Summary
The existing literature on preoperative spine tumor embolization is limited in size of patient cohorts and diversity of tumor histologies. This report presents our experience with preoperative embolization of hypervascular thoracic, lumbar, and sacral spinal column tumors in the largest series to date.
We conducted a retrospective review of 228 angiograms and 188 pre-operative embolizations for tumors involving thoracic, lumbar and sacral spinal column. Tumor vascularity was evaluated with conventional spinal angiography and was graded from 0 (same as normal adjacent vertebral body) to 3 (severe tumor blush with arteriovenous shunting). Embolic materials included poly vinyl alcohol (PVA) particles and detachable platinum coils and rarely, liquid embolics. The degree of embolization was graded as complete, near-complete, or partial. Anesthesia records were reviewed to document blood loss during surgery.
Renal cell carcinoma (44.2%), thyroid carcinoma (9.2%), and leiomyosarcoma (6.6%) were the most common tumors out of a total of 40 tumor histologies. Hemangiopericytoma had the highest mean vascularity (2.6) of all tumor types with at least five representative cases followed by renal cell carcinoma (2.0) and thyroid carcinoma (2.0). PVA particles were used in 100% of cases. Detachable platinum coils were used in 51.6% of cases. Complete, near-complete, and partial embolizations were achieved in 86.1%, 12.7%, and 1.2% of all cases, respectively. There were no new post-procedure neurologic deficits or other complications with long-term morbidity. The mean intra-operative blood loss for the hypervascular tumors treated with pre-operative embolization was 1745 cc.
Preoperative embolization of hypervascular thoracic, lumbar, and sacral spine tumors can be performed with high success rates and a high degree of safety at high volume centers.
Key words: spine, tumor, preoperative embolization, surgery
Introduction
The treatment of spinal metastases is mostly palliative with the goals of improving or maintaining neurologic function and spinal stability. It usually involves radiation therapy and/or spinal surgery. The treatment decision-making process can be broken down into four fundamental considerations referred to as NOMS: Neurologic (N) considerations include the degree of myelopathy and the degree of radiographic spinal cord compression; Oncologic (O) considerations primarily reflect the known radiosensitivity of the tumor; Mechanical instability (M) is broadly defined as movement-related pain and is level dependent; and Systemic disease (S) considerations include both the extent of disease and the medical comorbidities 1. The treatment of primary spinal tumors involves a similar decision process as well as considerations regarding curative resection that are pathology and case-dependent.
Preoperative embolization of hypervascular spinal tumors aims to reduce intraoperative blood loss 2,3. Furthermore, it also aims to reduce operative time, increase resectability of lesions, and improve visualization of the operative field. Arterial embolization of bone tumors (including tumors of the spine) was first described in 1975 4,5. Since then, there have been several reports in the literature describing embolization of spinal tumors prior to surgical resection 6-13. Many of these studies are limited by sample size and are lacking in diversity of tumor histologies. In this report, we present our experience with preoperative embolization of thoracic, lumbar, and sacral spine tumors in the largest series to date based on the limits of the literature search criteria utilized. We describe the technical aspects of successfully and safely performing the procedure and examine important anatomical and pathological considerations.
Methods
The study was approved by the institutional review board. A retrospective review of all peri-procedural records was conducted for spine angiograms/embolizations performed between January 2001 and December 2010. All patients with primary and metastatic thoracic, lumbar, and sacral spine tumors referred for spine angiography with the intention of embolization prior to surgical resection were included. These patients were referred based on tumor histology and/or imaging findings suspicious for hypervascularity. Histological criteria used as indications for preoperative angiography and embolization included benign hypervascular tumors (hemangiomas. aneurysmal bone cyst, giant cell tumor), primary malignant tumors (chordoma, osteosarcoma, chondrosarcoma, Ewing sarcoma, plasmacytoma, hemangiopericytoma), and metastatic tumors (renal cell carcinoma, thyroid carcinoma, hepatocellular carcinoma, breast cancer, angiosarcoma, melanoma, neuroendocrine tumors) of the spine. MR characteristics used as indications for preoperative angiography and embolization included intratumoral flow voids, hemorrhage and avid contrast enhancement 7, as these were considered indicative of hypervascularity.
All angiograms were reviewed for tumor vascularity and degree of embolization. Vascularity was graded as none (0, same as or less than the normal adjacent vertebral body), mildly increased (1, slightly more prominent than the normal vertebral body blush), moderately increased (2, considerable tumor blush without early arteriovenous shunting), or severely increased (3, intense tumor blush with early arteriovenous shunting) (Figures 1-4). The degree of embolization of a tumor feeder was recorded as complete if there was grade 0 or less of residual vascularity, near-complete if there was grade 1 of residual vascularity, and partial if there was grade 2 or more of residual vascularity. The angiograms were reviewed for type and number of identified tumor feeders, type and number of spinal artery feeders, type and number of embolized tumor feeders, type of embolic material used, and reasons for technical failure.
Figure 1.
Patient with epidermoid cyst at the T2 vertebral level. Catheter angiogram of the right T2, T3, and T4 segmental arteries (common origin) shows no increased vascularity (Grade 0) at the T2 level (arrow) compared to the normal T3 and T4 levels (arrowheads).
Figure 2.
Patient with leiomyosarcoma metastasis at the T12 vertebral level. Catheter angiogram of the right T12 segmental artery shows mildly increased vascularity (Grade 1) with tumor blush (arrow) that is slightly more prominent than the normal vertebral body.
Figure 3.
Patient with renal cell carcinoma metastasis at the T5 vertebral level. Catheter angiogram of the right T5 segmental artery shows moderately increased vascularity (Grade 2) with considerable tumor blush (arrow) without arteriovenous shunting.
Figure 4.
Patient with hemangiopericytoma at the T11 vertebral level. Catheter angiogram of the right T11 segmental artery shows severely increased vascularity (Grade 3) with intense tumor blush (arrow) and early arteriovenous shunting (arrowheads).
Post-procedural charts were reviewed for any post-angiogram/embolization complications. Surgical resection with or without spinal stabilization was typically performed within 24-72 hours of the embolization procedure. Surgical pathology reports were used to document final tumor pathology. Anesthesia records were reviewed to document blood loss during surgery. We performed a literature search of the MEDLINE database between the years 1975 and 2012. The date of the last search was April 2012. The following keywords were queried singly and in combination: spine, tumor, metastatic, primary, thoracic, lumbar, sacral, embolization, angiography, preoperative. The search was limited to studies published in English and ‘Humans' were specified as the study category.
Angiography Technique
Angiograms were performed using standard interventional neuroradiology techniques using 5 French catheters like the Cobra 2 (Terumo), and Simmons 1 (Terumo), via a transfemoral arterial access. Most thoracic procedures were performed with general anesthesia to achieve the best possible quality of images with transient apnea. Lumbar procedures were typically performed with moderate sedation, unless the patients were unable to tolerate being recumbent due to severe back pain. Heparin or other anticoagulants were not administered peri-procedurally. An aortogram was performed in one patient for whom catheterization of the segmental arteries was difficult secondary to diffuse atherosclerotic disease and prior extensive spinal instrumentation obscuring fluoroscopy. Diagnostic angiography included evaluation of thoracic and lumbar spinal segmental arteries at the level(s) of the tumor as well as at least one contiguous level above and below the tumor. For every lower thoracic and upper lumbar lesion identification of the artery of Adamkiewicz was always attempted. For L4 and L5 level lumbar tumors and sacral tumors, angiography included evaluation of the common, external, and internal iliac arteries and their branches, as well as selective angiogram of the median and lateral sacral arteries. For upper thoracic tumors, angiography included evaluation of the vertebral arteries, subclavian arteries, thyrocervical trunks, costocervical trunks, and supreme intercostal arteries. Post-embolization angiography included the artery embolized, the contralateral segmental artery at the same level, and ipsilateral segmental arteries at least one level above and one below.
Technical Aspects of Embolization
The criteria for embolization included the presence of significant tumor vascularity (grade 1 or higher) and the ability to achieve stable microcatheter position without risk of reflux into the aorta, carotid, or vertebral arteries. The presence of a spinal cord artery feeder (anterior or posterior spinal artery) arising from the same segmental artery as the tumor feeder and the presence of a dangerous anastomosis between adjacent segmental arteries were absolute contraindications for embolization of that particular feeder. There were two main methods of embolization. The first method involved selective catheterization of the posterior (dorsal) branch of the segmental artery using a microcatheter such as Echelon 10 (Covidien Neurovasular), Excelsior SL 10 (Stryker Neurovascular), and Prowler 10 (Cordis Neurovascular), and injection of PVA particles directly into the tumor feeder. The second method was employed when the tumor feeder could not be selectively catheterized. In these cases, the anterior (ventral) branch of the segmental artery was selectively catheterized with a microcatheter such as Echelon 10 (Covidien Neurovasular), Excelsior SL 10 (Stryker Neurovascular), and Prowler 10 (Cordis Neurovascular) and then occluded with detachable coils. The microcatheter was then retracted into the segmental artery, where PVA particles were injected with flow directed towards the posterior (dorsal) branch (Figures 5 and 6). Occlusion of the anterior (ventral) branch of the segmental artery was, in other words, used to divert particles into the posterior (dorsal) branch. In both methods, PVA particles were injected until stasis and absence of residual tumoral blush from the particular feeder. Liquid embolic materials (N-butyl cyano-acrylate, NBCA) were considered for use as an adjunct in cases of extreme hypervascularity and prominent arteriovenous shunting that did not improve significantly after particle embolization. Technical failure of embolization occurred when we were unable to achieve superselective catheterization of a small tumor feeder and/or stable catheter position, and embolization was not performed due to the risk of embolic complications. Provocative testing with a barbiturate or anesthetic or intra-operative neurophysiologic monitoring was never used.
Figure 5.
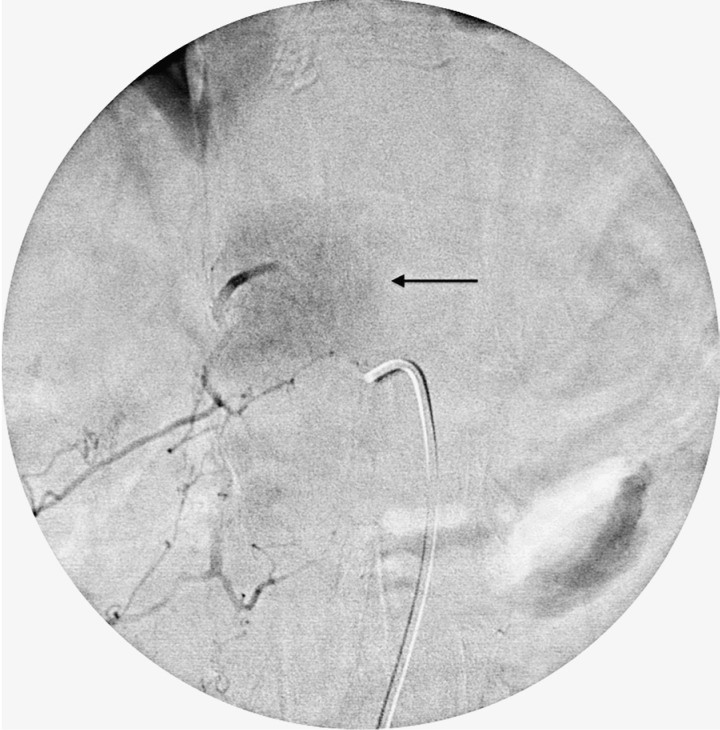
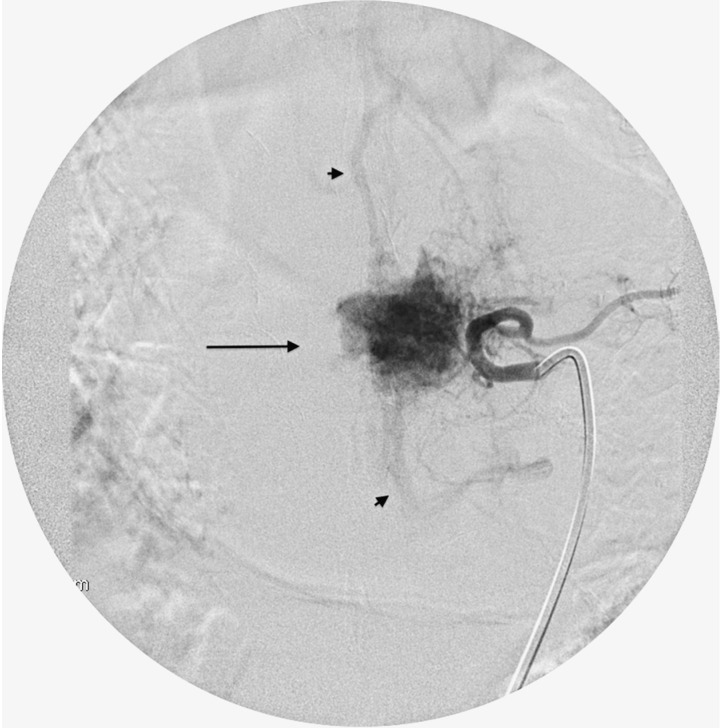
Patient with renal cell carcinoma metastasis at the T8 vertebral level. Catheter angiogram of the left T8 segmental artery shows severely increased vascularity (Grade 3) with intense tumor blush (arrow) and early arteriovenous shunting (arrowheads).
Figure 6.
Post embo.tif. Post embolization angiogram of the left T8 segmental artery shows complete resolution of the tumor blush. The embolization was performed with PVA particles infused via a microcatheter (arrow) into the T8 segmental artery after occlusion of the ventral branch with coils (arrowhead).
Results
A total of 228 spinal angiography procedures were performed in 199 patients (ages 14-91, 115 males and 84 females) with 40 different tumor types. The most common primary tumor type was renal cell carcinoma (44.2%) followed by thyroid carcinoma (9.2%) and leiomyosarcoma (6.6%). The tumors were located between the T1-S3 vertebral levels. Mean vascularity score of all lesions was 1.69. Hemangiopericytoma had the highest mean vascularity (2.6) of all tumor types of which there were at least five cases, followed by renal cell carcinoma (2.0), and thyroid carcinoma (2.0) (Table 1).
Table 1.
Tumor types and mean vascularity
| Vascularity < 1.0 (n= number of cases) |
1.0 < = Vascularity < 2.0 (n= number of cases) |
Vascularity >= 2.0 (n= number of cases) |
|---|---|---|
| Chordoma (n=2) | Aneurysmal bone cyst (n=7) | Breast (n=1) |
| Desmoid tumor (n=1) | Angiosarcoma (n=4) | Cholangiocarcinoma (n=2) |
| Epidermoid cyst (n=1) | Bladder (n=1) | Ewing's sarcoma (n=1) |
| Germ cell tumor (n=1) | Chondrosarcoma (n=2) | Hemangiopericytoma (n=6) |
| Liposarcoma (n=1) | Ganglioneuroma (n=1) | Lung cancer – adenocarcinoma (n=1) |
| Meningioma (n=1) | Giant cell tumor (n=2) | Lung cancer – squamous cell (n=1) |
| Hemangioma (n=12) | Multiple myeloma (n=1) | |
| Hepatocellular carcinoma (n=4) | Neurofibroma (n=1) | |
| Leiomyosarcoma (n=15) | Osteosarcoma (n=1) | |
| Lung cancer – small cell (n=1) | Ovarian (n=1) | |
| Melanoma (n=4) | Pheochromocytoma (n=1) | |
| Prostate (n=3) | Plasmacytoma (n=2) | |
| Sarcoma (unknown type) n=5 | Renal (104) | |
| Schwannoma (n=11) | Solitary fibrous tumor (n=1) | |
| Indeterminate tumor histology (n=3) | Thyroid (n=22) | |
There were 193 hypervascular tumors identified and 188 embolizations performed (32 Grade 1, 116 Grade 2, and 40 Grade 3). The number of tumor feeders embolized per case ranged from one to eight (mean 2.3). The most common tumor types embolized were renal cell carcinoma (48.4%), thyroid (11.2%), hemangioma (5.3%), schwannoma (5.3%), and leiomyosarcoma (4.8%). The mean vascularity for the embolized cases was 2.0 compared to mean vascularity of 0.4 in cases where no embolization was performed was 0.4.
PVA particles (150-250 microns) were used in all cases. Larger PVA particles (250-355 microns) were used as an adjunct in 16 cases where there was persistent tumor blush. In 51.6% of embolizations, detachable platinum coils were used in addition to PVA particles to occlude the ventral branch proximally. Liquid embolic materials (NBCA) were used in addition to PVA particles in three cases of renal cell carcinoma and one case of aneurysmal bone cyst with extreme hypervascularity (2.1%).
Complete, near-complete, and partial embolizations were achieved in 86.1%, 12.7%, and 1.2% of all cases, respectively. Complete, near-complete, and partial embolizations were achieved in 92.0%, 8.0%, and 0% of tumors embolized with coils and PVA particles and in 79.5%, 17.9%, and 2.6% of tumors embolized with PVA particles only. There was similar mean tumor vascularity between the tumors embolized with PVA only and those embolized with coils and PVA (1.93 compared to 1.98, respectively). Two of the four cases requiring NBCA were embolized completely and the other two cases were partially embolized. Complete embolization was achieved in 86.0% of renal cell carcinoma metastases, the most common tumor histology.
Sixteen patients had 18 repeat embolization procedures because of tumor recurrence requiring repeat surgery. The great majority (94%) of repeat embolizations for local recurrence followed complete embolization of the original lesion. The mean vascularity of tumors that recurred locally was 1.9; 83% of repeat embolizations of tumor recurrence were complete embolizations and the others were near-complete.
Five hypervascular tumors (mean vascularity 1.3) were not embolized at all because of a common origin of the tumor feeder and a radiculomedullary feeder to the anterior spinal artery from the same segmental artery (n=4) or due to inability to achieve good microcatheter position secondary to difficult anatomy (n=1). In 67 additional hypervascular tumors, some of the tumor feeders were not embolized (range 1-5; mean 1.7). These 67 tumors are categorized as partial embolizations. The most common reason for not embolizing a feeder was minimal degree of tumor enhancement from that particular feeder compared to other feeders and therefore insignificant contribution towards an otherwise significant hypervascular tumor blush (55.2% of feeders not embolized). Another 35.2% of feeders not embolized were excluded because a spinal cord artery was arising from the same segmental artery as that tumor feeder. Technical failure was the reason for not embolizing the other 9.6% of feeders not embolized.
Complete, near-complete, and partial embolizations were achieved in 86.0%, 12.9%, and 1.1% of tumors with all tumor feeders embolized. Similarly, complete, near-complete, and partial embolizations were achieved in 86.1%, 9.7%, and 1.4% of tumors with only some of the tumor feeders embolized.
There were only two minor complications in the 228 total procedures. One patient had a large groin hematoma that did not extend into the pelvis or abdomen and did not require any intervention other than prolonged monitoring. Another patient suffered a cardiac event secondary to anesthesia. Neither complication resulted in long-term morbidity. There were no major complications. Specifically, there were no cases of spinal cord ischemia, new post-procedural motor or sensory deficits, retroperitoneal hemorrhage, vessel dissection, skin or muscle necrosis, following either angiography or embolization.
The mean intra-operative blood loss for the 188 tumors that showed increased vascularity and were treated with pre-operative embolization was 1745cc (range 50-11900). The mean intra-operative blood loss for tumors treated with complete, near-complete and partial embolization was 1611, 2442 and 3750 cc respectively. Finally, the mean intra-operative blood loss for the 35 tumors that did not demonstrate increased vascularity on angiography (i.e. grade 0) was 1284 cc.
Discussion
Preoperative embolization of spinal tumors has been described in prior reports. However, these papers were limited by sample size with the majority of papers having approximately 10-30 patients and the largest previous series having 100 patients 10.
In this paper, we describe our experience with preoperative embolization of a variety of spinal column tumors in the largest series of patients reported so far, and we provide our results in terms of tumor vascularity, embolization technique, embolization outcomes and intraoperative blood loss. The decision to refer a patient for angiography and preoperative embolization was based on tumor histology or on MRI findings; these criteria are quite similar to those of other groups 8,13. Tumor histology was used as an independent predictor of hypervascularity and patients underwent angiography irrespective of MRI findings. On the other hand, MR findings highly suspicious of hypervascularity were used to refer a patient for angiography even if the tumor was not considered hypervascular histologically.
Renal cell carcinoma was by far the most common histology among the hypervascular tumors, with a mean vascularity score of 2.0 according to our grading system. The range of renal cell carcinoma vascularity scores included several tumors that demonstrated only mildly increased vascularity and some tumors that demonstrated no hypervascularity. While the role of prior therapies including radiation requires further investigation, it appears that not every renal cell carcinoma metastasis to the spine has significant hypervascularity. Other tumors with significant hypervascularity included thyroid carcinoma, hemangiopericytoma, hemangioblastoma, osteosarcoma, pheochromocytoma, cholangiocarcinoma metastasis, ovarian cancer, lung cancer (adenocarcinoma and squamous cell), and breast cancer. Though lung and breast cancer metastases are not typically thought to be hypervascular, there were few hypervascular lesions with these histologies in our series. The implication is that there is a spectrum of hypervascularity within these tumors and a need for a noninvasive way to assess the hypervascularity of spinal tumors. Prabhu et al. 7 previously noted that while the positive predictive value of MRI imaging criteria suggestive of significant vascularity in predicting angiographically confirmed vascularity was 77%, the absence of these criteria could not be used to reliably exclude actual tumor hypervascularity. Therefore, the current MRI criteria are not optimal and better imaging tools need to be developed. MR perfusion imaging appears to be a promising technique that requires further validation 14,15.
Embolization with PVA particles was performed in all cases. We used 150-250 microns particles because this size allows for occlusion of the arterioles and is effective in decreasing blood flow at the tumoral bed. PVA particles smaller than 150-250 microns were not used for embolization due to the risk of migration of particles through the capillary bed, as well as skin and muscle necrosis. Further embolization with larger PVA particles was necessary in a few cases because of persistent tumor blush following initial particle embolization. Coils were used in slightly more than half the cases to occlude the ventral branch proximally and direct the PVA particles into the tumor feeders from the dorsal branch. Because of rich collateral networks, skin necrosis secondary to occlusion of the ventral branch was never encountered in our series of patients. Given the safety and efficacy of this technique, ventral branch occlusion with coils should be used along with PVA particles when possible. Liquid embolic material was rarely used for embolization of intensely vascular lesions in select cases when PVA particles were not effective in decreasing tumor vascularity significantly. Liquid embolic materials have the ability to achieve greater penetration of the tumor vasculature and result in permanent occlusion 12, but are also more difficult to control and may result in inadvertent embolization.The vast majority of embolization procedures were successfully accomplished and achieved angiographic complete or near-complete embolization. A very high rate of angiographic complete embolization was seen even when some tumor blush feeders were not embolized, essentially identical to the rate of angiographic complete embolization when all tumor feeders were embolized (86.1% and 86% respectively). This most likely reflects the fact that most feeders that were not embolized did not contribute significantly to tumor vascularity and that embolization of the main tumor feeders is enough to achieve an effective embolization angiographically.
In cases of repeat embolization for tumor recurrence, tumor regrowth was not associated with prior incomplete embolization. In fact, the majority of tumor recurrences most commonly followed prior complete embolizations.
Given the lack of major procedure-related complications and post-procedural neurological deficits, our opinion is that preoperative embolization of spinal tumors can be performed safely and with great success in experienced hands with proper knowledge of the vascular anatomy. Meticulous evaluation of pre-embolization angiography is essential to identify, and therefore protect, radiculomedullary and spinal arteries. The presence of an artery supplying the spinal cord from the same segmental artery as arteries supplying the spinal cord, or the presence of a dangerous anastomosis between segmental arteries should not be missed, to avoid devastating and usually irreversible neurological complications of spinal cord ischemia. The decision on whether to embolize any or all feeders must be made based on the results of pre- and intra-embolization angiography and the risk/benefit ratio. This must be determined on a case-by-case basis.
To date, there has been no prospective randomized study determining the efficacy of pre-operative embolization for spinal tumors. Surgical resection of hypervascular spinal tumors is known to be associated with excessive hemorrhage and morbidity. In our view, any attempt at randomizing angiography and possible preoperative embolization for patients with hypervascular spine tumors would be unethical because highly vascular tumors cannot be safely operated without pre-operative embolization. There are many retrospective studies indicating reduced intra-operative hemorrhage in patients treated with pre-operative embolization. Berkefeld et al.16 reported the intraoperative blood loss in 69 patients with hypervascular tumors that underwent corpectomy. Patients treated with particle and particle-coil embolizations had significantly reduced hemorrhage (1800-1850 cc) compared to patients that were embolized with coils only or those that were not embolized at all. Similarly, Manke et al. 17 reported significantly reduced intraoperative hemorrhage in patients with spinal metastases from renal cancer that underwent pre-operative tumor embolization with PVA particles (1500 cc for complete and 2200 for partial embolizations) compared to patients treated surgically without pre-operative embolization (5000 cc). In a series of patients with metastatic renal cell carcinoma to the spinal column who underwent successful pre-operative embolization, Sundaresan et al. 18 reported average intraoperative blood loss of 2200 cc (range 50-4000 cc) during spinal surgery.
Even though intraoperative blood loss is commonly used as an outcome measure for the evaluation of the efficacy of pre-operative embolization, this is limited by the difficulty to quantify it accurately. In addition, there are many other variables that can affect the intraoperative blood loss, including surgical technique, operative approach, location of tumor, size of tumor, tumor histology, adjunctive intraoperative tools, and means of intraoperative blood loss assessment. With these limitations in mind, our results are very similar to prior studies where preoperative embolization was performed in smaller series 16-18. Our results also corroborate the fact that complete embolization is more effective in decreasing intraoperative blood loss compared to near-complete or partial embolization. In our cohort there were too few hypervascular lesions that weren't embolized at all to allow for comparison of intra-operative blood loss between embolized and non-embolized tumors.
Conclusion
Preoperative embolization of hypervascular tumors of the spine aims to reduce intraoperative blood loss and improve qualitative variables for the surgeon such as visibility and resectability. We have demonstrated that this procedure can be done with high success rates and a high degree of safety at high volume centers. Excluding spinal artery feeder origin from tumor feeders prior to embolization is of paramount importance to patient safety. Preoperative embolization was shown to be associated with less intraoperative blood loss.
References
- 1.Bilsky MH, Azeem S. NOMS framework for decision making in metastatic cervical spine tumors. Curr Opin Orthop. 2007;18(3):263–269. [Google Scholar]
- 2.Olerud C, Jónsson H, Löfberg A-M, et al. Embolization of spinal metastases reduces peroperative blood loss: 21 patients operated on for renal cell carcinoma. Acta Orthop Scand. 1993;64(1):9–12. doi: 10.3109/17453679308994517. [DOI] [PubMed] [Google Scholar]
- 3.Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: effect of preoperative particle embolization on intraoperative blood loss. Am J Neuroradiol. 2001;22(5):997–1003. [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman F, Casarella WJ, Dick HM, et al. Selective intra-arterial embolization of bone tumors. A useful adjunct in the management of selected lesions. Am J Roentgenol Radium Ther Nucl Med. 1975;123(1):130–139. doi: 10.2214/ajr.123.1.130. [DOI] [PubMed] [Google Scholar]
- 5.Hilal SK, Michelsen JW. Therapeutic percutaneous embolization for extra-axial vascular lesions of the head, neck, and spine. J Neurosurg. 1975;43(3):275–287. doi: 10.3171/jns.1975.43.3.0275. [DOI] [PubMed] [Google Scholar]
- 6.Shi HB, Suh DC, Lee HK, et al. Preoperative transarterial embolization of spinal tumor: Embolization techniques and results. Am J Neuroradiol. 1999;20(10):2009–2015. [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhu VC, Bilsky MH, Jambhekar K, et al. Results of preoperative embolization for metastatic spinal neoplasms. J Neurosurg. 2003;98(2) Suppl:156–164. doi: 10.3171/spi.2003.98.2.0156. [DOI] [PubMed] [Google Scholar]
- 8.Guzman R, Dubach-Schwizer S, Heini P, et al. Preoperative transarterial embolization of vertebral metastases. Eur Spine J. 2005;14(3):263–268. doi: 10.1007/s00586-004-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radeleff B, Eiers M, Lopez-Benitez R, et al. Transarterial embolization of primary and secondary tumors of the skeletal system. Eur J Radiol. 2006;58(1):68–75. doi: 10.1016/j.ejrad.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Wilson M, Cooke D, Ghodke B, et al. Retrospective analysis of preoperative embolization of spinal tumors. Am J Neuroradiol. 2010;31(4):656–660. doi: 10.3174/ajnr.A1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heran MK. Preoperative embolization of spinal metastatic disease: rationale and technical considerations. Semin Musculoskelet Radiol. 2011;15:135–142. doi: 10.1055/s-0031-1275596. [DOI] [PubMed] [Google Scholar]
- 12.Santillan A, Zink W, Lavi E, et al. Endovascular embolization of cervical hemangiopericytoma with Onyx-18: Case report and review of the literature. J Neurointerv Surg. 2011;3:304–307. doi: 10.1136/jnis.2010.003756. [DOI] [PubMed] [Google Scholar]
- 13.Ozkan E, Gupta S. Embolization of spinal tumors: vascular anatomy, indications, and technique. Tech Vasc Interv Radiol. 2011;14:129–140. doi: 10.1053/j.tvir.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Mazura JC, Patsalides A, Pauliah M, et al. Spinal blood flow maps: A minimally invasive alternative to spinal angiography in the evaluation of extramedullary spinal tumors; ASNR 50th Annual Meeting; 2012. p. 270. [Google Scholar]
- 15.Khadem NR, Karimi S, Peck KK, et al. Characterizing hypervascular and hypovascular metastases and normal bone marrow of the spine using dynamic contrast-enhanced MR imaging. Am J Neuroradiol. 2012;33:2178–2185. doi: 10.3174/ajnr.A3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkefeld J, Scale D, Kirchner J, et al. Hypervascular spinal tumors: Influence of the embolization technique on perioperative hemorrhage. Am J Neuroradiol. 1999;20:757–763. [PMC free article] [PubMed] [Google Scholar]
- 17.Manke C, Bretschneider T, Lenhart M, et al. Spinal metastases from renal cell carcinoma: Effect of preoperative particle embolization on intraoperative blood loss. Am J Neuroradiol. 2001;22:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 18.Sundaresan N, Choi IS, Hughes JE, et al. Treatment of spinal metastases from kidney cancer by presurgical embolization and resection. J Neurosurg. 1990;73(4):548–554. doi: 10.3171/jns.1990.73.4.0548. [DOI] [PubMed] [Google Scholar]