Abstract
Background
The linkage and barriers of linkage to facility-based HIV care from a mobile HIV testing unit have not previously been described.
Methods
A stratified random sample (N=192) was drawn of all eligible, newly-diagnosed HIV-infected individuals with a laboratory CD4 count result on a mobile unit between August 2008 and December 2009. All individuals with CD4 counts ≤ 350 cells/μl and 30% of individuals with CD4 counts > 350 cells/μl were sampled. Linkage to care was assessed during April to June 2010 in those that received their CD4 count result. A participant who accessed HIV care at least once after testing was regarded as having linked to care. Binomial regression models were used to identify clinical and socio-demographic factors associated with receiving a CD4 count result and linking to care.
Results
Forty-three (27%) individuals did not receive their CD4 count result. A lower CD4 count, being female and the availability of a phone number increased the likelihood of receiving this result. Follow-up was attempted in the remaining 145 individuals. Ten refused to participate and contact was unsuccessful in 42.4%. Linkage was 100% in patients with CD4 counts ≤ 200 cells/μl, 66.7% in individuals with CD4 counts of 201-350 cells/μl and 36.4% in those with CD4 counts > 350 cells/μl. A lower CD4 count, disclosure, presence of TB symptoms and unemployment increased the likelihood of linking to care.
Conclusion
Linkage to care was best among those eligible for ART. Interventions designed at improving linkage among employed individuals are urgently warranted.
Keywords: Mobile HIV testing unit, linkage to HIV and ART care, barriers to linkage to HIV care
INTRODUCTION
In 2008 an estimated 1.9 million deaths were attributable to HIV/AIDS in Sub-Saharan Africa. 1 HIV-infected individuals from this region usually present for care late and thus commence antiretroviral therapy (ART) with advanced immunodeficiency thereby increasing their risk for HIV-related mortality.2-7 An earlier HIV diagnosis followed by timely initiation of HIV care can help maximise health benefits. HIV testing remains the entry point to a continuum of HIV medical care and social support. 1 Over the past few years the number of HIV testing sites has been scaled-up significantly. 1 However, increasing testing is of little use without strategies to direct newly-diagnosed HIV positive (HIV+) patients to appropriate packages of HIV care. In South Africa, HIV+ adults with baseline CD4 counts ≤ 200 cells/μl or in WHO clinical stage IV are considered ART-eligible whereas those above these thresholds are required to remain in pre-ART care until eligible.
There is a dearth of published literature on retention in ART programmes yet few studies have examined linkage to HIV care. Nonetheless, recent studies have shown that linkage to care from traditional HIV testing points at stationary facilities is often inadequate and varies across sites. 8-13 Factors known to enhance enrolment to care at these sites include use of referral forms, transportation allowances and community escorts. 12 The main predictors of non-linkage to HIV and ART care from these facilities include unemployment 8, user-fees 8, distance of clinic 10,12, transport costs 12, lack of education 14, history of tuberculosis (TB) treatment 10, referral by healthcare providers 10 as well as being male 11, a young adult 11 or a TB patient 11. Noteworthy is that failure to access care is prominent among those with lower CD4 counts 8 and individuals self-referred for testing. 11 Successful interventions resulting in earlier diagnosis of HIV must also be accompanied by strategies that enhance linkage into HIV care in order to improve health outcomes.
Mobile HIV testing services have been shown to facilitate testing of individuals earlier in their stage of HIV infection and to be more accessible to hard-to-reach and high-risk populations. 15-19 Moreover, streamlined HIV counselling and testing (HCT) procedures offered by mobile services allow for a high number of individuals to be screened. 15-19 Evidence indicates that in comparison to stationary testing facilities, mobile testing units are cost-effective in diagnosing new cases of HIV. 19 However, ongoing HIV care may not be sustainable from mobile units therefore requiring patients to be referred to stationary facilities for the necessary follow-up care. Furthermore, it is possible that linkage to facility based care from these units is inadequate because of their mobile nature. 12
To date, no study has assessed the extent to which people diagnosed with HIV in mobile testing units’ link to HIV medical care at public healthcare facilities. Understanding the associated challenges of linkage to HIV care from mobile units is important if this mobile approach is to be considered for the expansion of HCT. The aim of this study was to assess the proportion and characteristics of individuals who accessed HIV care after testing HIV+ in a mobile testing unit. We investigated whether disease progression (as defined by CD4 count) influenced access to care, and we described the barriers of linkage to care.
METHODS
Study Design
Individuals diagnosed with HIV (for the first time) at the mobile unit during a 16 month period were identified retrospectively through mobile unit records. Those who received their laboratory CD4 count result were prospectively followed up to assess linkage to HIV care.
Setting and population
A nurse-run and counsellor-supported mobile testing unit, as described elsewhere 15, provided free HCT services to underserved communities within the Cape Metropolitan region, Western Cape, South Africa.
Client-initiated HCT was offered in combination with free screening for other chronic conditions (i.e. hypertension, diabetes and obesity) and TB to a predominantly black Xhosa-speaking African population. HCT was performed according to the Provincial Government guidelines. 20 Individuals who consented to rapid HIV testing and tested positive were subsequently clinically staged and underwent laboratory CD4 count testing.
After the completion of testing, individuals received a referral letter to take to a healthcare provider to facilitate their access to HIV care. Nurses detailed all conditions that the individual was referred for on this letter (i.e. HIV/ART care, concomitant medical problems and/or screening for other conditions). When CD4 counts became available (usually within 72 hours) patients were contacted telephonically and received their results. If contact numbers were unavailable, home visits were done or a letter was sent to the patient, requesting the individual to contact the counsellor for their result. Once contact was established, the CD4 count result was received and implications discussed. All HIV+ patients were encouraged to attend clinics for either HIV care or to start ART if eligible according to South African national guidelines 21 and other non HIV-related health services if necessary. The aforementioned procedures were followed for all HIV+ patients.
Sampling strategy
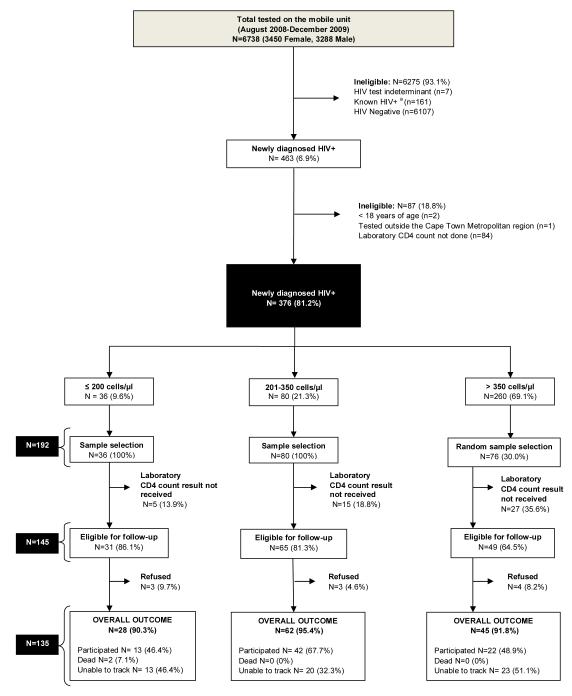
Records of all those who were newly-diagnosed with HIV between August 2008 and December 2009 were accessed and retrospectively reviewed to identify an eligible cohort (Fig.1). Only those who were newly-diagnosed with HIV (≥ 18 years of age) on the mobile unit within the Cape Metropolitan region and had a laboratory CD4 count done were eligible for follow-up. The majority of newly-diagnosed HIV+ individuals diagnosed on this mobile unit have CD4 counts > 350 cells/μl.15 Due to a higher proportion of patients in this stratum a stratified random sample was drawn. We sampled 100% of individuals with laboratory CD4 counts ≤ 350 and 30% of individuals with laboratory CD4 counts > 350 cells/μl.
Figure 1.
Schematic representation of study’s inclusion and exclusion criteria for each stage of the analysis, sampling procedure and overall outcome for each CD4 count strata. a) HIV+= HIV Positive
Follow-up for assessment of linkage to HIV care
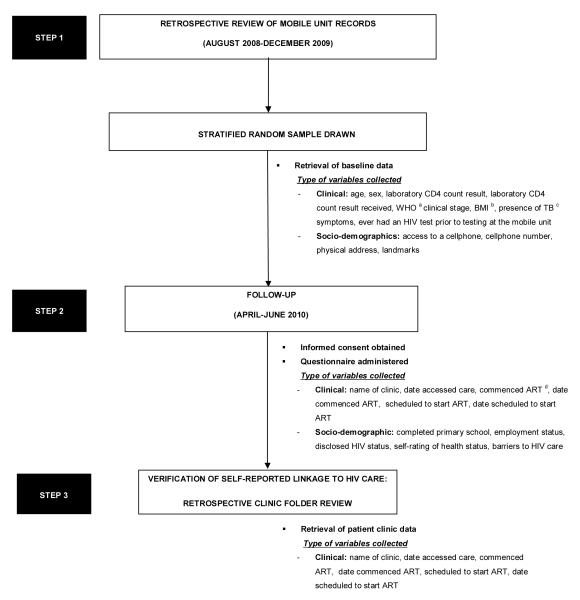
Eligible individuals who had previously received their CD4 count result were telephoned or interviewed in person between April and June 2010, to obtain informed consent (Fig. 2). At least 3 telephonic attempts were made to track the participant and if no contact number was available a home visit was conducted. A questionnaire was subsequently administered to those consenting to participate. Clinical and socio-demographic data were collected by mobile unit record reviews and interviews.
Figure 2.
Flow diagram illustrating study procedures, points of data collection and types of variables collected. a) WHO= World Health Organisation, b) BMI= Body Mass Index, c) TB= Tuberculosis and d) ART= Antiretroviral Therapy
Verification of self-reported linkage to HIV care
A retrospective clinic folder review was conducted at respective public healthcare facilities on participants who reported they linked to medical care, once additional consent was acquired for this process (Fig.2).
Ethics
Informed consent (written or verbal) was obtained from participants. Data collection and analysis was approved by the University of Cape Town Research Ethics Committee and the Provincial Government of the Western Cape Research Ethics committee.
Study definitions
Outcomes
Laboratory CD4 count result received
A newly-diagnosed HIV+ individual who tested at the mobile unit and was subsequently successfully contacted (telephonically or by home visit) and received their laboratory CD4 count test result.
Linked to care
A newly-diagnosed HIV+ individual who accessed HIV care at a public healthcare facility at least once after testing on the mobile unit.
Predictors
Presence of TB symptoms
A newly-diagnosed HIV+ patient who had one or more TB symptoms (i.e. cough or fever for > 2 weeks, weight loss of > 1.5 kgs in the last month, drenching night sweats, loss of appetite, blood stained sputum).
Disclosure of HIV status
A newly-diagnosed HIV+ patient who had disclosed his/her status to at least one other.
Statistical analysis
Data were double entered into EpiData Entry (Version 3.1) and analysis was carried out using STATA (Version 11.0, Stata Corporation. LP, College Station, TX, USA). Data were first explored via cross-tabulations and chi square tests. Total proportions were calculated taking sampling weights into account. Baseline characteristics of the stratified random sample (N=192) were used to identify correlates of receiving a laboratory CD4 count result through a binomial regression. Variables that had 10% significance in the bivariate analysis were included in the multivariate model. In addition, clinical and socio-demographic variables of all participants (N=77) were examined to ascertain those that best predicted linkage to care through a binomial regression, using bivariate analysis only. All variables in the regression analysis were controlled for laboratory CD4 count strata.
RESULTS
A total of 6738 records of individuals accessing the mobile service (51.2% female) between August 2008 and December 2009 were available (Fig.1). The overall prevalence of those newly-diagnosed with HIV was 6.9% (463/6738). Only 376 (81.2%) of these newly-diagnosed HIV+ individuals met the study’s inclusion criteria. A stratified random sample (N=192) was then drawn from this cohort (N=376): 36/36 individuals with CD4 counts ≤ 200 cells/μl, 80/80 individuals with CD4 counts between 201-350 cells/μl and 30% of individuals with CD4 counts > 350 cells/μl (76/260) were sampled.
Baseline characteristics of individuals in the stratified random sample
Of the 192 newly-diagnosed HIV+ individuals sampled, majority was women (60.5%) and the mean age was 34.8 years (Table 1). In total, 60.2% had tested for HIV previously. The mean CD4 count was 488.6 cells/μl, majority (60.4%) was WHO clinical stage I and the mean body mass index (BMI) was 26.7 kg/m2. Furthermore, TB symptoms occurred in 20.7% and only 18.2% of individuals could not provide a contact telephone number.
Table 1.
Description of baseline characteristics for those newly-diagnosed with HIV on the mobile unit and had a laboratory CD4 count done (N=192).
| Variable | Total N=192 % (95% CI) a |
CD4 ≤ 200 cells/μl (n=36) % (n) |
CD4 201-350 cells/μl (n=80) % (n) |
CD4 > 350 cells/μl (n=76) % (n) |
|---|---|---|---|---|
| Laboratory CD4 count result received | 72.8 (65.0-80.5) | 86.1 (31) | 81.3 (65) | 68.1 (49) |
| Access to a cellphone | 81.8 (75.2-88.4) | 91.7 (33) | 82.5 (66) | 80.3 (61) |
| Female | 60.5 (52.3-68.7) | 66.7 (24) | 57.5 (46) | 60.5 (46) |
| Mean age | 34.8 (33.1-36.5) | 37.1 | 36.2 | 34.1 |
|
Ever had an HIV test
prior to testing at the mobile unit |
60.2 (52.2-68.2) | 40.0 (14) | 48.1 (38) | 66.7 (50) |
| Mean laboratory CD4 count (cells/μl) | 488.6 (457.5-519.6) | 134.6 | 279.1 | 602.0 |
| WHO b clinical stage ( I vs. II, III and IV) | 60.4 (52.5-68.3) | 34.3 (12) | 45.6 (36) | 68.4 (52) |
| Mean BMI c | 26.7 (25.7-27.7) | 27.1 | 27.2 | 26.5 |
| Presence of TB d symptoms | 20.7 (14.4-27.1) | 41.7 (15) | 32.4 (24) | 14.1 (10) |
95% CI= 95% Confidence Interval,
WHO= World Health Organisation,
BMI= Body Mass Index and
TB= Tuberculosis
Predictors for receiving a laboratory CD4 count result
A total of 73% of individuals received their CD4 count (Table 1). Receipt of a CD4 count result was 0.82 times less likely for individuals with CD4 counts > 350 cells/μl compared to individuals with CD4 counts ≤ 350 cells/μl (95% CI: 0.69-0.98) (Table 2). Being a woman, having access to a cellphone and having lower CD4 counts were factors that increased the likelihood of receiving CD4 count results in the bivariate analysis. Results were similar in the multivariate analysis: individuals with a CD4 count > 350 cells/μl were 0.85 times less likely to receive their CD4 count result (95% CI: 0.73-0.99). Availability of a cellphone made a successful contact twice as likely.
Table 2.
Binomial regression models to assess the likelihood of receiving a laboratory CD4 count result among individuals newly-diagnosed with HIV on the mobile unit (N=145).
| Variable | Univariate analysis RR a (95% CI) b |
Bivariate analysis c RR (95% CI) |
Multivariate analysis c, d RR (95% CI) |
|---|---|---|---|
|
Laboratory CD4 count
(cells/μl) |
|||
| CD4 ≤ 350 | 1 | 1 | |
| CD4 > 350 | 0.82 (0.69-0.98) 1 | 0.85 (0.73-0.99) 4 | |
| Sex | |||
| Male | 1 | 1 | |
| Female | 1.19 (1.01-1.40) 2 | 1.09 (0.95-1.24) | |
| Age | |||
| ≤30 years | 1 | ||
| ≥ 31 years | 1.04 (0.88-1.24) | ||
| Has a cellphone | |||
| No | 1 | 1 | |
| Yes | 2.71 (1.58-4.65) 3 | 2.64 (1.54-4.53) 5 |
RR= Risk Ratio,
95% CI= 95% Confidence Interval,
all variables in the bivariate and multivariate analysis were adjusted for laboratory CD4 count
variables assessed in the multivariate analysis: laboratory CD4 count, sex and has a cellphone.
P= 0.032,
P= 0.041,
P= 0.001,
P=0.001
P= 0.032
Baseline characteristics of individuals lost to follow-up
Of the 145 individuals eligible for follow-up, 56 (42.4%) were untraceable, despite previously having been successfully contacted and received their CD4 count result (Fig.1). This included 13 with CD4 counts < 200 cells/μl (46.4%), 20 with CD4 counts in the mid range (32.3%) and 23 with CD4 counts > 350 cells/μl (51.1%). The majority were women (52.9%) with a mean age of 34.2 years, mean BMI of 26.5 kg/m2, mean CD4 count of 458.3 cells/μl and were in WHO clinical stage I. Moreover, TB symptoms were prevalent in 16% and most (95.1%) had access to a cellphone.
Characteristics of study participants
Seventy-seven newly-diagnosed HIV+ individuals who received their CD4 count result participated (Table 3). Most of these individuals completed primary school (84.8%). Unemployment was more prevalent in individuals with CD4 counts ≤ 200 cells/μl (76.9%) compared to individuals with CD4 counts >350 cells/μl. Of the 69.3% who disclosed their HIV status, only a small proportion (10.9%) felt stigmatised after disclosing. The majority of participants (73.8%) rated their health status as “strong”.
Table 3.
Description of clinical and socio-demographic data for newly-diagnosed HIV+ individuals that received their laboratory CD4 count result and participated (N=77).
| Variable | Total N= 77 % (95% CI) a |
CD4 ≤ 200 cells/μl (n=13) % (n) |
CD4 201-350 cells/μl (n=42) % (n) |
CD4 > 350 cells/μl (n=22) % (n) |
|---|---|---|---|---|
| Female | 72.0 (59.6-84.3) | 76.9 (10) | 69.1 (29) | 72.7 (16) |
| Mean age | 36.6 (33.7-39.6) | 39.1 | 36.6 | 36.2 |
| WHO b clinical stage ( I vs. II, III and IV) | 63.5 (50.9-76.1) | 53.9 (7) | 50.0 (21) | 72.7 (16) |
| Mean laboratory CD4 count (cells/μl) | 432.8 (387.2-478.4) | 123.4 | 288.0 | 567.1 |
| Mean BMI c | 28.2 (26.3-30.0) | 27.7 | 27.9 | 28.5 |
| Presence of TB d symptoms | 24.4 (13.7-35.1) | 53.9 (7) | 33.3 (13) | 14.3 (3) |
|
Ever had an HIV test prior to testing at
the mobile unit |
60.3 (47.0-73.7) | 58.3 (7) | 50.0 (21) | 66.7 (14) |
| Completed primary school | 84.8 (74.4-95.2) | 83.3 (10) | 90.5 (38) | 81.8 (18) |
| Unemployed | 50.1 (36.3-63.8) | 76.9 (10) | 50.0 (21) | 45.5 (10) |
| Informal dwelling | 64.4 (51.2-77.7) | 53.9 (7) | 69.1 (29) | 63.6 (14) |
| Disclosed one’s HIV status | 69.3 (56.5-82.1) | 69.2 (9) | 71.4 (30) | 68.2 (15) |
|
Self-rating of health status ( strong vs.
average and weak) |
73.8 (62.0-85.6) | 69.2 (9) | 69.1 (29) | 77.3 (17) |
| Linked to HIV care | 52.5 (39.4-65.6) e | 100.0 (13) | 66.7 (28) | 36.4 (8) |
| Mean time (months) from diagnosis to accessing HIV care |
2.2 (0.3-4.0) | 0.6 | 2.0 | 3.0 |
| On ART e | 69.2 (9) | N/A | N/A | |
| Mean time (months) from accessing care to starting ART |
1.9 (0.6-3.1) | 1.9 | N/A | N/A |
| Mean time (months) from diagnosis to starting ART |
4.3 (2.1-6.5) | 4.3 | N/A | N/A |
| Scheduled to start ART | 30.8 (4) | N/A | N/A | |
| Failed to link to HIV care | 47.5 (34.4-60.6) | 0 | 33.3 (14) | 63.6 (14) |
| Barriers to HIV care: | ||||
| Work during the day and cannot get time off to visit the clinic |
41.4 (21.9-61.0) | N/A | 25.0 (5) | 47.1 (8) |
| Clinic too far away from work | 15.7 (0.9-30.5) | N/A | 10.0 (2) | 17.7 (3) |
| Fear toxicity and side effects of ART |
12.6 (0.3-25.5) | N/A | 15.0 (3) | 11.8 (2) |
| Fear of disclosure of one’s HIV status/stigma associated with being HIV+ f /social isolation |
8.8 (3.4-20.9) | N/A | 0 | 11.8 (2) |
95% CI= 95% Confidence Interval,
WHO= World Health Organisation,
BMI= Body Mass Index,
TB= Tuberculosis,
ART= Antiretroviral Therapy
HIV+= HIV Positive
Linkage to HIV care
Overall, 52.5% (49) accessed HIV medical care (Table 3). There was an inverse relationship between CD4 count result and linkage to care: all of those with CD4 counts ≤ 200 cells/μl linked to care, however, 66.7% and 36.4% of those with CD4 counts between 201-350 cells/μl and > 350 cells/μl, respectively, linked to care. Among those who accessed care, the majority (71.0%) stated that the mobile unit’s referral letter facilitated their linkage to care at a public healthcare facility. The overall mean time from diagnosis to accessing HIV care was 2.2 months. Those with CD4 counts ≤ 200 cells/μl took on average less than a month (3 weeks) to access care whereas those with CD4 counts > 350 cells/μl took a mean time of 3 months. Of those with CD4 counts ≤ 200 cells/μl 69.2% started ART within 2 months of diagnosis and the remaining 30.8% were within the ART screening process.
Verification of self-reported linkage to HIV care
Among the 49 who linked to care, 43 (88%) provided additional consent to access their clinic folders at respective public healthcare facilities. Eight (19%) of these folders were untraceable. A validation of the 35 (81.4%) clinic folders which were traced showed that the sensitivity of self-reported linkage to care was excellent (100%).
Predictors of accessing HIV medical care
Those contactable patients with CD4 counts > 350 cells/μl were 0.49 times less likely to link to care compared to individuals with CD4 counts ≤ 350 cells/μl (95% CI: 0.27-0.87) (Table 4). In the bivariate analysis, individuals who had TB symptoms and who had disclosed their HIV status were more likely to link to care whereas those employed were less likely.
Table 4.
Binomial regression models to assess the likelihood of linking to care among newly-diagnosed HIV+ individuals that received a laboratory CD4 count (N=77).
| Variable | Univariate analysis RR a (95% CI) b |
Bivariate analysis c RR (95% CI) |
|---|---|---|
| Laboratory CD4 count (cells/ μl ) | ||
| CD4 ≤ 350 | 1 | |
| CD4 > 350 | 0.49 (0.27-0.87) 1 | |
| Sex | ||
| Male | 1 | |
| Female | 1.18 (0.81-1.72) | |
| Age | ||
| ≤30 years | 1 | |
| ≥ 31 years | 1.21 (0.83-1.77) | |
| WHO d clinical stage | ||
| II, III and IV | 1 | |
| I | 0.88 (0.65-1.18) | |
| Presence of TB e symptoms | ||
| No | 1 | |
| Yes | 1.45 (1.11-1.90) 2 | |
| Disclosed one’s HIV status | ||
| No | 1 | |
| Yes | 1.57 (0.99-2.48) 3 | |
| Self-rating of health status | ||
| Weak and average | 1 | |
| Strong | 1.00 (0.72-1.38) | |
| Dwelling | ||
| Informal | 1 | |
| Formal | 1.05 (0.78-1.42) | |
| Completed primary school | ||
| No | 1 | |
| Yes | 1.17 (0.66-2.08) | |
| Employment Status | ||
| Unemployed | 1 | |
| Employed | 0.72 (0.51-1.01) 4 |
RR= Risk Ratio,
95% CI= 95% Confidence Interval,
all variables in the bivariate analysis were adjusted for laboratory CD4 count,
WHO= World Health Organisation
TB= Tuberculosis
P= 0.014,
P= 0.007,
P= 0.054
P= 0.056
Barriers of linking to HIV care
The most common stated barrier (41.4%) to linking to care was the inability to access public healthcare facilities during working hours and many participants reported that they could not get time off work (Table 3). Other barriers included fear of toxicity and side effects of ART (12.6%) as well as stigma and fear of disclosure (8.8 %).
DISCUSSION
Losses can occur at critical phases in the cascade from HIV testing to care. This study highlights the challenges of contacting newly-diagnosed HIV+ individuals accessing a mobile testing service in order to inform them of their CD4 count result and the poor linkage to HIV care among those with higher CD4 counts. However, results also indicate that linkage to care among those eligible for ART from this mobile unit is higher than that reported in studies examining linkage from stationary facilities.
Approximately 27% of newly-diagnosed HIV+ individuals who underwent CD4 count testing at the mobile unit did not receive this test result. Failure to receive a baseline CD4 count could result in delays in entering into HIV care. Our findings indicate that a CD4 count result of ≤ 350 cells/μl, availability of a cellphone and being female increased one’s likelihood for receiving a CD4 count result. Other recently conducted studies in South Africa have shown poor return for CD4 count results particularly in patients with higher CD4 counts. 9, 10 In our study, the CD4 count variable was adjusted for in the model as it is conceivable that due to the urgency for ART-eligible patients to link to care, personnel may have placed more emphasis on following up this stratum of patients with their result, despite protocol, additionally, these patients may have felt unwell at the time of diagnosis therefore might have actively facilitated the receipt of their result. Overall, these data underscore the difficulties faced in following up clients after blood is drawn for laboratory CD4 testing and the value of point-of-care (POC) CD4 count testing on mobile units as well as in stationary facilities to attenuate the high attrition occurring between HIV diagnoses and receiving a CD4 count result.
All individuals diagnosed HIV+ on this mobile are given a referral letter to actively seek care. In our study linkage to care among the ART-eligible patients who could be traced was 100%, albeit a small sample size. This finding was unexpectedly higher than studies assessing linkage to care in stationary facilities as these found a 30-80% linkage among ART-eligible individuals. 8-13
We showed that there was a delay from time of diagnosis to initiation of ART in that 30.8% of these individuals were still waiting to commence ART after 2 months post diagnosis. This period of ART initiation delay is consistent with findings from South Africa (3-6 months) 8-11, Mozambique (3 months) 13 and Malawi (22 days after screening) 14. This indicates the need for further interventions, if recommendations of shortening treatment readiness programmes and prioritising those with advance disease are met. 8 In contrast, we found that linkage to care among those with higher CD4 counts was poor as more than 60% of those with CD4 counts > 350 cells/μl failed to access care. This is similar to the findings from two South African studies conducted in stationary services 9, 11, however there is a paucity of studies assessing linkage to care among ART-ineligible patients. More data and possibly better interventions in this category are required as test and treat strategies are contemplated for reduced population HIV transmission.
Our results also illustrate that having a higher CD4 count, no TB symptoms, not disclosing an HIV+ status and being employed increased one’s risk for not linking to care. Patients who are physically well may not perceive the need to register for follow-up care, especially those in the higher CD4 count bracket as evidenced in this study. Hence linkage to care at healthcare facilities maybe considerably higher than mobile services because patients presenting at these sites have advanced disease compared to those accessing mobile services. 15 Those patients with lower CD4 counts and TB symptoms were possibly more motivated to link to care because of their weakened physical condition at time of diagnosis or mobile unit staff may have put greater emphasis on the urgency to link to care based on their clinical results. Furthermore, unemployment was the highest among individuals with CD4 counts ≤ 200 cells/μl hence these individuals may have had time during the day to access clinics.
The main barriers of linkage to HIV and ART care from stationary facilities have been well established, these include transportation costs 12, being male 11 and having a low CD4 count 8. This is the first study, to our knowledge, that has assessed barriers to linkage from a mobile unit. The fact that clinics are inaccessible outside working hours was identified as a major barrier to linkage to care from a mobile unit. The mobile unit, unlike state run facilities does operate outside of traditional working hours and hence may have accessed clients who would not have tested due to difficulties with clinic operating hours. It is likely that those who access the mobile unit outside working hours and on weekends have difficulty in subsequently linking to care at a public healthcare facility. Thus, extending service hours to evenings and weekends at public clinics and establishing work-place programmes through mobile units might improve linkage to care among the working population. In addition, more thought into the type of messaging delivered to those with higher baseline CD4 counts and the type of services offered whilst waiting for ART eligibility are warranted. This study would suggest that at least adequate advice and support on disclosure of an HIV+ status might improve linkage to HIV wellness programmes.
Our findings are subject to several limitations. The small sample size limited the power to detect associations particularly in the group of individuals with CD4 counts ≤ 200 cells/μl. Furthermore, a multivariate analysis for the assessment of predictors of linkage to care could not be conducted due to insufficient power. Notably, we were unable to track 42.4% of the eligible participants despite multiple follow-up attempts which might have resulted in a biased estimate. The strata with < 200 and > 350 CD4 cells/μl had the greatest proportion of uncontactable patients. The challenges we encountered in following up HIV+ patients were exacerbated by the fact that this study was conducted 6-18 months after their HIV+ diagnosis and were similar to those highlighted in an earlier study conducted in South Africa. These include incorrect contact details, lost cellphones, disconnected cellphone numbers, changes in physical address. 8 It is likely that the proportion deceased in our study maybe underestimated as the high number of disconnected cellphone numbers is perhaps indicative of death particularly in the more advanced patients. 8 We were unable to trace 19% of patient folders to validate clinic attendance as these were either misplaced at the clinic or participants provided us with incorrect clinic information. Recall and social desirability bias may have also influenced findings pertaining to disclosure, clinic visit, reasons for failing to link to care as well as self-assessment of one’s current health and the mobile unit.
Strengths of the study included that self-reported linkage to HIV care was verified through clinic folder reviews and experienced HIV/AIDS counsellors conducted follow-ups. Trained bilingual counsellors were employed to minimise respondent bias and no incentives were received for participation. The mobile HIV testing services and HIV care at public healthcare facilities that these individuals accessed were free of charge therefore our results could be generalised to a similar setting.
In conclusion, findings from this study indicate that linkage to care from a mobile testing unit is feasible. In the cascade from HIV testing to care, one area of attrition is the delivery of CD4 count results which can be remedied with the use of reliable POC CD4 count testing. Linkage to HIV care was shown to be inversely related to CD4 count result.This will need to be addressed in the setting of test and treat interventions. Of note, ART-eligible patients linked to care best but this study also indicates delays in ART commencement among these patients which could adversely affect outcomes. Other areas for intervention include improving healthcare access for employed HIV-infected individuals, and ensuring health staff emphasise the need to link to care despite feeling well and having a high CD4 count.
ACKNOWLEDGEMENTS
We would like to acknowledge all those who sacrificed their time to participate in this study. We are indebted to the dedicated study staff, Tutu Tester Project and staff at Desmond Tutu HIV Foundation. We are also thankful to all public healthcare facilities within the Cape Metropolitan region that kindly assisted us with patient folder reviews.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1. [Date accessed 12 December 2009];Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2009. Available at: http://www.who.int/entity/hiv/pub/tuapr_2009_en.pdf.
- 2.Adam MA, Johnson LF. Estimation of adult antiretroviral treatment coverage in South Africa. S Afr Med J. 2009;99:661–667. [PubMed] [Google Scholar]
- 3.Cornell M, Technau K, Fairall L, et al. Monitoring the South African National Antiretroviral Treatment Programme, 2003-2007: the IeDEA Southern African collaboration. S Afr Med J. 2009;99:653–660. [PMC free article] [PubMed] [Google Scholar]
- 4.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;9543:1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 6.Amuron B, Namara G, Birungi J, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassett IV, Wang B, Chetty S, et al. Loss to care and death before antiretroviral therapy in Durban, South Africa. J Acquir Immune Defic Syndr. 2009;51:135–139. doi: 10.1097/qai.0b013e3181a44ef2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson BA, Brennan A, McNamara L, et al. Lost opportunities to complete CD4+ lymphocyte testing among individuals who tested positive for HIV in South Africa. Bull World Health Organ. 2010;88:675–680. doi: 10.2471/BLT.09.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS One. 2010;5:e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranzer K, Zeinecker J, Ginsberg P, et al. Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS One. 2010;5:e13801. doi: 10.1371/journal.pone.0013801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nsigaye R, Wringe A, Roura M, et al. From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc. 2009;12:31. doi: 10.1186/1758-2652-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micek MA, Gimbel-Sherr K, Baptista AJ, et al. Loss to Follow-Up of Adults in Public HIV Care Systems in Central Mozambique: Identifying Obstacles to Treatment. J Acquir Immune Defic Syndr. 2009;51:397–405. doi: 10.1097/QAI.0b013e3181ab73e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath N, Glynn JR, Saul J, et al. What happens to ART-eligible patients who do not start ART? Dropout between screening and ART initiation: a cohort study in Karonga Malawi. BMC Public Health. 2010;10:601. doi: 10.1186/1471-2458-10-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Schaik N, Kranzer K, Wood R, et al. Earlier HIV diagnosis: are mobile services the answer? S Afr Med J. 2010;100:671–674. doi: 10.7196/samj.4162. [DOI] [PubMed] [Google Scholar]
- 16.Matovu JKB, Makumbi FE. Expanding access to voluntary HIV counselling and testing in sub-Saharan Africa: alternative approaches for improving uptake, 2001-2007. Trop Med Int Health. 2007;12:1315–1322. doi: 10.1111/j.1365-3156.2007.01923.x. [DOI] [PubMed] [Google Scholar]
- 17.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing Barriers to Knowing HIV Status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41:218–224. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 18.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): A community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49:422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabbe K, Menzies N, Taegtmeyer M, et al. Increasing Access to HIV Counseling and Testing Through Mobile Services in Kenya: Strategies, Utilization, and Cost-Effectiveness. J Acquir Immune Defic Syndr. 2010;54:317–323. doi: 10.1097/QAI.0b013e3181ced126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. [Date accessed 15 February 2011];Western Cape Department of Health: HIV Counselling and Testing Protocol. 2009 Dec; Available at: http://dws.wcape.gov.za/pls/dmsv525/PubShowFolders?p_folder_id=17541.
- 21.Clinical Guidelines for the management of HIV and AIDS in adults and adolescents [Date accessed 29 October 2010];National Department of Health South Africa. 2010 Available at: http://www.doh.gov.za/docs/factsheets/guidelines/adult_art.pdf.