Abstract
Azatanavir is a protease inhibitor (PI) approved for the treatment of HIV-1 infection. Atazanavir is a substrate and inhibitor of cytochrome P450 isozyme 3A and an inhibitor and inducer of P-glycoprotein. It has similar virologic efficacy as efavirenz and ritonavir-boosted lopinavir in antiretroviral-naive individuals. Its impact on lipids is less than other PIs and it is suitable for those in whom hyperlipidemia is undesirable. Ritonavir boosting of atazanavir enhances the bioavailability of atazanavir but may result in some elevation of lipids and is recommended for treatment-experienced patients and those receiving efavirenz or tenofovir. Ritonavir-boosted atazanavir has similar antiviral activity as ritonavir-boosted lopinavir in both antiretroviral therapy-naive and -experienced patients. Atazanavir causes unconjugated bilirubinemia in over 40% of patients but results in less than 2% discontinuations. Atazanavir is licensed for once-daily use and atazanavir/ritonavir competes with lopinavir/ritonavir as the most commonly prescribed PI.
Keywords: atazanavir, hyperbilirubinemia, lipid profile, protease inhibitor, ritonavir
The development of the first HIV protease inhibitors (PIs) in 1995 revolutionized the management of HIV-1 infection [1,2]. A combination of existing nucleoside reverse transcriptase inhibitors with newly developed PIs had the ability to suppress circulating HIV viremia [2]. Subsequently, the development of non-nucleoside reverse transcriptase inhibitors (NNRTIs) broadened the range of available treatment options. Both PI- and NNRTI-treatment regimens changed the previously dismal prognosis of HIV-infected individuals to such an extent that the term ‘highly-active antiretroviral therapy’ (HAART) was coined and, subsequently, the history of the HIV epidemic has been characterized as consisting of the pre- and post-HAART eras. In industrialized settings, the use of PIs has contributed to substantial reductions in HIV-related morbidity and mortality [3–5]. In low and middle income countries (LMICs), the recent expansion in antiretroviral therapy (ART) availability using a public-health approach has been primarily based on an initial NNRTI-containing first-line regimen [6,7], because of an approximate sixfold price differential between NNRTIs and PIs. In 2007, approximately 2 million people were on ART, which represent 28% of those in need [6]. The switch rate from first- to second-line ART in LMICs is approximately 3% per annum, which will result in a growing demand for PI-containing regimens [8]. However, the present range of prices for second-line PI-containing ART regimens in LMICs is US$1000–2500 per year and the WHO estimates that by 2010, in the absence of price reductions, 90% of the cost of ART could be required to provide second-line drugs [8].
Overview of the market
Presently there are nine US FDA-approved PIs on the market, each with its own virologic potency, adverse effect profile and pharmacokinetic properties. In industrialized settings, the role of any specific PI in the treatment armamentarium depends largely on its comparative performance against other PIs, whereas in LMICs, global use will continue to be largely cost driven [9]. This review will explore the characteristics of atazanavir that will determine its positioning in the global expansion of ART both in developed and developing countries.
Introduction to the drug
Atazanavir is the sixth FDA-approved PI of the nine currently on the market. Atazanavir is the first once-daily PI licensed for the treatment of HIV-1 infection and should be used only as part of a combination therapy. Atazanavir is a highly potent azapeptide PI, which selectively inhibits the virus-specific processing of viral gag and gag–pol polyproteins in HIV-infected cells, thereby preventing the formation of mature virions [101]. In addition to being a potent PI in vitro, atazanavir has a distinct cross-resistance profile that does not confer resistance to other PIs. However, resistance to other PIs often confers clinically relevant resistance to atazanavir. While ritonavir (RTV)-boosted PIs are standard of care, unboosted proteases continue to have a limited role in exceptional circumstances. Unboosted atazanavir, given at a dosage of 400 mg once daily, has fewer adverse effects on lipid profiles than other available PIs and it has been categorized as an alternative PI regimen for patients with underlying disease risk factors, in whom hyperlipidemia is particularly undesirable [102,103]. Atazanavir has variable bioavailability and food decreases the coefficient of variability of AUC and Cmax of unboosted atazanavir by 50% and RTV-boosted atazanavir by 25% compared with the fasting state. RTV-boosted atazanavir is recommended in treatment-experienced patients or when coadministered with tenofovir or efavirenz. Recently, RTV-boosted atazanavir has been included as a preferred PI-based regimen recommended by the US Department of Health and Human Services (DHHS) [102] and remains an alternative PI recommended by the British HIV Association [103], and RTV-boosted atazanavir is now one of the recommended WHO PI-based second-line regimens [9].
Chemistry
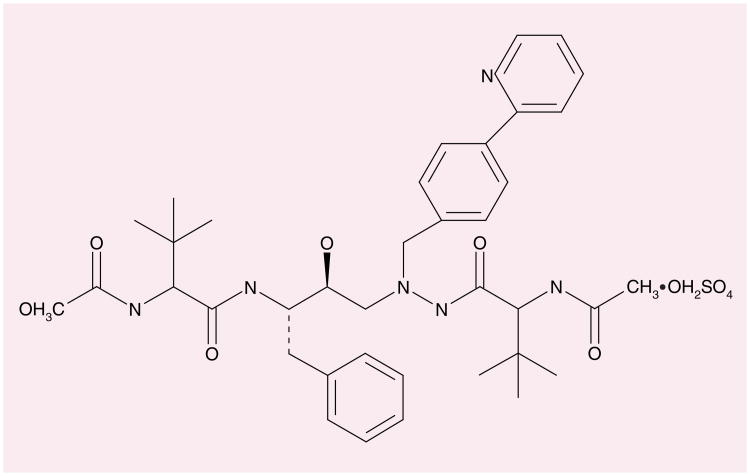
Atazanavir was developed by Bristol-Myers Squibb Company, Princeton, NJ, USA and is marketed under the trade name Reyataz™. Atazanavir sulphate is a white to pale yellow crystalline powder, which is relatively insoluble in water (4–5 mg/ml) with pH at saturation of 1.9 at 24°C. The molecular formula is C38H52N6O7•H2SO4 with a free base molecular weight of 704.9 [101]. The licensed formulation is heat stable. The structural formula is shown in Figure 1.
Figure 1.
Atazanavir.
Pharmacodynamics
In vitro antiviral activity
In cell culture, atazanavir has demonstrated anti-HIV-1 activity with a MIC50 in the 2–5 nM range. Atazanavir has lower and variable activity against HIV-2 isolates [10,11] and is not a recommended first-line PI for treatment of HIV-type II infection. Additive in vitro HIV-1 antiviral effect has also been demonstrated with other PIs, nucleoside reverse transcriptase inhibitors (NRTIs) and the HIV-1 fusion inhibitor (enfuvirtide) together with viral hepatitis agents, adefovir and ribavirin [101]. HIV-1 isolates with reduced susceptibility to atazanavir have been selected in vitro and isolated from patients receiving atazanavir or atazanavir/RTV. The I50L and N88S are major atazanavir-selected protease mutations. Treatment failure in PI-naive individuals taking unboosted atazanavir is characterized by the signature I50L mutation which is associated with an eightfold reduced susceptibility to atazanavir but may be associated with increased susceptibility to other PIs [12,13], probably related to increased in vitro binding affinity with the protease of other PI-resistant strains [14]. Flexibility of the molecular structure of atazanavir has been demonstrated by x-ray crystallography, which allows it to adopt different but effective binding conformations to proteases [15]. It remains to be confirmed whether the isolated I50L mutation potentially preserves future PI-treatment options. The N88S mutation has been reported in patients developing failure while receiving atazanavir/RTV and is associated with a tenfold-reduced susceptibility to atazanavir. In clinical studies of treatment-experienced patients, reduced susceptibility to atazanavir has been associated with multiple mutations, including V32I, M46I, I47V, G48V, I54V/L/M/T/A, V82A/T/F/S, I84V and L90M. These mutations generally have not been reported in patients receiving atazanavir or atazanavir/RTV and result in cross-resistance to other PIs [104].
Pharmacokinetics & metabolism
Atazanavir is rapidly absorbed after oral administration (Tmax 2.5 h) and demonstrates nonlinear pharmacokinetics, resulting in greater than dose-proportional increases in bioavailability (AUC and Cmax) over a dose range of 200–800 mg daily [101]. Administration of atazanavir with food enhances bioavailability and reduces pharmacokinetic variability [101]. Once absorbed, atazanavir is highly bound to plasma proteins α1-acid glycoprotein and albumin to similar extents (89 and 86%, respectively). Atazanavir is extensively metabolized by the hepatic cytochrome P450 (CYP) system to form two main inactive metabolites and is both a substrate and inhibitor of the CYP3A4 isoenzyme [16,101]. In vitro studies have also demonstrated that atazanavir is both an inhibitor and inducer of the P-glycoprotein ATP-dependent efflux pump, which has a wide cellular distribution and a broad substrate specificity, further increasing its potential for drug-drug interactions and variable pharmacokinetics in vivo [17]. Atazanavir should therefore be used with caution in patients taking strong CYP3A4 inhibitors, moderate or strong CYP3A4 inducers and major CYP3A4 substrates. Coadministration with drugs that induce CYP3A4, such as rifampicin, may decrease atazanavir plasma concentrations and reduce clinical effect, while drugs that inhibit CYP3A4 may elevate atazanavir levels and increase toxicity.
The mean elimination half-life of atazanavir 400 mg taken with food is approximately 7–8 h at steady state with 20 and 7% of active drug eliminated in feces and urine, respectively [101].
In vitro studies have indicated that a direct inhibition of UGT1A1-mediated bilirubin glucuronidation by free, nonproteinbound atazanavir gives a mechanistic rationale for dose-related hyperbilirubinemia [18]. Indinavir may similarly inhibit UGT1A and coadministration with atazanavir is not recommended [102].
Large inter- and intrapatient variability in atazanavir plasma concentrations have been demonstrated in population pharmacokinetic studies [19 –21], yet the same dose of atazanavir is currently administered regardless to differences in systemic blood and tissue disposition. The therapeutic range of atazanavir lies between 150 and 850 ng/ml [21,102]; however, plasma levels in the absence of RTV have been reported to be frequently lower than the target Cmin of 150 ng/ml in both patients [20,25] and substance [22]. The wide interpersonal variability in atazanavir exposure has been considered an indication for twice daily dosing or therapeutic drug monitoring [23]. However, no significant relationship has been established between atazanavir plasma trough concentration (Cmin) and antiviral response in patients starting atazanavir without PI mutations [23,24]. The wide variability in atazanavir exposure strongly supports the preferable use of RTV-boosted atazanavir in PI-experienced individuals.
Clinical efficacy studies
Tolerability and efficacy studies have been undertaken for atazanavir at doses between 200 and 600 mg once daily in ART-naive individuals [25,26] and comparative studies of atazanavir 400 mg with nelfinavir/efavirenz and lopinavir/RTV have also been carried out [27–29]. Atazanavir/RTV (300/100 mg) has been compared with unboosted atazanavir, fosamprenavir and lopinavir/RTV [30–35]. Further studies have explored the use of RTV-boosted atazanavir as monotherapy in previously virologically suppressed patients [36–38] and in combination with other PIs [39–43].
Study AI424-007 was a 48-week dose-ranging, safety and efficacy comparative trial of atazanavir (200, 400 and 500 mg once daily) in combination with didanosine and stavudine in antiretroviral-naive subjects with a comparator arm of nelfinavir (750 mg three-times a day) [25]. Viral responses and CD4 recovery were comparable across all four arms of the study. Unconjugated hyperbilirubinemia occurred in each of the atazanavir arms but was more frequent in the 500-mg cohort. In marked contrast to the nelfinavir comparator arm, lipid changes were neutral at all atazanavir doses [25].
Study AI424-008 compared atazanavir (400 and 600 mg once daily) in combination with lamivudine and stavudine against nelfinavir (1250 mg twice daily) in ART-naive patients [26]. At 48 weeks, the CD4 cell rise and the proportion of patients with viral suppression lower than 50 copies/ml were similar across all arms, although the proportion of patients with lower than 400 copies/ml in the atazanavir 600-mg arm was significantly higher than the comparator arm. Lipid changes were again neutral in both atazanavir arms in contrast to the nelfinavir arm. Although unconjugated bilirubinemia was reported in 58% of the higher-dose atazanavir (600 mg) arm, it resulted in treatment discontinuation in only four patients [26].
Study AI424-044 was a long-term efficacy, safety and tolerability atazanavir continuation study of individuals completing the earlier nelfinavir comparative phase (AI424-008) with viral loads of less than 10,000 copies/ml [27]. Lipid parameters remained stable in those continuing atazanavir and improved in those switching from nelfinavir to atazanavir. Atazanavir was demonstrated to be safe, tolerable and effective during extended use and in patients switched from nelfinavir [27].
Study I424-034 was a randomized study which demonstrated comparable efficacy and tolerability of atazanavir 400 mg or efavirenz 600 mg administered once daily in combination with zidovudine plus lamivudine twice daily in treatment-naive patients [28]. At week 48, HIV RNA levels were lower than 400 copies/ml in 70% of patients receiving atazanavir and 64% of patients receiving efavirenz. Immunological responses were similar in both treatment arms. However, atazanavir-treated patients did not demonstrate significant increases in total cholesterol, fasting low-density lipoprotein (LDL) cholesterol, fasting triglycerides, fasting glucose or insulin levels and atazanavir-linked bilirubin elevations resulted in treatment discontinuation in less than 1% of participants.
Study AI424-043 compared atazanavir (400 mg once daily) with lopinavir/RTV (400/100 mg twice daily), each together with two NRTIs in PI-experienced subjects [29]. Both regimens were equally effective in subjects who had no baseline NRTI mutations. However, lopinavir/RTV resulted in a significantly greater reduction in HIV RNA than unboosted atazanavir (p < 0.001) at week 48. Secondary efficacy end points also favored lopinavir/RTV. However, atazanavir resulted in either no change or decreases in fasting LDL cholesterol, total cholesterol and fasting triglycerides, whereas lopinavir/RTV resulted in increases. While both treatments demonstrated good antiviral efficacy, relatively greater antiviral suppression was observed with lopinavir/RTV.
Study AI424-089 was a randomized, open-label, multicenter, comparative study of efficacy and safety of atazanavir/RTV (300/100 mg) and atazanavir (400 mg) in combination with lamivudine and stavudine in antiretroviral-naive patients. The primary end point for this noninferiority study was the proportion of patients with less than 400 and less than 50 copies/ml. At 96 weeks, discontinuations were similar in both arms (17 and 18%, respectively) but the response rate was slightly higher with 94% having less than 400 copies/ml and 84% less than 50 copies/ml in the boosted arm compared with 87% having less than 400 copies/ml and 76% less than 50 copies/ml in the unboosted arm. No subjects with virologic failure in the 300/RTV arm developed ontreatment phenotypic resistance, compared with four subjects in the 400 arm. Treatment-emergent major International AIDS Society (IAS)-defined protease substitutions associated with phenotypic resistance were I50L and I50L + N88N/S. The other two subjects with phenotypic resistance at virologic failure did not have major protease substitutions as defined by the IAS [44]. Phenotypic and genotypic NRTI resistance was observed more frequently in the unboosted arm (11v1). Increases in lipids were similar in both regimens and were consistent with ‘known return to health effect’ in treatment-naive individuals started on ART. The study demonstrated a trend for less virologic failure and acquisition of resistant mutations in the atazanavir/RTV treatment arm [31].
The Assessment of Lescol in Renal Transplantation (COL103952) randomized, open-label, HDL 48-week study compared once-daily regimens; atazanavir/RTV (300/100 mg) with fosamprenavir/RTV (1400/100 mg) together with tenofovir/emitricitabine (300/200 mg) in antiretroviral-naive patients [32]. Equal numbers of patients (n = 53) were randomized to each arm with 49 of the atazanavir and 45 of the fosamprenavir arms completing the study. Primary study end points included the proportion with HIV RNA less than 400 and less than 50 copies/ml and CD4 cell change from baseline. At 48 weeks, the virologic and immunologic responses were similar across both arms. Nonsignificant differences were seen in fasting triglycerides and cholesterol levels but the study was not powered for these secondary end points. Treatment-related adverse events were more common in the atazanavir/RTV arm (57 vs 15%), predominantly due to grade 2–4 hyperbilirubinemia [32].
The CASTLE study is a large, open-label, randomized study comparing atazanavir/RTV (300/100 mg once daily) with lopinavir/RTV (400/100 mg twice daily) in ART-naive patients. The study randomized 440 and 443 patients to the atazanavir/RTV and lopinavir/RTV arms, respectively. At 48 weeks, 78 and 76% achieved the primary end point of fewer than 50 copies/ml with discontinuations of 9 and 13% in the atazanavir and lopinavir containing arms, respectively. The atazanavir/RTV arm was associated with significantly lower increases in total cholesterol, triglycerides and non-high-density lipoprotein cholesterol. These preliminary results demonstrated noninferiority of virologic and immunologic responses, with both lower incidence of gastrointestinal events and lipid abnormalities in the atazanavir arm [33].
Study AI424-045 evaluated atazanavir/RTV (300/100 mg) once daily, atazanavir/saquinavir (400/1200 mg) once daily and lopinavir/RTV (400/100 mg) twice daily, each with tenofovir (300 mg) once daily and another NRTI in treatment-experienced HIV-infected patients [45]. An interim analysis found the atazanavir/saquinavir arm to be both virologically and immunologically inferior to the other arms and was discontinued at 24 weeks. The primary end point was time-averaged reduction in HIV RNA levels from baseline, with secondary objectives including safety and lipid changes through 96 weeks. The randomized numbers starting therapy were n = 120 for atazanavir/RTV and n = 123 for lopinavir/RTV. Discontinuation rates were comparable across arms and, at 96 weeks, 132 patients were available for evaluation with 72% with viral RNA levels of less than 50 copies/ml in both arms. Median total cholesterol level changes were -5 and +9 mg/dl and fasting triglycerides -40 and 40 mg/dl for the atazanavir/RTV and lopinavir/RTV arms, respectively. Once-daily atazanavir/RTV demonstrated noninferiority to lopinavir/rotonavir with respect to efficacy and safety associated with significant reductions in total cholesterol and fasting triglycerides in treatment experienced patients over 96 weeks [35].
Monotherapy studies
The long-term adverse effects, expense and difficulty of adherence to antiretroviral regimens have led to studies of simpler maintenance therapies. Maintenance therapy with atazanavir/RTV alone is a possible option because of low pill burden, once-daily dosing, safety and unique resistance profile. An initial pilot study of virologically suppressed adults on non-PI containing regimens, switched therapy to atazanavir/RTV (300/100 mg) and discontinued NRTIs 6 weeks later [36]. At 24 weeks, three of 34 patients experienced virologic failure without acquired PI-resistance mutations. These preliminary data suggested that simplified maintenance therapy with atazanavir/RTV alone might be a possible strategy for maintaining virologic suppression in carefully selected patients with HIV-infection. A similar strategy was explored in a second study, which included monitoring of cerebrospinal fluid (CSF) and semen viral load [37]. After 24 weeks, three out of 20 patients had measurable viral loads in CSF and two out of 15 in semen, despite viral suppression in plasma. Ongoing viral replication, together with low drug penetration into CSF (CSF/blood ratio: 0.9%), served as a caution that atazanavir monotherapy may not suppress viral replication in sanctuary sites. A third study of atazanavir/RTV monotherapy treatment simplification was prematurely discontinued because of five cases of early virologic failure among the first 15 patients recruited [38]. The authors concluded that atazanavir/RTV as maintenance monotherapy in HIV-1 infection might not be as potent as conventional ART.
PI-combination studies
The interaction between atazanavir and other PIs is being explored to maximise activity against PI-resistant viruses and as a class-sparing strategy. Coadministration with amprenavir results in increased amprenavir levels but approximately 40% lower atazanavir exposure and has not been pursued [39]. By contrast, atazanavir greatly enhances the exposure of saquinavir but the pharmacokinetics of atazanavir remain unchanged [40]. However, the dual combination of atazanavir and saquinavir was found to be less efficacious than either lopinavir/RTV or atazanavir/RTV [34]. The triple combinations of atazanavir/saquinavir/RTV [41], atazanavir/fosamprenavir/RTV [42] and atazanavir/lopinavir/RTV [43,45], have been shown to be both tolerable and efficacious in clinical practice. Experience of boosted double PI regimens is limited and some patients may not achieve enhanced therapeutic levels [46]; therefore, careful selection of patients and therapeutic drug monitoring may be required.
Atazanavir in pregnancy
Atazanavir is categorized as a FDA category B antiretroviral, indicating that animal studies have revealed no evidence of harm to the fetus; however, there have been concerns about infant hyperbilirubinemia. Due to a lack of well-controlled safety studies, the current DHHS 2008 treatment guidelines do not recommend routine use in pregnancy. Physiological changes, which are most marked in the third trimester, may result in lower PI AUC and Cmin values [47] for both unboosted PIs indinavir and nelfinavir [48] and for the RTV-boosted PIs saquinavir [49] and lopinavir [50]. By contrast, a retrospective case review study of 31 women who received atazanavir during pregnancy reported mean trough levels above therapeutic levels for wild-type virus in all but one individual [51]. Intensive pharmacokinetic studies of atazanavir/RTV (300/100 mg) in pregnancy showed no statistical differences in antepartum and postpartum AUC, Cmax and Cmin were 40-fold higher than the IC90 for wild-type HIV-1 with undetectable viral load throughout the study [52]. The overall exposure to atazanavir in the third trimester of pregnancy when boosted with RTV appeared similar to the nonpregnant state and dose adjustment is not required in pregnancy. A case series of 33 pregancies with exposure to atazanavir/RTV reported good tolerability and immunologic and virologic responses together with lack of any early infant morbidity as a result of in utero exposure to atazanavir [50].
Transplacental passage of atazanavir
In contrast to NRTIs, which freely cross the placenta by means of passive diffusion [53], the transplacental passage of PI's is limited by their large molecular size, high protein binding and placental P-glycoprotein backward transportation [53–57]. Concurrent measurement of maternal venous and fetal cord drug concentrations have shown variable and very limited transfer of nelfinavir, saquinavir, lopinavir, amprenavir and RTV [54–57]. Studies of women taking boosted atazanavir/RTV (300/100 mg) showed mean ratio of cord/maternal concentrations between 0.13 and 0.23 [52,58], with cord concentrations higher than the IC90 for wild-type HIV [52]. Atazanavir, unlike other PIs, crosses the placenta and when boosted with RTV achieves measurable concentrations in the fetus that are lower than maternal levels, although the risks and benefits of these atazanavir levels in the newborn are uncertain at present.
Atazanavir levels in genital secretions
The capacity to achieve therapeutic levels of anrtiretrovirals in genital secretions may have major implications for sexual transmission of HIV and for pre-exposure (PrEP) and postexposure prophylactic (PEP) strategies. However, low drug exposure in the genital tract may also contribute to higher viral shedding and the development of viral resistance. A study of genital tract atazanavir levels in eight women receiving atazanavir/RTV (300/100 mg once daily), reported the ratio of AUC0–24 between genital secretions and blood of 0.16 and 0.18 after first dose and at steady state, respectively [59], and achieved genital atazanavir Cmin levels within the therapeutic range for wild-type HIV-1 and further studies of atazanavir for use in PrEP and PEP may be warranted [59].
HIV viral RNA levels in semen are highest soon after seroconversion and subsequently decline to lower but stable levels, which are considerably less than those in blood over the following months [60]. An exploratory study of drug penetration into seminal fluid detected atazanavir in seminal plasma samples; however, the penetration was limited and variable and the median seminal to blood plasma ratio was only 0.1 resulting in subtherapeutic levels [61]. This preliminary study demonstrated that atazanavir, in contrast to most other PIs, penetrates seminal plasma but that the penetration is very limited and variable [61]. The significance of low atazanavir levels in semen for transmission of resistant virus is unknown.
Susceptibility of HIV-2 proteases to atazanavir
Protease inhibitors have provided a key component of ARTs for HIV/AIDS, where the majority of HIV/AIDS cases are due to HIV-1. HIV-2 is endemic to West Africa; however, due to the relatively small number of AIDS patients infected with HIV-2, there has been no specific PI development against HIV-2 protease. HIV-2 proteases share only 50% sequence identity with HIV-1 protease and have lower relative binding affinity for PIs than HIV-1 protease [11,62,63]. Phenotypic susceptibility of HIV-2 isolates to a panel of PIs reported atazanavir to be less potent than saquinavir, lopinavir and tipranavir [10], and atazanavir should not be considered as a first-line option for HIV-2 treatment.
Drug–drug interactions
As with other PIs, atazanavir is a substrate and moderate inhibitor of the CYP system, in particular CYP3A4 and CYP2C9, and clinically significant drug interactions include (but are not limited to) antacids, proton pump inhibitors (PPIs), histamine type 2 (H2) receptor antagonists, tenofovir, diltiazem, irinotecan, simvastatin, lovastatin, PDE5 inhibitors, St John's wort and warfarin [101].
Tenofovir
Tenofovir has significant pharmacokinetic interaction with atazanavir. Coadministration of atazanavir (400 mg) increases the AUC0–24, Cmax and Cmin of tenofovir (300 mg) by 14–24% and decreases the AUC 0–24, Cmax and Cmin of atazanavir by 20–40% [105]. Reports of the impact of tenofovir (300 mg) on the pharmaco kinetics of atazanvir/RTV have been somewhat variable. In heavily pretreated HIV-1 patients started on atazanavir/RTV, the addition of tenofovir resulted in a significant decrease in the atazanavir AUC from 0 to 24 h (AUC0–24 ratio: 0.75; 90% confidence interval: 0.58–0.97; p = 0.05) but only a nonsignificant trend for decreased Cmin[64]. A population pharmacokinetic study of patients receiving atazanavir/RTV, tenofovir significantly increased atazanavir clearance but with no significant decrease in mean Cmin [65]. By contrast, a standardized therapeutic drug monitoring study of adult HIV-1-infected patients receiving boosted atazanavir and NRTIs showed no differences in plasma atazanavir concentrations individuals matched for gender, ethnicity, weight and US CDC status either receiving tenofovir or not [66].
Acid-reducing agents
Atazanavir requires an acidic gastric pH for dissolution and clinically significant drug interactions occur between atazanavir and antacids, PPIs and H2 -receptor antagonists [67,68]. Therefore concomitant medications which raise gastric pH, such as antacids, H2-receptor antagonists (famotidine and ranitidine) and PPIs markedly reduce atazanavir bioavailability [68]. PPI usage is frequent among HIV-infected individuals and may be acquired without prescription over the counter in USA [69]. Polymorphisms of CYP2C19, by which all PPIs are metabolized, may account for highly variable concentrations of omeprazole and lansoprazole [70,71]. Concurrent PPIs cause a significant reduction in bioavailability of atazanavir, resulting in 94% reduction in AUC and Cmax[72]. Coadministration of PPIs and unboosted atazanavir should be avoided as significant reduction in atazanavir levels can be expected. Atazanavir boosting with RTV and temporal separation of administration with PPIs decreases the effect on atazanavir pharmacokinetic parameters. Virologic and immunologic outcomes appear stable when boosted atazanavir is used in a limited case series of HIV-positive patients [73]. Acid-reducing agents coadministered with atazanavir should be used only with extreme caution, with careful dose separation and therapeutic drug monitoring should be considered [68].
Rifamycins
Rifamycins are essential drugs for the treatment of active TB disease, with rifabutin the preferred rifamycin in HIV/TB-coinfected patients, since it has fewer and less-severe interactions with antiretroviral therapy than rifampicin [102]. However, in resource-poor settings, rifampicin is widely used because it is both cheap and available in fixed-dose combinations with other anti-TB agents. Rifampicin is a potent inducer of CYP oxidative enzymes (although it itself is not a substrate) as well as the P-glycoprotein transport system. All PIs are substrates of CYP3A4, so their metabolic clearance may be modified by CYP inducers or inhibitors. Coadministration of rifampicin with all currently marketed HIV PIs results in a low PI exposure, with the exception of full strength RTV. Atazanvir is a weak inhibitor of P-glycoprotein and undergoes metabolism by CYP3A, which generates metabolites lacking antiviral activity [101]. Unboosted atazanavir, even at increased dosage (400 mg twice daily), did not maintain adequate plasma exposure when coadministered with rifampin in HIV-positive subjects [74]. Atazanavir/RTV/rifampicin (400/200/600 mg) is safe and well tolerated but results in lower atazanavir Cmin than achieved by atazanavir 400 mg or atazanavir/RTV (300/100 mg) alone and a two-to threefold increase in rifampicin and desacetyl-rifampicin exposure (AUC) [106,107]. Presently, rifampicin should not be coadministered with atazanavir and RTV because of reduced atazanavir plasma concentrations.
The PIs, particularly if pharmacologically boosted with RTV, markedly increase serum concentrations and toxicity of rifabutin [74] and its active metabolite [73]. The dose of rifabutin should be decreased when used with PIs to 150 mg every other day or three-times weekly [106]. The exposure of atazanavir/RTV is not significantly affected by the coadministration of rifabutin and no dose adjustment is required. Rifabutin levels would be subtherapeutic if the patient stopped taking the PI, therefore, adherence to the PI should be reinforced and ensured if a supervised dose of PI is taken at the same time as the directly observed dose of TB treatment [106].
Raltegravir
The integrase inhibitor raltegravir is not a substrate of the CYP enzymes and is eliminated predominantly via the UGT1A1-mediated glucuronidation pathway. Atazanavir is an inhibitor of UGT1A1 and coadministration may increase raltregravir levels. However atazanavir/RTV coadministered with raltegravir does not require dose adjustment of raltegravir [107].
Safety & tolerability
Patients who receive atazanavir generally experience similar rates of adverse events compared with patients receiving comparator regimens with the exception of hyperbilirubinemia, which occurs frequently.
Hepatic complications
Atazanavir is principally metabolized by the liver; caution should be exercised when administering this drug to patients with hepatic impairment because atazanavir concentrations may be increased. Patients with underlying hepatitis B/C viral infections or marked elevations in transaminases prior to treatment may be at increased risk for developing further transaminase elevations, including hepatic decomposition. In controlled studies of atazanavir (400–600 mg once daily), the proportion of hepatitis grade 3 and 4 elevations among patients seropositive for hepatitis B and C has been similar (14–17%) to comparator arms [26,28,101]. In study AI424-045, 25% of patients seropositive for hepatitis B and C at study entry and treated with atazanavir/RTV (300/100 mg) once, developed grade 3 elevations of alanine transferase (ALT) [29, 101]. However, in a large, uncontrolled study of hepatitis-coinfected patients, only 6% developed grade 3 and 4 ALT elevations and baseline hepatic fibrosis was not found to be a risk factor for ALT elevation [75]. Hepatitis coinfection has not been shown to have significant effects on atazanavir trough levels [76]; however, liver function tests should be monitored in patients with hepatitis B or C infection.
Atazanavir is commonly associated with an increased risk of asymptomatic hyperbilirubinemia, due to competitive inhibition of UGT1A1, although resulting in fewer than 2% discontinuations of atazanavir therapy. In controlled studies, no difference in frequency of bilirubin elevations was noted between hepatitis B and or C seropositive and seronegative patients. Atazanavir is, however, contraindicated in infants under 3 months of age because of possible kernicterus.
Cardiac complications
Atazanavir may be preferentially indicated compared with other PIs in those patients with increased cardiovascular risk factors. The neutral lipid profile of atazanavir results in a lowering of plasma lipids, together with a modest reduction in normalized-for-age cardiovascular risk score, after switching to atazanavir from other PIs [77]. In contrast to other PIs, atazanavir does not impair glucose transport and does not exacerbate heart failure in a mouse model of dilated cardiomyopathy [78]. However, atazanavir has been shown to prolong the PR interval of the electro cardiogram of patients 1 month after switching from other PIs [79]. A further analysis of 75 patients 1 year after enrolling in an atazanavir-expanded access programme reported that 56 individuals had a median increase in QRS interval of 5 ms. New asymptomatic bundle blocks were also observed in four patients and one further patient receiving the β-blocker atenolol developed first-degree atrioventricular block. Increases in PR interval were also noted after 1 year but these did not reach statististical significance [80]. Pharmacokinetic studies between atazanavir and the β-blocker atenolol demonstrated no significant pharmacokinetic interaction and with the calcium channel blocker diltiazem an approximate doubling of diltiazem AUC, Cmax and Cmin levels with no effect on atazanavir levels [101]. Interaction with other drugs that prolong the PR interval, including β-blockers, verapamil and digoxin, are unknown. An additive effect of atazanavir and these drugs, therefore, cannot be excluded and caution should be exercised when atazanavir is given concurrently with these drugs, especially those that are metabolized by CYP3A (e.g., verapamil).
Renal complications
Acute renal failure due to insterstitial nephritis has been reported in patients receiving atazanavir [81–83]. Nephrolithiasis also appears to be associated with atazanavir use [84,85] and may result in acute renal failure [86]. Approximately 7% of an atazanavir dose in excreted unchanged in the urine and solubility increases with lower pH [101]. Urinary calculi have been reported to contain up to 40–100% of atazanavir by weight [87]. Atazanavir is not appreciably cleared during hemodialysis [101,88]. Severe renal disease does not appear to change mean drug exposures but is associated with marked interindividual variability in Cmin and, therefore, therapeutic drug monitoring may be warranted [88].
Lipid changes
Switching studies have allowed comparison of the impact of atazanavir and other PI-based therapies on lipid profiles. The SWAN study (AI424-097) randomized virally suppressed patients receiving stable PI-based regimens to either continue or switch the PI to atazanavir 400 mg per day or atazanavir/RTV 300/100 mg once daily, if tenofovir was included in the NRTI backbone. Patients switching to atazanavir-containing regimens maintained virological suppression but had significantly improved changes in total cholesterol and fasting triglycerides [89]. The ATAZIP study randomized patients on stable lopinavir/RTV-based therapy to either continue or switch the PI to atazanavir/RTV 300/100 mg per day. The atazanavir/RTV arm of the study was noninferior to lopinavir/RTV with respect to viral suppression and tolerability but was associated with significant improvements in total cholesterol and fasting triglycerides [90].
Body fat redistribution
Protease inhibitor treatment has been linked to the development of lipodystrophy manifested by central fat accumulation and peripheral wasting. However, a prospective randomized comparative study of efavirenz or atazanavir in combination with zidovudine and lamivudine in ART-naive patients assessed body-fat changes with computed tomography and dual-energy x-ray absorptiometry (DEXA) and found no abmormal fat redistribution in either treatment arm after 48 weeks [91]. A further prospective, randomized, comparative study of atazanavir or atazanavir/RTV in combination with stavudine and lamivudine found no clinically significant difference in body composition between either study arms as assessed by computed tomography, DEXA and questionnaire [92]. Longer-term studies will be needed to exclude an impact of long-term use of either atazanavir or atazanavir/RTV on body composition.
Conclusion
Unboosted atazanavir has extremely variable drug exposures but remains a preferred PI for those with increased cardiovascular risks and in those whom elevation of lipids would be disadvantageous. Atazanavir/RTV efficacy is comparable to lopinavir/RTV and side effects are tolerable and generally manageable. Raised unconjugated bilirubin levels are inconvenient but not generally a drug-limiting toxicity. Once-daily dosing is convenient when combined with once-daily NRTIs, such as tenofovir, making it a preferred option for initial therapy in industrialized settings and preferred second-line therapy in LMICs.
Expert commentary
Atazanavir has similar virologic efficacy to efavirenz for drugnaive patients, shares the benefit of a once-daily regimen and has the advantage of a neutral impact on lipids. Disadvantages include less clinical experience of its use, the common side effect of hyperbilirubinemia and the lack of a coformulation with once-daily NRTIs. In ART-experienced patients, RTV-boosted atazanavir has similar virological activity to RTV-boosted lopinavir, which is its main market competitor. The benefits of a relatively neutral lipid profile are somewhat diminished by the addition of RTV to atazanavir, and the current lack of a coformulation with RTV and frequent hyperbilirubinemia are both disadvantageous. Use of atazanavir in LMICs is further hampered by the lack of a regimen for use together with standard rifampicin-based anti-TB therapy [106]. PI use in the global roll-out program in LMICs will continue to be determined predominantly by cost. With economies of scale and market competition, a coformulation of atazanavir with heat-stable RTV could achieve a significant price advantage [77], with subsequent increasing market share.
Five-year view
The DHHS 2008 treatment guidelines [102] and the WHO expert panel on second-line ART [9] have recently upgraded atazanavir/RTV from an alternative PI regimen to preferred PI status. Future updates of other major guidelines will most probably follow these recommendations, thereby increasing market penetration in industrialized countries and LMICs. The lipidneutral characteristics of atazanavir will increase in importance with increased survival of HIV-infected patients at risk of cardiovascular events. In LMICs, the global numbers of patients requiring PI-based regimens will continue to grow at a rate of approximately 3% per annum. Atazanavir/RTV has efficacy and tolerability similar to its main competitors; however, it is likely that costing will determine market penetration in LMIC settings. With economies of scale, atazanavir/RTV combinations are anticipated to be 40% or cheaper than competitor PIs, based on requirements for less active pharmaceutical ingredients per dose [9,93]. Atazanavir is heat stable and the WHO has declared wider availability of coformulation atazanavir, together with heat-stable RTV, to be an urgent priority [8,9]. National programs will consider adopting atazanavir/RTV as a preferred PI option in their national second-line protocols as prices decrease and heat-stable formulations of RTV become more widely available.
Table 1.
Atazanavir and ritonavir-boosted atazanavir efficacy studies.
| Study | Number and time (weeks) | Patient population | Atazanavir regimen (mg) | NNRTIs | Comparator regimen | Results | Ref. |
|---|---|---|---|---|---|---|---|
| AI424-007 | n = 420 48 |
ART-naive | 200 400 500 |
Didanosine Stavudine | NLF 750 mg t.i.d. | Viral and CD4 responses comparable across four arms resulted in unconjugated bilirubinemia in all arms and 12% in 500 mg arm. NLF resulted in 31% elevated lipids and 61% diarrhea | [30] |
| AI424-008 | n = 467 48 |
ART-naive | 400 600 |
Lamivudine Stavudine | NLF 1250 mg b.i.d. | Viral and CD4 responses comparable in all three arms. NLF resulted in 56% diarrhea and 33% decrease in those with desirable cholesterol levels | [31] |
| AI424-044 | n = 346 72 |
Roll over from AI424-008 | 400 600 |
Lamivudine Stavudine | Switch from NLF | Switch from NLF resulted in improved lipid profile and maintained viral suppression with long-term tolerability | [32] |
| AI424-034 | n = 810 48 |
ART-naive | 400 | Zidovudine Lamivudine | Efavirenz 600 mg once daily | Comparable efficacy of both arms. NB efavirenz virologic response somewhat lower than reported in prior studies | [33] |
| AI424-089 | n = 199 96 |
ART-naive | 400 | Lamivudine Stavudine-ER | RTV 300/100 mg once daily | ARV/RTV arm had nonstatistically significant trend for improved viral responses and significantly higher total cholesterol levels | [36] |
| COL103952 ‘ALERT’ | n = 94 48 |
ART-naive | RTV 300/100 | Tenofovir Emitricitabine | Fosamprenavir-APV/RTV 1400/100 mg once daily | Similar virological, CD4 cell count and lipid changes in both arms | [38] |
| Castle study | n = 878 48 |
ART-naive | RTV 300/100 | Tenofovir Emitricitabine | Tenofovir Emitricitabine | Ongoing 96-week study. Similar virologic and immunological outcomes. Diarrhea and lipid abnormalities lower in RTV arm | [39] |
| AI424-043 | n = 290 48 |
PI-experienced | 400 | Optimized background therapy | LPV/RTV 400/100 mg b.i.d. | LPV/RTV greater reduction in HIV RNA but increased lipids. RTV and LPV/RTV similar virologic responses in those without NRTI mutations at baseline | [34] |
| AI424-045 | n = 358 96 |
Failed two or more HAART regimens | RTV 300/100 | Tenofovir + one NRTI | SQV 400/1200 mg LPV/RTV 400/100 mg b.i.d. | SQV discontinued early by Data Safety Monitoring Board. RTV and LPV/RTV have similar virological efficacy. LPV/RTV associated with 9% increased cholesterol and 30% triglycerides and 13% grade 2–4 diarrhea. RTV associated with grade 3–4 bilirubin elevation in 53% | [40,41] |
APV: Amprenavir; ARV: Antiretroviral therapy; b.i.d.: Twice daily; ER: Extended release; HAART: Highly-active antiretroviral therapy; LPV: Lopinavir; NLF: Nelfinavir; NNRTI: Non-nucleoside reverse transcriptase inhibitor; NRTI: Nucleoside reverse transcriptase inhibitor; PI: Protease inhibitor; RTV: Ritonavir; SQV: Saquinavir; t.i.d.: Three-times daily.
Key issues.
Atazanavir is an azapeptide HIV-1 protease inhibitor (PI) registered for once-daily use, which has limited activity against HIV-2 proteases. Atazanavir formulation is heat stable.
Unboosted atazanavir, unlike many other PIs, has a neutral effect on lipids and switching from other PIs to atazanavir can improve an individual's lipid profile. Furthermore, unboosted atazanavir is the preferred PI in high cardiac-risk patients.
Atazanavir is both a substrate and inducer of CYP3A4, and an inducer and inhibitor of the P-glycoprotein ATP-dependent pump.
Drug–drug interactions are frequent with other drugs metabolized by CYP3A4.
Bioavailability is enhanced and variability decreased by food. Ritonavir boosting enhances bioavailabilty and improves variability.
Unconjugated hyperbilirubinemia occurs in 40% of patients, however, side effects are tolerable and generally manageable.
I50L is the signature mutation in PI-naive patients failing atazanavir. An isolated I50L mutation maintains susceptibility to other PIs.
The efficacy of ritonavir-boosted atazanavir and lopinavir are comparable. Tenofovir lowers atazanavir plasma levels by 20–40% and ritonavir boosting of atazanavir is recommended with tenofovir.
The dose of active constituent (300 mg) is lower than other PIs, increasing the potential for price reductions.
Ritonavir-boosted atazanavir is a US Department of Health and Human Services guidelines preferred PI regimen and is one of two preferred PIs recommended by the WHO for national antiretroviral therapy programs.
Footnotes
Financial & competing interests disclosure: The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Schwarcz SK, Hsu LC, Vittinghoff E, Katz MH. Impact of protease inhibitors and other antiretroviral treatments on acquired immunodeficiency syndrome survival in San Francisco, California, 1987–1996. Am J Epidemiol. 2000;152(2):178–185. doi: 10.1093/aje/152.2.178. [DOI] [PubMed] [Google Scholar]
- 2.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1 EuroSIDA Study Group. Lancet. 1998;352(9142):1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Vittinghoff E, Scheer S, O'Malley P, et al. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179(3):717–720. doi: 10.1086/314623. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Towards Universal Access: Scaling-up Priority HIV/AIDS Interventions in the Health Sector. WHO HIV Department; Geneva, Switzerland: 2007. [Google Scholar]
- 7.Gilks CF, Crowley S, Ekpini R, et al. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Report on WHO/UNAIDS Meeting on Forecasting ARV Needs Up to 2010: (Draft) WHO HIV Department; Geneva, Switzerland: 2006. [Google Scholar]
- 9.WHO. Report on Prioritizing Second-Line Antiretroviral Drugs for Adults and Adolescent: a Public Health Approach. WHO HIV Department; Geneva, Switzerland: 2007. [Google Scholar]
- 10.WHO. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. WHO HIV Department; Geneva, Switzerland: 2006. [PubMed] [Google Scholar]
- 11.Desbois D, Roquebert B, Peytavin G, et al. In vitro phenotypic susceptibility of HIV-2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother. 2008;52(4):1545–1548. doi: 10.1128/AAC.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brower ET, Bacha UM, Kawasaki Y, Freire E. Inhibition of HIV-2 protease by HIV-1 protease inhibitors in clinical use. Chem Biol Drug Des. 2008;71(4):298–305. doi: 10.1111/j.1747-0285.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 13.Brower ET, Bacha UM, Kawasaki Y, Freire E. Inhibition of HIV-2 protease by HIV-1 protease inhibitors in clinical use. Chem Biol Drug Des. 2008;71(4):298–305. doi: 10.1111/j.1747-0285.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 14.Gong YF, Robinson S, Rose RE, et al. In vitro resistance profile of the human immunodeficiency virus type 1 protease inhibitor BMS-232632. Antimicrob Agents Chemother. 2000;44:2319–2326. doi: 10.1128/aac.44.9.2319-2326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colonno R, Rose R, McLaren C, Thiry A, Parkin N, Friborg J. Identification of I50L as the signature atazanavir (ATV)-resistance mutation in treatment-naive HIV-1-infected patients receiving-containing regimens. J Infect Dis. 2004;189:1802–1810. doi: 10.1086/386291. [DOI] [PubMed] [Google Scholar]
- 16.Klei Herbert E, Kish Kevin, et al. X-ray crystal structures of human immunodeficiency virus type 1 protease mutants complexed with atazanavir. J Virol. 81(17):9525–9535. doi: 10.1128/JVI.02503-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busti AJ, Hall RG, Margolis DM. Atazanavir for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2004;24(12):1742–1747. doi: 10.1592/phco.24.17.1732.52347. [DOI] [PubMed] [Google Scholar]
- 18.Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33(6):764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos. 2005;33(11):1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 20.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(11):3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moltó J, Blanco A, Miranda C, et al. Variability in non-nucleoside reverse transcriptase and protease inhibitor concentrations among HIV-infected adults in routine clinical practice. Br J Clin Pharmacol. 2006;62(5):560–566. doi: 10.1111/j.1365-2125.2006.02694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins N, Zingman BS, Slish J, et al. Factors associated with altered pharmacokinetics in substance users and non-substance users receiving lopinavir and atazanavir. Am J Addict. 2007;16(6):488–494. doi: 10.1080/10550490701641256. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez de Requena D, Bonora S, Cavechia I, et al. Atazanavir trough is associated with efficacy and safety at 24 weeks: definition of therapeutic range; Presented at: 6th International Workshop on Clinical Pharmacology of HIV Therapy; Québec, Canada. 28–30 April 2005; Abstract 60. [Google Scholar]
- 24.Cleijsen RM, van de Ende ME, Kroon FP, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007;60(4):897–900. doi: 10.1093/jac/dkm298. [DOI] [PubMed] [Google Scholar]
- 25.Winston A, Bloch M, Carr A, et al. Atazanavir trough plasma concentration monitoring in a cohort of HIV-1-positive individuals receiving highly active antiretroviral therapy. J Antimicrob Chemother. 2005;56(2):380–387. doi: 10.1093/jac/dki235. [DOI] [PubMed] [Google Scholar]
- 26.Sanne I, Piliero P, Squires K, Thiry A, Schnittman S AI424-007 Clinical Trial Group. Results of a Phase 2 clinical trial at 48 weeks (AI424-007): a dose-ranging, safety, and efficacy comparative trial of atazanavir at three doses in combination with didanosine and stavudine in antiretroviral-naive subjects. J Acquir Immune Defic Syndr. 2003;32(1):18–29. doi: 10.1097/00126334-200301010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Murphy RL, Sanne I, Cahn P, et al. Dose-ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral-naive subjects: 48-week results. AIDS. 2003;17(18):2603–2614. doi: 10.1097/00002030-200312050-00007. [DOI] [PubMed] [Google Scholar]
- 28.Wood R, Phanuphak P, Cahn P, et al. Long-term efficacy and safety of atazanavir with stavudine and lamivudine in patients previously treated with nelfinavir or atazanavir. J Acquir Immune Defic Syndr. 2004;36(2):684–692. doi: 10.1097/00126334-200406010-00005. [DOI] [PubMed] [Google Scholar]
- 29.Squires K, Lazzarin A, Gatell JM, et al. Comparison of once-daily atazanavir with efavirenz, each in combination with fixed-dose zidovudine and lamivudine, as initial therapy for patients infected with HIV. J Acquir Immune Defic Syndr. 2004;36(5):1011–1019. doi: 10.1097/00126334-200408150-00003. [DOI] [PubMed] [Google Scholar]
- 30.Cohen C, Nieto-Cisneros L, Zala C, et al. Comparison of atazanavir with lopinavir/ritonavir in patients with prior protease inhibitor failure: a randomized multinational trial. Curr Med Res Opin. 2005;10:1683–1692. doi: 10.1185/030079905x65439. [DOI] [PubMed] [Google Scholar]
- 31.Johnson VA, Brun-Vézinet F, Clotet B, et al. Drug resistance mutations in HIV-1. Top HIV Med. 2003;11(6):215–221. [PubMed] [Google Scholar]
- 32.Malan N, Krantz E, David N, et al. Efficacy and safety of atazanavir-based therapy in antiretroviral naive HIV-1 infected subjects both with and without ritonavir: 96-week results from AI424-089; Presented at: 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Sydney, Australia. 22–25 July 2007; Abstract WEPEB024. [Google Scholar]
- 33.Smith KY, Weinberg WG, DeJesus E, et al. Fosamprenavir or atazanavir once daily boosted with ritonavir 100 mg, plus tenofovir/emtricitabine, for the initial treatment of HIV infection, 48-week results of ALERT. AIDS Res Ther. 2008;5(1):5. doi: 10.1186/1742-6405-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Efficacy and safety of once-daily atazanavir/ritonavir compared with twice daily lopinavir/ritonavir each in combination with tenofovir and emtricitabine ARV-naive HIV-1-infected subjects The CASTLE study, 48-week results; Presented at: 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 25–28 February 2008. [Google Scholar]
- 35.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19(7):685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 36.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20(5):711–718. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 37.Swindells S, DiRienzo AG, Wilkin T, et al. Regimen simplification to atazanavir–ritonavir alone as maintenance antiretroviral therapy after sustained virologic suppression. JAMA. 2006;296(7):806–814. doi: 10.1001/jama.296.7.806. [DOI] [PubMed] [Google Scholar]
- 38.Vernazza P, Daneel S, Schiffer V, et al. The role of compartment penetration in PI-monotherapy: the atazanavir–ritonavir monomaintenance (ATARITMO) trial. AIDS. 2007;21(10):1309–1315. doi: 10.1097/QAD.0b013e32814e6b1c. [DOI] [PubMed] [Google Scholar]
- 39.Karlstrom O, Josephson F, Sonnerborg A. Early virologic rebound in a pilot trial of ritonavir-boosted atazanavir monotherapy. J Acquir Immune Defic Syndr. 2007;44:417–422. doi: 10.1097/QAI.0b013e31802e2940. [DOI] [PubMed] [Google Scholar]
- 40.Guffanti M, DePascalis CR, Seminari E, et al. Pharmacokinetics of amprenavir given once or twice a day when combined with atazanavir in heavily pre-treated HIV-positive patients. AIDS. 2003;217(18):2669–2671. doi: 10.1097/00002030-200312050-00017. [DOI] [PubMed] [Google Scholar]
- 41.Seminari E, Guffanti M, Villani P. Steady-state pharmacokinetics of atazanavir given alone or in combination with saquinavir hard-gel capsuals or amprenavir in HIV-infected patients. Eur J Clin Pharmacol. 2005;61(7):545–549. doi: 10.1007/s00228-005-0966-x. [DOI] [PubMed] [Google Scholar]
- 42.Winston A, Mallon PW, Satchell C, et al. The safety, efficacy, and pharmacokinetic profile of a switch in antiretroviral therapy to saquinavir, ritonavir, and atazanavir alone for 48 weeks and a switch in the saquinavir formulation. Clin Infect Dis. 2007;44(11):1475–1483. doi: 10.1086/517507. [DOI] [PubMed] [Google Scholar]
- 43.Khanlou H, Bhatti L, Fathing C. Interaction between atazanavir and fosamprenavir in the treatment of HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):124–125. doi: 10.1097/01.qai.0000192003.00530.9c. [DOI] [PubMed] [Google Scholar]
- 44.Malan D, Krantz E, Wirtz D, et al. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2008;47(2):161–167. doi: 10.1097/QAI.0b013e31815ace6a. [DOI] [PubMed] [Google Scholar]
- 45.Ribera E, Azuaje C, Lopez RM, et al. Atazanavir and lopinavir/ritonavir: pharmacokinetics, safety and efficacy of a promising double-boosted protease inhibitor regimen. AIDS. 2006;12(8):1131–1139. doi: 10.1097/01.aids.0000226953.56976.ad. [DOI] [PubMed] [Google Scholar]
- 46.Gilliam BL, Chan-Tack KM, Qaqish RB, Rode RA, Fantry LE, Redfeld RR. Successful treatment with atazanavir and lopinavir/ritonavir combination therapy in protease inhibitor-susceptible and proteaseinhibitor-restant HIV-infected patients. AIDS Patient Care STDS. 2006;20(11):745–759. doi: 10.1089/apc.2006.20.745. [DOI] [PubMed] [Google Scholar]
- 47.von Hentig N, Kaykhin P, Stephan C, et al. Decrease of atazanavir and lopinavir plasma concentrations in a boosted double human immunodeficiency virus protease inhibitor salvage regimen. Antimicrob Agents Chemother. 2008;52(6):2273–2275. doi: 10.1128/AAC.01565-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43(15):1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 49.Kosel BW, Beckerman KP, Hayashi S, Homma M, Aweeka FT. Pharmacokinetics of nelfinavir and indinavir in HIV-1-infected pregnant women. AIDS. 2003;17:1195–1199. doi: 10.1097/00002030-200305230-00011. [DOI] [PubMed] [Google Scholar]
- 50.Acosta EP, Bardeguez A, Zorrilla CD, et al. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48:430–436. doi: 10.1128/AAC.48.2.430-436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stek AM, Mirochnick M, Capparelli M, et al. Reduced lopinavir exposure during pregnancy AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 52.Natha M, Hay P, Taylor G, et al. Atazanavir use in pregnancy: a report of 33 cases; Presented at: 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. 25–28 February 2007; Poster 750. [Google Scholar]
- 53.Ripamonti D, Cattaneo D, Maggiolo F, et al. Atazanavir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS. 2007;21(18):2409–2415. doi: 10.1097/QAD.0b013e32825a69d1. [DOI] [PubMed] [Google Scholar]
- 54.Chappuy H, Tréluyer JM, Jullien V, et al. Maternal–fetal transfer and amniotic fluid accumulation of nucleoside analogue reverse transcriptase inhibitors in human immunodeficiency virus-infected pregnant women. Antimicrob Agents Chemother. 2004;48:4332–4336. doi: 10.1128/AAC.48.11.4332-4336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marzolini C, Rudin C, Decosterd LA, et al. Transplacental passage of protease inhibitors at delivery. AIDS. 2002;16(6):889–893. doi: 10.1097/00002030-200204120-00008. [DOI] [PubMed] [Google Scholar]
- 56.Gingelmaier A, Kurowski M, Kästner R, et al. Placental transfer and pharmacokinetics of lopinavir and other protease inhibitors in combination with nevirapine at delivery. AIDS. 2006;20(13):1737–1743. doi: 10.1097/01.aids.0000242820.67001.2c. [DOI] [PubMed] [Google Scholar]
- 57.Mirochnick M, Dorenbaum A, Holland D, et al. Concentrations of protease inhibitors in cord blood after in utero exposure. Pediatr Infect Dis J. 2002;21(9):835–838. doi: 10.1097/00006454-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Chappuy H, Tréluyer JM, Rey E, et al. Maternal–fetal transfer and amniotic fluid accumulation of protease inhibitors in pregnant women who are infected with human immunodeficiency virus. Am J Obstet Gynecol. 2004;191(2):558–562. doi: 10.1016/j.ajog.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 59.Lechelt M, Lyons F, Clarke A, Magaya V, Issa R, de Ruiter A. Human placental transfer of atazanavir: a case report. AIDS. 2006;20(2):307. doi: 10.1097/01.aids.0000202653.49020.dd. [DOI] [PubMed] [Google Scholar]
- 60.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21(14):1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–1730. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Leeuwen E, Ter Heine R, van der Veen F, Repping S, Beijnen JH, Prins JM. Penetration of atazanavir in seminal plasma of men infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2007;51(1):335–337. doi: 10.1128/AAC.00774-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Witvrouw M, Pannecouque C, Switzer WM, Folks TM, De Clercq E, Heneine W. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and post exposure prophylaxis. Antivir Ther. 2004;9(1):57–65. [PubMed] [Google Scholar]
- 64.Masse S, Lu X, Dekhtyar T, et al. In vitro selection and characterization of human immunodeficiency virus type 2 with decreased susceptibility to lopinavir. Antimicrob Agents Chemother. 2007;51(9):3075–3080. doi: 10.1128/AAC.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taburet AM, Piketty C, Chazallon C, et al. Interactions between atazanavir–ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48(6):2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dailly E, Tribut O, Tattevan P, et al. Influence of tenofovir, nevirapine and evavirenz on ritonavir-boosted atazanavir pharmacokinetics in HIV-infected individuals. Eur J Clin Pharmacol. 2006;62(7):523–526. doi: 10.1007/s00228-006-0122-2. [DOI] [PubMed] [Google Scholar]
- 67.Von Hentig N, Dauer B, Haberi A, et al. Tenofovir comedication does not impair steady-state pharmacokinetics of ritonavir-boosted atazanavir in HIV-infected adults. Eur J Clin Pharmacol. 2007;63(10):935–940. doi: 10.1007/s00228-007-0344-y. [DOI] [PubMed] [Google Scholar]
- 68.Beique L, Giguere P, la Porte C, Angel J. Interactions between protease inhibitors and acid-reducing agents: a systematic review. HIV Med. 2007;8:335–345. doi: 10.1111/j.1468-1293.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 69.Fulco PP, Vora UB, Bearman GM. Acid suppressive therapy and the effects on protease inhibitors. Ann Pharmacother. 2006;40(11):1974–1983. doi: 10.1345/aph.1H022. [DOI] [PubMed] [Google Scholar]
- 70.van Lunzen J, Liess H, Arastéh K, Walli R, Daut B, Schürmann D. Concomitant use of gastric acid-reducing agents is frequent among HIV-1-infected patients receiving protease inhibitor-based highly active antiretroviral therapy. HIV Med. 2007;8(4):220–225. doi: 10.1111/j.1468-1293.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 71.Chong E, Ensom MH. Pharmacogenetics of the proton pump inhibitors: a systemic review. Pharmacotherapy. 2003;23:460–471. doi: 10.1592/phco.23.4.460.32128. [DOI] [PubMed] [Google Scholar]
- 72.Robinson M, Horn J. Clinical pharmacology of proton pump inhibitors: what the practicing physician needs to know. Drugs. 2003;63:2739–2754. doi: 10.2165/00003495-200363240-00004. [DOI] [PubMed] [Google Scholar]
- 73.Tomilo DL, Smith PF, Ogundele AB, et al. Inhibition of atazanavir oral absorption by lansoprazole gastric acid suppression in healthy volunteers. Pharmacotherapy. 2006;26:341–346. doi: 10.1592/phco.26.3.341. [DOI] [PubMed] [Google Scholar]
- 74.Antoniou T, Yoong D, Beique L, et al. Impact of acid-suppressive therapy on virologic response to atazanavir-based regimens in antiretroviral-experienced patients: a case series. J Acquir Immune Defic Syndr. 2005;39(1):126–128. doi: 10.1097/01.qai.0000160518.12957.c0. [DOI] [PubMed] [Google Scholar]
- 75.Acosta EP, Kendall MA, Gerber JG, et al. Effect of concomitantly administered rifampin on the pharmacokinetics and safety of atazanavir administered twice daily. Antimicrob Agents Chemother. 2007;51(9):3104–3110. doi: 10.1128/AAC.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pineda JA, Santos J, Rivero A, et al. Liver toxicity of antiretroviral combinations including atazanavir/ritonavir in patients coinfected with HIV and hepatitis viruses: impact of pre-existing liver fibrosis. J Antimicrob Chemother. 2008;61(4):925–932. doi: 10.1093/jac/dkn045. [DOI] [PubMed] [Google Scholar]
- 77.Slish J, Ma Q, Zingman BS, et al. Assessing the impact of substance use and hepatitis coinfection on atazanavir and lopinavir trough concentrations in HIV-infected patients during therapeutic drug monitoring. Ther Drug Monit. 2007;29(5):560–565. doi: 10.1097/FTD.0b013e31806db8ae. [DOI] [PubMed] [Google Scholar]
- 78.Colafigli M, Di Giambenedetto S, Bracciale L, Tamburrini E, Cauda R, De Luca A. Cardiovascular risk score change in HIV-1-infected patients switched to an atazanavir-based combination antiretroviral regimen. HIV Med. 2008;9(3):172–179. doi: 10.1111/j.1468-1293.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 79.Hruz PW, Yan Q, Struthers H, Jay PY. HIV protease inhibitors that block GLUT4 precipitate acute, decompensated heart failure in a mouse model of dilated cardiomyopathy. FASEB J. 2008;22(7):2161–2167. doi: 10.1096/fj.07-102269. [DOI] [PubMed] [Google Scholar]
- 80.Busti AJ, Tsikouris JP, Peeters MJ, et al. A prospective evaluation of the effect of atazanavir on the QTc interval and QTc dispersion in HIV-positive patients. HIV Med. 2006;7(5):317–322. doi: 10.1111/j.1468-1293.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 81.Gianotti N, Guffanti M, Galli L, et al. Electrocardiographic changes in HIV-infected drug-experienced patients being treated with atazanavir. AIDS. 2007;21(12):1648–1651. doi: 10.1097/QAD.0b013e32826fbc6a. [DOI] [PubMed] [Google Scholar]
- 82.Brewster UC, Perazella MA. Acute interstitial nephritis associated with atazanavir, a new protease inhibitor. Am J Kidney Dis. 2004;44(5):E81–E84. [PubMed] [Google Scholar]
- 83.Möddel M, Pfammatter R, Varga Z, Keusch G. Acute tubulointerstitial nephritis in HIV infection. Praxis (Bern 1994) 2006;95(23):949–951. doi: 10.1024/0369-8394.95.23.949. [DOI] [PubMed] [Google Scholar]
- 84.Schmid S, Opravil M, Moddel M, et al. Acute interstitial nephritis of HIV-positive patients under atazanavir and tenofovir therapy in a retrospective analysis of kidney biopsies. Virchows Arch. 2007;450(6):665–670. doi: 10.1007/s00428-007-0418-3. [DOI] [PubMed] [Google Scholar]
- 85.Change HR, Pella PM. Atazanavir urolithiasis. N Engl J Med. 2006;355:2158–2159. doi: 10.1056/NEJMc061892. [DOI] [PubMed] [Google Scholar]
- 86.Pacanowski J, Poirier JM, Petit I, Meynard JL, Girard PM. Atazanavir urinary stones in an HIV-infected patient. AIDS. 2006;20:2130–2131. doi: 10.1097/01.aids.0000247571.88256.90. [DOI] [PubMed] [Google Scholar]
- 87.Moriyama Y, Minamidate Y, Yasuda M, et al. Acute renal failure due to bilateral ureteral stone impaction in an HIV-positive patient. Urol Res. 2008;36(5):275–277. doi: 10.1007/s00240-008-0147-3. [DOI] [PubMed] [Google Scholar]
- 88.Anderson PL, Lichtenstein KA, Gerig NE, Kiser JJ, Bushman LR. Atazanavir-containing renal calculi in an HIV-infected patient. AIDS. 2007;21:1060–1061. doi: 10.1097/QAD.0b013e3280c56ae1. [DOI] [PubMed] [Google Scholar]
- 89.Chan-Tack KM, Truffa MM, Struble KA, Birnkrant DB. Atazanavir-associated nephrolithiasis: cases from the US Food and Drug Administration's adverse event reporting system. AIDS. 2007;21(9):1215–1218. doi: 10.1097/QAD.0b013e32813aee35. [DOI] [PubMed] [Google Scholar]
- 90.Gatell J, Salmon-Ceron D, Lazzarin A, et al. Efficacy and safety of atazanavir-based highly active antiretroviral therapy in patients with virologic suppression switched from a stable, boosted or unboosted protease inhibitor treatment regimen: the SWAN study (AI424-097) 48-week results. Clin Infect Dis. 2007;44:1484–1492. doi: 10.1086/517497. [DOI] [PubMed] [Google Scholar]
- 91.Mallolas J, Podzamczer D, Domingo P, et al. Efficacy and safety of switching from lopinavir/r (LPV/r) to atazanavir/r (/r) in patients with virological suppression receiving a LPV/r containing HAART: the ATAZIP study; Presented at: 4th IAS Conference on HIV pathogenesis and Treatment; Sydney, Australia. 22–25 July 2007; Abstract WEPEB117LB. [Google Scholar]
- 92.Jemsek JG, Arathoon E, Arlotti M, et al. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin Infect Dis. 2006;42:273–280. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- 93.McGrath D, Frederick D, Wirtz V, et al. Body composition changes in ART-naive subjects treated with atazanavir or atazanavir/ritonavir-based once daily HAART: 48-week computed tomography and DEXA data; Presented at: 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. 25–28 February 2007; Poster 804. [Google Scholar]
- 94.Steinbrook R. Closing the affordability gap for drugs in low-income countries. N Engl J Med. 2007;35(20):1996–1999. doi: 10.1056/NEJMp0706918. [DOI] [PubMed] [Google Scholar]
Websites
- 101. [Accessed 28 March 2008];Reyataz (atazanavir sulphate) US package insert. www.fda.gov/cder/foi/label/2007/021567s014lbl.pdf.
- 102.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; Jan 29, 2008. [Accessed 28 March 2008]. www.aidsinfo.nih.gov/contentfiles/adultandadolescentgl.pdf. [Google Scholar]
- 103. [Accessed 27 July 2008];British HIV Association (BHIVA) guidelines for the treatment of HIV-infected adults with antiretroviral therapy. 2008 www.bhiva.org/files/file1030835.pdf.
- 104. [Accessed 27 August 2008];Stanford University HIV drug resistance database. http://hivdb.stanford.edu/GRIP/.html.
- 105. [Accessed 28 March 2008];VIREAD®(tenofovir disoproxil fumarate) Patient information Leaflet. www.gilead.com/pdf/viread_pi.pdf.
- 106.CDC. Managing drug interactions in the treatment of HIV-related tuberculosis. 2007 www.cdc.gov/tb/tb_hiv_drugs/default.htm.
- 107. [Accessed 28 March 2008];Raltegravir prescribing information. www.merck.com/product/usa/pi_circulars/i/isentress/isentress_pi.pdf.