Abstract
Upregulation of Toll-like receptor 2 (TLR2) plays a critical role in inflammation associated with ischemia/reperfusion–induced tissue damage. OPN-305 is the first humanized IgG4 monoclonal antibody against TLR2 in development and is intended for the prevention of reperfusion injury following renal transplantation and other indications. A phase I, single-center, prospective randomized, double-blind, placebo-controlled study was performed to evaluate single ascending doses of OPN-305 in 41 healthy male subjects (age range: 19–58 years) randomized to OPN-305 or placebo across six cohorts. OPN-305 was well tolerated across all doses, with no elevations in endogenous cytokines. A dose-proportional increase in maximum serum concentration (Cmax) was observed, with area under the curve increasing in a greater-than-dose-proportional manner with increasing elimination half-life. OPN-305 produced full TLR2 receptor blockade on CD14+CD45+ cells (monocytes), from 14 (0.5 mg/kg) to >90 (10 mg/kg) days, with a linear effect on the duration of inhibition of interleukin-6 release after TLR2 stimulation.
OPN-305 is a humanized IgG4 monoclonal antibody against Toll-like receptor (TLR)2 being developed by Opsona Therapeutics. It is believed to function by targeting the ligand-binding site on the TLR2 receptor, thereby preventing heterodimerization of the receptor with TLR1 or TLR6. OPN-305 antagonizes TLR2-dependent cytokine production.1 OPN-305 contains a mutation in the hinge region that prevents Fab arm switching in vivo; the molecule is stable and retains its monospecificity for TLR2. Rather than using traditional complimentarity determining region grafting, OPN-305 was generated using a proprietary method of humanization known as “Composite Antibody Technology,” developed by Antitope (Cambridge, UK). Using this method, composite variable region sequences guided by the reference antibody, OPN-301, contained in human antibody sequence data banks were sourced to form V-region sequences composing fully human sequences. These segments were then modeled in silico to identify constraining sequences within the murine parent sequences. Finally, candidate sequences were screened in silico for potential human leukocyte antigen–binding sites, and any resulting T-cell epitopes identified were removed.
The initial target indication for OPN-305 is delayed graft function (DGF). This is the most common complication in the immediate post-transplantation period, affecting 25–35% of all patients who receive a cadaveric donor graft.2,3
The upregulation of TLR2 and its ligation by either exogenous or endogenous danger signals has been demonstrated to play a critical role in the inflammatory cascade that exacerbates tissue damage after reperfusion.4 TLR2 mRNA is constitutively expressed by tubular epithelial cells in the murine kidney and increases following ischemia, driving a TLR2-mediated increase in cytokines.5,6,7 Putative endogenous ligands for TLR2, such as heat shock proteins and necrotic cells, also increase following ischemic injury.1,8,9,10 The extravasation of immune cells and secretion of proinflammatory cytokines after reperfusion of transplanted organs are well characterized and are believed to exacerbate DGF and organ rejection.
OPN-305 specifically targets and blocks TLR2 with the aim of preventing DGF by minimizing the sequelae of ischemia/reperfusion injury by tempering the innate immune response following reperfusion.
Given the high degree of conservation of TLR2 sequence homology across species (e.g., human and cynomolgus monkey TLR2 share absolute identity of 96.18%), OPN-305 and OPN-301, the murine monoclonal parent antibody from which it is derived, have been effective in a number of animal models including ischemia/reperfusion injury in mice1 and pigs.11 In addition, in vitro data indicate that OPN-305 antagonizes TLR2 signaling in mice,1 pigs,11 cynomolgus monkeys, and human cells (see Supplementary Data and Supplementary Figure S1 online). These data, together with data from the toxicology studies performed in mice, monkeys, and the first-in-human phase I study, support the starting dose of OPN-305 for clinical development.
The aim of this first-in-human phase I study was to provide an initial assessment of the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics (PD) of OPN-305 after infusing single ascending i.v. doses in healthy adult subjects. The study characterized the various doses and infusion times in healthy subjects as a prelude to initiating trials in patients.
Results
Demographics
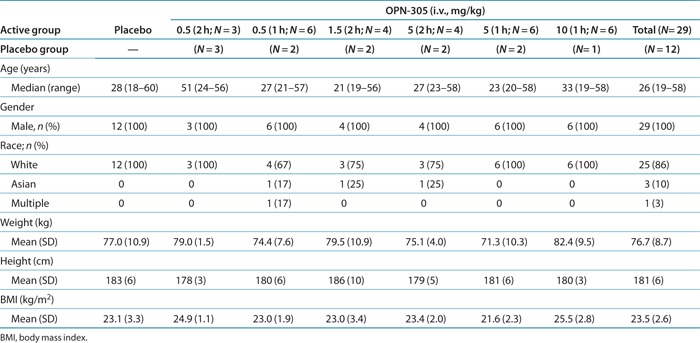
Summary demographic information on the 41 subjects enrolled in the study is provided in Table 1. All subjects were men. All subjects in the placebo group and all but one in the OPN-305 groups were white. The median age was similar in both the placebo group (28 years; range: 18–60 years) and OPN-305 groups (26 years; range: 19–58 years). The median age of subjects in the 2-h i.v. dose (0.5 mg/kg) group was higher than those in the other treatment groups (51 years; range: 24–56 years; mean: 44 years).
Table 1. Summary statistics of demographic and baseline characteristics (intention-to-treat population).
Pharmacodynamic evaluation
Receptor occupancy. Receptor occupancy (RO) was determined using an assay based on fluorescence-activated cell sorting. This assay determined the total amount of OPN-305 bound in the patient's sample and used an excess of exogenously added OPN-305 to determine the unbound expression level of TLR2 on the cells. These values were used to determine the RO at each time point.
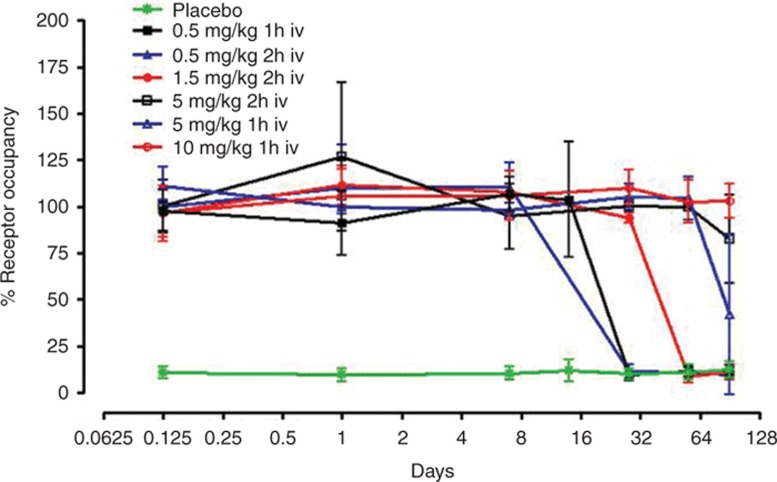
The mean percentage change in RO from baseline over time is shown by treatment in Table 2 and Figure 1. Treatment with OPN-305 was associated with almost complete RO in all subjects, at all doses, by 1 h after the end of infusion. Full RO continued for at least 14 days at all doses, with the RO at the highest dose exceeding 90 days. The duration of the infusion did not have any substantial effect on magnitude or duration of RO.
Table 2. Mean percentage receptor occupancy (RO) on blood monocytes and corresponding whole-blood assay IL-6 inhibition.
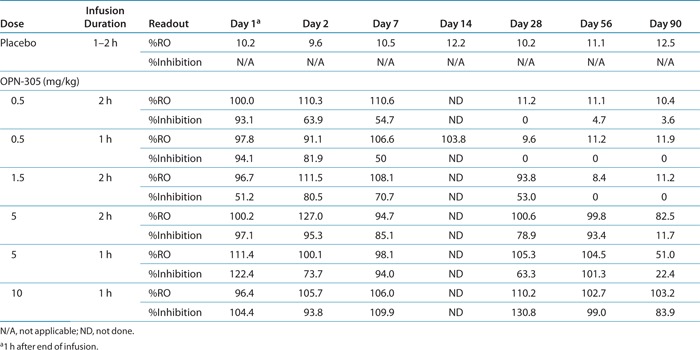
Figure 1.
The duration of TLR2 receptor occupancy is dependent on the dose of OPN-305 administered. The assay background is shown in green, as determined in placebo-treated patients. TLR2, Toll-like receptor 2.
Eleven subjects had a RO level ≥70% at the 90-day follow-up visit. These subjects were all in the 5 or 10 mg/kg dose groups. At approximately 1 year after dosing, the TLR2 receptor levels in all subjects were confirmed as having returned to baseline. No additional relevant medical history or adverse events were reported during that period.
Interleukin-6 inhibition. Typical PD assays use a direct in vivo measurement of a molecule on the signaling pathway of interest. In the case of TLR signaling, adapter molecules and subsequent transcription factor activation are initiated only in the presence of an inflammatory signal. Thus, binding with an antagonistic antibody will not activate these pathways. Inhibition of this pathway in the absence of an inflammatory signal elicits no effect on downstream molecules. To combat this, we developed an ex vivo blood assay, whereby a stimulus (heat-killed Listeria monocytogenes) was added in order to induce interleukin (IL)-6 expression. Inhibition of IL-6 after administration of OPN-305 was used as the PD measurement. Inhibition at each time point was measured relative to induction of IL-6 before the administration of OPN-305 in each patient.
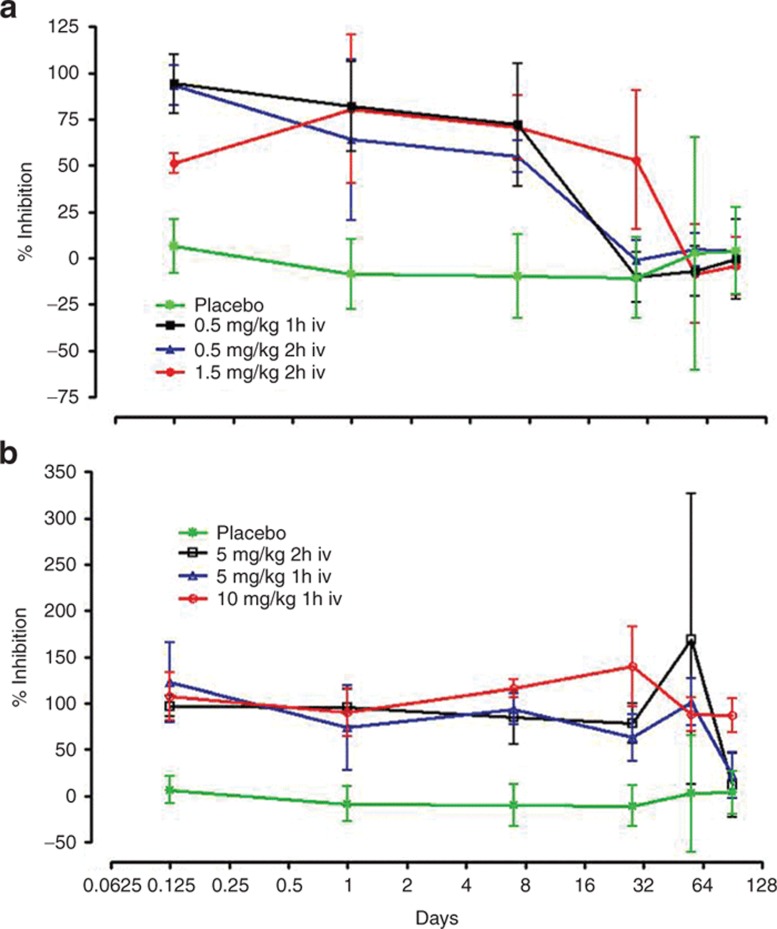
The mean percentage change from baseline in the IL-6 stimulation index over time is shown by treatment in Table 2 and Figure 2. Single-dose treatment at all dose levels resulted in significant inhibition of IL-6 stimulation. The lowest dose (0.5 mg/kg) provided >90% inhibition for 3 h after the end of infusion and >50% inhibition at day 14, returning to baseline by day 28. The highest dose, 10 mg/kg, resulted in >65% inhibition of IL-6 stimulation for 90 days or longer in all subjects. The length of infusion had no impact on the degree of inhibition of stimulated IL-6 production. This figure excludes data from subject 401 (in the placebo group), who had an unexpected high value at day 90 (219.0%), thought to be due to switching of tubes, but this could not be confirmed. If other factors specifically relating to the subject were to play a significant role, we would most likely observe elevated responses at all time points rather than at one isolated time point.
Figure 2.
The duration of the inhibitory effect of OPN-305 on HKLM-induced IL-6 is dependent on the administered dose. (a) 0.5 and 1.5 mg/kg doses, (b) 5 and 10 mg/kg doses. HKLM, heat-killed Listeria monocytogenes.
White blood cell counts. There was no trend in mean changes from baseline for basophils, eosinophils, leukocytes, lymphocytes, monocytes, or neutrophils over time for any dose group as compared with the placebo group, and no dose effect was noted.
Pharmacokinetic results
A summary of the PK parameters following single i.v. infused doses of OPN-305 are presented in Figure 3 and Table 3.
Figure 3.
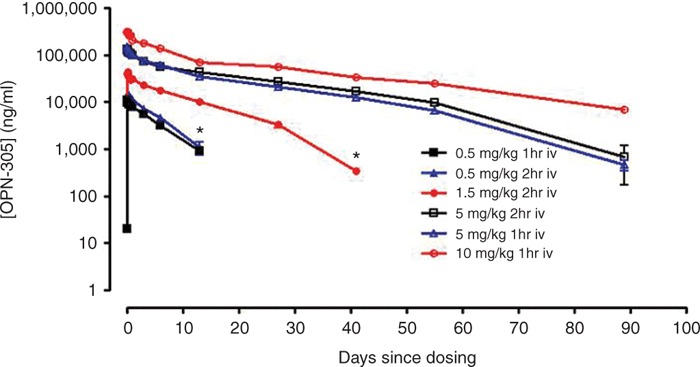
Cmax and clearance time of OPN-305 are dependent on the administered dose. Infusion duration does not affect the pharmacokinetics. *OPN-305 was below the limit of detection of the assay from this time point on. Cmax, maximum serum concentration.
Table 3. Summary statistics for OPN-305 serum pharmacokinetic parameters by treatment.
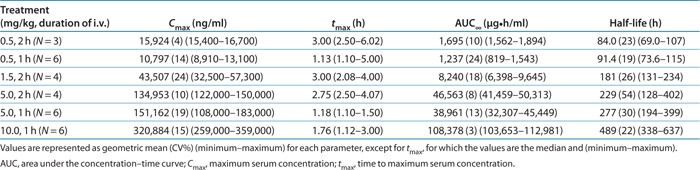
Following 2-h i.v. infusion, OPN-305 achieved rapid peak serum levels, with median tmax (time to reach maximum serum concentration (Cmax)) similar across doses of 0.5, 1.5, and 5 mg/kg, ranging from 2.75 to 3.00 h. The same pattern was seen following 1-h i.v. infusion, with median tmax similar across doses of 0.5, 5, and 10 mg/kg, ranging from 1.13 to 1.76 h. Following attainment of Cmax), serum concentrations of OPN-305 declined in a biphasic manner. The mean serum terminal elimination half-life (t1/2) was dose dependent and ranged from 84 to 489 h. A consistent observation in subjects dosed at 5 mg/kg was a more rapid decline in the t1/2 between 56 and 91 days. A similar decline was observed between 28 and 42 days in subjects dosed with 1.5 mg/kg who had quantifiable serum OPN-305 concentrations at 42 days.
In general, the results suggest that the PK parameters Cmax and the areas under the curve (AUCs), namely, AUClast (AUC for concentration–time curve up to the last time point with a concentration above the lower limit of quantification) and AUC∞ (AUC for concentration–time curve up to the last time point with a concentration above the lower limit of quantification extrapolated to infinity), increased in magnitude with increasing dose, with AUC increasing in a greater-than-dose-proportional manner. PK profiles were similar regardless of infusion time. Cmax and AUC were somewhat higher at 0.5 mg/kg when the subject was dosed for 2 h as compared with dosing for 1 h; however, because the 2-h infusion was performed in only three subjects (as compared with n = 6 for 1 h), any apparent differences must be treated with caution. Serum concentrations of OPN-305 at the two lowest dosages, 0.5 and 1.5 mg/kg, were below the limit of detection of the assay (20 ng/ml) at days 28 and 42, respectively.
Safety results
This phase I study was blinded and in keeping with the strict rules regarding the reporting of adverse events. These events are reported regardless of the treatment received, i.e., they may be from the placebo or the active group, with the investigator's assessment of causality (which was blinded to treatment). A total of 42 treatment-emergent adverse events (TEAEs) were reported in 24 of 41 subjects in all the dose groups and the placebo group during the 90 days following treatment.
Most of the TEAEs were mild, self-limiting, and not considered to be treatment related by the investigator. The frequency of TEAEs was similar between OPN-305-treated (59% of subjects) and placebo-treated (58%) subjects. There was no relationship with dose, with the lowest rate of TEAEs (17%) being reported in the highest dose group (10 mg/kg).
Only 3 of the 42 TEAEs were considered to be drug related by the investigator (one event each of headache in the placebo and the 5 mg/kg groups and one case of frequent bowel movements in the 5 mg/kg group).
The most frequent TEAEs were headache, nasopharyngitis, abdominal pain, and dizziness/postural dizziness, occurring throughout the 90-day follow-up period after treatment. All other TEAEs occurred as single events in individual subjects (Table 4).
Table 4. Treatment-emergent adverse events by preferred term by treatment group.
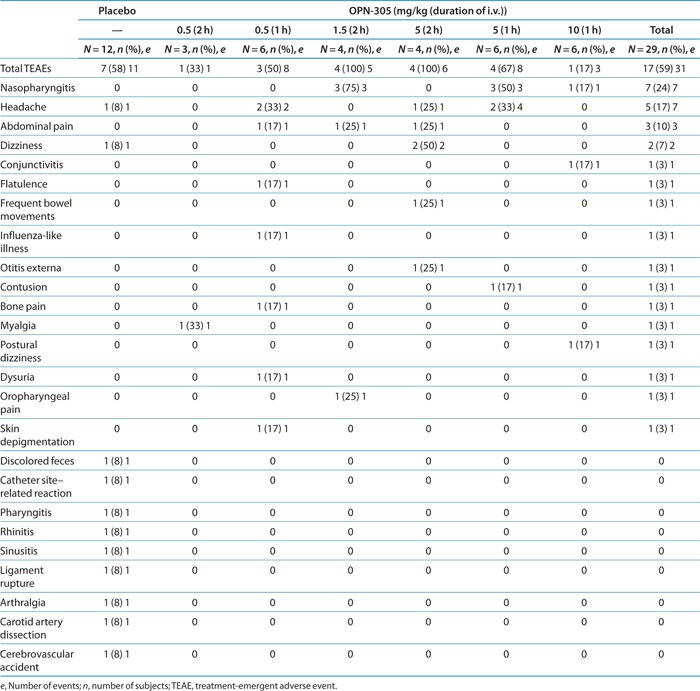
Four moderate TEAEs were reported, three in the placebo group and one in a subject treated with OPN-305 at a dose of 0.5 mg/kg for 2 h.
Serious TEAEs were reported in the placebo group only (two subjects, three events). No severe or fatal TEAEs were reported.
No clinically significant values were observed with respect to clinical chemistry, hematology, coagulation, special proteins, vital signs, physical examinations, and immunogenicity. No notable trends were noticed in electrocardiogram results, and in particular, there was no evidence of an effect on the QTc interval. No infusion reactions were observed in the OPN-305-treated subjects. No effects on endogenous cytokines were observed.
Discussion
The primary objective of the study was to assess the safety and tolerability of single ascending i.v. infused doses of OPN-305 in healthy subjects. As expected, based on what is known from models of TLR2 blockade, OPN-305 was well tolerated, with no apparent relationship with dose for events, and no infusion-related adverse events were reported. No elevations in endogenous cytokines were noted at any time during the study. OPN-305 exhibited a very weak immunogenic response, with only 1 of 29 treated subjects developing a low-titer positive antibody response, which was nonneutralizing. One of 12 placebo-treated subjects also returned a low-titer positive anti–OPN-305 antibody response that was nonneutralizing, in line with the false-positive rate of 5% set for the assay. Therefore, antibody formation to OPN-305 did not affect PK or PD data interpretation. It is noteworthy that a novel method of humanization was applied in the development of OPN-305, which included in silico screening for potential human leukocyte antigen–binding sites, resulting in the removal of potentially immunogenic T-cell epitopes.
In general, Cmax increased in a dose-proportional manner; AUC increased in a greater-than-dose-proportional manner, with a longer elimination half-life as the dose increased, possibly due to saturation of TLR2 sites in cells/tissues at high serum concentrations. At lower serum concentrations, the main clearance mechanism for OPN-305 would be the fast turnover of these cells. However, when TLR2 sites are saturated and high levels of free antibody predominate, normal catabolic clearance of antibodies would define the predominant, longer, half-life. This may also explain why, when serum OPN-305 concentrations decline at later time points, an apparently shorter elimination half-life is observed, for example, between 56 and 91 days, in subjects dosed at 5 mg/kg.
Increasing the dose of OPN-305 had a linear effect on the duration of ≥100% TLR2 blockade on leukocytes, and there was full RO at the higher doses for longer than the normal life of a leukocyte, indicating that new cells are probably also blocked during production exposure to the still present OPN-305. In healthy volunteers, the lowest dose tested (0.5 mg/kg) provided >100% RO for between 14 and 28 days, which is the critical time for the prevention of DGF in renal transplant patients, the reperfusion injury occurring when and soon after the allograft is perfused, with DGF diagnosed within 7 days after transplantation. As in vivo models5 have suggested that there may be upregulation of TLR2 expression in inflammatory conditions associated with ischemia/reperfusion, higher doses may be required in patients in order to reach 100% RO for the same duration as seen in these healthy volunteers.
The duration of inhibition of IL-6 release after ex vivo TLR2 stimulation increased with increasing doses of OPN-305. At the lowest dosage (0.5 mg/kg), greater than 50% inhibition of IL-6 release was observed up to 7–14 days after infusion, whereas at 10 mg/kg, 84% inhibition was observed even on day 90. Data analysis suggested that RO may still be maximal at OPN-305 serum concentrations for which IL-6 inhibition is less than 80%, but, in effect, inhibition is reasonably correlated with RO.
The chronic application of agents such as cyclosporin A, one of the two calcineurin inhibitors routinely prescribed for prevention of rejection in solid organ transplantation, has been associated with renal injury and has also been correlated with upregulation of TLR2 expression on renal tubular cells.12 OPN-301 (the murine version of OPN-305) treatment of mice following renal transplantation significantly improved their kidney function, which correlated with histopathological changes.1 Therefore, it is anticipated that blocking TLR2 before reperfusion following renal transplantation would reduce the inflammatory process that contributes to DGF.
This study has demonstrated that OPN-305 is effective in occupying TLR2 at the chosen doses and blocking the potential downstream TLR2-mediated inflammatory response in an ex vivo whole-blood assay. In addition, this can be achieved with a single infusion to cover the period of greatest risk for reperfusion injury and with no significant toxicity, which would be important when used in conjunction with the immunosuppressive regimens required after organ transplantation.
The dose of OPN-305 can be adjusted, not only to provide the short-term blockade required to ameliorate reperfusion injury but also because it has the potential to provide a serum concentration that would be sufficient to block TLR2 responses for several months, which would be of value in TLR2-mediated chronic disease.
In conclusion, all OPN-305 doses were well tolerated, and a dose of 0.5 mg/kg given as a 1-h infusion was adequate to provide 100% RO of TLR2 and 80% inhibition of IL-6 release after ex vivo stimulation of whole-blood samples, for up to 14 days in healthy subjects. This would be the maximum duration that would be required to prevent reperfusion injury. OPN-305 holds considerable promise as a well-tolerated and efficacious treatment for prevention of reperfusion injury in kidney and other solid organ transplants.
Methods
Study design. The study was a first-in-human phase I, single-center, randomized, double-blind (site staff, patients, and sponsor), placebo-controlled study designed to evaluate sequential single ascending i.v. infused doses of OPN-305 in healthy male subjects. This is typical for phase I, and as we have not performed reproductive toxicology, we considered this to be best practice.
This study was conducted at a single center (PRA International, Clinical Research Unit, University Medical Centre Groningen, The Netherlands) in accordance with the Declaration of Helsinki. The protocol was approved by the institutional review board with jurisdiction over that site; all subjects gave written informed consent before being enrolled in the study.
Eligible subjects were randomized to receive either OPN-305 or matched placebo in a 1:1, 2:1, or 3:1 design in one of six sequential dose cohorts (cohorts 1–6). A randomization code list for assigning randomization numbers to treatment with OPN-305 or placebo was prepared by the Biometric Department. The formulation used for active and placebo groups is a buffer solution at pH 5.5 containing 25 mmol/l sodium citrate, 125 mmol/l sodium chloride, and 6 mmol/l citric acid in 0.01% wt/vol polysorbate 20. This is a typical formulation currently used for other monoclonals, such as Herceptin, Xolair, Raptiva, and Avastin.13 Subjects were assigned to treatment by allocation of the next sequential randomization number in each dose cohort. Investigational medicinal product was dosed by i.v. infusion using a syringe or infusion pump for 2 h in dose cohorts 1–3 (0.5, 1.5, and 5 mg/kg, respectively) and for 1 h in dose cohorts 4–6 (5, 10, and 0.5 mg/kg, respectively).
After the initial 4-day inpatient period, subjects made regular clinic visits for up to 90 days for blood sampling to determine the t1/2 of OPN-305. Safety measurements, including electrocardiogram study, vital signs testing, clinical biochemistry tests, hematology, urinalysis dipstick, and serum cytokine evaluations were conducted throughout the study. Blood samples were analyzed for OPN-305 concentrations and immunogenicity (binding and neutralizing antibodies against OPN-305). Additional identified markers (ex vivo whole-blood assay, target RO, and total white blood cell count) were also investigated.
Dose cohorts followed one another sequentially based on safety review and available PK and PD markers of maximal effect.
A total of 41 subjects were randomized to OPN-305 or placebo across six dose cohorts as outlined in Table 1; each subject participated in one cohort only and was analyzed according to assigned cohort.
Subjects included were men aged 18–60 years, in good health as determined by past medical history, physical examination, vital signs, 12-lead electrocardiogram, and laboratory tests at screening. Dosing occurred between June and October 2011, with follow-up completed in January 2012.
Safety and tolerability were evaluated from adverse events, with particular emphasis on the occurrence of infection, infusion/immunological reactions, physical examinations, vital signs, 12-lead electrocardiograms, clinical laboratory parameters, and inflammatory cytokines (tumor necrosis factor-α, IL-1β, IL-6, and interferon-γ).
PD was measured by total white blood cell count, ex vivo whole-blood assay (% inhibition of IL-6), and target RO (% TLR2 saturation on CD14+CD45+ cells).
RO assay. Fluorescence-activated cell sorting was used to determine TLR2 RO. Human whole blood was collected aseptically in K3-EDTAtetraacetic acid tubes (Greiner Bio-One, Monroe, NC, USA) and processed immediately. A cocktail containing PerCP (peridinin–chlorophyll protein complex)–conjugated mouse anti–human-CD45 and allophycocyanin-conjugated mouse anti–human-CD14 antibodies (BD Biosciences, Breda, The Netherlands) in the presence and absence of OPN-305 was added to the blood. Following incubation and washing, mouse anti–human IgG4-fluorescein isothiocyanate(Southern Biotech) was added. Cells were lysed, washed, and fixed. Mean fluorescence intensity of CD14+CD45+OPN-305+ cells was determined using a fluorescence-activated cell sorting Calibur cytometer (Becton Dickinson), using appropriate compensation. Data acquisition and analysis were performed using BD CellQuest software. Percentage RO was determined by comparison of the binding signal in the sample to the signal in the same sample when excess OPN-305 had been added.
IL-6 inhibition assay. Whole-blood samples were incubated with the TLR2 agonist heat-killed Listeria monocytogenes (Invitrogen) in the presence and absence of a saturating concentration of OPN-305, determined to be 10 μg/ml. Heat-killed L. monocytogenes induces secretion of IL-6 in human blood. Following stimulation, supernatant was collected and stored at nominal <−70 °C; following freeze–thaw, IL-6 was measured using a commercially available ELISA kit (IL-6 Duoset, RnD Systems, UK). IL-6 inhibition was determined by comparing OPN-305-saturated and unsaturated replicates. Data were expressed at each time point as a function of the maximal inhibition observed before OPN-305 administration, i.e., at time zero.
PK assay. The PK assay was a quantitative sandwich ELISA immunoassay. Histidine-tagged TLR2 (RnD Systems, UK) immobilized on HisGrab nickel-coated microtiter plates (Pierce, UK) was used to capture OPN-305 in human serum after blocking with 1% bovine serum albumin in phosphate-buffered saline. Captured OPN-305 was detected by addition of mouse anti–human IgG4 horseradish peroxidase conjugate and visualized with tetramethylbenzidine (Sigma, UK) after stopping the reaction with H2SO4. The ELISA plates were read at a wavelength of 450 nm, with 650 nm as reference, and processed with Watson 7.2 LIMS software (Thermo Scientific, Waltham, MA) using a four-parameter curve fit and 1/Y2 weighting factor. Plates were washed between successive steps.
PK was evaluated by measurement of serum drug concentrations at specified times after administration, which were used to determine the PK parameters, including Cmax, tmax, t½, AUC∞, and AUClast.
Statistical methods. Placebo infusions of 1 and 2 h were pooled, and summary statistics were calculated by treatment and placebo subjects. Unless otherwise indicated, continuous variables were summarized with the following descriptive statistics: n (number of observations), (arithmetic) mean, SD, minimum value, median, and maximum value. Categorical data were summarized with frequencies and percentages. Percentages by categories were based on the number of subjects exposed within a treatment. Descriptive statistics were presented with the same precision as the original data. Missing data were not imputed, except for start and end times of adverse events and serum OPN-305 concentrations less than the lower limit of quantification. PK/PD analysis was performed to attempt to correlate serum concentrations of OPN-305 with blood PD markers (TLR2 RO and IL-6 inhibition) measured at the same time in the same subjects. PK/PD variables were estimated using WinNonlin Enterprise (Version 6.2), Pharsight (St Louis, MO).
Detection of OPN-305 antibodies. Samples were screened for the presence of total and neutralizing antibodies to OPN-305 using bridging and competitive immunoassay formats, respectively.
Author Contributions
M.R., R.M.M., and M.H.T. wrote the manuscript. M.R., R.M.M., M.H.T., and V.P. designed the research. M.R., R.M.M., M.H.T., V.P., P.M., L.M., B.K., P.G., W.M., C.M., P.R., and M.B. analyzed the data. J.v.d.W.d.R., C.M., and M.B. performed the research.

Acknowledgments
This study was sponsored by Opsona Therapeutics, Republic of Ireland. The sponsor supplied the study treatment solution for infusion. Opsona Therapeutics provided payments to the investigating site to perform the study and assay receptor occupancy. Opsona also provided payments to the named vendors and consultants for assay PK and analysis of data. The authors additionally acknowledge the support from Petra Schuilenga-Hut, for the organization and logistics of the study, and Peter McGuirk, for review of the manuscript. The conduct of this study has been part funded by the European Union Seventh Framework programme (FP7/2007-2013) under grant agreement 261468.
M.R., B.K., W.M., and P.G. are full-time employees of Opsona Therapeutics. M.H.T., R.M.M., P.M., V.P., P.R., and L.M. are consultants to Opsona Therapeutics. J.v.d.W.d.R., M.B., and C.M. are paid by Opsona Therapeutics to perform research and analyze data.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/cpt
Supplementary Material
References
- Farrar C.A., et al. Inhibition of TLR2 promotes graft function in a murine model of renal transplant ischemia-reperfusion injury. FASEB J. 2012;26:799–807. doi: 10.1096/fj.11-195396. [DOI] [PubMed] [Google Scholar]
- Jochmans I., et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann. Surg. 2010;252:756–764. doi: 10.1097/SLA.0b013e3181ffc256. [DOI] [PubMed] [Google Scholar]
- Andrés A., et al. NI2A Study Group A randomized trial of basiliximab with three different patterns of cyclosporin A initiation in renal transplant from expanded criteria donors and at high risk of delayed graft function. Clin. Transplant. 2009;23:23–32. doi: 10.1111/j.1399-0012.2008.00891.x. [DOI] [PubMed] [Google Scholar]
- Arslan F., Keogh B., McGuirk P., Parker A.E. TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010;2010:704202. doi: 10.1155/2010/704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T.G., et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-gamma and TNF-alpha mediated up-regulation during inflammation. J. Immunol. 2002;168:1286–1293. doi: 10.4049/jimmunol.168.3.1286. [DOI] [PubMed] [Google Scholar]
- Meldrum K.K., Meldrum D.R., Meng X., Ao L., Harken A.H. TNF-alpha-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H540–H546. doi: 10.1152/ajpheart.00072.2001. [DOI] [PubMed] [Google Scholar]
- Anders H.J., Banas B., Schlöndorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J. Am. Soc. Nephrol. 2004;15:854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- Asea A., et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Li M., et al. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J. Immunol. 2001;166:7128–7135. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- Padanilam B.J. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am. J. Physiol. Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- Arslan F., et al. Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs. Circ. Cardiovasc. Interv. 2012;5:279–287. doi: 10.1161/CIRCINTERVENTIONS.111.967596. [DOI] [PubMed] [Google Scholar]
- Lim S.W., et al. Cyclosporine-induced renal injury induces toll-like receptor and maturation of dendritic cells. Transplantation. 2005;80:691–699. doi: 10.1097/01.tp.0000173594.69089.a0. [DOI] [PubMed] [Google Scholar]
- Daugherty A.L., Mrsny R.J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 2006;58:686–706. doi: 10.1016/j.addr.2006.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.