Abstract
Purpose
To determine if escalated radiation dose using hypofractionation significantly reduces biochemical and/or clinical disease failure (BCDF) in men treated primarily for prostate cancer.
Patients and Methods
Between June 2002 and May 2006, men with favorable- to high-risk prostate cancer were randomly allocated to receive 76 Gy in 38 fractions at 2.0 Gy per fraction (conventional fractionation intensity-modulated radiation therapy [CIMRT]) versus 70.2 Gy in 26 fractions at 2.7 Gy per fraction (hypofractionated IMRT [HIMRT]); the latter was estimated to be equivalent to 84.4 Gy in 2.0 Gy fractions. High-risk patients received long-term androgen deprivation therapy (ADT), and some intermediate-risk patients received short-term ADT. The primary end point was the cumulative incidence of BCDF. Secondarily, toxicity was assessed.
Results
There were 303 assessable patients with a median follow-up of 68.4 months. No significant differences were seen between the treatment arms in terms of the distribution of patients by clinicopathologic or treatment-related (ADT use and length) factors. The 5-year rates of BCDF were 21.4% (95% CI, 14.8% to 28.7%) for CIMRT and 23.3% (95% CI, 16.4% to 31.0%) for HIMRT (P = .745). There were no statistically significant differences in late toxicity between the arms; however, in subgroup analysis, patients with compromised urinary function before enrollment had significantly worse urinary function after HIMRT.
Conclusion
The hypofractionation regimen did not result in a significant reduction in BCDF; however, it is delivered in 2.5 fewer weeks. Men with compromised urinary function before treatment may not be ideal candidates for this approach.
INTRODUCTION
Prostate cancer is often treated with radiotherapy (RT) alone or in combination with androgen-deprivation therapy (ADT). A number of studies have shown that biochemical failure (BF) rates are reduced by escalating radiation dose above conventional doses of 68 to 70 Gy to doses of 76 to 79 Gy.1,2 Dose escalation has become standard for treatment using external-beam RT, along with the use of intensity-modulated RT (IMRT), to better limit high doses in normal tissues.
Prostate cancer has a low growth fraction, proliferates slowly, and has a long potential doubling time.3,4 These attributes are more typical of normal tissues that exhibit late reactions to irradiation.5 Late-reacting tissues have a broad shoulder and steep falloff in cell survival curves, which have been characterized as having a low α/β ratio (α and β describe the linear and quadratic components of the cell survival curve). A majority of studies have estimated the α/β ratio for prostate cancer to be low, at approximately 1.5 Gy,6,7 whereas that for the surrounding normal tissues has been estimated to be > 3 Gy. For the rectum, it is reportedly > 5.0 Gy.8,9 A low α/β ratio indicates greater sensitivity to higher radiation doses per fraction (hypofractionation), suggesting a therapeutic advantage using hypofractionation for prostate cancer. At the time this trial was designed, preliminary prospective data on hypofractionation supported this approach.10
The hypothesis of the current study was that additional increases in dose above the equivalent of 76 Gy in conventional 2-Gy fractions using IMRT (mean doses > 78 Gy11) would result in additional reductions in biochemical and/or clinical disease failure (BCDF) and that hypofractionation could be used to accomplish this without increasing toxicity.
PATIENTS AND METHODS
Design Overview
Using data available in the Fox Chase Cancer Center database,12 we estimated that the equivalent of an increase of 8.4 Gy in 2-Gy fractions from 76.0 to 84.4 Gy would result in 15% fewer BCDFs. The intent was to include patients with primarily intermediate- to high-risk features. A sample size of 300 assessable patients equally randomly assigned would achieve 90% power to detect a hazard ratio of 0.46, when the proportions with evidence of BCDF for the two arms at 4 years after the last patient was entered were 30% and 15% for conventionally fractionated (CIMRT) and hypofractionated (HIMRT) IMRT, respectively, at a .05 significance level using a two-sided log-rank test.13 The anticipated response was consistent with our prior randomized dose-escalation study using conventional fractionation, in which 70 Gy to the isocenter was increased to 78 Gy in 2.0-Gy fractions.1 Using an α/β ratio of 1.5 Gy for prostate cancer, the administration of 70.2 Gy in 2.7-Gy fractions was expected to be equivalent to 84.4 Gy in 2.0-Gy fractions. The normal tissues would receive the equivalent of 77.2 Gy in 2.0-Gy fractions, using an α/β ratio of 5.0 Gy.
Patients
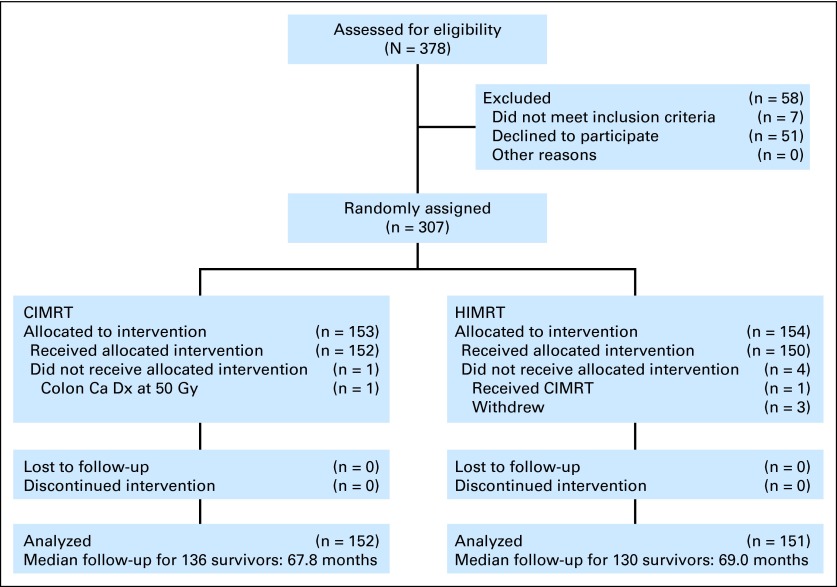
We enrolled 307 patients between 2002 and 2006 (Fig 1), and 303 were assessable, with 152 randomly assigned to receive CIMRT and 151 to receive HIMRT. The use of long-term ADT was planned for 24 months in those classified as high risk per the protocol (prostate-specific antigen [PSA] > 20 ng/mL; Gleason score [GS] of 8 to 10, ≥ cT3, or GS7 ≥ four biopsy cores positive). For patients considered to be at less than high risk, short-term ADT was planned for up to 4 months of ADT starting ≤ 4 months before random assignment. All but two patients were enrolled and treated at the Fox Chase Cancer Center.
Fig 1.
CONSORT diagram. Ca, cancer; CIMRT, conventional fractionated intensity-modulated radiotherapy; Dx, diagnosis; HIMRT, hypofractionated intensity-modulated radiotherapy.
Patients were stratified by pre-enrollment initial PSA ≤ 10 versus > 10 to 20 versus > 20 ng/mL, GS 5 to 7 versus 8 to 10, and high risk versus lower risk. A prepopulated block randomization sheet was used for assignment by the office of protocol research. An initial PSA ≤ 80 ng/mL was required.
RT
The technique used was described previously.11 Briefly, planning for IMRT involved the fusion of 1.5T magnetic resonance (without an endorectal coil) images to computed tomography scan images. Patients were positioned supine. The first clinical target volume (CTV) included the prostate and proximal seminal vesicles (CTV1), and this was the only CTV for favorable- to intermediate-risk patients. The distal seminal vesicles (CTV2) and pelvic lymph nodes (CTV3) were treated in those with high-risk disease.
There was concern at the outset about potential increased toxicity with HIMRT, so the dose constraints for the normal tissues were conservatively calculated using the same α/β ratio as for the prostate (1.5 Gy). In addition, the planning target volumes (PTVs) to the corresponding CTVs were tighter in the HIMRT arm (7 mm everywhere, 3 mm posteriorly v 8 mm everywhere, 5 mm posteriorly). We reasoned that the 90% isodose line in the HIMRT patients would include the same volume as the prescription isodose line in the CIMRT patients.11 The dose of 95% of the PTV (D95%) was to be the prescription dose or higher. For patients in arm I, the PTV1 was to receive 76 Gy, and the PTV2 and PTV3 were to receive 56 Gy, all in 38 fractions. For arm II, the PTV1 was to receive 70.2 Gy, and the PTV2 and PTV3 were to receive 50 to 52 Gy (most received 50 Gy), all in 26 fractions.
For arm I, the rectal constraints were that ≤ 17% and ≤ 35% of the rectum should receive ≥ 65 Gy (V65 Gy) and ≥ 40 Gy (V40 Gy), respectively. The bladder constraints included V65 Gy and V40 Gy of ≤ 25% and ≤ 50%, respectively. For arm II, the rectal constraints included V50 Gy and V31 Gy of ≤ 17% and ≤ 35%, and the bladder V50 Gy and V31 Gy would be ≤ 25% and ≤ 50%. As described previously,11 the constraints for arm II were probably overly strict, and variations were more often seen as compared with arm I. An absolute variation of 7.5% in the constraints was allowed. Concerning the target doses and organ-at-risk constraints, there were no protocol violations. Corrections for day-to-day, interfraction, and uncertainty in prostate position were achieved using ultrasound image guidance.
Toxicity Assessment
Protocol toxicity was measured using modified LENT (Late Effects of Normal Tissues)/RTOG (Radiation Therapy Oncology Group) criteria based on those described by Hanlon et al14 and Pollack et al11 (Appendix Table A1, online only), which are similar to, yet more detailed than, those described in the Common Terminology Criteria for Adverse Events (version 4).15
Statistical Analysis
The primary end point was the cumulative incidence of BCDF, timed from treatment start to earliest failure or start of salvage therapy (ADT, cryosurgery, or prostatectomy). Clinical failure included local failure (local progression or prostate biopsy proof of disease with rising PSA) or regional/distant metastasis (radiographic or pathologic). Per protocol, a modification of the ASTRO (American Society for Radiation Oncology) definition16 of BF (ie, three consecutive PSA rises) was used, such that dips between rises were allowed. Results obtained using the BF definition of nadir plus 2 ng/mL17 are also reported.
Death without BCDF was treated as a competing risk if PSA was current to within 3 months; otherwise, patients were censored as failure free at the date of last PSA. Similarly, failure-free surviving patients were censored at the date of last contact if PSA was current within 3 months or otherwise at the date of last PSA. Gray's test was used to compare incidence of BCDF by treatment arm.18 Regression models included age, T category, GS, initial PSA, percent adenocarcinoma positive tissue on diagnostic biopsy, risk group, and use of ADT and were fitted using the Fine and Gray19 method. Multivariable models were adjusted for treatment arm, with additional covariates selected after consideration of univariate significance and collinearity. Cumulative incidence was also used to estimate prostate cancer or other cause of death, and the Kaplan-Meier method was used to estimate overall survival (OS).20
Adverse GI and genitorurinary (GU) reactions were analyzed by prevalence and incidence. Prevalence, by severity (grade), was calculated as a simple proportion for pre-existing reactions (baseline); those at 3, 6, 12, and 18 months of RT treatment start using events within 3 months; and at years 2 through 6 using events within 6 months. Fisher's exact test was used to compare prevalence distributions by treatment arm. Comparison at different times (eg, baseline v 5 years) was made within each arm using the Wilcoxon signed rank test (all grades) or McNemar's test (grades 0 to 1 v ≥ 2).
For incidence, we considered grade ≥ 2 reactions occurring ≥ 3 months after the end of RT. Determination of competing-risk deaths and censoring times was based on whether follow-up for toxicity was current to within 3 months. The incidence of late adverse reactions was compared by treatment arm using Gray's test, with Fine and Gray regression used for multivariable analysis.
All analyses were carried out in the intention-to-treat population using two-sided 5% significance levels for hypothesis tests and 95% CIs for estimation based on the log-log transform method. Analyses involving competing risks were performed using R software (version 2.14; R Foundation for Statistical Computing. Vienna, Austria). All other analyses were carried out in SAS software (version 9.2; SAS Institute, Cary, NC).
RESULTS
Patients
Patients were evenly distributed between the arms (Table 1) in terms of age, T category, GS, initial PSA, planned short- or long-term ADT (Table 2; Appendix Table A2, online only), and protocol risk group plus corresponding use of ADT. The median short- and long-term ADT durations were 3.9 and 24.1 months, respectively.
Table 1.
Patient Demographic, Clinical, and Pathologic Characteristics by Treatment Arm
| Characteristic | Arm I: CIMRT (n = 152) |
Arm II: HIMRT (n = 151) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .436 | ||||
| 45 to 54 | 13 | 8.6 | 10 | 6.6 | |
| 55 to 64 | 47 | 30.9 | 43 | 28.5 | |
| 65 to 74 | 62 | 40.8 | 75 | 49.7 | |
| ≥ 75 | 30 | 19.7 | 23 | 15.2 | |
| Mean | 66.9 | 66.7 | |||
| SD | 8.4 | 7.6 | |||
| T category | .767 | ||||
| T1 | 59 | 38.8 | 61 | 40.4 | |
| T2 | 77 | 50.7 | 71 | 47.0 | |
| T3 | 16 | 10.5 | 19 | 12.6 | |
| GS | .960 | ||||
| 6 | 51 | 33.5 | 53 | 35.1 | |
| 7 | 72 | 47.4 | 70 | 46.4 | |
| 8 to 10† | 29 | 19.1 | 28 | 18.5 | |
| Pretreatment PSA, ng/mL | .889 | ||||
| < 10 | 99 | 65.1 | 95 | 62.9 | |
| 10 to 20 | 40 | 26.3 | 41 | 27.2 | |
| > 20 | 13 | 8.6 | 15 | 9.9 | |
| Pretreatment biopsy PPT, % | .679 | ||||
| ≤ 20 | 41 | 27.7 | 48 | 32.0 | |
| > 20 | 107 | 72.3 | 102 | 68.0 | |
| Unknown | 4 | 1 | |||
| Protocol risk group and planned duration of ADT | .701 | ||||
| Intermediate; none‡ | 81 | 53.3 | 83 | 55.0 | |
| Intermediate; short term§ | 20 | 13.2 | 15 | 9.9 | |
| High; long term‖ | 51 | 33.5 | 53 | 35.1 | |
Abbreviations: ADT, androgen deprivation therapy; CIMRT, conventional fractionated intensity-modulated radiotherapy; GS, Gleason score; HIMRT, hypofractionated intensity-modulated radiotherapy; NCCN, National Comprehensive Cancer Network; PPT, percent positive tissue; PSA, prostate-specific antigen; SD, standard deviation.
Fisher's exact test.
Gleason grade 5, as a component of GS 8 to 10, was seen in five and 10 patients in CIMRT and HIMRT arms, respectively, and there was no statistical difference.
Patients who did not receive ADT were intermediate risk per protocol classification, including 45 with favorable risk per NCCN criteria and one patient later determined to have GS 8 (high risk) on basis of biopsy review.
Short-term ADT patents were intermediate risk per protocol and included one favorable risk per NCCN criteria.
Long-term ADT patients were high risk per protocol, including 18 (CIMRT, seven; HIMRT, 11) GS7 patients with ≥ four positive cores and no other high risk factors (17 intermediate risk and one favorable per NCCN criteria).
Table 2.
Relative Risk of BCDF Estimated From Multivariable Regression
| Characteristic | Model A* |
Model B† |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted ASTRO |
Nadir Plus 2 |
Adjusted ASTRO |
Nadir Plus 2 |
|||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| T category | ||||||||||||
| T2 v T1 | 1.68 | 0.92 to 3.08 | .093 | 1.52 | 0.76 to 3.02 | .234 | Not included | Not included | ||||
| T3 v T1 | 2.83 | 1.18 to 6.77 | .020 | 2.53 | 0.97 to 6.60 | .059 | ||||||
| GS | ||||||||||||
| 7 v 6 | 2.17 | 1.16 to 4.05 | .016 | 2.89 | 1.39 to 6.02 | .005 | Not included | Not included | ||||
| 8 to 10 v 6 | 3.24 | 1.32 to 7.96 | .010 | 4.80 | 1.72 to 13.41 | .003 | ||||||
| Initial PSA, ng/mL | ||||||||||||
| 10 to 20 v < 10 | 2.34 | 1.34 to 4.10 | .003 | 1.52 | 0.76 to 3.07 | .239 | Not included | Not included | ||||
| > 20 v < 10 | 4.42 | 1.95 to 10.05 | < .001 | 5.27 | 2.20 to 12.64 | < .001 | ||||||
| Actual duration of ADT | ||||||||||||
| Short term (≤ 6.5 months) v no ADT | 0.55 | 0.20 to 1.51 | .249 | 0.54 | 0.17 to 1.73 | .299 | Not included | Not included | ||||
| Long term (> 6.5 months) v no ADT | 0.51 | 0.21 to 1.23 | .136 | 0.33 | 0.12 to 0.91 | .033 | ||||||
| Protocol risk group and planned ADT | ||||||||||||
| Intermediate risk plus ADT v no ADT | Not included | Not included | 0.99 | 0.40 to 2.41 | .974 | 1.02 | 0.39 to 2.72 | .962 | ||||
| High risk plus ADT v no ADT | Not included | Not included | 1.60 | 0.96 to 2.67 | .073 | 1.38 | 0.77 to 2.46 | .281 | ||||
| Protocol treatment random assignment | ||||||||||||
| HIMRT v CIMRT | 1.19 | 0.71 to 1.99 | .503 | 1.43 | 0.80 to 2.58 | .231 | 1.10 | 0.68 to 1.80 | .699 | 1.38 | 0.79 to 2.40 | .255 |
NOTE. Five-year BCDF cumulative incidence estimates by risk group using protocol-adjusted ASTRO BF definitions for CIMRT and HIMRT, respectively, were: intermediate, no ADT: 19.5% (95% CI, 11.2 to 29.5) and 17.8% (95% CI, 9.9 to 27.6; P = .76); intermediate with short-term ADT: 15.0% (95% CI, 3.6 to 34.0) and 26.2% (95% CI, 4.7 to 55.5; P = .77); and high with long-term ADT: 26.1% (95% CI, 14.2 to 39.7) and 32.1% (95% CI, 19.1 to 45.8; P = .43).
Abbreviations: ADT, androgen deprivation therapy; ASTRO, American Society for Radiation Oncology; BCDF, biochemical or clinical disease failure; CIMRT, conventional fractionated intensity-modulated radiotherapy; GS, Gleason score; HIMRT, hypofractionated intensity-modulated radiotherapy; HR, hazard ratio; PSA, prostate-specific antigen.
Fine and Gray test adjusted for individual components of risk, treatment, and actual length of ADT (short-term ADT defined as ≤ 6.5 months ADT; long term as > 6.5 months). Of 35 patients for whom short-term ADT was planned, three received long term; of 104 patients for whom long term was planned, two received short term.
Fine and Gray test adjusted for patient groups defined by protocol risk classification and use of ADT and radiation treatment. Model B avoids collinearity, which occurs in model A because length of ADT is related to risk factors. In model B, relative risk estimates are from comparisons of 35 intermediate-risk patients who received ADT (planned on enrollment, intent to treat, short-term ADT) with 164 intermediate-risk patients not administered ADT and of 104 high-risk patients who received ADT (planned on enrollment, intent to treat, long-term ADT) with 164 intermediate-risk patients not administered ADT, all after adjustment for radiation treatment. Neither age (categorical, median cut point, or continuous) nor percent positive tissue on pretreatment biopsy was significant on univariate analysis (data not shown).
BCDF End Points and Survival
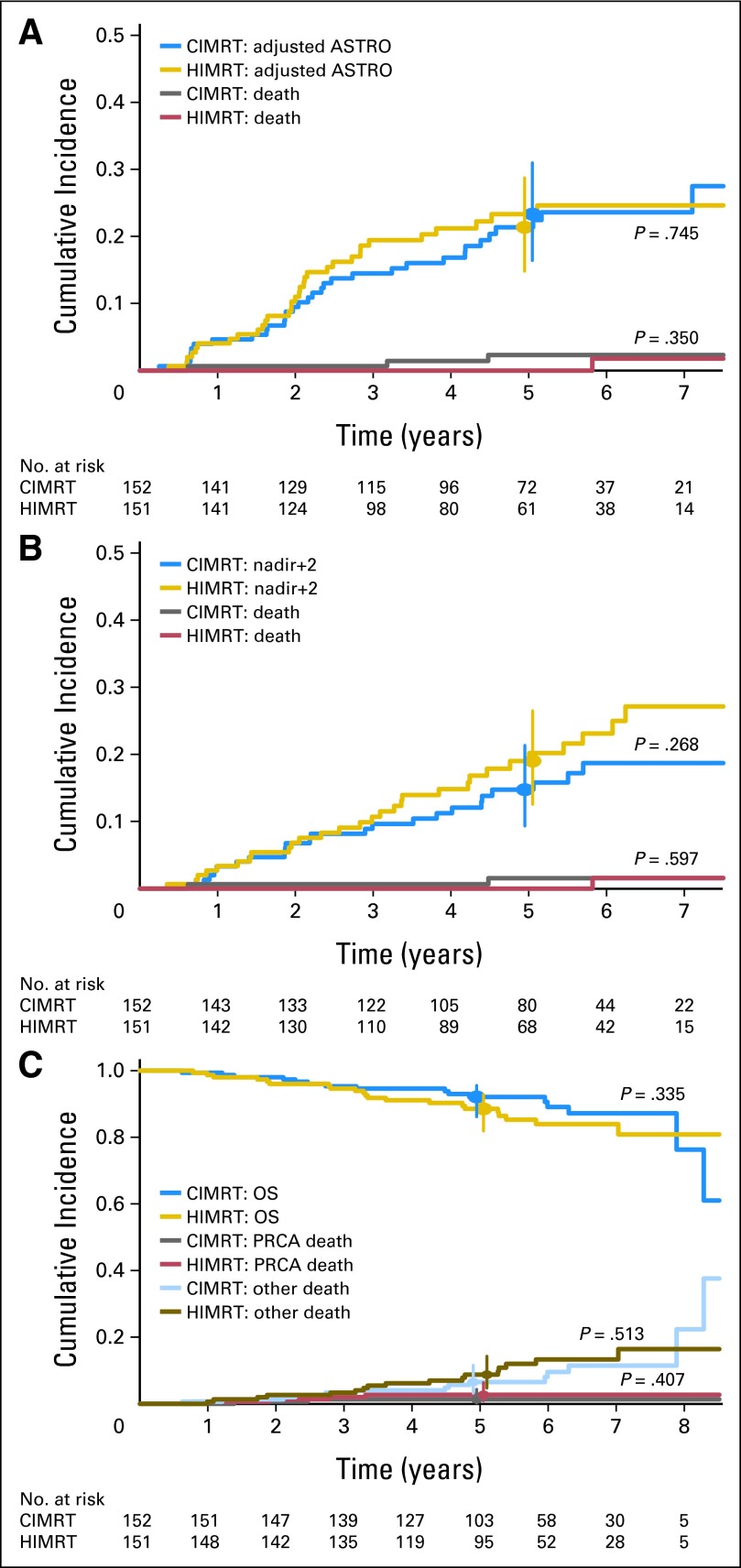
Figure 2 displays the BCDF cumulative incidence plots adjusted for the competing risk of death. There was no statistically significant difference in BCDF between the arms using the protocol definition of BCDF (Fig 2A); the 5-year rates were 21.4% for CIMRT (95% CI, 14.8% to 28.7%) versus 23.3% for HIMRT (95% CI, 16.4% to 31.0%). Using the strict ASTRO BF16 definition, BCDF results were nearly identical (data not shown). Likewise, there was no statistically significant difference between the arms using the nadir plus 2 BF17 definition (Fig 2B).
Fig 2.
Incidence of biochemical or clinical disease failure (BCDF) using (A) protocol-adjusted ASTRO (American Society for Radiation Oncology) and (B) nadir plus 2 criteria for biochemical failure and (C) overall survival (OS) and incidence of prostate cancer death (PRCA) and death resulting from other causes. P values compare treatment arms using Gray's test for cumulative incidence of BCDF and PRCA; log-rank test was used for OS. The 5-year rates for BCDF using the protocol-adjusted ASTRO definition of biochemical failure were 21.4 (95% CI, 14.8 to 28.7) and 23.3 (95% CI, 16.4 to 31.0) for conventional fractionated intensity-modulated radiotherapy (CIMRT) and hypofractionated intensity-modulated radiotherapy (HIMRT), respectively. The 5-year rates for BCDF using the nadir plus 2 definition of biochemical failure were 14.8 (95% CI, 9.3 to 21.4) and 19.0 (95% CI, 12.6 to 26.5) for CIMRT and HIMRT, respectively. Vertical bars depict 95% CIs.
There was no difference between the treatment arms in local (three in CIMRT, five in HMIRT; P = .5) or distant (six in CIMRT, eight in HIMRT; P = .6) failure. There was also no difference in prostate cancer–specific mortality, death resulting from other cause, or OS (Fig 2C). In multivariable analysis of BCDF (Table 2), treatment arm was not significant, whereas T category, GS, initial PSA, and duration of ADT (nadir plus 2 definition only) were significant. Risk group and planned length of ADT were tested separately to avoid collinearity with clinicopathologic factors determining risk group (Table 2, Model B column) and were not significant.
Toxicity
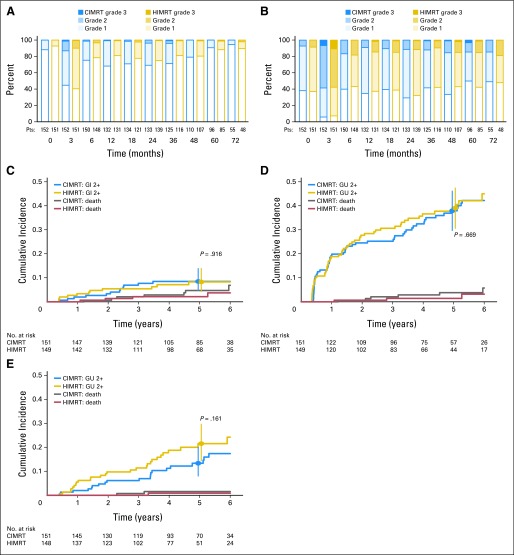
In the prevalence plots (Figs 3A and 3B), a predominance of grade 1 acute GI reactions (Fig 3A, 3-month post-RT values) was observed, without difference between the arms (P = .57). By 6 months, GI reactions had declined, and no difference was found between baseline and 5-year GI effects based on 96 CIMRT (P = .29) and 85 HIMRT (P = .49) patients. The overall (crude) incidences of grade 0, 1, 2, and 3 worst late GI reactions were 18.5%, 58.9%, 20.5%, and 2.0% for CIMRT versus 28.2%, 53.7%, 16.1%, and 2.0% for HIMRT (P = .39 comparing grade ≥ 2 rates of 22.5% v 18.1%; Fig 3C).
Fig 3.
(A, B) Prevalence and (C, D, E) incidence of (A, C) GI and (B, D, E) genitourinary (GU) adverse effects. (A, B) Prevalence plots display baseline, acute (3 months), and late reactions (≥ 6 months). (C, D, E) Cumulative incidence plots display first grade ≥ 2 toxicity occurring ≥ 3 months after completing irradiation using (C, D) original GI and GU definitions and (E) amended GU criteria. P values compare adverse reactions by treatment arm using Gray's test. Vertical bars depict 95% CIs. CIMRT, conventional fractionated intensity-modulated radiotherapy; HIMRT, hypofractionated intensity-modulated radiotherapy.
In terms of GU function, the prevalence plots (Fig 3B) revealed that many patients had compromised function at baseline mainly because of urinary frequency-urgency syndrome. A substantial increase in acute GU grade ≥ 2 adverse effects was observed, which did not differ by treatment arm (P = .58). Although a reduction in the prevalence of adverse effects was evident by 6 months, the 5-year rates of grade ≥ 2 GU effects were higher than baseline in both arms (CIMRT, 14.6% v 5.2%; P = .029 and HIMRT, 15.3% v 10.6%; P = .371).
For the protocol definition of GU toxicity11,14 (Appendix Table A1, online only), an increase of greater than once-daily use of tamsulosin 0.4 mg (dominant alpha blocker used) is coded as a grade 2 reaction. The overall (crude) incidences of grade 0, 1, 2, and 3 worst late GU reactions were 2.0%, 50.3%, 44.4%, and 3.3% for CIMRT versus 3.4%, 51.7%, 40.9%, and 4.0% for HIMRT. Liberal use of an alpha blocker during RT is common practice and substantially influences the incidence of grade ≥ 2 toxicity. The 5-year cumulative risks of grade ≥ 2 GU adverse effects were 37.9% (95% CI, 29.7% to 46.1%) for CIMRT and 39.1% (95% CI, 30.6% to 47.4%) for HIMRT (Fig 3D). The coding criteria were modified such that any alpha blocker use and occasional non-narcotic medication for dysuria were coded as grade 1 (Appendix Table A1, online only). The revised plot (Fig 3E) shows that grade ≥ 2 reactions are much less when considered this way. The 5-year revised cumulative risks of grade ≥ 2 late GU adverse effects for the CIMRT and HIMRT patients were 13.4% (95% CI, 8.0% to 20.1%) and 21.5% (95% CI, 14.4% to 29.6%) with no overall difference (P = .16).
Baseline factors were examined for association with onset of late GU toxicity. The International Prostate Symptom Score (IPSS),21 a 35-point questionnaire assessing urinary function, is routinely used in clinical practice and has been related to late GU toxicity using pretreatment cut points of 10 to 15.22,23 Setting the cut point at 12, to correspond with the upper quartile for our study patients, revealed a strong association with grade ≥ 2 late reactions for the whole group (P = .003). Univariate analyses with the same clinicopathologic variables described in Table 1 identified age and high risk as significant, with T category as marginally significant. Consideration of additional factors identified pelvic lymph node treatment and some dose-volume relationships, namely organ volumes above the high–constraint dose marker (> 25%) for the bladder and low-dose marker (> 35%) for the rectum as significant. There was no association with prostate volume, bladder volume, or prostate dose over median (CIMRT, 8,136 Gy; HIMRT, 7,515 Gy).
Regression models in Table 3 are by treatment arm based on evidence of variation in effect of IPSS by tests for interaction using the original (P = .028) and amended GU toxicity criteria (P = .056). None of the factors examined were significant in the arm I patients. For arm II, IPSS > 12, age ≥ 67 years (original criteria), and pelvic lymph node treatment (amended criteria; done for high risk with four exceptions) were associated with increased risk of late grade ≥ 2 toxicity.
Table 3.
Multivariable Analyses of Late Grade ≥ 2 GU Adverse Reactions
| Treatment | Original Criteria |
Amended Criteria |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | HR | 95% CI | P* | No. | % | HR | 95% CI | P | |
| Arm I (CIMRT) | ||||||||||
| Pretreatment IPSS | ||||||||||
| ≤ 12 | 121 | 80.1 | Reference | — | — | 121 | 79.6 | Reference | — | — |
| > 12 | 30 | 19.9 | 1.17 | 0.64 to 2.14 | .608 | 30 | 20.4 | 1.25 | 0.47 to 3.33 | .660 |
| Arm II (HIMRT) | ||||||||||
| Pretreatment IPSS | ||||||||||
| ≤ 12 | 115 | 77.2 | Reference | — | — | 114† | 76.7 | Reference | — | — |
| > 12 | 34 | 22.8 | 2.54 | 1.44 to 4.48 | .001 | 34 | 23.3 | 4.41 | 1.97 to 9.88 | < .001 |
| Nodal treatment | ||||||||||
| No | 98 | 64.9 | Reference | — | — | 97† | 64.7 | Reference | — | — |
| Yes | 51 | 35.1 | 1.62 | 0.96 to 2.74 | .070 | 51 | 35.3 | 2.44 | 1.09 to 5.45 | .030 |
| Age, years | ||||||||||
| ≤ 67 | 78 | 52.3 | Reference | — | — | 77† | 52.0 | Reference | — | — |
| > 67 | 71 | 47.7 | 1.91 | 1.09 to 3.36 | .024 | 71 | 48.0 | 1.51 | 0.69 to 3.30 | .302 |
NOTE. IPSS was most important factor identified in univariate tests performed using original criteria for GU reactions and full-analysis set of 300 patients. Univariate analysis of clinicopathologic factors identified age and high risk as significant and T stage as marginally significant; there was no association with treatment arm, Gleason score, or PSA. Consideration of additional factors identified IPSS, pelvic lymph nodes treated, and excess volume irradiated in relation to bladder high-dose constraint marker (> 25%) and rectum low-dose constraint marker (> 35%) as significant, whereas there was no association with prostate volume, bladder volume, prostate dose > median (CIMRT, 8,136 Gy; HIMRT, 7,515 Gy) or excess volume irradiated in relation to bladder low-dose constraint marker (> 50%) or rectum high-dose constraint marker (> 17%). Factors that remained significant in multivariable analysis treatment arm models are shown.
Abbreviations: CIMRT, conventional fractionated intensity-modulated radiotherapy; GU, genitourinary; HIMRT, hypofractionated intensity-modulated radiotherapy; HR, hazard ratio; IPSS, International Prostate Symptom Score.
Fine and Gray test.
Missing GU data by amended criteria for one HIMRT patient.
Figure 4 displays the cumulative incidence of grade ≥ 2 GU toxicity subdivided by IPSS (cut point of 12) and radiation treatment arm. The CIMRT arm had comparable rates regardless of IPSS (P = .63), whereas HIMRT patients with a pretreatment IPSS > 12 had significantly increased grade ≥ 2 late GU reactions compared with those with an IPSS of ≤ 12 (P ≤ .001), which was observed for both the protocol (Fig 4A) and revised (Fig 4B) GU late reaction definitions.
Fig 4.
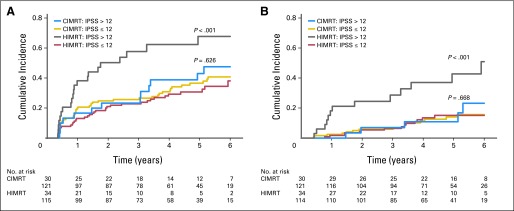
Cumulative incidence of late grade ≥ 2 genitourinary (GU) toxicity subdivided by treatment arm (conventional fractionated intensity-modulated radiotherapy [CIMRT] v hypofractionated intensity-modulated radiotherapy [HIMRT]) and International Prostate Symptom Score (IPSS) at a cut point of 12. Results using (A) original protocol definition of GU toxicity and (B) amended criteria are shown. P value determined using Gray's test.
DISCUSSION
With more precise methods for delivering RT to the prostate, such as IMRT, there has been revitalized interest in applying hypofractionation to improve tumor control based on radiobiologic considerations and patient convenience and to reduce health care costs. We designed the randomized trial described herein to test whether increasing RT dose to the equivalent of 8.4 Gy in 2-Gy fractions using hypofractionation would significantly reduce BCDF. Because there was no statistically significant difference between the treatment arms in BCDF, the results are not sufficient to reject the null hypothesis. This hypothesis was based on evidence that: one, the α/β ratio for prostate cancer is lower, at approximately 1.5 Gy,7 than that for the surrounding normal tissues, at approximately 5 Gy8,9; and two, there would be a reduction in BF of 15% with an 8.4-Gy dose escalation in the population studied. These assumptions may have contributed to the findings. Although the vast majority of the evidence supports an α/β ratio for prostate cancer in the 1.5-Gy range, some reports have indicated that the α/β ratio is much higher.24 Factors that may contribute to this disparity include the heterogeneity of prostate cancer, hypoxia, use of BF as an end point without establishing local tumor control, and use of ADT as an adjunct to irradiation. We used a risk-stratified approach for the addition of short-term ADT (intermediate risk) or long-term ADT (high risk); however, we did account for effects from ADT on BCDF when we planned the trial using data from a large retrospective database available at Fox Chase Cancer Center. Of note, BCDF was lower than expected in the CIMRT arm and higher than expected in the HIMRT arm. The GS shift,25 combined with modern planning using MRI and IMRT delivery techniques using image guidance, may have contributed in the CIMRT arm, along with the foibles of the α/β ratio in the HIMRT arm.
Another example where the α/β ratio seems to have fallen short is in the randomized trial reported by Arcangeli et al,26 wherein the α/β ratio–derived primary end point was toxicity. The dose fractionation schedules of the conventional (80 Gy in 40 fractions) and hypofractionation (62 Gy in 20 fractions) arms were hypothesized to be isoeffective, whereas normal tissue toxicity was hypothesized to be lower in the hypofractionation patients. All patients received ADT for 9 months. Although BF rates were as predicted (not statistically different), normal tissue toxicity rates were not statistically different. Their findings were also not sufficient to reject the null hypothesis.
In our trial, the PTV margin was smaller for the HIMRT patients, which could have contributed to the findings. We have reported a randomized prostate cancer standard fractionation randomized trial of 70 versus 78 Gy, in which the 78-Gy patients were treated with tighter margins to accommodate dose escalation.1,27 The dose escalation results in this prior standard fractionation trial1,27 and the hypofractionation trial described here should be interpreted in the context of such planned technical differences between the arms.
There was no significant difference in BCDF between the treatment arms in our hypofractionation trial, and hypofractionation patients received 12 fewer daily RT treatments without significant increases in overall GU or GI toxicity. Prevalence studies indicated that at 5 years after treatment, GU and GI reactions were not much different from baseline. Nonetheless, we had concerns because the incidence of maximum GU reactions was much greater than that for GI. As a consequence, factors associated with GU toxicity were examined. Baseline IPSS questionnaire results were strongly associated with grade ≥ 2 late reactions in arm II patients only (Table 3; Fig 4). Although this subgroup analysis suggests that the hypofractionation regimen described is most appropriate for men without substantial baseline urinary dysfunction, the trial was not designed to specifically address this question, and confirmation is needed.
Supplementary Material
Acknowledgment
We thank Lawrence R. Coia, MD, and Rajesh V. Iyer, MD, of Community Medical Center, Toms River, NJ, for contributing patients to the trial; Theresa White-Marino, RN, and Elaine Callahan, RN, for protocol support; and Ruth Peter, RN, ThM, for database management.
Glossary Terms
- Prostate-specific antigen (PSA):
A protein produced by cells of the prostate gland, the blood level of PSA is used as a tumor marker for men who may be suspected of having prostate cancer. Most physicians consider 0 to 4.0 ng/mL as the normal range. Levels of 4 to 10 and 10 to 20 ng/mL are considered slightly and moderately elevated, respectively. PSA levels have to be complemented with other tests to make a firm diagnosis of prostate cancer.
Appendix
Table A1.
Chronic Radiation Toxicity Grading
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| GI | Excess bowel movements twice baseline or need for ≤ two anti diarrheals per week; slight rectal discharge or bleeding not requiring pads or medication | > Two antidiarrheals per week; ≤ two coagulations for bleeding; temporary steroids per suppositories or enema for symptoms/ulceration of ≤ 1 month; ≤ two dilatations; mucous discharge requiring < two pads per day; infrequent use of sanitary pads; non-narcotic or narcotic medication for pain once per day for < 1 month; regular non-narcotic or occasional narcotic for pain | > Two antidiarrheals per day for > 1 month; one blood transfusion or > two coagulations for bleeding; steroids per suppositories or enema for > 1 month; hyperbaric oxygen treatment for ulceration or bleeding; > two dilations; sanitary pads ≥ two per day for > 1 month; narcotic use > once per day for > 1 month | Fistula or obstruction requiring surgery; > one blood transfusion |
| GU | Nocturia twice baseline or non-narcotic medication (eg, alpha blocker) once per day increase over baseline; microscopic hematuria; light mucosal atrophy and minor telangiectasia; dysuria not requiring medication; incontinence or dribbling not requiring sanitary pad (over baseline) | Frequency ≤ once every hour requiring alpha blocker > once per day increase over baseline; nocturia > 2× baseline; generalized telangiectasias; macroscopic hematuria requiring ≤ two cauterizations; dysuria requiring medication (non-narcotic > once per day or narcotic for pain ≤ once per day over baseline); ≤ two dilations; Foley or self-catheter for ≤ 2 weeks; incontinence requiring ≤ two pads (over baseline) | Frequency > once every hour or dysuria requiring narcotics > one per day; nocturia more frequent than once every hour; reduction in bladder capacity (150 cm3); ≥ one blood transfusion or > two cauterizations for bleeding; narcotic use of > once per day; hyperbaric oxygen, Foley or self-catheter for > 2 weeks; urethrotomy, TURP, or > two dilatations; incontinence requiring > two sanitary pads (over baseline | Gross hematuria requiring > one blood transfusion; severe hemorrhagic cystitis or ulceration requiring urinary diversion and/or cystectomy |
| GU (revised) | Nocturia twice baseline or medication (eg, alpha blocker) increase over baseline; hematuria not requiring intervention; light mucosal atrophy and minor telangiectasia; dysuria or pain requiring occasional non-narcotic medication; incontinence or dribbling not requiring sanitary pad (over baseline) | Frequency ≤ every hour; nocturia > 2× baseline; generalized telangiectasias; hematuria requiring ≤ two cauterizations; pain requiring regular anti-inflammatory agent, anesthetic or antispasmodic, or occasional narcotic; stricture requiring ≤ two dilatations; Foley or self-catheter for ≤ 2 weeks; incontinence requiring ≤ two sanitary pads (over baseline) | Frequency or nocturia > hourly; dysuria and/or pain requiring regular narcotic use; reduction in bladder capacity (150 cm3); ≥ one blood transfusion or > two cauterizations for bleeding; hyperbaric oxygen treatment; Foley or self-catheter for > 2 weeks; urethrotomy, TURP, or > two dilatations; incontinence requiring > two sanitary pads (over baseline) or artificial sphincter | Gross hematuria requiring > one blood transfusion; severe hemorrhagic cystitis or ulceration requiring urinary diversion and/or cystectomy |
NOTE. Bold font indicates differences in GU and GU (revised) toxicity definitions. No patient had grade 4 or 5 (death) complication.
Abbreviations: GU, genitourinary; TURP, transurethral resection of prostate.
Table A2.
Duration of ADT by Treatment Arm and Protocol Risk Group
| Protocol Risk Group* | Duration of Initial ADT (months) |
|||||
|---|---|---|---|---|---|---|
| No. of Patients | Minimum | Q1 | Median | Q3 | Maximum | |
| CIMRT | ||||||
| Intermediate | 20 | 1.0 | 2.2 | 3.7 | 4.1 | 24.0 |
| High | 51 | 7.0 | 23.8 | 24.1 | 25.1 | 34.3 |
| HIMRT | ||||||
| Intermediate | 15 | 0.6 | 3.1 | 4.0 | 5.5 | 24.0 |
| High | 53 | 3.0 | 14.1 | 24.0 | 25.1 | 72.9 |
Abbreviations: ADT, androgen deprivation therapy; CIMRT, conventional fractionated intensity-modulated radiotherapy; HIMRT, hypofractionated intensity-modulated radiotherapy; Q1, first quartile; Q3, third quartile.
Intent for those classified as intermediate risk per protocol definition, and who began ADT before protocol entry, was administration of short-term ADT ≤ 4 months duration (three patients received ADT > 6.5 months). Intent for those classified as high risk per protocol definition was administration of long-term ADT of 2 years duration (two patients received ADT < 6.5 months; one received ADT > 35 months).
Footnotes
Processed as a Rapid Communication manuscript; see accompanying editorial on page 3849
Supported by National Cancer Institute Grants No. CA101984-01 and CA-00692 and Florida Biomed Bankhead Coley Grant No. 09BW11.
Presented in part at the 53rd Annual Meeting of the American Society for Radiation Oncology, Miami Beach, FL, October 2-6, 2011.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00062309.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Alan Pollack, GE Healthcare (C), Calypso (C); Robert G. Uzzo, WILEX (C); Mark K. Buyyounouski, Augmenix (C), GE Healthcare (C) Stock Ownership: None Honoraria: Alan Pollack, Varian Medical Systems, Accuray, Siemens Healthcare Research Funding: Alan Pollack, Varian Medical Systems, Siemens Healthcare; Benjamin Movsas, Varian Medical Systems, Philips Healthcare; Mark K. Buyyounouski, Varian Medical Systems Expert Testimony: None Patents: Mark K. Buyyounouski, Amersys, UpToDate Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Alan Pollack, Eric M. Horwitz, Robert A. Price, Charlie Ma
Financial support: Alan Pollack
Provision of study materials or patients: Alan Pollack, Richard E. Greenberg
Collection and assembly of data: Alan Pollack, Eric M. Horwitz, Robert A. Price, Radka Stoyanova, Benjamin Movsas, Richard E. Greenberg, Robert G. Uzzo
Data analysis and interpretation: Alan Pollack, Gail Walker, Eric M. Horwitz, Steven Feigenberg, Andre A. Konski, Radka Stoyanova, Benjamin Movsas, Mark K. Buyyounouski
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Al-Mamgani A, van Putten WL, Heemsbergen WD, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;72:980–988. doi: 10.1016/j.ijrobp.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 3.Khoo V, Pollack A, Cowen D, et al. Relationship of Ki-67 labeling index to DNA-ploidy, S-phase fraction, and outcome in prostate cancer treated with radiotherapy. Prostate. 1999;41:166–172. doi: 10.1002/(sici)1097-0045(19991101)41:3<166::aid-pros3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Haustermans K, Hofland I, Poppel H, et al. Cell kinetic measurements in prostate cancer. Int J Radiat Oncol Biol Phys. 1997;37:1067–1070. doi: 10.1016/s0360-3016(96)00579-2. [DOI] [PubMed] [Google Scholar]
- 5.Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 1999;44:747–748. doi: 10.1016/s0360-3016(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 6.Miralbell R, Roberts SA, Zubizarreta E, et al. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 7.Dasu A, Toma-Dasu I. Prostate alpha/beta revisited: An analysis of clinical results from 14,168 patients. Acta Oncol. 2012;51:963–974. doi: 10.3109/0284186X.2012.719635. [DOI] [PubMed] [Google Scholar]
- 8.Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013–1015. doi: 10.1016/j.ijrobp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Tucker SL, Thames HD, Michalski JM, et al. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kupelian PA, Reddy CA, Klein EA, et al. Short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy per fraction) for localized prostate cancer: Preliminary results on late toxicity and quality of life. Int J Radiat Oncol Biol Phys. 2001;51:988–993. doi: 10.1016/s0360-3016(01)01730-8. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A, Hanlon AL, Horwitz EM, et al. Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eade TN, Hanlon AL, Horwitz EM, et al. What dose of external-beam radiation is high enough for prostate cancer? Int J Radiat Oncol Biol Phys. 2007;68:682–689. doi: 10.1016/j.ijrobp.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 14.Hanlon AL, Schultheiss TE, Hunt MA, et al. Chronic rectal bleeding after high dose conformal treatment of prostate cancer warrants modification of existing morbidity scales. Int J Radiat Oncol Biol Phys. 1997;38:59–63. doi: 10.1016/s0360-3016(97)00234-4. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009.
- 16.Cox J, Grignon D, Kaplan R, et al. Consensus statement: Guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 17.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1143. [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 21.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia: The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549–1557. doi: 10.1016/s0022-5347(17)36966-5. discussion 1564. [DOI] [PubMed] [Google Scholar]
- 22.Locke J, Ellis W, Wallner K, et al. Risk factors for acute urinary retention requiring temporary intermittent catheterization after prostate brachytherapy: A prospective study. Int J Radiat Oncol Biol Phys. 2002;52:712–719. doi: 10.1016/s0360-3016(01)02657-8. [DOI] [PubMed] [Google Scholar]
- 23.Malik R, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer: Urinary outcomes for men with high International Prostate Symptom Scores (IPSS) Int J Radiat Oncol Biol Phys. 2011;80:1080–1086. doi: 10.1016/j.ijrobp.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Nahum AE, Movsas B, Horwitz EM, et al. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: Implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57:391–401. doi: 10.1016/s0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 25.Chism DB, Hanlon AL, Troncoso P, et al. The Gleason score shift: Score four and seven years ago. Int J Radiat Oncol Biol Phys. 2003;56:1241–1247. doi: 10.1016/s0360-3016(03)00268-2. [DOI] [PubMed] [Google Scholar]
- 26.Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–1178. doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: Results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53:1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.