suggests that ARID1A plays an important role in the pathogenesis of type I endometrial carcinomas. McConechy et al. [44] used a nine-gene mutation profile to subclassify endometrial carcinomas. The nine genes included ARID1A, PPP2R1A, PTEN, PIK3CA, KRAS, CTNNB1, TP53, BRAF, and PPP2R5C. By target enrichment sequencing, each endometrial carcioma subtype exhibits a distinct mutation profile. Mutation of ARID1A was detected in 46.7% low-grade endometrioid carcinoma, 60% high-grade endometrioid carcinoma, 10.8% serous carcinoma, and 23.8% carcinosarcoma. The frequency of mutation of ARID1A is significantly different between endometrioid carcinoma and serous carcinoma. Interetingly, the molecular profile of carcinosarcomas showed either endometrioid type (mutation of PTEN and ARID1A) or serous type (mutation of TP53 and PPP2R1A). The relationship between loss of ARID1A and MSI in endometrial endometrioid carcinoma has been recently demonstrated [45,46]. In one report [45], and loss of ARID1A is significantly more frequent in sporadic MSI than in Lynch syndrome. The authors concluded that ARID1A is a causative gene instead of a target gene of MSI by the role in epigenetic silencing of the MLH1 gene in endometrial carcinoma. In another report [46], loss of ARID1A is associated with mismatch repair deficiency. In vitro functional study showed that ARID1A controls tumor growth by collaboration with p53 and regulates p53 downstream targets [26]. By examining tumor samples, mutations of ARID1A and TP53 were mututally exclusive in ovarian clear cell carcinomas, and uterine carcinomas [46] endometrial endometrioid carcinomas. Immunohistochemically, loss of ARID1A and mutant-like p53 expression was found to be nearly mutually exclusive in endometrial carcinomas [45,46].
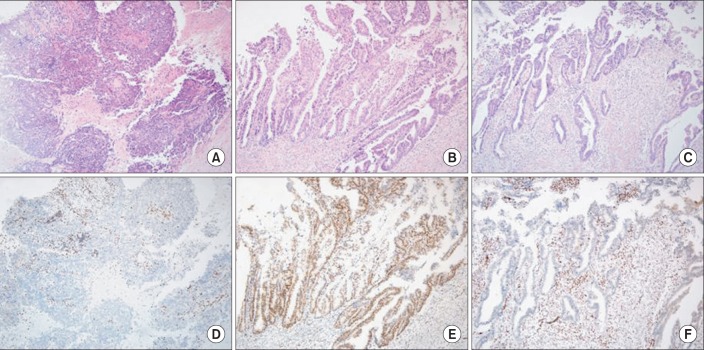
Besides genomic study, immunohistochemistry provided an opportunity for the evaluation of heterogeneity in endometroid carcinomas. Clonal loss of ARID1A immunoreactivity in a discrete tumor area against a background of ARID1A positive tumor cells was observed in some tumors with ARID1A mutation, suggesting mutations arising from subclones within these tumors. On the other hand, tumors with wild-type ARID1A did not exhibit any pattern of clonal loss [29]. This phenomenon was further elucidated in a recent study, showing clonal loss of ARID1A immunoreactivity in 16% complex atypical hyperplasia, 24% of low-grade endometrioid carcinoma, and 10% of high-grade endometrioid carcinma, while complete loss of ARID1A was found in 0% of complex atypical hyperplasia, 25% of low-grade endometrioid carcinoma, and 43% of high-grade endometrioid carcinoma (Fig. 1) [47]. Thus, ARID1A plays important role not only in tumor initiation, but also in tumor progression.
Fig. 1.
Immunohistochemical study of an endometrial endometrioid carcinoma with coexisting high-grade (A) and low-grade (B, C) components. The high-grade component is negative for ARID1A (D) while the low-grade component has areas with retained ARID1A staining (E) and areas with clonal loss of ARID1A (F) (A-F, ×200).
3. Alteration of ARID1A and tumor behavior in gynecologic cancers
Several studies had investigated the behavior of tumors showing ARID1A alterations. Maeda el al. [28] found no significant difference between ARID1A negative and positive cases in terms of histopathologic features, age, clinical stage, or overall survival in 149 ovarian clear cell carcinomas. Similarly, Yamamoto et al. [34] also found no correlation of ARID1A immunoreactivity with any clinicopathological parameters except the higher association with adjacent endometriosis in 90 ovarian clear cell carcinomas. Lowery et al. [48] examined 212 ovarian clear cell and endometrioid carcinomas and found no relationship between loss of ARID1A and stage, grade, survival or epidemiological variables. In endometrial cancers, Fadare et al. [49] showed that loss of ARID1A was found in 22.7% of endometrial clear cell carcinomas, and cases with loss of ARID1A were significantly high in late stages, but later on they found no association with reduced overall or progression-free survival [50]. On the other hand, loss of ARID1A was reported to be associated with reduced progression-free survival and chemoresistance in ovarian clear cell carcinoma [51] and deep myometrial invasion in endometrial carcinoma [52]. The differences of the impact on prognosis may be due to different antibodies used, sample size variation, and probably other co-factors that affect the behavior of the tumors.
CONCLUSION
Disorganized chromatin structure is known to be associated with the appearance of various abnormal phenotypes, including cancer [53,54]. ARID1A, a gene participated in chromatin remodeling, is an emerging tumor suppressor gene. Accumulating evidence has reported somatic inactivating mutations of ARID1A and loss of its expression in many types of human cancers, especially in endometrium-derived tumors, including ovarian clear cell carcinomas, ovarian endometrioid carcinomas and uterine endometrioid carcinomas. The high prevalence of somatic mutations in those ovarian and endometrial cancers indicates a pivotal role of ARID1A in their development. Understanding the roles of ARID1A in the pathogenesis of endometrium-derived tumors is fundamental for future translational studies aimed at designing new diagnostic tests for early detection and identifying critical molecular targets for new therapeutic interventions.
ACKNOWLEDGMENTS
The study was supported by Taiwan National Science Council (NSC) 100-2320-B-002-081 and NIH/NCI CA165807.
Footnotes
No potential conflict of interest relevant to this article was reported.
This article was solicited and has not been peer reviewed.
References
- 1.Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653–1668. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- 2.Van Rechem C, Boulay G, Leprince D. HIC1 interacts with a specific subunit of SWI/SNF complexes, ARID1A/BAF250A. Biochem Biophys Res Commun. 2009;385:586–590. doi: 10.1016/j.bbrc.2009.05.115. [DOI] [PubMed] [Google Scholar]
- 3.Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A. 2008;105:6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biegel JA, Kalpana G, Knudsen ES, Packer RJ, Roberts CW, Thiele CJ, et al. The role of INI1 and the SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the workshop on childhood atypical teratoid/rhabdoid tumors. Cancer Res. 2002;62:323–328. [PubMed] [Google Scholar]
- 6.Biegel JA, Pollack IF. Molecular analysis of pediatric brain tumors. Curr Oncol Rep. 2004;6:445–452. doi: 10.1007/s11912-004-0075-5. [DOI] [PubMed] [Google Scholar]
- 7.Bourdeaut F, Freneaux P, Thuille B, Lellouch-Tubiana A, Nicolas A, Couturier J, et al. hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol. 2007;211:323–330. doi: 10.1002/path.2103. [DOI] [PubMed] [Google Scholar]
- 8.Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: correlation with poor prognosis. Cancer Res. 2003;63:560–566. [PubMed] [Google Scholar]
- 9.Fukuoka J, Fujii T, Shih JH, Dracheva T, Meerzaman D, Player A, et al. Chromatin remodeling factors and BRM/BRG1 expression as prognostic indicators in non-small cell lung cancer. Clin Cancer Res. 2004;10:4314–4324. doi: 10.1158/1078-0432.CCR-03-0489. [DOI] [PubMed] [Google Scholar]
- 10.Chunder N, Mandal S, Basu D, Roy A, Roychoudhury S, Panda CK. Deletion mapping of chromosome 1 in early onset and late onset breast tumors: a comparative study in eastern India. Pathol Res Pract. 2003;199:313–321. doi: 10.1078/0344-0338-00423. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Zhao YL, Li Y, Fletcher JA, Xiao S. Genomic and functional evidence for an ARID1A tumor suppressor role. Genes Chromosomes Cancer. 2007;46:745–750. doi: 10.1002/gcc.20459. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Nagl NG, Jr, Flowers S, Zweitzig D, Dallas PB, Moran E. Expression of p270 (ARID1A), a component of human SWI/SNF complexes, in human tumors. Int J Cancer. 2004;112:636. doi: 10.1002/ijc.20450. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cornen S, Adelaide J, Bertucci F, Finetti P, Guille A, Birnbaum DJ, et al. Mutations and deletions of ARID1A in breast tumors. Oncogene. 2012;31:4255–4256. doi: 10.1038/onc.2011.598. [DOI] [PubMed] [Google Scholar]
- 16.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agrawal N, Jiao Y, Bettegowda C, Hutfless SM, Wang Y, David S, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2:899–905. doi: 10.1158/2159-8290.CD-12-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streppel MM, Lata S, Delabastide M, Montgomery EA, Wang JS, Canto MI, et al. Next-generation sequencing of endoscopic biopsies identifies ARID1A as a tumor-suppressor gene in Barrett's esophagus. Oncogene. 2013 Jan 14; doi: 10.1038/onc.2012.586. [Epub]. http://dx.doi.org/10.1038/onc.2012.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 20.Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, et al. Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer. Proc Natl Acad Sci U S A. 2012;109:E252–E259. doi: 10.1073/pnas.1114817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Deng Q, Wang Q, Li KY, Dai JH, Li N, et al. Exome sequencing of hepatitis B virus-associated hepatocellular carcinoma. Nat Genet. 2012;44:1117–1121. doi: 10.1038/ng.2391. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet. 2012;44:760–764. doi: 10.1038/ng.2291. [DOI] [PubMed] [Google Scholar]
- 24.Sausen M, Leary RJ, Jones S, Wu J, Reynolds CP, Liu X, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2013;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulino-Roth L, Wang K, MacDonald TY, Mathew S, Tam Y, Cronin MT, et al. Targeted genomic sequencing of pediatric Burkitt lymphoma identifies recurrent alterations in antiapoptotic and chromatin-remodeling genes. Blood. 2012;120:5181–5184. doi: 10.1182/blood-2012-06-437624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan B, Wang TL, Shih IeM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan B, Gao M, Wu CH, Wang TL, Shih IeM. Functional analysis of in-frame indel ARID1A mutations reveals new regulatory mechanisms of its tumor suppressor functions. Neoplasia. 2012;14:986–993. doi: 10.1593/neo.121218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda D, Mao TL, Fukayama M, Nakagawa S, Yano T, Taketani Y, et al. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–5128. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, et al. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veras E, Mao TL, Ayhan A, Ueda S, Lai H, Hayran M, et al. Cystic and adenofibromatous clear cell carcinomas of the ovary: distinctive tumors that differ in their pathogenesis and behavior: a clinicopathologic analysis of 122 cases. Am J Surg Pathol. 2009;33:844–853. doi: 10.1097/PAS.0b013e31819c4271. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25:615–624. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]
- 32.Ayhan A, Mao TL, Seckin T, Wu CH, Guan B, Ogawa H, et al. Loss of ARID1A expression is an early molecular event in tumor progression from ovarian endometriotic cyst to clear cell and endometrioid carcinoma. Int J Gynecol Cancer. 2012;22:1310–1315. doi: 10.1097/IGC.0b013e31826b5dcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto S, Tsuda H, Takano M, Iwaya K, Tamai S, Matsubara O. PIK3CA mutation is an early event in the development of endometriosis-associated ovarian clear cell adenocarcinoma. J Pathol. 2011;225:189–194. doi: 10.1002/path.2940. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. PIK3CA mutations and loss of ARID1A protein expression are early events in the development of cystic ovarian clear cell adenocarcinoma. Virchows Arch. 2012;460:77–87. doi: 10.1007/s00428-011-1169-8. [DOI] [PubMed] [Google Scholar]
- 36.Wu CH, Mao TL, Vang R, Ayhan A, Wang TL, Kurman RJ, et al. Endocervical-type mucinous borderline tumors are related to endometrioid tumors based on mutation and loss of expression of ARID1A. Int J Gynecol Pathol. 2012;31:297–303. doi: 10.1097/PGP.0b013e31823f8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda D, Shih IeM. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv Anat Pathol. 2013;20:45–52. doi: 10.1097/PAP.0b013e31827bc24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vercellini P, Crosignani P, Somigliana E, Vigano P, Buggio L, Bolis G, et al. The 'incessant menstruation' hypothesis: a mechanistic ovarian cancer model with implications for prevention. Hum Reprod. 2011;26:2262–2273. doi: 10.1093/humrep/der211. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi K, Mandai M, Toyokuni S, Hamanishi J, Higuchi T, Takakura K, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]
- 40.Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 41.Liang H, Cheung LW, Li J, Ju Z, Yu S, Stemke-Hale K, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012;22:2120–2129. doi: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, Velasco A, et al. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. J Clin Pathol. 2009;62:777–785. doi: 10.1136/jcp.2008.056101. [DOI] [PubMed] [Google Scholar]
- 43.Cheung LW, Hennessy BT, Li J, Yu S, Myers AP, Djordjevic B, et al. High frequency of PIK3R1 and PIK3R2 mutations in endometrial cancer elucidates a novel mechanism for regulation of PTEN protein stability. Cancer Discov. 2011;1:170–185. doi: 10.1158/2159-8290.CD-11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosse T, Ter Haar NT, Seeber LM, Diest PJ, Hes FJ, Vasen HF, et al. Loss of ARID1A expression and its relationship with PI3K-Akt pathway alterations, TP53 and microsatellite instability in endometrial cancer. Mod Pathol. 2013 May 24; doi: 10.1038/modpathol.2013.96. [Epub]. http://dx.doi.org/10.1038/modpathol.2013.96. [DOI] [PubMed] [Google Scholar]
- 46.Allo G, Bernardini MQ, Wu RC, Shih IM, Kalloger S, Pollett A, et al. ARID1A loss correlates with mismatch repair deficiency and intact p53 expression in high-grade endometrial carcinomas. Mod Pathol. 2013 Jul 26; doi: 10.1038/modpathol.2013.144. [Epub]. http://dx.doi.org/10.1038/modpathol.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mao TL, Ardighieri L, Ayhan A, Kuo KT, Wu CH, Wang TL, et al. Loss of ARID1A expression correlates with stages of tumor progression in uterine endometrioid carcinoma. Am J Surg Pathol. 2013;37:1342–1348. doi: 10.1097/PAS.0b013e3182889dc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowery WJ, Schildkraut JM, Akushevich L, Bentley R, Marks JR, Huntsman D, et al. Loss of ARID1A-associated protein expression is a frequent event in clear cell and endometrioid ovarian cancers. Int J Gynecol Cancer. 2012;22:9–14. doi: 10.1097/IGC.0b013e318231f140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadare O, Renshaw IL, Liang SX. Does the loss of ARID1A (BAF-250a) expression in endometrial clear cell carcinomas have any clinicopathologic significance? A pilot assessment. J Cancer. 2012;3:129–136. doi: 10.7150/jca.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fadare O, Gwin K, Desouki MM, Crispens MA, Jones HW, 3rd, Khabele D, et al. The clinicopathologic significance of p53 and BAF-250a (ARID1A) expression in clear cell carcinoma of the endometrium. Mod Pathol. 2013;26:1101–1110. doi: 10.1038/modpathol.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katagiri A, Nakayama K, Rahman MT, Rahman M, Katagiri H, Nakayama N, et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol. 2012;25:282–288. doi: 10.1038/modpathol.2011.161. [DOI] [PubMed] [Google Scholar]
- 52.Werner HM, Berg A, Wik E, Birkeland E, Krakstad C, Kusonmano K, et al. ARID1A loss is prevalent in endometrial hyperplasia with atypia and low-grade endometrioid carcinomas. Mod Pathol. 2013;26:428–434. doi: 10.1038/modpathol.2012.174. [DOI] [PubMed] [Google Scholar]
- 53.Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 2013;3:35–43. doi: 10.1158/2159-8290.CD-12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Papamichos-Chronakis M, Peterson CL. Chromatin and the genome integrity network. Nat Rev Genet. 2013;14:62–75. doi: 10.1038/nrg3345. [DOI] [PMC free article] [PubMed] [Google Scholar]