To the Editor:
Since 1982, 112 patients newly diagnosed with primary CNS lymphoma (PCNSL) have been treated at Oregon Health and Science University with methotrexate (intra-arterial)–based chemotherapy in conjunction with osmotic blood-brain barrier disruption (BBBD) without whole-brain irradiation. We previously published survival outcomes associated with this first-line therapy.1,2 Pretreatment (baseline) neuropsychological and neuroimaging evaluation has routinely been conducted in patients with PCNSL treated with BBBD. This correspondence summarizes recent findings regarding neuropsychological and neuroimaging outcomes in 26 long-term survivors of PCNSL treated with this therapy.
From February 2009 to February 2011, we prospectively evaluated long-term survivors of PCNSL to assess changes in neuropsychological scores and the association with pretreatment and long-term neuroimaging outcomes. Survivors who were a minimum of 2 years post diagnosis and in complete remission after BBBD treatment were evaluated with neuropsychological tests and brain magnetic resonance (MR) or computed tomography (CT) imaging.3 Complete disease remission was required to assess neurotoxicity without the confounding presence of infiltrative, often multifocal CNS disease. Neuropsychological scores were available on 24 of 26 long-term survivors; pretreatment scores were not available on one of the 24 survivors.
Neuropsychological tests included in a test battery that was developed by international PCNSL investigators were used to evaluate neurotoxicity.4 The tests measure attention/executive function (Wechsler Adult Intelligence Scale—III Digit Span subtest [Digits Forward, Digits Backward], Trail Making Test [TMT] A and B), and verbal memory (Hopkins Verbal Learning Test—Revised). For neuroimaging, the axial section with the largest enhancing tumor was selected on pretreatment contrast-enhanced T1-weighted MR or CT images, and abnormal MR T2 hyperintensity or CT hypodensity around enhancing tumor was assessed in the same plane. The increase, decrease, or stable appearance and size of lesions at completion of BBBD and long term were compared with pretreatment measurements. A multivariable linear mixed model was used to assess changes in neuropsychological least squares mean scores, and the association between pretreatment and long-term outcomes was assessed using Pearson's correlation coefficient.
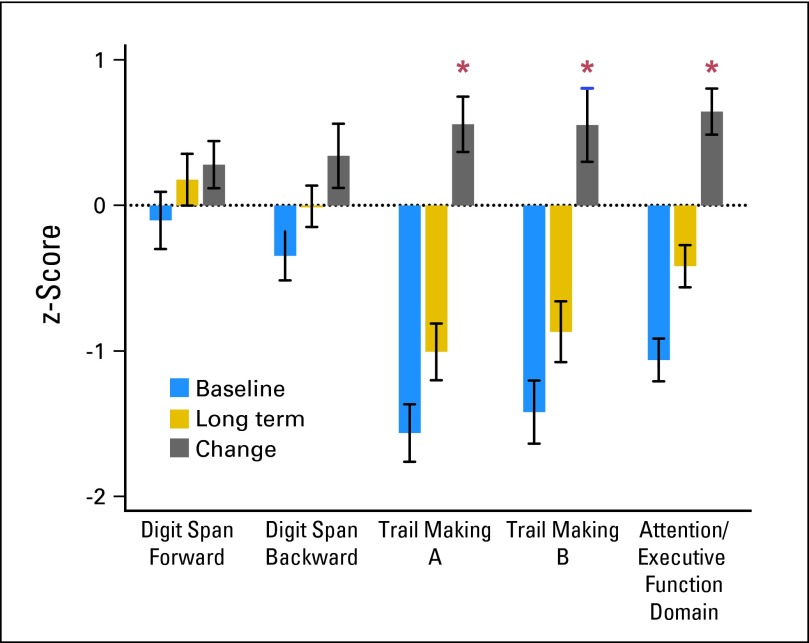
The median age at diagnosis was 50 years. Eight patients (33%) were age 60 years or older; seven (29%) had a Karnofsky performance score of less than 70. The median interval from diagnosis to long-term evaluation was 12 years (minimum, 2 years; maximum, 26 years); eight survivors (33%) were evaluated 15 years or more after diagnosis. There was significant improvement in TMT A (P = .0085), TMT B (P = .0411), and attention/executive function domain (P < .001) from pretreatment to long term (Fig 1 and Appendix Figure A1A, online only). There were no significant changes in Digits Forward, Digits Backward, and verbal memory. The majority showed stable or improved cognitive status at long term (Appendix Fig A1B, online only). Before diagnosis, 17 patients were working, two were college students, four were retired, and one was unemployed as a result of psychomotor slowing and depression. Of the 17 who were working and the two students, at long term, 11 had retired, six were employed (including the students), and two were disabled as a result of orthopedic problems. Of eight survivors evaluated 15 years or more after diagnosis, five were working in high-level occupations as a surgeon, attorney, registered nurse, law enforcement officer, and optician. Three were retired.
Fig 1.
Baseline and long-term cognitive outcomes. Raw test scores were converted to z-scores that were based on normative values demographically adjusted to age. A domain score was obtained by averaging all test z-scores in each domain, for each participant. The z-score (mean ± SD) across survivors at baseline (pretreatment), long-term follow-up, and the change score are shown. The asterisks indicate statistical significance. There was improvement in Trail Making A, P = .0085; Trail Making B, P = .0411; and Attention/Executive Function domain, P < .001.
On neuroimaging, the total T2 MR hyperintensities or CT hypodensities decreased or resolved by the end of treatment in 75% of survivors. Total T2 MR hyperintensities or CT hypodensities did not change from the end of treatment to long term. There was no association between neuropsychological scores and neuroimaging pretreatment and long term.
With a median follow-up of 12 years, comparison of pretreatment and long-term scores indicates preserved cognitive function in the majority of survivors. We are not aware of studies that have evaluated cognition and neuroimaging with such lengthy follow-up, showing preserved or improved cognitive functioning in this rare cancer. Our data showed significant improvement in TMT A and B, which measure speed of information processing and complex set shifting. The improvement in TMT B, which measures executive functioning, is likely an important component of why survivors were able to return to high-level occupations. Nonetheless, verbal learning and memory did not significantly improve, and some survivors had long-term cognitive limitations. This reinforces the importance of identifying rehabilitation strategies for survivors with residual cognitive deficits.
The importance of prospective pretreatment and long-term evaluation to understand cognitive effects of cancer treatment has been established.5–7 However, few studies have included pre- and post-treatment psychometric evaluation, and data regarding long-term impact of most treatments are sparse. As PCNSL treatment regimens intensify, whether by high-dose chemotherapy with or without autologous stem-cell transplantation, or by BBBD, durable remission rates and survival are expected to increase. Increased survival in combination with intact cognitive abilities is widely acknowledged as a critical benchmark for determining the optimum therapy for this disease.1–3,8
Supplementary Material
ACKNOWLEDGMENT
Presented in part at the 11th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 15-18, 2011 (abstr 036), and at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 3-7, 2011 (abstr 2085) and June 1-5, 2012 (abstr 2040). Funding was provided by National Institutes of Health Grant No. 5RO1 CA137488 (E.A.N.), The Walter S. and Lucienne Driskill Foundation (E.A.N.), and János Bolyai Research Scholarship of the Hungarian Academy of Sciences (E.D.).
Appendix
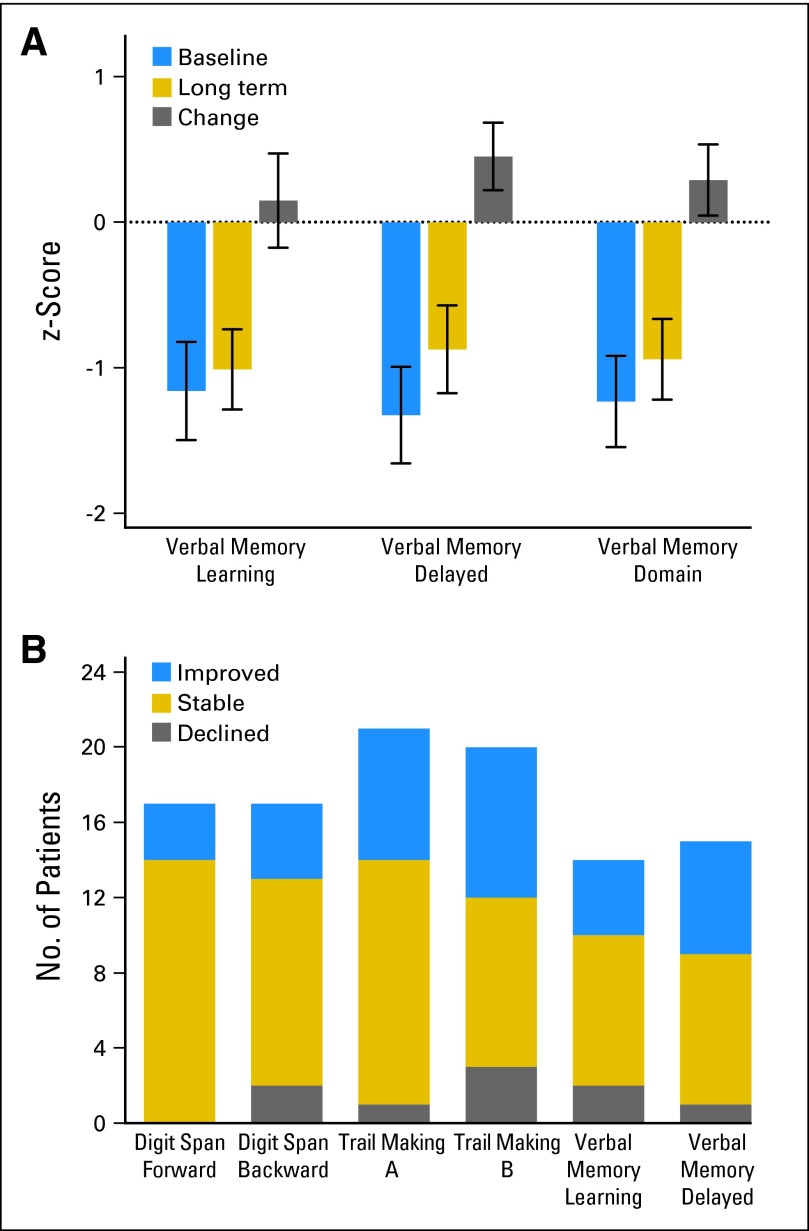
Fig A1.
(A) The z-score (mean ± SD) across survivors at baseline (pretreatment), long-term follow-up, and the change score for Verbal Memory Learning, Verbal Memory Delayed, and for Verbal Memory Domain are shown. There was no significant change from baseline to long-term follow-up. (B) Number of patients who declined (z-score declined 1 SD or more), were stable (z-score remained within 1 SD of baseline score), and improved (z-score improved 1 SD or more) from baseline (pretreatment) to long-term follow-up for the following tests: Digit Span Forward, Digit Span Backward, Trail Making A, Trail Making B, Verbal Memory Learning, and Verbal Memory Delayed.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.Neuwelt EA, Goldman DL, Dahlborg SA, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: Prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9:1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 2.Angelov L, Doolittle ND, Kraemer DF, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J Clin Oncol. 2009;27:3503–3509. doi: 10.1200/JCO.2008.19.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doolittle ND, Korfel A, Lubow MA, et al. Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81:84–92. doi: 10.1212/WNL.0b013e318297eeba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correa DD, Maron L, Harder H, et al. Cognitive functions in primary central nervous system lymphoma: Literature review and assessment guidelines. Ann Oncol. 2007;18:1145–1151. doi: 10.1093/annonc/mdl464. [DOI] [PubMed] [Google Scholar]
- 5.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 6.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: A review of published studies and recommendations for future research. J Clin Oncol. 2007;25:2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 7.Abrey LE. The impact of chemotherapy on cognitive outcomes in adults with primary brain tumors. J Neurooncol. 2012;108:285–290. doi: 10.1007/s11060-012-0807-6. [DOI] [PubMed] [Google Scholar]
- 8.Batchelor TT. Flying solo: Chemotherapy without radiation for primary CNS lymphoma. J Clin Oncol. 2013;31:3051–3053. doi: 10.1200/JCO.2013.48.9138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.