Summary
We describe a case of brain cortical reorganization after embolization of a large right temporal arteriovenous malformation. A comprehensive imaging protocol, including functional magnetic resonance imaging (fMRI), cortical thickness analysis and 320-row computed tomography (CT) perfusion was used to provide information on brain plasticity and potential steal phenomenon.
A 25-year-old man known for a right temporal grade V Spetzler-Martin classification arteriovenous malformation (AVM) presented with left progressive hemiparesis.
He underwent functional 3T magnetic resonance imaging (fMRI), cortical thickness analysis, and CT perfusion (CT 320 row, Aquilion ONE, Toshiba, Tokyo, Japan) before and after endovascular treatment. The results were compared to look for modifications in brain perfusion and organization.
An improvement in the left hemiparesis and a reorganization of motor function were observed after endovascular treatment.
Modifications in the angioarchitecture and perfusion of an extensive AVM may be accompanied by a functional and structural reorganization of the brain. The location in the so-called eloquent regions may not be sufficient to explain the wide spectrum of symptoms that these patients can present. A more comprehensive approach considering a global involvement of the brain in patients with large AVMs is suggested to achieve the best treatment strategy and to stage treatment in incurable AVMs.
Key words: intracranial arteriovenous malformation, functional neuroimaging, brain perfusion
Introduction
Brain arteriovenous malformations (AVMs) are characterized by a direct communication between arteries and an abnormal venous system without interposing capillaries 1. The mean age at presentation for AVMs is approximately 35-40 years 2,3. AVMs may arise de novo, regress or even reappear 4,5.
The pathogenesis of AVMs, their evolution and the possible influence of environmental factors on their morphology are still unknown 2. Their variability in size, hemodynamic features and vascular components make an accurate determination of their natural history difficult 6,7.
Given that AVMs are embedded in the brain, functionally important tissue may be displaced by the lesion or enmeshed in it 8,9. Conventional neuroimaging alone is not sufficient to describe the overall AVM picture, since, even if it can provide a precise anatomical localization, it does not offer any information about functional organization. Functional MRI (fMRI) has been shown to be an effective means of studying eloquently localized vascular malformations 10,11. However, this tool cannot be sufficient to explain completely the complex clinical picture and evolution of patients with AVMs. In fact, the hemodynamic features – such as high or low flow – which are essential in the understanding of these lesions, can bias the findings of functional MRI.
The so-called “steal phenomenon” - described as both the relative hypoperfusion due to shunting of blood and decreased perfusion pressure caused by venous hypertension in the brain tissue adjacent to the AVM area - has been considered a possible cause of neurological symptoms 12. Despite evidence provided by neuropsychological studies following treatment 13 and by PET studies before and after endovascular procedures 14,15, the debate on the significance of the “steal phenomenon” in AVMs remains open and a consensus has not been achieved in the literature.
The complementary use of imaging techniques, such as functional MRI, anatomical MRI (e.g. cortical thickness analysis) and perfusion studies may help to integrate more information about brain functioning and hemodynamics 16.
We describe here the case of a patient with a right temporal AVM who initially presented with a motor and sensory deficit. A comprehensive imaging protocol allowed us to illustrate the close relation between brain function reorganization and hemodynamic modifications. The role of different imaging techniques in describing brain plasticity is also discussed.
Case Report
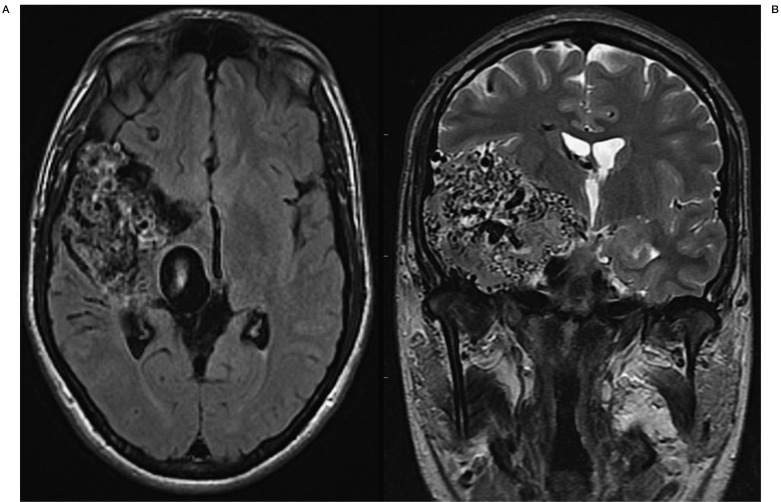
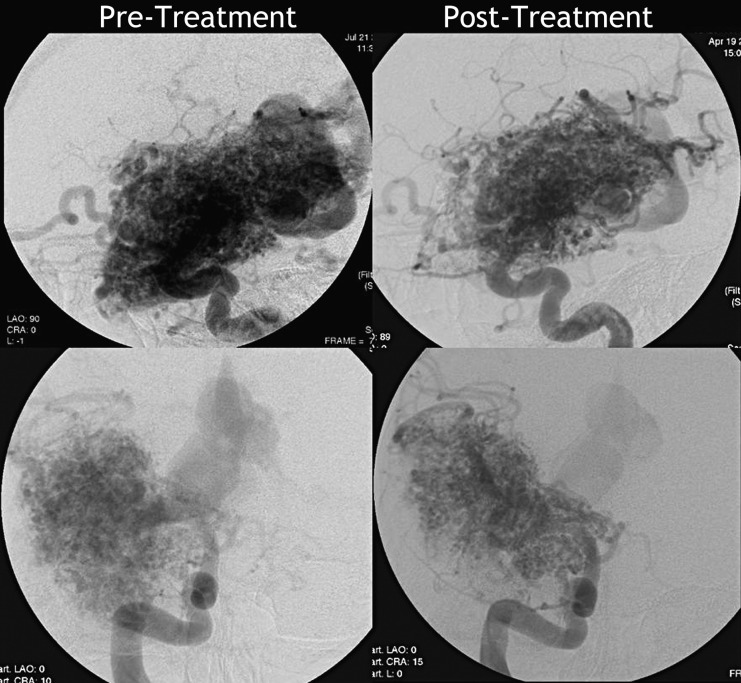
A 25-year-old right-handed man known for a large right temporal AVM (Spetzler-Martin grade V) was seen in our clinic in 2010 after deterioration of his neurological condition. The AVM had been discovered at nine years of age during a screening examination due to familiar history of vascular malformations 17. The nidus of the AVM measured 5.5×7×4 cm and the lesion appeared to be fed by several branches mainly of the right middle cerebral artery (Figures 1 and 2). The ectatic venous drainage was both superficial, and deep through the basal vein of Rosenthal (Figures 1 and 2). The AVM appeared to be relatively stable during follow-ups in 2002 and 2006.
Figure 1.
MR axial T1-weighted (left) and coronal T2-weighted (right) images of the patient showing the grade V Spetzler-Martin right occipito-parieto-temporal AVM.
Figure 2.
Digital subtraction angiography before and after treatment (upper row, lateral view; lower row, postero-anterior view) showing the AVM nidus fed by several branches of the right MCA and with an ectatic venous drainage both superficial and deep through the basal vein of Rosenthal. Reduction in the size and density of the nidus is seen in the post-embolization images in comparison to the pre-treatment study, in particular in the antero-superior and inferior regions of the lesion.
In May 2010, the patient presented at our Institution complaining of progressive and almost constant weakness and sensory problems of the left hemibody and fatigue. These symptoms had been known for more than ten years, but they were transient and of short duration. The patient had also reported episodes of complicated migraines and speech difficulties.
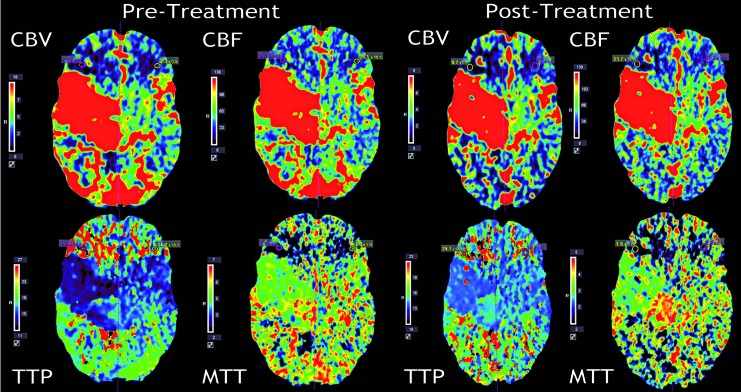
The neurological examination was characterized by left hemibody hypoesthesia and hemiparesis (strength 3/5 both at the left upper and lower limb). The MRI showed that the appearance and overall size of the AVM had not significantly changed since 2002. A dilation of the venous drainage was responsible for considerable mass effect at the level of the mesencephalon; the signal intensity of perilesional brain parenchyma appeared normal. A perfusion CT study was obtained by using the multidetector 320 row CT scanner Aquilion ONE (Toshiba, Tokyo, Japan) with a combination CTA/CBP protocol. The examination was performed with intravenous injection through the right antecubital vein using an 18G Cathlon needle of 60 ml of Isovue 370 (Iopamidol) (Bracco Diagnostics Inc, Milan, Italy) at the rate of 5 cc/s. The post-processing analysis was performed with Vitrea Perfusion software (Vital Images, Minnetonka, MN, USA) to obtain maps of cerebral blood flow (CBF) and volume (CBV), mean transit time (MTT) and time to peak (TTP). The arterial input and the venous output function regions of interest were manually selected at the left internal carotid terminus and at the straight sinus respectively at the time of their maximal contrast density. The two hemispheres were compared to look for increase or decrease in the perfusion parameters. From this comparison, CBV, CBF and MTT appeared decreased and the TTP increased in the area surrounding the lesion, in particular in the right inferior frontal lobule (Figure 3) and in the right temporo-parieto-occipital region compared to the contralateral side.
Figure 3.
CT perfusion study performed before (left) and after treatment (right). The pre-treatment data show decreased CBV, CBF, MTT and increased TTP in the right inferior frontal region in comparison to the contralateral corresponding area. In the post-treatment maps, no significant change is seen in CBV, CBF and MTT in comparison to pre-treatment, while a more symmetric pattern in TTP map is visible.
Taking into account the clinical presentation and the CT perfusion findings, a “steal phenomenon” was hypothesized to be the origin of the worsening symptoms.
The therapeutic approach determined by the multidisciplinary team was to first treat the patient with staged embolization and then to consider either radiosurgery or conventional surgery. A first embolization session took place. The post embolization angiogram revealed a reduction of the anterosuperior and inferior aspects of the nidus (Figure 2). The clinical examination after the first embolization showed a slight partial improvement in muscular power.
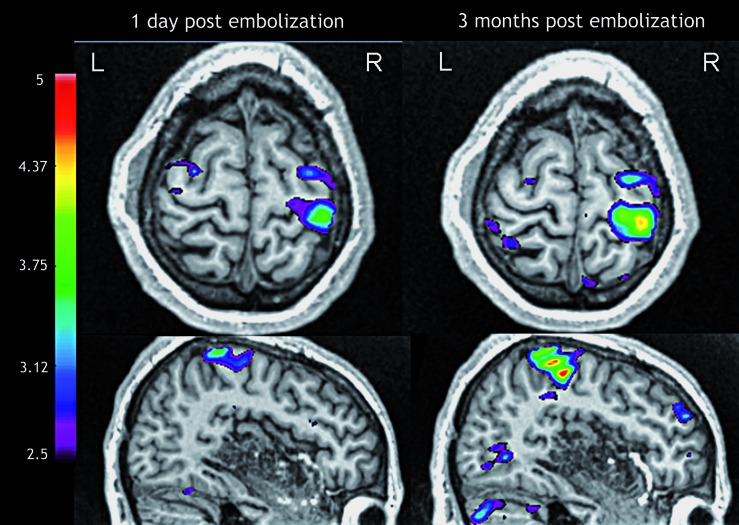
The patient underwent a second embolization ten weeks later, achieving significant reduction of the nidus size (Figure 2). A significant improvement in motor strength was noticed immediately after the procedure and a first fMRI (Siemens 3T Trio MRI system) was performed the following day. For the motor task, the patient was instructed to move the left and right hand, left and right foot, and tongue repeatedly a rate of approximately 1 Hz (TR=4, N=45). For the sensory task, the patient was stimulated with a brush on the left and right hand, left and right foot, and face, also at a rate of approximately 1 Hz. The activations in response to movement (Figure 4) and to sensory stimulation of the left hand and foot were in the expected areas.
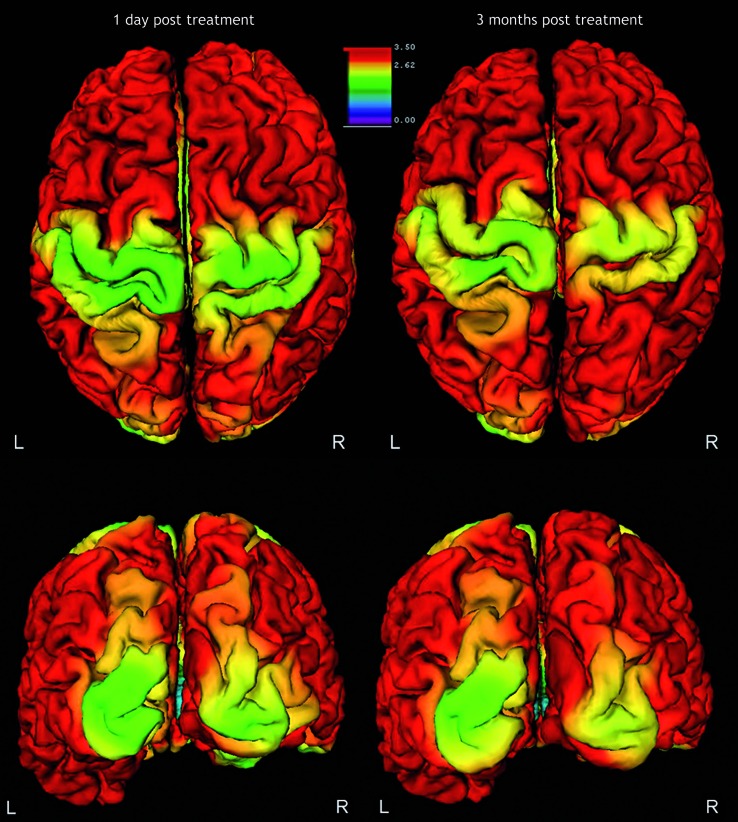
Figure 4.
Functional MRI during left hand motor task one day post-embolization and three months post-embolization. An increase in the size and BOLD signal intensity is seen in the study performed three months after the treatment in comparison to the previous one.
Three months later the motor power had further improved both at the upper and lower limb. The patient was able to climb some steps and walk for a moderate distance without fatigue. The fMRI was repeated and showed an increase in the blood oxygen level-dependent (BOLD) signal and in the area of activation in comparison to the previous examination during the motor tasks for the left hand (Figure 4) and foot.
Cortical thickness analysis was performed using the acquired 3T MR images pre and post-embolization. This was achieved by classifying brain tissue 18,19 and applying deformable meshes to extract the white and gray boundary and the pial surface 20,21. Subsequently, native space cortical thickness, measured as the distance between two corresponding points from each cortical surface was computed throughout the cortex 22-25. The analysis showed an increase in cortical thickness in the primary motor area, occipital and inferior frontal regions, particularly of the affected side, when comparing the first examination with the subsequent one performed three months later (Figure 5).
Figure 5.
Cortical thickness analysis performed one day and three months after embolization. The images show an apparent increased thickness in both the motor and occipital cortex, particularly of the affected side.
At that time, three months after the second embolization session, the patient noticed a visual deficit in the left superior visual field. The deficit was severe enough to affect the patient's capacity to drive. The neuro-ophthalmological evaluation confirmed the presence of left upper quadrantanopia, which had already been detected in 2010, even though the patient was not aware of the deficit.
A long-term follow-up CT perfusion study was performed 16 months after the last embolization, using the same examination technique, protocol and rate of injection as in the initial study. No significant qualitative or quantitative changes in the pattern of perfusion were seen in CBV, CBF and MTT maps in comparison to the pre-treatment study (Figure 3).
In contrast with the initial clinical picture of pronounced hemiparesis and almost complete lack of left hand use, the current clinical picture is characterized by fluctuating mild left hemibody weakness and left superior quadrantanopia.
Discussion
Our case report represents an example of the prompt positive impact that the staged endovascular treatment may have on the clinical status of patients with large complex arteriovenous malformations. In fact, in our patient the staged embolization improved his motor and sensory function, with results being evident immediately after the first session of treatment.
Four to 12 percent of patients harboring an AVM present progressive or fluctuating neurological deficits 26. Complex and extensive AVMs present more often with focal progressive deficits rather than hemorrhage as seems to occur in small lesions. This unstable clinical presentation can be best explained by the steal phenomenon whose evolution can be influenced by the endovascular treatment both in our case and in previous reports 27-31. Hence, it is important to provide high level perfusion studies that can document the hypoperfused areas and their changes over time 26,27,32.
The reversibility of neurological signs and perfusion abnormalities has been shown in some cases following endovascular treatment. In our patient we were not able to demonstrate that significant changes in the perfusion status of the sensory motor cortex accompanied the clinical recovery 33,34. However, we believe that the clinical improvement of the hemisyndrome observed immediately after treatment cannot be explained other than by a rapid improvement in the cerebral perfusion status.
To our knowledge, this is among the first papers 30 describing perfusion analysis results obtained using the newly developed 320-row CT scanner. As reported by Kim et al. 30, one of the possible patterns of perfusion in patients with AVM includes the co-existing decrease of CBV, CBF and MTT, as we found in our case. Kim et al. hypothesized that these findings may indicate a sump effect from the contiguous normal parenchyma 30. The clinical improvement observed in our patient following treatment supports the hypothesis that this pattern of perfusion (decreased CBV, CBF and MTT) represents a functional arterial steal as opposed to an ischemic steal or, in other words, that the perilesional tissue is viable and can benefit from treatment 30. It is difficult to hypothesize why the follow-up CT perfusion did not demonstrate any significant quantitative changes in spite of the clinical improvement. We believe that the large size of this AVM and its high flow could play a role in the CT perfusion data. Specifically, in spite of a clinically successful embolization, the amount of residual lesion and more importantly the high speed of the AVM shunt precluded a quantifiable improvement in CBV, CBF and MTT. Work is still needed to determine how tissue functionality changes in relation to perfusion status. For this reason we are in the process of collecting and analyzing the CTP data before and after embolization of all the patients with AVM treated at our Institution to assess whether the size and hemodynamics of the lesion may play a role in the CTP results. Overall, the perfusion imaging findings described here suggest that, despite the advances in this technique, there are still limitations to the use of CT perfusion in the clinical setting.
Our findings suggest to clinicians the need to take into consideration more than the classical one-to-one correspondence between location and function, because an extensive AVM can affect the perfusion of even remote regions as multiple territories are likely involved. Our case study confirms what others have pointed out, i.e., that the hypoperfused regions can be perilesional, more remote and even on the contralateral side 26,35,36.
Despite the role of the steal physiology, the clinical evolution of our case cannot be explained exclusively by the improved cerebral perfusion. Other mechanisms, partly secondary to reperfusion, probably took place and accounted for the modification in the symptoms.
When comparing the fMRI three months after the second embolization with the corresponding images taken one day after the intervention, we observe an increase in size of the blood oxygen level-dependent (BOLD) signal of the area of activation during motor tasks on the affected side. We speculate that the differences between the two functional examinations may reflect the changes in motor function, although we cannot corroborate our hypothesis with data on the autoregulatory reserve because we did not perform a cerebrovascular reactivity study 37. Our data obtained by cortical thickness analysis may further support this hypothesis in that we observed a modification in cortical thickness in the same area that showed an increase in activation during the motor tasks.
A correlation between cortical thickness and functional activation has been already reported in patients with stroke 38. Our study supports the hypothesis that functional and structural plasticity are coupled in the recovery of cerebrovascular diseases 38. We can speculate that a better perfusion of the previously hypoperfused left pre-motor area may have lead to functional and structural changes that are a likely explanation for the increased activation on the fMR images and for the apparently increased gray matter thickness. As previously reported by Fierstra et al., 39 the reversal of a steal phenomenon can be paralleled by an increase in cortical thickness following therapeutic revascularization of steno-occlusive disease. Whether in our case the cortical thickness changes are the basis for or the consequence of the clinical improvement is debatable. Some methodological concerns related to cortical thickness analysis (i.e. the assumption that all tissue can be characterized as either white matter, gray matter, CSF, or a partial volume of these classes) should also be taken into account. We speculate that the increased cortical thickness three months after endovascular treatment is attributable to the improved motor function consequent to the better perfusion. It is still unclear whether a three month interval is a sufficient time to observe structural changes, and it would be of interest to continue to follow the patient with functional and anatomical imaging in order to confirm the extent of the changes we have observed, although it has been demonstrated that motor learning 40 and training 41 processes in healthy subjects can induce structural changes in grey matter, and that a three month interval of training is sufficient to provide anatomical changes 40. In our patient, an increased cortical thickness was also observed in the occipital region and in the orbito-frontal cortex, areas unrelated to the motor function. The restored better perfusion and, for the orbito-frontal cortex, the even partial removal of the steal phenomenon in the same area may have contributed to the observed increase in cortical thickness.
The recovery from the visual neglect is a particularity of this case. It could be speculated that the involvement of Meyer's loop in the right hemisphere was responsible for the left upper quadrantanopia clinically observed 42. The patient remained unaware of this deficit probably due to an involvement of the white matter tracts responsible for visual awareness – the inferior fronto-occipital fasciculus, which runs in the depth of the temporal lobe, and the arcuate fasciculus and the superior longitudinal fasciculus which connect the frontal and parietal regions. Disconnections 43 and hypoperfusion of white matter tracts have been shown to cause visual awareness deficits 44. The T2 FLAIR hyperintense signal in that area suggests the presence of gliosis and supports this hypothesis. The embolization could have led to a restored functionality of the cells constituting the networks responsible for visual awareness, therefore allowing the patient to become aware of his visual deficit. As suggested in Urbanski et al. 44, it is possible that a large lesion in the temporal lobe could cause a disconnection mechanism leading to neglect. This hypothesis could explain the observed findings but it cannot clarify the relationship between newly reperfused areas with previous gliosis and changes in clinical status. This area did not require a significant structural change in the AVM to have a clinical impact, probably suggesting the presence of reperfusion at a microvascular level 44.
Our case demonstrates that a comprehensive assessment of complex and extensive AVMs using fMRI and CT perfusion could allow a mapping of endangered areas that may require more urgent treatment. However, it is only with more studies that we will be able to understand how these tools can be used to characterize AVMs and orient the treatment choice. In addition, the importance of meticulous correlation of imaging findings and clinical examination preceding and following any intervention in complex and extensive AVMs offers an encouraging prospect for tailored reduction of the nidus.
Conclusion
We have shown how the combined use of various imaging techniques can facilitate the comprehension of brain plasticity and hemodynamics in patients with AVM undergoing endovascular treatment. We suggest that valuable information on functional and anatomical brain plasticity in patients with complex AVM can be gained by using a more systematic and extensive imaging protocol, combining perfusion with comprehensive analyses of functional MRI, and anatomical MRI (e.g. cortical thickness analysis and white matter tractography).
Acknowledgments
We thank the patient for his participation in the study. This study has been supported by grants from Government of Canada Centres of Excellence for Commercialization and Research (CECR) and from the Natural Sciences and Engineering Research Council of Canada (NSERC). Roberta La Piana received a fellowship grant from the Montreal Neurological Institute.
References
- 1.Yasargil MG. Microneurosurgery. Volume IIIA. New York: Thieme; 1987. [Google Scholar]
- 2.Leblanc GG, Golanov E, Awad IA, et al. Biology of vascular malformations of the brain. Stroke. 2009;40(12):e694–702. doi: 10.1161/STROKEAHA.109.563692. Epub 2009 Oct 15. doi: 10.1161/STROKEAHA.109.563692. PubMed PMID: 19834013. PubMed Central PMCID: PMC2810509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H, Sidney S, McCulloch CE, et al. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38(9):2430–2437. doi: 10.1161/STROKEAHA.107.485573. Epub 2007/08/04. doi: 10.1161/STROKEAHA.107.485573. PubMed PMID: 17673729. [DOI] [PubMed] [Google Scholar]
- 4.Minakawa T, Tanaka R, Koike T, et al. Angiographic follow-up study of cerebral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery. 1989;24(1):68–74. doi: 10.1227/00006123-198901000-00011. Epub 1989/01/01. PubMed PMID: 2927601. [DOI] [PubMed] [Google Scholar]
- 5.Du R, Hashimoto T, Tihan T, et al. Growth and regression of arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia. Case report. J Neurosurg. 2007;106(3):470–477. doi: 10.3171/jns.2007.106.3.470. Epub 2007/03/21. doi: 10.3171/jns.2007.106.3.470. PubMed PMID: 17367071. [DOI] [PubMed] [Google Scholar]
- 6.Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg. 1998;24:131–214. doi: 10.1007/978-3-7091-6504-1_4. Epub 1999/03/02. PubMed PMID: 10050213. [DOI] [PubMed] [Google Scholar]
- 7.Alkadhi H, Kollias SS, Crelier GR, et al. Plasticity of the human motor cortex in patients with arteriovenous malformations: a functional MR imaging study. Am J Neuroradiol. 2000;21(8):1423–1433. Epub 2000/09/26. PubMed PMID: 11003274. [PMC free article] [PubMed] [Google Scholar]
- 8.Lazar RM, Marshall RS, Pile-Spellman J, et al. Anterior translocation of language in patients with left cerebral arteriovenous malformation. Neurology. 1997;49(3):802–808. doi: 10.1212/wnl.49.3.802. Epub 1997/09/26. PubMed PMID: 9305344. [DOI] [PubMed] [Google Scholar]
- 9.Geibprasert S, Pongpech S, Jiarakongmun P, et al. Radiologic assessment of brain arteriovenous malformations: what clinicians need to know. Radiographics. 2010;30(2):483–501. doi: 10.1148/rg.302095728. [DOI] [PubMed] [Google Scholar]
- 10.Ducreux D, Desal H, Bittoun J, et al. Diffusion, perfusion and activation functional MRI studies of brain arteriovenous malformations. J Neuroradiol. 2004;31(1):25–34. doi: 10.1016/s0150-9861(04)96876-9. Epub 2004/03/18. PubMed PMID: 15026729. [DOI] [PubMed] [Google Scholar]
- 11.Gabarros A, Young WL, McDermott MW, et al. Language and motor mapping during resection of brain arteriovenous malformations: indications, feasibility, and utility. Neurosurgery. 2011;68(3):744–752. doi: 10.1227/NEU.0b013e318207a9a7. Epub 2011/02/12. doi: 10.1227/NEU.0b013e318207a9a7. PubMed PMID: 21311300. [DOI] [PubMed] [Google Scholar]
- 12.Ellis MJ, Armstrong D, Dirks PB. Large vascular malformation in a child presenting with vascular steal phenomenon managed with pial synangiosis. J Neurosurg Pediatr. 2011;7:15–21. doi: 10.3171/2010.10.PEDS10388. [DOI] [PubMed] [Google Scholar]
- 13.Mahalick DM, Ruff RM, Heary RF , et al. Preoperative versus postoperative neuropsychological sequelae of arteriovenous malformations. Neurosurgery. 1993;33(4):563–570. doi: 10.1227/00006123-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kaminaga T, Hayashida K, Iwama T, et al. Hemodynamic changes around cerebral arteriovenous malformation before and after embolization measured with PET. J Neuroradiol. 1999;26(4):236–241. Epub 2000/04/28. PubMed PMID: 10783551. [PubMed] [Google Scholar]
- 15.Kaminaga T, Hayashida K, Iwama T, et al. Hemodynamic changes around a cerebral arteriovenous malformation before and after embolization measured with PET. Clin Nucl Med. 1999;24(10):813. doi: 10.1097/00003072-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Neumann-Haefelin T, Moseley ME, Albers GW. New magnetic resonance imaging methods for cerebrovascular disease: Emerging clinical applications. Ann Neurol. 2000;47(5):559–570. doi: 10.1002/1531-8249 (200005)47:5<559:: aid-ana2>3.0.co;2-s. [PubMed] [Google Scholar]
- 17.Leblanc R, Melanson D, Wilkinson RD. Hereditary neurocutaneous angiomatosis. J Neurosurg. 1996;85(6):1135–1142. doi: 10.3171/jns.1996.85.6.1135. [DOI] [PubMed] [Google Scholar]
- 18.Zijdenbos A, Forghani R, Evans AC. Automatic quantification of MS lesions in 3D MRI brain data sets: Validation of INSECT. LNCS. 1998;1496:439. [Google Scholar]
- 19.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23(1):84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. Epub 2005/05/18. doi: 10.1016/j.neuroimage.2005.03.036. Pub Med PMID: 15896981. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald D, Kabani N, Avis D, et al. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12(3):340–356. doi: 10.1006/nimg.1999.0534. Epub 2000/08/17. doi: 10.1006/nimg.1999.0534. PubMed PMID: 10944416. [DOI] [PubMed] [Google Scholar]
- 22.Robbins SM. Anatomical standardization of the human brain in euclidean 3-space and on the cortical 2-manifold. Technical Report PhD thesis, School of Computer Science, McGill University, Montreal, Quebec, Canada. Proceedings; 19th International Conference, IPMI 2005; July 10-15, 2005; Glenwood Springs, CO, USA. Springer; 2005. [Google Scholar]
- 23.Ad-Dab’bagh Y, Singh V, Robbins S (eds), et al. Native space cortical thickness measurement and the absence of correlation to cerebral volume. Proceedings; 11th Annual Meeting of the Organization for Human Brain Mapping; Toronto. 2005. [Google Scholar]
- 24.Lyttelton O, Boucher M, Robbins S, et al. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Boucher M, Whitesides S, Evans A. Depth potential function for folding pattern representation, registration and analysis. Med Image Anal. 2009;13(2):203–214. doi: 10.1016/j.media.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Taylor CL, Selman WR, Ratcheson A. Steal affecting the central nervous system. Neurosurgery. 2002;50(4):679–688. doi: 10.1097/00006123-200204000-00002. discussion 88-9. Epub 2002/03/21. PubMed PMID: 11904017. [DOI] [PubMed] [Google Scholar]
- 27.Manchola IF, De Salles AA, Foo TK, et al. Arteriovenous malformation hemodynamics: a transcranial Doppler study. Neurosurgery. 1993;33(4):556–562. doi: 10.1227/00006123-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Carrillo GJG, Hogg JP. Intracranial vascular lesions and anatomical variants all residents should know. Curr Probl Diagn Radiol. 2010;39(3):91–109. doi: 10.1067/j.cpradiol.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Okabe T, Meyer JS, Okayasu H, et al. Xenon-enhanced CT CBF measurements in cerebral AVM’s before and after excision. J Neurosurg. 1983;59(1):21–31. doi: 10.3171/jns.1983.59.1.0021. [DOI] [PubMed] [Google Scholar]
- 30.Kim DJ, Krings T. Whole-brain perfusion CT patterns of brain arteriovenous malformations: A pilot study in 18 patients. Am J Neuroradiol. 2011;32(11):2061–2066. doi: 10.3174/ajnr.A2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaminaga T, Hayashida K, Iwama T, et al. Hemodynamic changes around a cerebral arteriovenous malformation before and after embolization measured with PET. Clin Nucl Med. 1999;24(10):813. doi: 10.1097/00003072-199910000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Batjer HH, Devous MD Sr, Meyer YJ, et al. Cerebrovascular hemodynamics in arteriovenous malformation complicated by normal perfusion pressure breakthrough. Neurosurgery. 1988;22(3):503–509. doi: 10.1227/00006123-198803000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Sugita M, Takahashi A, Ogawa A, et al. Improvement of cerebral blood flow and clinical symptoms associated with embolization of a large arteriovenous malformation: case report. Neurosurgery. 1993;33(4):748–751. doi: 10.1227/00006123-199310000-00030. [DOI] [PubMed] [Google Scholar]
- 34.Abla AA, Dumont TM, Kan P, et al. Stroke intervention for middle cerebral artery thrombus in a young patient with an ipsilateral Spetzler-Martin grade V arteriovenous malformation. J Neurointerv Surg. 2012 doi: 10.1136/neurintsurg-2011-010164. Epub 2012/02/22. doi: 10.1136/neurintsurg-2011-010164. PubMed PMID: 22345144. [DOI] [PubMed] [Google Scholar]
- 35.Homan RW, Devous MD, Sr, Stokely EM, et al. Quantification of intracerebral steal in patients with arteriovenous malformation. Arch Neurol. 1986;43(8):779–785. doi: 10.1001/archneur.1986.00520080027015. Epub 1986/08/01. PubMed PMID: 3488052. [DOI] [PubMed] [Google Scholar]
- 36.Kader A, Young WL. The effects of intracranial arteriovenous malformations on cerebral hemodynamics. Neurosurg Clin N Am. 1996;7(4):767–81. [PubMed] [Google Scholar]
- 37.Mikulis DJ. Functional cerebrovascular imaging in brain ischemia: permeability, reactivity, and functional MR imaging. Neurosurg Clin N Am. 2005;15(3):667–80. doi: 10.1016/j.nic.2005.08.001. xii. Epub 2005/12/20. doi: 10.1016/j.nic.2005.08.001. PubMed PMID: 16360596. [DOI] [PubMed] [Google Scholar]
- 38.Schaechter JD, Moore CI, Connell BD, et al. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129(Pt 10):2722–2733. doi: 10.1093/brain/awl214. Epub 2006 Aug 18. [DOI] [PubMed] [Google Scholar]
- 39.Fierstra J, Maclean DB, Fisher JA, et al. Surgical revascularization reverses cerebral cortical thinning in patients with severe cerebrovascular steno-occlusive disease. Stroke. 2011;42(6):1631–1637. doi: 10.1161/STROKEAHA.110.608521. [DOI] [PubMed] [Google Scholar]
- 40.Draganski B, Gaser C, Busch V, et al. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427(6972):311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 41.Maguire EA, Gadian DG, Johnsrude IS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97(8):4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofer S, Karaus A, Frahm J. Reconstruction and dissection of the entire human visual pathway using diffusion tensor MRI. Front Neuroanat. 2010;4:15. doi: 10.3389/fnana.2010.00015. doi: 10.3389/fnana.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, et al. White matter (dis)connections and gray matter (dys)functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex. 2008;44(8):983–995. doi: 10.1016/j.cortex.2008.03.006. Epub 2008/07/08. doi: 10.1016/j.cortex.2008.03.006. PubMed PMID: 18603235. [DOI] [PubMed] [Google Scholar]
- 44.Urbanski M, Thiebaut de Schotten M, Rodrigo S, et al. Brain networks of spatial awareness: evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2008;79(5):598–601. doi: 10.1136/jnnp.2007.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]