Abstract
Purpose
To determine the likelihood of long-term amenorrhea after treatment with chemotherapy in women with breast cancer who carry a BRCA1 or BRCA2 mutation.
Patients and Methods
We conducted a multicenter survey of 1,954 young women with a BRCA1 or BRCA2 mutation who were treated for breast cancer. We included premenopausal women who were diagnosed with invasive breast cancer between 26 and 47 years of age. We determined the age of onset of amenorrhea after breast cancer for women who were and were not treated with chemotherapy, alone or with tamoxifen. We considered chemotherapy-induced amenorrhea to have occurred when the patient experienced ≥ 2 years of amenorrhea, commencing within 2 years of initiating chemotherapy, with no resumption of menses.
Results
Of the 1,426 women who received chemotherapy, 35% experienced long-term amenorrhea. Of the 528 women who did not receive chemotherapy, 5.3% developed long-term amenorrhea. The probabilities of chemotherapy-induced amenorrhea were 7.2% for women diagnosed before age 30 years, 33% for women age 31 to 44 years, and 79% for women diagnosed after age 45 years (P trend < .001). The probability of induced amenorrhea was higher for women who received tamoxifen than for those who did not (52% v 29%; P < .001).
Conclusion
Age at treatment and use of tamoxifen are important predictors of chemotherapy-induced amenorrhea in women who carry a BRCA1 or BRCA2 mutation. The risk of induced long-term amenorrhea does not seem to be greater among mutation carriers than among women who do not carry a mutation.
INTRODUCTION
In premenopausal women with breast cancer, the benefits of treatment must be balanced by the effects of treatment on fertility and quality of life. Among the concerns of young women with breast cancer are premature menopause and infertility.1 Long-term effects of treatment include osteoporosis, urogenital dysfunction, cardiovascular disease, and cognitive impairment.2 Many women with a BRCA1 or BRCA2 mutation who are diagnosed with breast cancer will seek the expertise of a fertility specialist, who may recommend oocyte cryopreservation or embryo preservation before treatment. This is based on the expectation that a high proportion of these women will be rendered amenorrheic by chemotherapy and the hope that fertility can be preserved through intervention. It has been proposed that many BRCA mutation carriers who do not receive chemotherapy will experience premature menopause.3 It is important that patients and clinicians have accurate information regarding the probabilities of induced and natural menopause and the factors that predict amenorrhea in this high-risk group.
At birth, each ovary contains approximately 300,000 oocytes. The number of residual follicles diminishes with each menstrual cycle, and the number of remaining follicles determines, in part, a woman's capability for fertility and is predictive of time to menopause. At the age of 51 years, only approximately 1,000 follicles remain.4,5 Women treated for breast cancer are at risk of ovarian failure after chemotherapy because of depletion of follicles. The incidence of chemotherapy-induced amenorrhea depends on the patient's age and the chemotherapy agent used.6
It has been proposed that women with a BRCA1 mutation may be more sensitive to the effects of chemotherapy on ovarian follicle depletion than women without a mutation.3 This hypothesis is based on a study of ovarian reserve of patients with breast cancer attending a fertility clinic. Little is known about the risk of induced amenorrhea in women with a BRCA1 or BRCA2 mutation. Our objective was to estimate the probability of chemotherapy-induced amenorrhea for premenopausal women with breast cancer and a BRCA1 or BRCA2 mutation. A secondary objective was to compare the age-specific probabilities of experiencing chemotherapy-induced amenorrhea in mutation carriers and noncarriers. Other members of the Hereditary Breast Cancer Clinical Study Group are listed in the Appendix (online only).
PATIENTS AND METHODS
Patients were selected from a database of 13,004 BRCA1 and BRCA2 mutation carriers and 2,451 noncarriers. Study patients were identified at one of 62 participating centers in seven countries. These women sought testing for BRCA1 and BRCA2 mutations because of a personal or family history of breast or ovarian cancer. The institutional review boards of the host institutions approved the study. All patients provided written informed consent. Mutation detection was performed using a range of techniques, but all nucleotide sequences were confirmed by direct sequencing of DNA. A woman was enrolled onto the BRCA carrier group when the molecular analysis established that she was a carrier of a deleterious mutation in BRCA1 or BRCA2. All study patients completed a baseline questionnaire. The questionnaire requested information on family and personal histories of cancer, reproductive and medical histories, and date and cause of menopause. For women with breast cancer, information was collected on types of therapy administered, including chemotherapy (yes v no), hormonal therapy, and ovarian ablation. We did not have details on type of chemotherapy.
In our study, we included mutation carriers from the parent study, who were diagnosed with invasive breast cancer between 26 and 47 years of age and who were premenopausal at the time of diagnosis. The date of breast cancer was considered the date of surgery. We considered the definition of induced menopause as ≥ 2 years of amenorrhea commencing within 2 years of chemotherapy with no resumption of menses. We considered natural amenorrhea to have occurred when the onset of amenorrhea was > 2 years after breast cancer treatment. For women who did not receive chemotherapy, we considered the onset of amenorrhea within 2 years of the initiation of treatment to be the relevant comparison period (in this sense, we used the term induced amenorrhea throughout the text for comparison purposes, but given the absence of chemotherapy, we recognize that these are likely to be examples of natural menopause). As a consequence of this definition, we excluded carriers if they had experienced menopause before breast cancer diagnosis (n = 1,084), if they were age > 47 years at diagnosis (n = 255), if they had undergone an oophorectomy (bilateral or unilateral) or hysterectomy within 2 years of diagnosis (n = 213), or if the follow-up period did not extend to 2 years from the onset of amenorrhea (n = 1,202). We also excluded 102 carriers who developed ovarian cancer or a second primary breast cancer within 2 years of breast cancer diagnosis, 338 carriers for whom genetic test results were of uncertain clinical significance, and 554 carriers for whom information on key variables was missing (ie, date of last menstrual period, date of breast cancer diagnosis, or date of breast cancer treatment). In total, 1,954 BRCA carriers with breast cancer were eligible.
We included 167 noncarrier women with breast cancer as a comparison group. These were women who had been assessed for family history and had undergone genetic testing for BRCA1 and BRCA2 mutations in the Narod laboratory in Toronto between 1995 and 2012 and were found to be negative for both mutations. These women completed the same lifestyle/medical questionnaire as did the carrier women, and the exclusion criteria were identical.
Statistical Analysis
We estimated the proportion of women who experienced induced amenorrhea as the number of women who experienced amenorrhea of ≥ 2 years duration after chemotherapy with no resumption of menses, divided by the total number of women. Relevant subgroups included chemotherapy use (yes v no), BRCA1 or BRCA2 mutation, age at diagnosis (age of surgery), and use of adjuvant hormonal therapy (tamoxifen). Between-group comparisons were assessed for significance using the t test.
In a secondary analysis, carrier and noncarrier patients were compared for menopause by comparing of the probabilities of induced amenorrhea by age at treatment. The comparisons were tested for statistical significance using the Cochran-Mantel-Haenszel test for nonzero correlation.
RESULTS
The median age at diagnosis for carriers was 37.3 years (range, 26 to 47 years; Table 1). All patients reported experiencing menstrual cycles at the time of breast cancer diagnosis. Of the 1,954 mutation carriers, 1,506 (77%) carried a BRCA1 mutation, 436 (22%) carried a BRCA2 mutation, and 12 (0.6%) carried both mutations. Of the 1,954 carrier patients, 1,426 (73%) received chemotherapy. In total, 478 carrier patients received tamoxifen, of whom 410 received both tamoxifen and chemotherapy (86%).
Table 1.
Patient Characteristics by Use of Chemotherapy (BRCA1 and BRCA2 carriers combined)
| Characteristic | No Chemotherapy (n = 528) |
Chemotherapy (n = 1,426) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Year of birth | < .001 | ||||
| Mean | 1948.4 | 1957.4 | |||
| Range | 1910-1978 | 1914-1982 | |||
| Age at diagnosis, years | .37 | ||||
| Mean | 37.1 | 37.4 | |||
| Range | 26-47 | 26-47 | |||
| BRCA1 | .10 | ||||
| Mean | 36.7 | 37.3 | |||
| Range | 26-47 | 26-47 | |||
| BRCA2 | .35 | ||||
| Mean | 38.4 | 37.9 | |||
| Range | 26-47 | 26-47 | |||
| Mutation status | .09 | ||||
| BRCA1 | 391 | 25.9 | 1,115 | 74.0 | |
| BRCA2 | 135 | 30.9 | 301 | 69.0 | |
| Country of residence | < .001 | ||||
| United States | 183 | 497 | |||
| Poland | 91 | 382 | |||
| Canada | 148 | 324 | |||
| Israel | 22 | 52 | |||
| Austria | 16 | 50 | |||
| Italy | 18 | 31 | |||
| France | 13 | 32 | |||
| Other | 37 | 58 | |||
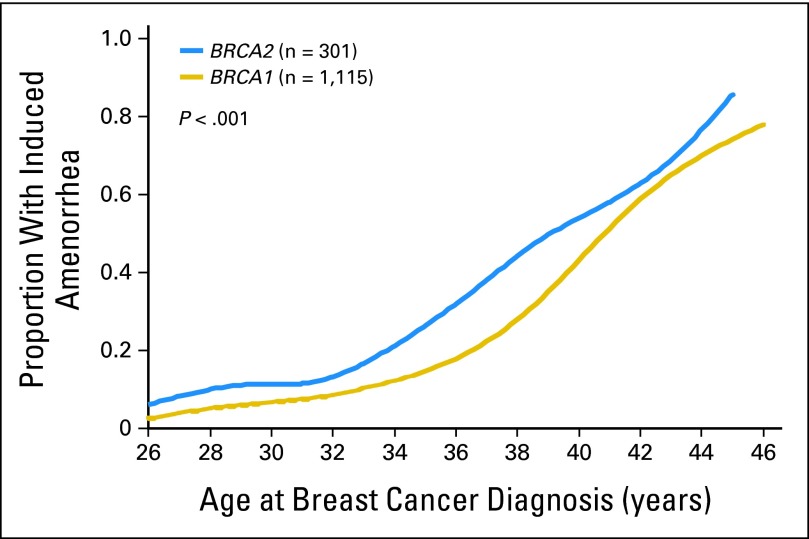
Of the 1,426 carriers who received chemotherapy, 35.6% experienced induced amenorrhea, and of the 528 women who did not receive chemotherapy, 5.3% experienced induced amenorrhea (P < .001 for difference). The probability of induced amenorrhea increased with age at diagnosis (Table 2). Of the carrier patients who received chemotherapy, the probability of chemotherapy-induced amenorrhea was 7.2% for women diagnosed before age 30 years, 33% for women diagnosed from age 31 to 44 years, and 79% for women diagnosed at or after age 45 years (P trend < .001). The probability of chemotherapy-induced amenorrhea was significantly higher for BRCA2 carriers than for BRCA1 carriers (46.8% v 32.7%; P < .001; Table 3; Fig 1). The average age at diagnosis of BRCA2 carriers was 1 year older than that of BRCA1 carriers (38 v 37 years); after adjustment for age at diagnosis, the difference remained significant (P < .001). By age 38 years, 50% of BRCA2 carriers were expected to experience amenorrhea after chemotherapy, compared with the age of 40 years expected for BRCA1 carriers. More BRCA2 carriers than BRCA1 carriers received tamoxifen (41.4% v 16.1%); therefore, we restricted the comparison to women who did not receive tamoxifen; in this subgroup, the probability of chemotherapy-induced amenorrhea was 36.6% for BRCA2 carriers and 27.8% for BRCA1 carriers (P = .04).
Table 2.
Probability of Induced or Natural Amenorrhea Among Women Treated With Chemotherapy by Age at Treatment (BRCA1 and BRCA2 combined)
| Age at Treatment (years) | Induced Amenorrhea* |
Natural Amenorrhea† |
Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| 26 | 0 | 0.0 | 39 | 100.0 | 39 |
| 27 | 2 | 8.0 | 23 | 92.0 | 25 |
| 28 | 2 | 6.9 | 27 | 93.1 | 29 |
| 29 | 5 | 11.1 | 40 | 88.9 | 45 |
| 30 | 4 | 9.3 | 39 | 90.7 | 43 |
| 31 | 3 | 5.1 | 56 | 94.9 | 59 |
| 32 | 3 | 4.3 | 67 | 95.7 | 70 |
| 33 | 13 | 15.1 | 73 | 84.9 | 86 |
| 34 | 15 | 19.5 | 62 | 80.5 | 77 |
| 35 | 12 | 14.1 | 73 | 85.9 | 85 |
| 36 | 16 | 22.2 | 56 | 77.8 | 72 |
| 37 | 15 | 22.1 | 53 | 77.9 | 68 |
| 38 | 26 | 29.2 | 63 | 70.8 | 89 |
| 39 | 35 | 37.6 | 58 | 62.4 | 93 |
| 40 | 40 | 41.2 | 57 | 58.8 | 97 |
| 41 | 36 | 59.0 | 25 | 41.0 | 61 |
| 42 | 45 | 65.2 | 24 | 34.8 | 69 |
| 43 | 44 | 59.5 | 30 | 40.5 | 74 |
| 44 | 42 | 76.4 | 13 | 23.6 | 55 |
| 45 | 56 | 78.9 | 15 | 21.1 | 71 |
| 46 | 42 | 79.3 | 11 | 20.8 | 53 |
| 47 | 52 | 78.8 | 14 | 21.2 | 66 |
| Total | 508 | 35.6 | 918 | 64.4 | 1,426 |
Amenorrhea onset ≤ 2 years of breast cancer diagnosis with no resumption of menses.
Amenorrhea onset ≥ 2 years after breast cancer diagnosis.
Table 3.
Probability of Induced Amenorrhea by Chemotherapy and Gene Mutation Status
| Chemotherapy | Amenorrhea in BRCA1 Carriers |
Amenorrhea in BRCA2 Carriers |
Amenorrhea in BRCA1 and BRCA2 Carriers Combined |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No | 22 of 391 | 5.6 | 6 of 135 | 4.4 | 28 of 526 | 5.3 |
| Yes | 364 of 1,115 | 32.7 | 141 of 301 | 46.8 | 505 of 1,416 | 35.6 |
| Total | 386 of 1,506 | 25.6 | 147 of 436 | 33.7 | 533 of 1,942 | 27.4 |
NOTE. Twelve women had both mutations and were excluded.
Fig 1.
Proportion of patients with induced amenorrhea by age at diagnosis; BRCA1 versus BRCA2; all patients received chemotherapy.
We also evaluated the impact of tamoxifen use on the probability of induced amenorrhea for carrier women who did and did not receive chemotherapy (Table 4). Of 518 mutation carriers who did not receive chemotherapy, those who received tamoxifen had a 5.3% probability of induced amenorrhea, compared with 5.8% for those who did not use tamoxifen (P = .9; 10 carrier patients were missing tamoxifen data and were excluded). Of 1,403 BRCA carriers who received chemotherapy, those who received tamoxifen had a 52% probability of induced amenorrhea, compared with 29% for those who did not receive tamoxifen (P < .001; 33 carrier patients were missing tamoxifen data and were excluded).
Table 4.
Probability of Induced Amenorrhea by Chemotherapy and Tamoxifen Use
| Treatments Received | Induced Amenorrhea |
Natural Amenorrhea |
Total | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No chemotherapy; no tamoxifen | 24 | 5.3 | 426 | 94.6 | 450 |
| No chemotherapy; tamoxifen | 4 | 5.8 | 64 | 94.1 | 68 |
| Chemotherapy; no tamoxifen | 288 | 29.0 | 705 | 71.0 | 993 |
| Chemotherapy; tamoxifen | 214 | 52.2 | 196 | 47.8 | 410 |
| Total | 536 | 1,418 | 1,954 | ||
NOTE. Information on tamoxifen was missing for 33 patients.
We also evaluated the probability of induced menopause in 167 noncarrier patients with breast cancer who otherwise met the study criteria. Of the 167 noncarrier patients, 100 women (59%) received chemotherapy, and of these, 49 (49%) developed chemotherapy-induced amenorrhea. We compared the age-specific probabilities of induced amenorrhea among chemotherapy-treated patients for the 1,426 mutation carriers and 100 noncarriers (Fig 2). We found no significant difference between the age-specific probabilities of chemotherapy-induced amenorrhea for the two groups (P = .18). Furthermore, there was no significant difference when BRCA1 carriers were compared with noncarrier controls (P = .10) or when BRCA2 carriers were compared with noncarrier controls (P = .50). Tamoxifen had been administered to 24% of carriers and 38% of noncarriers. Results were similar when women who received tamoxifen were excluded (data not shown).
Fig 2.
Proportion of patients with induced amenorrhea by age at diagnosis; mutation carriers versus noncarriers; all patients received chemotherapy.
We also addressed the question of whether women with a BRCA1 or BRCA2 mutation who were treated with chemotherapy and who experienced return of menses underwent menopause earlier than women who did not receive chemotherapy. Among all women who reached age 56 years, the mean age of menopause was 45.4 years for those who received chemotherapy and resumed menses and was 49.0 years for those who did not receive chemotherapy (P < .001), a difference of 3.6 years. Among women who had a BRCA1 mutation and reached age 56 years, the mean age of menopause was 45.5 years for those who received chemotherapy and 48.7 years for those who did not receive chemotherapy (P < .001). Among women who had a BRCA2 mutation and reached age 56 years, the mean age of menopause was 45.2 years for those who received chemotherapy and 49.9 years for those who did not receive chemotherapy (P = .003).
DISCUSSION
Chemotherapy can induce transient or permanent amenorrhea. Definitions of chemotherapy-induced amenorrhea vary7; we defined it as ≥ 2 years of amenorrhea commencing within 2 years after chemotherapy with no resumption of menses. Patients with breast cancer for whom amenorrhea lasts > 24 months after chemotherapy are unlikely to resume menses.7–9
The postmenopausal ovary is characterized by atrophy of the cortex and depletion of the follicles.4,6,10 Chemotherapy can target the oocyte directly (germ cells) or can induce oocyte death indirectly via damage to somatic cells (granulosa cells).9 Ovarian cytotoxicity is irreversible; women are born with a fixed number of germ cells, and these are not replenished.9 Ovarian dysfunction after chemotherapy depends on patient age and type of chemotherapy received.9,11,12 High-risk agents include alkylating agents; medium-risk agents include platinum, anthracycline antibiotics, and taxoids; and low-risk agents include vinca plant alkaloids, some anthracycline antibiotics such as bleomycin, and antimetabolites such as methotrexate, fluorouracil, and mercaptopurine. In our study, we did not have access to data regarding specific chemotherapy regimens.
We show here that the probability of chemotherapy-induced amenorrhea in women who carry a BRCA1 or BRCA2 mutation increases steadily with age at treatment; the probability of induced amenorrhea was 7% for women age ≤ 30 years, 12% for women age 31 to 35 years, and 52% for women age ≥ 36 years. It does not seem that BRCA mutation carriers are at particularly high risk of chemotherapy-induced menopause. The results are similar to those of previous studies that evaluated for sporadic breast cancer cases.13–16 Forty-five percent of women with breast cancer who received CMF (cyclophosphamide, methotrexate, and fluorouracil) or a similar protocol experienced amenorrhea 1 year after treatment when age 35 to 40 years, in contrast with 28% of women age < 35 years.18 The risk of amenorrhea resulting from a single-agent or combination regimen has been estimated to be up to 80% for patients diagnosed at age ≥ 40 years, compared with 60% if the woman is age 30 to 39 years and 20% if the patient is age < 30 years at treatment.19,20 Rzepka-Górska et al21 found a tendency for premature menopause in BRCA1 mutation carriers; the mean age at menopause for BRCA1 mutation carriers was 45.5 years, compared with 48.2 years for noncarriers. In this study, 45 of the 81 BRCA1 carriers had breast cancer, and it is difficult to distinguish if the age of onset of menopause among BRCA1 carriers was influenced by treatment or by BRCA1 status per se. We also found that among women who had transient amenorrhea after chemotherapy and then resumed menses, the eventual age of amenorrhea was advanced, by 3.2 years in BRCA1 carriers and 4.7 years in BRCA2 carriers. A similar effect was reported by Partridge et al22 in patients with breast cancer from the general public who did and did not receive chemotherapy.
Oktay et al3 performed ovarian stimulation in 126 young women with breast cancer using letrozole and gonadotropins to cryopreserve embryos or oocytes. Ovarian reserve was measured by total oocyte yield and incidence of poor response (< four oocytes retrieved after ovarian stimulation). They found a higher fraction of poor responders among BRCA1 carriers than noncarriers (four [33.3%] of 12 v one [3.3%] of 33; P = .014). The sample size of mutation carriers was small, and it is premature to make clinical recommendations based on this article. We found a statistically significantly higher proportion of women experiencing amenorrhea if they had a BRCA2 versus BRCA1 mutation, but for neither subgroup was the probability of amenorrhea higher than that of the noncarrier population. There is no clear biologic rationale for why a BRCA1 germline mutation should be associated with early menopause; Oktay et al propose that because DNA repair is deficient in patients with BRCA mutations, oocytes might be more prone to DNA damage.
We also evaluated the impact of tamoxifen on amenorrhea. In the absence of chemotherapy, there was no association between use of tamoxifen and onset of long-term amenorrhea, but tamoxifen augmented the toxicity of chemotherapy. Several other results support our findings.13,22 In the NSABP (National Surgical Adjuvant Breast and Bowel Project) B-30 trial23 708 premenopausal patients were treated with anthracyclines and docetaxel. The rate of chemotherapy-induced amenorrhea was strongly influenced by the addition of tamoxifen (29% v 52%; P = .003). Perez-Fidalgo et al13 reported that in younger patients, tamoxifen contributes to the delayed recovery of menses. Jung et al24 reviewed 241 premenopausal patients with breast cancer who underwent adjuvant CMF or flourouracil, doxorubicin, and cyclophosphamide therapy and found that the addition of tamoxifen to chemotherapy increased the incidence of chemotherapy-induced amenorrhea from 48% to 64% (P = .02). Fornier et al25 in a cohort of women with breast cancer age ≤ 40 years found the incidence of chemotherapy-induced amenorrhea to be 13% for patients treated only with chemotherapy and 17% for patients treated with chemotherapy and tamoxifen. The mechanism is unclear; however, tamoxifen increases circulating estrogen, which may induce a negative feedback of the hypothalamic-ovarian axis.25 The opposite effect has been noted in several patients treated with an aromatase inhibitor. Aromatase inhibitors lead to an increase in the secretion of pituitary gonadotrophins, and when used as adjuvant hormonal therapy, they can sometimes induce a resumption of menses. Smith et al26 found that of 45 women with breast cancer age 39 to 52 years who had ceased menses after chemotherapy, 12 women (27%) had a resumption of ovarian function after starting an aromatase inhibitor.
Several strategies have been used to preserve fertility in cancer survivors who receive chemotherapy, including embryo and oocyte cryopreservation.12 Gonadotropin-releasing hormone (GnRH) has been used to suppress the ovaries during chemotherapy to protect the pool of follicles.11 The SWOG (Southwest Oncology Group) 0230 randomized clinical trial will evaluate the rates of ovarian failure with and without GnRH in premenopausal women with hormone receptor–negative breast cancer. In the United Kingdom, OPTION (Ovarian Protection Trial in Premenopausal Breast Cancer Patients) is also assessing the impact of chemotherapy and the protective effect of GnRH on ovarian function at 12 months after breast cancer treatment.12,29,30
Young age at menopause has been linked to several adverse health outcomes, including osteoporosis,31cardiovascular disease,32 and overall mortality.33 Finch et al34 reported that in the short term after surgically induced menopause, quality of life seemed to be similar before and after oophorectomy in BRCA1 and BRCA2 carriers; however, vasomotor symptoms and declines in sexual functioning were common. Hormone replacement therapy is not recommended after a diagnosis of breast cancer, leaving limited options for the management of menopause in this group of patients. We have recently shown that oophorectomy is associated with improved survival in BRCA1 carriers treated for early-onset breast cancer.35 It is important to establish whether the preservation of ovarian function in young women with breast cancer and a BRCA mutation has an adverse impact on disease recurrence.
In conclusion, BRCA mutation carriers age > 35 years who receive chemotherapy for breast cancer are at high risk of developing long-term amenorrhea, but the probability of induced amenorrhea does not seem to be greater than that in the noncarrier population, and BRCA1 or BRCA2 status per se should not be an indication for referral to a fertility clinic. If tamoxifen is added to chemotherapy, the risk of amenorrhea is much higher. Women with breast cancer who wish to preserve fertility should be aware of the synergistic impact of chemotherapy and tamoxifen on inducing long-term amenorrhea and possibly menopause. Future studies should evaluate the impact of induced amenorrhea and ovarian preservation on survival in women with breast cancer and a BRCA1 or BRCA2 mutation.
Acknowledgment
We thank Roxana Bucur for administrative support.
Appendix
Other members of the Hereditary Breast Cancer Clinical Study Group:
Jacek Gronwald, Cezary Cybulski, Tomasz Huzarski, Andre Robidoux, Kenneth Offit, William D. Foulkes, Dominique Stoppa-Lyonnet, Ruth Gershoni-Baruch, Claudine Isaacs, Siranoush Manoukian, Leigha Senter, Barry Rosen, Susan Randall, Rochelle Demsky, Andrea Eisen, Lovise Bordeleau, Jeffrey Weitzel, Olufunmilayo I. Olopade, Beth Karlan, Judy Garber, Dawna Gilchrist, Howard Saal, Charis Eng, Fergus Couch, Gareth Evans, Ava Kwong, Pal Moller, Lovise Maehle, Eitan Friedman, Wendy McKinnon, Marie Wood, Mary Daly, Joanne L. Blum, Albert Chudley, Seema Panchal, Jane McLennan, Barabara Pasini, Gad Rennert, John Lunn, Taya Fallen, Daniel Rayson, Dana Zakalik, Ophira Ginsburg, Edmond Lemire, Wendy Meschino, Tuya Pal, Susan Vadaparampil, David Euhus, Josephine Wagner Costalas, Talia Donenberg, Raluca N. Kurz, Susan Friedman (on behalf of FORCE), Kevin Sweet, Carey A. Cullinane, Robert E. Reilly, Annie Venne, Joanne Kotsopoulos, Aletta Poll, Sonia Nanda, Kelly Metcalfe, Alejandra Ragone, and Marcia Llacuachaqui.
Footnotes
See accompanying article on page 3920
Written on behalf of the Hereditary Breast Cancer Clinical Study Group.
Supported by the Canadian Breast Cancer Research Alliance, the Canadian Cancer Research Society Research Initiative, Fonds de Recherche en Santé du Québec, and National Institutes of Health Grant No. R01 CA74415.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Adriana Valentini, Amy Finch, Parviz Ghadirian, Ellen Greenblatt, Steven A. Narod
Administrative support: Christian Singer
Provision of study materials or patients: Jan Lubiński, Charmaine Kim-Sing, Henry T. Lynch, Susan L. Neuhausen, Christian Singer, Steven A. Narod
Collection and assembly of data: Adriana Valentini, Jan Lubiński, Tomasz Byrski, Parviz Ghadirian, Charmaine Kim-Sing, Henry T. Lynch, Susan L. Neuhausen, Christian Singer, Steven A. Narod
Data analysis and interpretation: Adriana Valentini, Amy Finch, Tomasz Byrski, Peter J. Ainsworth, Ping Sun, Steven A. Narod
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22:4174–4222. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 2.Daniel JM. Estrogens, estrogen receptors, and female cognitive aging: The impact of timing. Horm Behav. 2013;63:231–237. doi: 10.1016/j.yhbeh.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K, Kim JY, Barad D, et al. Association of BRCA1 mutations with occult primary ovarian insufficiency: A possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–244. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility. ed 7. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 5.Strauss JF, III, Robert L. Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. ed 6. Philadelphia, PA: Elsevier Saunders; 2009. [Google Scholar]
- 6.Fleischer RT, Vollenhoven BJ, Weston GC. The effects of chemotherapy and radiotherapy on fertility in premenopausal women. Obstet Gynecol Surv. 2011;66:248–254. doi: 10.1097/OGX.0b013e318224e97b. [DOI] [PubMed] [Google Scholar]
- 7.Clemons M, Simmons C. Identifying menopause in breast cancer patients: Considerations and implications. Breast Cancer Res Treat. 2007;104:115–120. doi: 10.1007/s10549-006-9401-y. [DOI] [PubMed] [Google Scholar]
- 8.Bines J, Oleske DM, Cobleigh MA, et al. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 9.Meirow D, Biederman H, Anderson RA, et al. Toxicity of chemotherapy and radiation on female reproduction. Clin Obstet Gynecol. 2010;53:727–739. doi: 10.1097/GRF.0b013e3181f96b54. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf) 1981;14:245–255. doi: 10.1111/j.1365-2265.1981.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 11.Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012;26:379–390. doi: 10.1016/j.bpobgyn.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Kim CH, Jeon GH. Fertility preservation in female cancer patients. ISRN Obstet Gynecol. doi: 10.5402/2012/807302. [epub ahead of print on January 26, 2012] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Fidalgo JA, Roselló S, García-Garré E, et al. Incidence of chemotherapy-induced amenorrhea in hormone-sensitive breast cancer patients: The impact of addition of taxanes to anthracycline-based regimens. Breast Cancer Res Treat. 2010;120:245–251. doi: 10.1007/s10549-009-0426-x. [DOI] [PubMed] [Google Scholar]
- 14.Morgan S, Anderson RA, Gourley C, et al. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–535. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RA, Cameron DA. Pretreatment serum anti-Müllerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab. 2011;96:1336–1343. doi: 10.1210/jc.2010-2582. [DOI] [PubMed] [Google Scholar]
- 16.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 17. Reference deleted.
- 18.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Kil WJ, Chun M. Chemotherapy-related amenorrhea in premenopausal women with breast cancer. Menopause. 2009;16:98–103. doi: 10.1097/gme.0b013e3181844877. [DOI] [PubMed] [Google Scholar]
- 20.Torino F, Barnabei A, De Vecchis L. Recognizing menopause in women with amenorrhea induced by cytotoxic chemotherapy for endocrine-responsive early breast cancer. Endocr Relat Cancer. 2012;19:R21–R33. doi: 10.1530/ERC-11-0199. [DOI] [PubMed] [Google Scholar]
- 21.Rzepka-Górska I, Tarnowski B, Chudecka-Głaz A, et al. Premature menopause in patients with BRCA1 gene mutation. Breast Cancer Res Treat. 2006;100:59–63. doi: 10.1007/s10549-006-9220-1. [DOI] [PubMed] [Google Scholar]
- 22.Partridge A, Gelber S, Gelber RD, et al. Age of menopause among women who remain premenopausal following treatment for early breast cancer: Long-term results from International Breast Cancer Study Group Trials V and VI. Eur J Cancer. 2007;43:1646–1653. doi: 10.1016/j.ejca.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Swain SM, Land SR, Ritter MW, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113:315–320. doi: 10.1007/s10549-008-9937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung M, Shin HJ, Rha SY, et al. The clinical outcome of chemotherapy-induced-amenorrhea in premenopausal young patients with breast cancer with long-term follow-up. Ann Surg Oncol. 2010;17:3259–3268. doi: 10.1245/s10434-010-1172-3. [DOI] [PubMed] [Google Scholar]
- 25.Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. doi: 10.1002/cncr.21385. [DOI] [PubMed] [Google Scholar]
- 26.Smith IE, Dowsett M, Yap YS, et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: Caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 27. Reference deleted.
- 28. Reference deleted.
- 29.Huser M, Crha I, Ventruba P, et al. Prevention of ovarian function damage by a GnRH analogue during chemotherapy in Hodgkin lymphoma patients. Hum Reprod. 2008;23:863–868. doi: 10.1093/humrep/den005. [DOI] [PubMed] [Google Scholar]
- 30.Pagani O, Partridge A, Korde L, et al. Pregnancy after breast cancer: If you wish, ma'am. Breast Cancer Res Treat. 2011;129:309–317. doi: 10.1007/s10549-011-1643-7. [DOI] [PubMed] [Google Scholar]
- 31.Wellons M, Ouyang P, Schreiner PJ, et al. Early menopause predicts future coronary heart disease and stroke: The multi-ethnic study of atherosclerosis. Menopause. 2012;19:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shuster LT, Rhodes DJ, Gostout BS, et al. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65:161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8:229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 34.Finch A, Narod SA. Quality of life and health status after prophylactic salpingo-oophorectomy in women who carry a BRCA mutation: A review. Maturitas. 2011;70:261–265. doi: 10.1016/j.maturitas.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Huzarski T, Byrski T, Gronwald J, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013;31:3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]