Abstract
Genetic diversity and the way a species is introduced influence the capacity of populations of invasive species to persist in, and adapt to, their new environment. The diversity of introduced populations affects their evolutionary potential, which is particularly important for species that have invaded a wide range of habitats and climates, such as European gorse, Ulex europaeus. This species originated in the Iberian peninsula and colonised Europe in the Neolithic; over the course of the past two centuries it was introduced to, and has become invasive in, other continents. We characterised neutral genetic diversity and its structure in the native range and in invaded regions. By coupling these results with historical data, we have identified the way in which gorse populations were introduced and the consequences of introduction history on genetic diversity. Our study is based on the genotyping of individuals from 18 populations at six microsatellite loci. As U. europaeus is an allohexaploid species, we used recently developed tools that take into account genotypic ambiguity. Our results show that genetic diversity in gorse is very high and mainly contained within populations. We confirm that colonisation occurred in two stages. During the first stage, gorse spread out naturally from Spain towards northern Europe, losing some genetic diversity. During the second stage, gorse was introduced by humans into different regions of the world, from northern Europe. These introductions resulted in the loss of rare alleles but did not significantly reduce genetic diversity and thus the evolutionary potential of this invasive species.
Keywords: Colonisation, invasive species, polyploidy, population genetic structure
Introduction
Invasive species represent one of the most important causes of loss of biodiversity across the world (Walker and Steffen, 1997; Pimentel et al., 2000; Millennium Ecosystem Assessment, 2005). Understanding the mechanisms leading to invasion is thus important for the prevention and control of biological invasions and for the conservation of biodiversity (Stockwell et al., 2003). A critical stage in the invasion of an exotic species is its introduction, as the way introduction occurs can affect the capacity of the species to persist in, and to adapt to, its new environment. The number of propagules introduced influences the magnitude of the founder effect, the possibility of finding mates, the level of inbreeding, the demography of the colony and hence genetic drift (Nei et al., 1975; Dlugosch and Parker, 2008a). The introduction and bringing into contact of genotypes originating from differentiated populations from the native range can create new trait combinations (for example, Lavergne and Molofsky, 2007). Therefore the number of introduction events, the number of propagules and their origin together determine the amount of genetic diversity in introduced populations of a given species, with consequences for response to selection (Müller-Schärer and Steinger, 2004; Dlugosch and Parker, 2008a). A single introduction of a few individuals will lead to a loss of diversity in the introduced populations compared with the native populations (for example, Hypericum canariense, Dlugosch and Parker, 2008b), whereas large and/or multiple introductions may produce similar diversity to that of native populations (for example, Centaurea stoebe micranthos, Marrs et al., 2008). In extreme cases, the introduction of propagules coming from different populations can lead to greater diversity in the introduced populations than in the native populations (for example, Anolis sagrei, Kolbe et al., 2004). Without the help of humans, the colonisation of new territories (in the native range or after introduction) mainly occurs gradually, and we usually observe a decline in genetic diversity due to successive founder effects (Hewitt, 2000). In fact, colonising populations only contain a fraction of the genetic diversity of the source population, and because they are often of small size, populations undergo considerable drift after colonisation (Nei et al., 1975).
The study of neutral genetic diversity makes it possible to retrace the routes of introduction and colonisation of a species and to estimate the genetic diversity introduced (for example Kopp et al., 2012). This in turn enables inference on the evolutionary potential of populations and also to find out whether non-selective processes (for example, founder effects) have contributed to the evolution of the introduced populations (Amsellem et al., 2000; Lavergne and Molofsky, 2007; Keller and Taylor, 2008). In invasive species, several studies have therefore aimed to compare diversity in introduced and native populations. Reviews of comparative diversity in plants by Bossdorf et al. (2005) and Dlugosch and Parker (2008a) reveal that most of these studies show that genetic diversity of the introduced populations is similar to, or greater than, that of native populations. The increase in diversity is attributed by the authors to multiple introductions from multiple sources, which can provide the introduced populations with strong evolutionary potential (for example, Kolbe et al., 2004; Marrs et al., 2008; Calsbeek et al., 2011; Hahn et al., 2012). In the studies that revealed a loss of diversity in the introduced populations (for example, Rubus alceifolius, Amsellem et al., 2000), post-introduction evolution was sometimes also observed (for example, Hypericum canariense, Dlugosch and Parker, 2008b). The capacity of species to respond to selection seems therefore to be a determining factor in their invasive success (Lee, 2002; Lee and Gelembiuk, 2008). This can explain why polyploids are very common among invasive plants (reviewed in te Beest et al., 2012). In fact in such species, in particular in allopolyploids (resulting from the hybridisation of different species), individuals bear numerous alleles per locus and can show fixed heterozygosity, increasing the genetic diversity introduced and reducing genetic drift (Ellstrand and Schierenbeck, 2000; te Beest et al., 2012).
The evolutionary potential of introduced populations is potentially very important for species that have invaded a very wide range of habitats and climates, possibly even wider than in their native range. This is the case for gorse, Ulex europaeus, which has invaded very different geographical areas. This shrub originated in Europe, where it is found at sea level on the Atlantic coast, from Spain to Denmark (Tutin et al., 1968). In invaded regions, it is present across a wide range of latitudes, from the equator to 50°N and 54°S, and at altitudes from 0 to >3500 metres. Its introduction has been mainly deliberate: gorse was used in Europe in pastoral practices (for example, to make hedges or forage; Calvel, 1809; Cubas, 1999), and it was introduced into European colonies mainly in the nineteenth century for agricultural uses (Darwin, 1839; Gay, 1846; Mack, 1991; Parsons and Cuthbertson, 2001). It is now considered by the International Union for Conservation of Nature as one of the world's worst invasive species (Lowe et al., 2000), causing problems in many regions in every continent, such as on the west coast of north and south America, Hawaii, the island of Reunion, Australia and New Zealand (Holm et al., 1997). Thus, to characterise genetic diversity of gorse in its native and invaded ranges would allow us to better understand the nature of the ecological and evolutionary processes behind the invasive success of an introduced species.
In spite of the large number of studies on gorse in both the native and invaded regions, no study of its neutral genetic diversity has yet been undertaken. One of the reasons is that gorse is an allohexaploid species (2n=6x=96 chromosomes; Misset and Gourret, 1996), which complicates studies of its nuclear diversity (Clark and Jasieniuk, 2011). Also, very low cytoplasmic diversity has been found in the Ulex genus (Cubas et al., 2005; Kader Ainouche, personal communication). However, it has been shown that the phenotypic diversity is great, both in the native range and in the invaded regions (Hill et al., 1991; Tarayre et al., 2007; Hornoy et al., 2011), and that it has a genetic basis (Atlan et al., 2010). The study of genetic diversity in allopolyploids like U. europaeus has now become possible using methods recently developed for nuclear markers, such as allozymes or microsatellites (Obbard et al., 2006; Falush et al., 2007). These methods allow inference on neutral genetic diversity and the genetic structure of allopolyploid populations.
The objective of this study is to obtain information on the action of neutral processes and on genetic diversity in populations of U. europaeus, to infer how it was introduced into the invaded regions and the evolutionary potential of the introduced populations. The questions asked were (i) what is the diversity and the genetic structure of populations in the native range? (ii) what is the diversity and genetic structure of populations in the invaded range? and (iii) what can we conclude about the modes of introduction and colonisation of gorse?
Materials and methods
Study species
Gorse, U. europaeus ssp. europaeus, is a perennial plant that can live up to 30 years, and the adult plant can reach several metres high and wide (Chater, 1931; Lee et al., 1986). Flowers are hermaphroditic and pollinated by large insects, such as honeybees or bumblebees (Bowman et al., 2008). Seed dispersal occurs primarily by ejection from the pod within a few metres around the mother plant, but seeds can be further dispersed by ants, water, humans, mammals and, possibly, birds and wind (Ridley, 1930; Moss, 1959; Hill et al., 1996). Seeds are very persistent in the seed bank (Hill et al., 1996, 2001) and may germinate over a period of up to 30 years (Moss, 1959; Zabkiewicz, 1976).
The genus Ulex belongs to the Genistae tribe. It is a young genus (four or five million years old) within which little cytoplasmic variation has yet been found (Cubas et al., 2005; Kader Ainouche, personal communication). The Iberian peninsula (Spain and Portugal) is regarded as the centre of diversification of the Ulex genus, because it hosts a dozen Ulex species, with various ploidy levels (Feoli-Chiapella and Cristofolini, 1981). Three of these species are found outside the Iberian Peninsula, but U. europaeus ssp. europaeus is the only species found outside Europe. This species is hexaploid (2n=6x=96 chromosomes; Misset and Gourret, 1996) and originated from hybridisation between a tetraploid and a diploid ancestor belonging to two different Ulex lineages (Ainouche et al., 2003, 2009).
Population sampling
Seeds used in this study were collected from 1999 to 2009 in gorse populations from its native range and from invaded regions (see Table 2). In Europe, seeds were sampled in three regions where gorse is very common: north western Spain, Brittany (western France), and Scotland. In the invaded range, we chose different regions where gorse is a serious weed and for which we could get seeds: Chile, New Zealand, Reunion (Indian Ocean), and the west coast of the USA. We aimed at sampling three populations per region, to estimate population diversity as well as regional genetic structure. However, we got only two samples from Chile and one from California, resulting in a total of 18 populations analysed. Although Reunion is an overseas department of France, in the following we use ‘Reunion' to refer to the island and ‘France' to refer to metropolitan France, for the sake of simplicity. Population names were encoded with the first letters representing the region it comes from: SPA for Spain, FRA for France, SCO for Scotland, CHI for Chile, NZE for New Zealand, REU for Reunion, and USA for California, USA. Populations FRA1, FRA2 and FRA3 correspond to populations BCC, BCV and BKE, respectively, in Hornoy et al. (2011, 2012); SCO1, SCO2, SCO3 correspond to SBA, SCR, SST; REU1, REU2, REU3 correspond to RLB, RMA, RPB; and NZE1, NZE2, NZE3 correspond to ZAU, ZCH, ZWE, respectively.
In each population, gorse pods were collected separately from 30 individuals (except for Chilean populations where seeds were bulked per population). Seeds were kept for at least four months at 4 °C to break dormancy. Then for each population, seeds from each individual were allowed to germinate and grow to small seedlings. One seedling per mother plant was retained for further analysis. DNA was extracted from aerial parts of these seedlings. We were able to extract and analyse 17–25 samples per population (see Table 2).
Microsatellite analysis
Aerial parts were ground in liquid nitrogen with a pestle and mortar, and total genomic DNA was then extracted with a NucleoSpin Plant II Kit (Macherey-Nagel, Hoerdt, France), following the manufacturer's recommendations. DNA quality was checked on a 2% agarose gel, and extract samples were assayed using a ND-1000 spectrophotometer (NanoDrop, Illkirch, France).
Eight nuclear microsatellite loci were developed from U. europaeus ssp. europaeus samples from western France by GIS (Genetic Identification Services, Chatsworth, CA, USA), and we optimised PCR conditions for each locus (Table 1). We obtained clear results from six of them. All six appeared to be highly polymorphic and displayed 1–6 alleles per individual, consistent with the hexaploidy of gorse.
Table 1. Characteristics of the six microsatellite loci used in the study.
| Locus | Primers | Dye | [Primers] (μM) | Tm (°C) | Allele size range (bp) | Number of alleles | H′T | F′ST |
|---|---|---|---|---|---|---|---|---|
| A110 | F: 5′-CTATGGTGAATTTGTGATACAC-3′ | PET | 0.35 | 52 | 128–152 | 22 | 4.40 | 0.092 |
| R: 5′-ACCTTGTTGCATCTTTACC-3′ | ||||||||
| A125 | F: 5′-GCATATACATACCCGAGGTAAG-3′ | NED | 0.26 | 58 | 152–232 | 53 | 6.54 | 0.096 |
| R: 5′-AACCTGATGAAATGCACTATTC-3′ | ||||||||
| B4 | F: 5′-GGGCTCTGGCTCTGATAC-3′ | 6-FAM | 0.20 | 53 | 101–137 | 13 | 1.35 | 0.136 |
| R: 5′-TTGGATTAACCAACTTTCCTC-3′ | ||||||||
| B104 | F: 5′-GAACCTTATTCACTGGAATCTG-3′ | VIC | 0.30 | 53 | 122–188 | 30 | 4.13 | 0.122 |
| R: 5′-CCCTTTTCTTTCCTTTCTTAAC-3′ | ||||||||
| B123 | F: 5′-AATTTGCCTGACATTGTTACTC-3′ | NED | 0.22 | 53 | 206–269 | 48 | 6.35 | 0.116 |
| R: 5′-AGACCGTGTTCATTATGGTTAG-3′ | ||||||||
| C12 | F: 5′-GGAAAATGGGAAGTTCTAAGG-3′ | VIC | 0.30 | 50 | 120–320 | 16 | 1.77 | 0.155 |
| R: 5′-CCACAGAATTGAGGCAGTC-3′ |
H′T: phenotype-based diversity statistic of Obbard et al. (2006) calculated across all individuals.
F′ST: phenotype-based differentiation statistic of Obbard et al. (2006) between all 18 populations.
PCR reactions were performed separately for each locus in 25 μl containing 12.5 μl of 2X GoTaq Colorless Master Mix (Promega, Charbonnières, France), 0.20–0.35 μM of each primer (Table 1) and 150 ng template DNA made up to 25 μl with water. PCR included an initial denaturation step at 94 °C for 3 min, then 35 cycles with 40 s denaturation at 94 °C, 40 s hybridisation of primers at Tm (Table 1) and 30 s elongation at 72 °C, with a final elongation step at 72 °C for 4 min, using a Mastercycler epgradient S (Eppendorf, Le Pecq, France).
For each locus, the forward primer was labelled with a fluorescent dye (Table 1). After PCR and before electrophoresis, two multiplexes were done: multiplex A involved loci A110, A125 and C12; and multiplex B involved loci B4, B104 and B123. For each multiplex, 1.15 μl of each PCR product was added to 10 μl Hi-Di Formamide (Applied Biosystems, Carlsbad, CA, USA), containing 3% GeneScan -500 LIZ Size Standard (Applied Biosystems). Electrophoresis was then performed for each multiplex in an ABI PRISM 3130x genetic analyser (Applied Biosystems—Hitachi, Carlsbad, CA, USA), using POP-7 polymer (Applied Biosystems). Electrophoretic profiles were captured with the software ABI 3130xl Data Collection (Applied Biosystems), and allele scoring was performed manually in GeneMapper v4.1 (Applied Biosystems).
Data analysis
In polyploid species (for example, hexaploid), it is impossible to deduce the exact genotype at a microsatellite locus from the number of bands observed, because each allele may be present in several copies so that ambiguity often exists between several possible genotypes. For example, the genotype of a hexaploid individual carrying alleles A, B and C could be AABBCC, AAAABC, ABCCCC, and so on. Although methods have been developed to infer allele copy number from peak dosage (Esselink et al., 2004), they are impracticable in high-order polyploids such as U. europaeus (Helsen et al., 2009). Because allele and genotype frequencies cannot be estimated, it is not possible to use classical diversity and structure statistics, such as H and FST. Instead we used phenotype-based methods recently developed for polyploids (Obbard et al., 2006; Falush et al., 2007) that have proven useful in comparing genetic diversity and genetic structure at microsatellite loci between regions in plant species (for example, Hamilton and Eckert, 2007; Marrs et al., 2008).
Estimation of genetic diversity
Diversity was estimated within populations, regions and within native and invaded ranges as: the mean number of alleles found per locus, the number of alleles found across the six loci (Ae) and the number of private alleles (found in only one population, or region, or range). These statistics are not affected by genotype ambiguity. We also used the recently developed phenotype-based diversity statistic H′, which is defined as the number of alleles by which pairs of individuals differ averaged over the loci (Obbard et al., 2006). H′ was compared among regions (and ranges) by randomising populations between regions or ranges 1000 times, and the P-value was the probability that the observed difference in diversity was >95% of all randomised differences. Diversity statistics and permutation tests were computed with the FDASH software (Obbard et al., 2006).
Estimation of genetic structure
The F′ST value is a differentiation statistic based on H′: after estimating H′ at the population and at the region levels, F′ST was computed as 1−(mean H′pop)/H′reg. This estimate is analogous to and behaves like FST (Obbard et al., 2006). The F′ST values were compared between ranges with 1000 permutations using FDASH. To test whether geographical distance reflected genetic distance, we performed a regression of pairwise F′ST between populations on their pairwise geographical distances and tested its significance with Mantel's test (1000 permutations), using GENALEX 6.41 (Peakall and Smouse, 2006).
We determined the hierarchical structure of genetic diversity among regions, populations and individuals with an analysis of molecular variance (Excoffier et al., 1992) in GENALEX 6.41. To do so, we considered each allele as a single locus with two states, present or absent. Based on this presence/absence matrix, the genetic variation was partitioned between regions, populations and individuals (Huff et al., 1993). Differentiation statistics (ϕ-statistics; Excoffier et al., 1992) were computed for each hierarchical level and were tested for significance by 1000 permutations of individuals in the data set, in GENALEX 6.41.
Genetic structure across native and introduced populations was investigated using the Bayesian clustering algorithm developed by Pritchard et al. (2000), which is now available for polyploids in the software STRUCTURE version 2.3.2 (Falush et al., 2007). This method aims to cluster individuals in K genetic groups, using the multilocus genotypes of individuals. We performed five independent runs with different proposals for K, testing each possible K from 1 to 18 using 100 000 iterations after a burn-in period of 50 000 iterations. All runs were conducted with the admixture model, and assuming correlated allele frequencies (Pritchard et al., 2000; Falush et al., 2003), without previous information on the population of origin of the individuals. To ensure convergence of the Markov Chain Monte Carlo estimates, the consistency of results was checked for the five replicates performed for each value of K. The most probable number of clusters (K) was then determined using the change in log likelihood of data between successive values of K, as described in Evanno et al. (2005).
Finally, to sum up and visually represent genetic variation between populations, we used pairwise F′ST values, computed with FDASH, to perform a principal coordinate analysis (PCoA) with GENALEX 6.41. This method produces a few axes containing most of the genetic variation in the data set and separates the populations. We also used the recently developed Discriminant Analysis of Principal Components (DAPC), which is a multivariate analysis that identifies and describes clusters of genetically related individuals (Jombart et al., 2010).
Results
General analysis
Across the 412 genotyped individuals that could be analysed, we found 182 different alleles across the six loci. The total number of alleles per locus varied from 13 to 53, and H′ ranged from 1.35 to 6.35 depending on the locus (Table 1). The estimates of genetic differentiation were of the same order of magnitude for all loci, with F′ST varying from 9.6 to 15.5% (Table 1). In the population analysis, we provide only the values averaged for all loci.
Some diversity statistics, such as the number of alleles found, can be sensitive to sample size. We thus performed all the analyses both with the total number of individuals per population and for only 17 random individuals per population (the lowest sample size in our data set). As expected, the diversity statistics were slightly lower with only 17 individuals per population relative to the entire data set, but the observed patterns of difference between populations, regions and ranges were exactly the same, so results from the complete data set are presented.
Genetic diversity
The total number of alleles across the six loci (Ae) ranged from 55 alleles in REU1 to 109 alleles in SPA1. Total H′ was 4.12, ranging from 2.74 in REU1 to 4.36 in SPA1 (Table 2). Values of Ae and H′ were strongly correlated (RSpearman=0.91, P<0.0001). Hereafter, we mainly discuss the results of the number of alleles and private alleles, which are not affected by genotype ambiguity.
Table 2. Location of populations and genetic diversity at the population, region and range levels.
| GPS coordinates | N | Ae | Private alleles | H′ | F′ST | |
|---|---|---|---|---|---|---|
| Native range | 202 | 179 | 60 | 4.25 | 0.099 | |
| Spain | 72 | 156 | 47 | 4.29 | 0.045 | |
| SPA1 | 43.7°N–07.8°W | 25 | 109 | 10 | 4.36 | |
| SPA2 | 42.9°N–08.5°W | 24 | 108 | 12 | 3.90 | |
| SPA3 | 42.9°N–07.1°W | 23 | 97 | 13 | 3.91 | |
| France | 71 | 115 | 7 | 4.14 | 0.032 | |
| FRA1a | 48.1°N–04.5°W | 25 | 90 | 1 | 4.08 | |
| FRA2a | 48.0°N–01.6°W | 22 | 73 | 1 | 3.69 | |
| FRA3a | 48.0°N–03.2°W | 24 | 91 | 3 | 4.21 | |
| Scotland | 59 | 91 | 0 | 3.66 | 0.048 | |
| SCO1a,b | 57.1°N–02.5°W | 17 | 70 | 0 | 3.61 | |
| SCO2a,b | 56.1°N–02.6°W | 24 | 75 | 0 | 3.65 | |
| SCO3a,b | 56.0°N–03.9°W | 18 | 61 | 0 | 3.14 | |
| Invaded range | 210 | 122 | 3 | 3.91 | 0.113 | |
| Chile | 47 | 88 | 0 | 3.76 | 0.148 | |
| CHI1b | 37.6°S–73.6°W | 23 | 63 | 0 | 2.84 | |
| CHI2b | 39.8°S–73.2°W | 24 | 69 | 0 | 3.53 | |
| Reunion | 72 | 87 | 1 | 3.41 | 0.079 | |
| REU1a | 21.1°S–55.6°E | 25 | 55 | 1 | 2.74 | |
| REU2a | 21.1°S–55.4°E | 24 | 69 | 0 | 3.38 | |
| REU3a | 21.2°S–55.6°E | 23 | 64 | 0 | 3.21 | |
| New Zealand | 67 | 103 | 1 | 4.18 | 0.026 | |
| NZE1a,b | 36.3°S–175.1°E | 25 | 85 | 1 | 4.13 | |
| NZE2a | 43.6°S–172.5°E | 23 | 86 | 0 | 4.22 | |
| NZE3a | 41.3°S–174.9°E | 19 | 69 | 0 | 3.76 | |
| USA | ||||||
| USA1 | 39.4°N–123.8°W | 24 | 66 | 0 | 3.32 | |
| Total | 412 | 182 | 4.12 | 0.119 | ||
Abbreviation: GPS, Global Positioning System.
N: number of genotyped individuals.
Ae: number of alleles found across the six loci.
Private alleles is the number of alleles, across the six loci, that are found only in the considered subsample.
H′: phenotype-based diversity statistic of Obbard et al. (2006).
F′ST: phenotype-based differentiation statistic of Obbard et al. (2006).
Seeds used in Hornoy et al. (2011).
Seeds collected and used by Buckley et al. (2003).
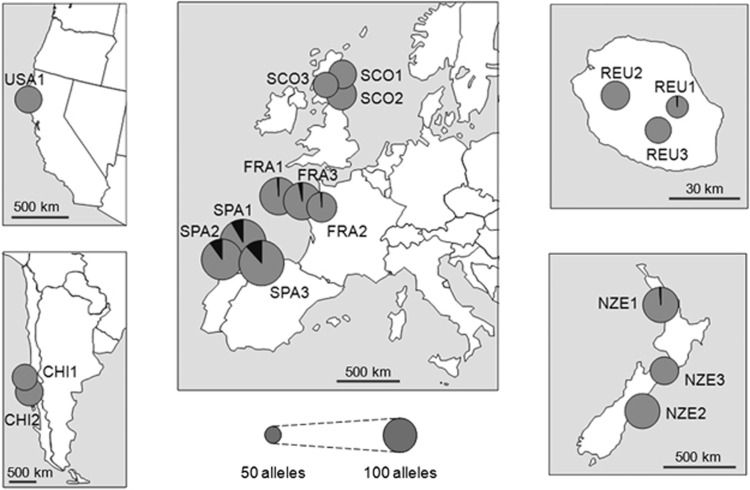
In Europe, we found 179 different alleles, ranging from 61 in SCO3 to 109 in SPA1. Regional Ae displayed a gradient from south to north Europe, with 156 alleles in Spain, 115 in France and 91 in Scotland (Figure 1). The mean number of alleles per locus within populations differed significantly among these three regions (Spain: 17.47, France: 14.25, Scotland: 11.74; 1000 permutations, P<0.001). When considering the 182 alleles of the whole data set, 60 were private to Europe, of which 47 were private to Spain, 7 were private to France and none were private to Scotland (Table 2). When reducing the data set to European regions only, we found 57 alleles private to Spain, 8 alleles private to France and 2 alleles private to Scotland, revealing the same northward gradient as for total numbers of alleles found.
Figure 1.
Map of genetic diversity of the populations sampled. Circles represent the total number of alleles found across the six loci, in each population. The black area within each circle represents the number of private alleles. See also Table 2.
In the invaded range, we found 122 different alleles, ranging from 55 in REU1 to 86 in NZE2. Regional Ae was similar in populations from Chile, Reunion and California but was much higher in New Zealand (Table 2). Out of the 182 different alleles found across the whole data set, only three were private to the invaded range, one being private to Reunion and one to New Zealand (Figure 1). The mean number of alleles per locus found within populations was lower in the introduced populations (11.70 alleles) than in Europe (14.69 alleles). This difference was significant (FDASH, 1000 permutations, P=0.02).
Genetic structure
In Europe, population differentiation within regions, as estimated with F′ST, ranged from 0.032 to 0.048 (Table 2). Pairwise comparisons of F′ST between populations varied greatly (mean pairwise F′ST=0.060±0.034) but were not influenced by geographical distance between populations (Mantel's test: R=−0.037; 1000 permutations, P=0.43). Analysis of molecular variance revealed that in Europe 90.83% of genetic variation occurred within populations, whereas <5% of variation occurred between populations within regions and <5% occurred between regions (Table 3).
Table 3. Results of the analysis of molecular variance within each range.
| Source | Variance | Percentage of total | ϕ-Statistics | |
|---|---|---|---|---|
| Native range | ||||
| Among regions | 0.641 | 4.26 | ϕRT=0.043 | *** |
| Among populations within regions | 0.740 | 4.91 | ϕPR=0.051 | *** |
| Within populations | 13.685 | 90.83 | ϕPT=0.092 | *** |
| Invaded range | ||||
| Among regions | 0.186 | 1.32 | ϕRT=0.013 | *** |
| Among populations within regions | 1.428 | 10.08 | ϕPR=0.102 | *** |
| Within populations | 12.547 | 88.60 | ϕPT=0.114 | *** |
***P=0.001.
In the invaded regions, F′ST ranged from 0.026 to 0.148 and was not significantly different from the native range (native range: F′ST=0.099, invaded range: F′ST=0.113; 1000 permutations, P=0.74). Analysis of molecular variance revealed that 88.60% of genetic variation occurred within populations, whereas 10.08% occurred between populations within regions and only 1.32% occurred between regions. In both ranges, ϕ-statistics were highly significant, meaning that genetic variation observed between populations and between regions was significantly different from zero.
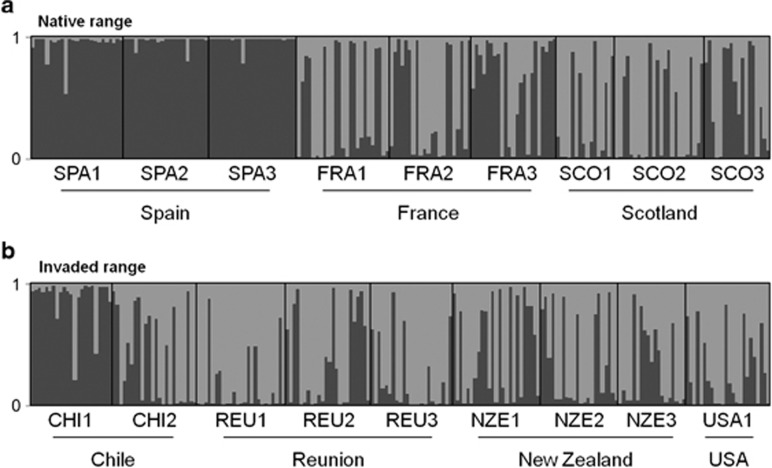
The population clustering algorithm of STRUCTURE resulted in the most probable number of clusters being K=2. In the native range, individuals from Spain were all very strongly assigned to the first cluster, while populations from France and Scotland displayed mixed assignment to the two clusters (Figure 2a). In the invaded range, Chilean population CHI1 was mainly assigned to the Spanish cluster, while other introduced populations displayed mixed assignment to the two clusters (Figure 2b). In short, populations can be divided into two groups, the first group including the Spanish populations and the Chilean population CHI1 and the second group including all other populations.
Figure 2.
(a, b) Assignment probabilities of membership to the two inferred clusters, based on the multilocus genotypes. Each individual is represented as a vertical line with proportional assignment to cluster 1 in dark grey and proportional assignment to cluster 2 in light grey. Vertical black lines separate the individuals from the different sampled populations.
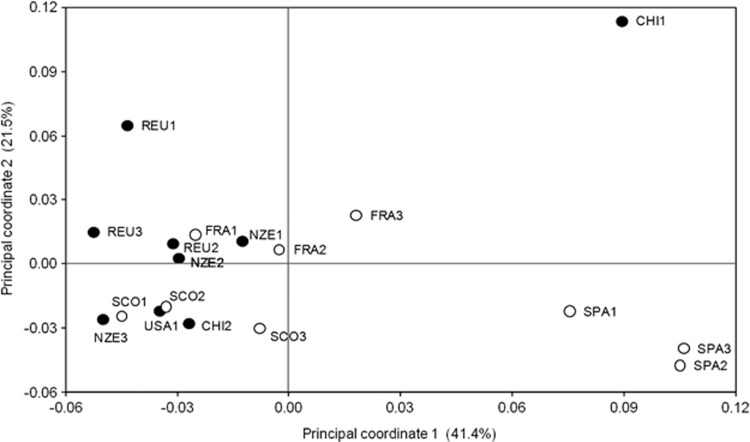
PCoA based on pairwise F′ST comparisons between populations produced two axes explaining 41.40% and 21.50% of the genetic variation, respectively (Figure 3). Considering the two-dimensional space of genetic variation, there was great genetic variation in Europe, mainly between Spain on one hand and France and Scotland on the other. Genetic variation between introduced populations was lower, except that CHI1 appeared very different from the other introduced populations. The first axis separated the three populations of Spain and population CHI1 of Chile from the other populations (Figure 3), consistent with the results of STRUCTURE. The second axis separated population CHI1 of Chile and population REU1 of Reunion from a main group that contains all the other populations. In the European populations of that group, the second axis separated France from Scotland. In the introduced populations of that group, no clear pattern appeared (Figure 3). DAPC identified five genetic clusters: one comprising nearly all individuals from Spanish populations, one comprising nearly all individuals from the Chilean population CHI1, and the three other clusters comprising a mix of the remaining individuals. Projection of the individuals on the first two principal components revealed a pattern roughly similar to the one found with the PCoA: the cluster corresponding to the Spanish populations and the cluster corresponding to CHI1 outlied, while the rest of the individuals were pooled in the same group.
Figure 3.
PCoA based on pairwise F′ST between the sampled populations. Open circles, native populations; closed circles, introduced populations.
As results from PCoA, DAPC and from STRUCTURE revealed that Spain formed a group differentiated from the other European populations, we compared genetic diversity by separating Spain from the France–Scotland group. The total number of alleles (Ae) found was 156 in Spain (N=72), 122 in France-Scotland (N=130) and 122 in the invaded range (N=210). When reducing the number of samples to a random subset of 72 (the minimum sample size in the three compared groups), we found 156 alleles in Spain, 110 in France–Scotland and 104 in the invaded range. The mean number of alleles per locus within populations was 17.47 in Spain, 13.13 in France–Scotland and 11.70 in the invaded range. It was significantly higher in Spain than in France–Scotland (FDASH, 1000 permutations, P=0.007) and than in the invaded range (1000 permutations, P<0.0001). By contrast, the difference between France–Scotland and the invaded range was not significant (1000 permutations, P=0.21).
Discussion
Although the study of neutral genetic diversity in polyploids can be complicated by genotype ambiguity (Clark and Jasieniuk, 2011), recently developed methods have proven useful in estimating genetic diversity and how it is structured geographically (for example, Hamilton and Eckert, 2007; Marrs et al., 2008). Here, we found very congruent patterns of genetic diversity and structure between different methods. Further, these results were also congruent with the existing historical data on gorse colonisation and history of introduction, giving us high confidence in the results of these analyses.
Genetic diversity and structure in Europe
In the native range of U. europaeus ssp. europaeus, we found very high neutral genetic diversity within populations. This may result from the allohexaploid nature of gorse, which means that each individual can carry up to six alleles at each locus. However, other studies on allohexaploid species have found a genetic diversity (H′) less than that found in gorse (for example, in Geum triflorum, Hamilton and Eckert, 2007; and in Festuca arundinacea, Sharifi Tehrani et al., 2009). The fact that gorse is allogamous and a perennial species (living up to 30 years; Lee et al., 1986) can also explain the high diversity observed (Nybom, 2004). Also, gorse has a very large seed bank (several thousand seeds per square metre; Richardson and Hill, 1998), which is long-lived (retaining a high germination capacity for 30 years; Moss, 1959). Genetic diversity is thus maintained in two perennial reservoirs, the plants and the seeds, increasing the effective population size and limiting the loss of diversity by genetic drift (Loveless and Hamrick, 1984; Honnay et al., 2008; Lundemo et al., 2009).
Genetic diversity and the number of private alleles are the greatest in Spain. They decrease strongly from Spain to France and less from France to Scotland. This south-north gradient can be explained by the fact that the Iberian peninsula is the centre of origin of U. europaeus (Feoli-Chiapella and Cristofolini, 1981). The smaller genetic diversity observed towards the north of Europe is in agreement with the colonisation (or recolonisation) of Europe by gorse from the Iberian peninsula. This colonisation probably took place 10 000 years ago when the icecap began its retreat from Europe. The sea level at that time allowed a land bridge between the north of Spain, western France and the British isles, allowing Iberian species to colonise Europe, despite the Pyrenees (Hewitt, 2000). In fact, the colonisation of Europe by gorse has mainly occurred since the Neolithic, with deforestation by humans and the expansion of the use of heathlands for agricultural purposes (Webb, 1998). In France, pollen profiles from Brittany show that gorse really increased in abundance since the bronze age, at the expense of forest trees (van Zeist, 1963, 1964). This (re)colonisation would have resulted in a loss of important genetic diversity in founding populations because of successive bottlenecks (Hewitt, 1996). Heather (Calluna vulgaris), a heathland plant often associated with gorse, shows a similar pattern of decreasing diversity from Spain to Scotland, also suggesting a loss of diversity during the colonisation of Europe from the Iberian peninsula (Mahy et al., 1997, 1999).
However, at the within-region level, differentiation between populations is rather small (<5% within each European region), suggesting that subsequent gene flow between established populations has been high and/or genetic drift has been weak. Further, hexaploidy and the longevity of gorse and its seed bank increase the effective size of the populations and limit the effects of drift, thus slowing down the differentiation of populations (Loveless and Hamrick, 1984; Honnay et al., 2008). The absence of isolation by distance between the European populations may result from these factors. However, the allopolyploid character of gorse can also create an artefact that prevents the detection of such a relationship. Indeed, the genome of U. europaeus is formed from the hybridisation of at least two parental species with differentiated genomes. As a consequence, alleles belonging to two different parental genomes within an individual may be more different than alleles belonging to the same parental genome in two different individuals, whatever the distance between the populations they belong to.
Introduced genetic diversity
In general, the genetic diversity found in the introduced populations is less than that of the European populations. However, this difference only applies if Spain is included in the comparison, and our data seem to show that the introduced populations studied (except CHI1 from Chile) are genetically much closer to the France–Scotland group than that of Spain. Also, introductions from France and Great Britain are in accord with the historical data. In fact, gorse would likely have been imported into Reunion by the French, who colonised the island from 1665 (Gay, 2007), into New Zealand by the British before 1835 (Darwin, 1839; Isern, 2007) and onto the American west coast from Ireland before 1912 (Pryor and Dana, 1952). The case of Chile is unusual, as it was colonised mainly by the Spaniards (Loveman, 2001), but gorse would have been introduced there at least by the English botanist John Miers at the beginning of the nineteenth century, as reported in the flora of Gay (1846). Gorse might also have been introduced by German settlers (including many farmers) who massively colonised the south of Chile in the second half of the nineteenth century (Young, 1974). Multiple origins of gorse populations in Chile could explain why its two populations relate to two different European genetic groups. A complex origin of Chilean populations was also observed for another invasive species native to Europe, scotch broom Cytisus scoparius (Kang et al., 2007).
The evolution of genetic diversity at the time of the introduction can be observed by comparing the introduced populations with those of the France–Scotland group. Native and introduced ranges exhibit similar diversities, suggesting that there had been no significant loss of diversity when gorse was introduced into the invaded regions studied. This probably results partly from the deliberate nature of the introduction of gorse for agricultural purposes (Opazo, 1930; Pryor and Dana, 1952; Mack, 1991; Isern, 2007). Gorse seeds were even sold or distributed, for example, in the United States (Mack, 1991) and New Zealand (Lee et al., 1986; Myers and Bazely, 2003). Further, in New Zealand planting of gorse hedges was encouraged by the governors (Isern, 2007). These massive introductions and exchanges can explain the weak differentiation we observed between populations of this region. The great genetic diversity found outside Europe implies that most of the introduced populations have not experienced a major bottleneck and that the evolutionary potential of these populations may be quite similar to that of the native populations. In fact, although the introduced populations contain fewer total alleles and fewer private alleles, the distribution of quantitative traits in populations is relatively insensitive to the loss of rare alleles (Dlugosch and Parker, 2008a). In the native range of gorse, there is substantial genetic variance for traits linked to growth, phenology and reproduction (Atlan et al., 2010). The present results suggest that this large variability was introduced in the invaded regions, which matches well with the high phenotypic diversity observed in the invaded regions (Hill et al., 1991; Hornoy et al., 2011).
In summary, both the history of introduction and the genetic properties of gorse likely resulted in the high genetic (this study) and phenotypic (Hornoy et al., 2011) diversity of introduced populations: multiple and/or massive introductions have contributed to the introduction of a relatively large number of individuals, each of them carrying a high number of alleles due to their allopolyploidy. By promoting the evolutionary potential and reducing inbreeding depression, this introduced genetic diversity may contribute to the persistence and rapid adaptation of gorse to a large geographical and climatic range. These results stress again the relevance of considering polyploidy as an important trait in invasion models and in management, as polyploids benefit from reduced genetic impact of bottlenecks, a high evolutionary potential, and other traits facilitating invasiveness (Lee, 2002; te Beest et al., 2012).
Conclusion
Our results indicate a colonisation of gorse from its centre of origin, the Iberian peninsula, in two stages. During the first stage, the species spread naturally from Spain towards France and Great Britain, probably in the Neolithic. This colonisation of northern Europe was accompanied by a significant loss of genetic diversity. However, the initial diversity was such that the diversity in northern Europe is still considerable. During the second stage, mainly from the nineteenth century, gorse was deliberately introduced by humans into different regions of the world. These introductions would have been large and mainly from northern Europe. They could have involved the loss of rare alleles but without significantly reducing genetic diversity and therefore the evolutionary potential of the species.
The tools developed recently for the study of polyploids have therefore allowed important information to be obtained on gorse's genetic diversity, even though it is impossible to characterise the genotypes. These results, coupled with the historical data, have made it possible to retrace numerous elements of the history and of the genetic consequences of the worldwide introduction of an invasive, whose allopolyploidy and absence of cytoplasmic diversity make a classical phylogeographical study fundamentally difficult.
Data archiving
Data deposited in the Dryad repository: doi:10.5061/dryad.g776j.
Acknowledgments
We thank Louis Parize, Guillaume Cossard, Stéphane Dréano and Dominique Vallet for technical assistance, Kristina Schierenbeck and Magui Basanta for providing seed samples, Eric Petit, Alex Baumel, Julie Jaquiéry, Jean-Francois Arnaud, Annie Guiller and Kader Ainouche for helpful discussions and comments on the manuscript.
The authors declare no conflict of interest.
References
- Ainouche A, Bayer RJ, Cubas P, Misset M-T.2003Phylogenetic relationships within tribe Genistae (Papilionoideae) with special reference to genus UlexIn: Klitgaard BB, Bruneau A (eds).Advances in Legume Systematics Part 10, Higher Level Systematics Royal Botanical Garden: Kew, UK; 239–252. [Google Scholar]
- Ainouche A, Mahé F, Affagard M, Ainouche ML, Misset M-T.2009Molecular evidence for an allopolyploid origin of the invasive European gorse, Ulex europaeus subsp. europaeus (Fabaceae, Genistae)Abstract book of the International Conference on Polyploidy, Hybridization and Biodiversity. University of Rennes 1: Rennes, France pp202
- Amsellem L, Noyer JL, Le Bourgeois T, Hossaert-McKey M. Comparison of genetic diversity of the invasive weed Rubus alceifolius Poir. (Rosaceae) in its native range and areas of introduction, using amplified fragment length polymorphism (AFLP) markers. Mol Ecol. 2000;9:443–455. doi: 10.1046/j.1365-294x.2000.00876.x. [DOI] [PubMed] [Google Scholar]
- Atlan A, Barat M, Legionnet AS, Parize L, Tarayre M. Genetic variation in flowering phenology and avoidance of seed predation in native populations of Ulex europaeus. J Evol Biol. 2010;23:362–371. doi: 10.1111/j.1420-9101.2009.01908.x. [DOI] [PubMed] [Google Scholar]
- Bossdorf O, Auge H, Lafuma L, Rogers WE, Siemann E, Prati D. Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia. 2005;144:1–11. doi: 10.1007/s00442-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Bowman G, Tarayre M, Atlan A. How is the invasive gorse Ulex europaeus pollinated during winter? A lesson from its native range. Plant Ecol. 2008;197:197–206. [Google Scholar]
- Buckley YM, Downey P, Fowler SV, Hill RL, Memmott J, Norambuena H, et al. Are invasives bigger? A global study of seed size variation in two invasive shrubs. Ecology. 2003;84:1434–1440. [Google Scholar]
- Calsbeek B, Lavergne S, Patel M, Molofsky J. Comparing the genetic architecture and potential response to selection of invasive and native populations of reed canarygrass. Evol Appl. 2011;4:726–735. doi: 10.1111/j.1752-4571.2011.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvel E. Mémoire sur l′ajonc ou genêt épineux. Marchant: Paris, France; 1809. [Google Scholar]
- Chater EH. A contribution to the study of the natural control of gorse. Bull Entomol Res. 1931;22:225–235. [Google Scholar]
- Clark LV, Jasieniuk M. POLYSAT: an R package for polyploid microsatellite analysis. Mol Ecol Resources. 2011;11:562–566. doi: 10.1111/j.1755-0998.2011.02985.x. [DOI] [PubMed] [Google Scholar]
- Cubas P.1999Ulex LIn: Castroviejo S (ed.). Flora Iberica: Plantas vasculares de la Peninsula Iberica e Islas Baleares Real Jardin Botanico: Madrid, Spain; 212–239. [Google Scholar]
- Cubas P, Pardo C, Tahiri H. Genetic variation and relationships among Ulex (Fabaceae) species in southern Spain and northern Morocco assessed by chloroplast microsatellite (cpSSR) markers. Am J Bot. 2005;92:2031–2043. doi: 10.3732/ajb.92.12.2031. [DOI] [PubMed] [Google Scholar]
- Darwin C. Voyages of the Adventure and Beagle. London; 1839. [Google Scholar]
- Dlugosch KM, Parker IM. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol. 2008a;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Dlugosch KM, Parker IM. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol Lett. 2008b;11:701–709. doi: 10.1111/j.1461-0248.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants. Proc Natl Acad Sci USA. 2000;97:7043–7050. doi: 10.1073/pnas.97.13.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esselink GD, Nybom H, Vosman B. Assignment of allelic configuration in polyploids using the MAC-PCR (microsatellite DNA allele counting-peak ratios) method. Theor Appl Genet. 2004;109:402–408. doi: 10.1007/s00122-004-1645-5. [DOI] [PubMed] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes. 2007;7:574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoli-Chiapella L, Cristofolini G. Serological contributions to the systematics of Ulex (Genisteae—Fabaceae) and allied genera. Nord J Bot. 1981;1:723–729. [Google Scholar]
- Gay C. Historia Fisica y Politica de Chile. Botanica: Paris, France; 1846. [Google Scholar]
- Gay J-C. L′outre-mer Francais, un Espace Singulier. Belin: Paris, France; 2007. [Google Scholar]
- Hahn MA, Buckley YM, Müller-Schärer H. Increased population growth rate in invasive polyploid Centaurea stoebe in a common garden. Ecol Lett. 2012;15:947–954. doi: 10.1111/j.1461-0248.2012.01813.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Eckert CG. Population genetic consequences of geographic disjunction: a prairie plant isolated on Great Lakes alvars. Mol Ecol. 2007;16:1649–1660. doi: 10.1111/j.1365-294X.2007.03241.x. [DOI] [PubMed] [Google Scholar]
- Helsen P, Verdyck P, Tye A, Van Dongen S. Low levels of genetic differentiation between Opuntia echios varieties on Santa Cruz (Galapagos) Plant Syst Evol. 2009;279:1–10. [Google Scholar]
- Hewitt GM. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc. 1996;58:247–276. [Google Scholar]
- Hewitt GM. The genetic legacy of the Quaternary ice ages. Nature. 2000;405:907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- Hill RL, Gourlay AH, Barker RJ. Survival of Ulex europaeus seeds in the soil at three sites in New Zealand. N Z J Bot. 2001;39:235–244. [Google Scholar]
- Hill RL, Gourlay AH, Lee WG, Wilson JB. Forty-ninth New Zealand Plant Protection Conference. New Zealand Plant Protection Society: Rotorua: New Zealand; 1996. Dispersal of seeds under isolated gorse plants and the impact of seed-feeding insects; pp. 114–118. [Google Scholar]
- Hill RL, Gourlay AH, Martin L. Seasonal and geographic variation in the predation of gorse seed, Ulex europaeus L., by seed weevil Apion ulicis Forst. N Z J Zool. 1991;18:37–43. [Google Scholar]
- Holm L, Doll J, Holm E, Pancho J, Herberger J. World Weeds: Natural Histories and Distribution. John Wiley and Sons, Inc.: New York, NY, USA; 1997. [Google Scholar]
- Honnay O, Bossuyt B, Jacquemyn H, Shimono A, Uchiyama K. Can a seed bank maintain the genetic variation in the above ground plant population. Oikos. 2008;117:1–5. [Google Scholar]
- Hornoy B, Atlan A, Tarayre M, Dugravot S, Wink M. Alkaloid concentration of the invasive plant species Ulex europaeus in relation to geographic origin and herbivory. Naturwissenschaften. 2012;99:883–892. doi: 10.1007/s00114-012-0970-9. [DOI] [PubMed] [Google Scholar]
- Hornoy B, Tarayre M, Hervé M, Gigord L, Atlan A. Invasive plants and enemy release: evolution of trait means and trait correlations in Ulex europaeus. PLoS ONE. 2011;6:e26275. doi: 10.1371/journal.pone.0026275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff DR, Peakall R, Smouse PE. RAPD variation within and among natural populations of outcrossing buffalograss [Buchloe¨ dactyloides (Nutt.) Engelm.] Theor Appl Genet. 1993;86:927–934. doi: 10.1007/BF00211043. [DOI] [PubMed] [Google Scholar]
- Isern TD. A good servant but a tyrannous master: gorse in New Zealand. Soc Sci J. 2007;44:179–186. [Google Scholar]
- Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genetics. 2010;11:94. doi: 10.1186/1471-2156-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Buckley YM, Lowe AJ. Testing the role of genetic factors across multiple independent invasions of the shrub Scotch broom (Cytisus scoparius) Mol Ecol. 2007;16:4662–4673. doi: 10.1111/j.1365-294X.2007.03536.x. [DOI] [PubMed] [Google Scholar]
- Keller SR, Taylor DR. History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol Lett. 2008;11:852–866. doi: 10.1111/j.1461-0248.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- Kolbe JJ, Glor RE, Schettino LR, Lara AC, Larson A, Losos JB. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;431:177–181. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- Kopp KC, Wolff K, Jokela J. Natural range expansion and human-assisted introduction leave different genetic signatures in a hermaphroditic freshwater snail. Evol Ecol. 2012;26:483–498. [Google Scholar]
- Lavergne S, Molofsky J. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Natl Acad Sci USA. 2007;104:3883–3888. doi: 10.1073/pnas.0607324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CE. Evolutionary genetics of invasive species. Trends Ecol Evol. 2002;17:386–391. [Google Scholar]
- Lee CE, Gelembiuk GW. Evolutionary origins of invasive populations. Evol Appl. 2008;1:427–448. doi: 10.1111/j.1752-4571.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WG, Allen RB, Johnson PN. Succession and dynamics of gorse (Ulex europaeus L.) communities in the Dunedin Ecological District South Island, New Zealand. N Z J Bot. 1986;24:279–292. [Google Scholar]
- Loveless MD, Hamrick JL. Determinants of genetic structure in plant populations. Annu Rev Ecol Syst. 1984;15:65–95. [Google Scholar]
- Loveman B. Chile: the Legacy of Hispanic Capitalism. Oxford University Press: New York, NY, USA; 2001. [Google Scholar]
- Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the World's worst invasive alien species. A selection from the global invasive species database, The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN) 2000. p. 12.
- Lundemo S, Falahati-Anbaran M, Stenoien HK. Seed banks cause elevated generation times and effective population sizes of Arabidopsis thaliana in northern Europe. Mol Ecol. 2009;18:2798–2811. doi: 10.1111/j.1365-294X.2009.04236.x. [DOI] [PubMed] [Google Scholar]
- Mack RN. Commercial seed trade: an early disperser of weeds in the United States. Econ Bot. 1991;45:257–273. [Google Scholar]
- Mahy G, Ennos RA, Jacquemart AL. Allozyme variation and genetic structure of Calluna vulgaris (heather) populations in Scotland: the effect of postglacial recolonization. Heredity. 1999;82:554–660. doi: 10.1046/j.1365-2540.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- Mahy G, Vekemans X, Jacquemart A-L, de Sloover J-R. Allozyme diversity and genetic structure in South-Western populations of heather, Calluna vulgaris. New Phytol. 1997;137:325–334. doi: 10.1046/j.1469-8137.1997.00811.x. [DOI] [PubMed] [Google Scholar]
- Marrs RA, Sforza R, Hufbauer RA. Evidence for multiple introductions of Centaurea stoebe micranthos (spotted knapweed, Asteraceae) to North America. Mol Ecol. 2008;17:4197–4208. doi: 10.1111/j.1365-294x.2008.03903.x. [DOI] [PubMed] [Google Scholar]
- Millennium Ecosystem Assessment . Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute: Washington, DC, USA; 2005. [Google Scholar]
- Misset M-T, Gourret J-P. Flow cytometric analysis of the different ploidy levels observed in the genus Ulex L. (Faboideae-Genistae) in Brittany (France) Botanica Acta. 1996;109:72–79. [Google Scholar]
- Moss GR. The Gorse Seed Problem. Proceedings of the twelfth New Zealand weed control conference. The New Zealand Weed Control Conference Inc.: Wellington: New Zealand; 1959. pp. 59–64. [Google Scholar]
- Müller-Schärer H, Steinger T.2004Predicting evolutionary change in invasive, exotic plants and its consequences for plant-herbivore interactionsIn: Ehler LE, Sforza R, Mateille T (eds).Genetics, Evolution and Biological Control CABI: Wallingford, UK; 137–162. [Google Scholar]
- Myers JH, Bazely D. Ecology and Control of Introduced Plants. Cambridge University Press: Cambridge, UK; 2003. pp. 1–13. [Google Scholar]
- Nei M, Maruyama T, Chakraborty R. The bottleneck effect and genetic variability in populations. Evolution. 1975;29:1–10. doi: 10.1111/j.1558-5646.1975.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Nybom H. Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol. 2004;13:1143–1155. doi: 10.1111/j.1365-294X.2004.02141.x. [DOI] [PubMed] [Google Scholar]
- Obbard DJ, Harris SA, Pannell JR. Simple allelic-phenotype diversity and differentiation statistics for allopolyploids. Heredity. 2006;97:296–303. doi: 10.1038/sj.hdy.6800862. [DOI] [PubMed] [Google Scholar]
- Opazo RG. Monografía Cultural de las Diversas Plantas Agrícolas. Santiago, Chile; 1930. [Google Scholar]
- Parsons WT, Cuthbertson EG. Noxious weeds of Australia. CSIRO Publishing: Collingwood, Australia; 2001. [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D, Lach L, Zuniga R, Morrison D. Environmental and economic costs of nonindiginous species in the United States. BioScience. 2000;50:53–65. [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor MR, Dana RH. Gorse control. Calif Dept Agric Bull. 1952;41:43–45. [Google Scholar]
- Richardson RG, Hill RL. The biology of Australian weeds. 34. Ulex europaeus L. Plant Prot Q. 1998;13:46–58. [Google Scholar]
- Ridley HN. L. Reeve and Co., Ltd.: Ashford, UK; 1930. The dispersal of plants throughout the world. [Google Scholar]
- Sharifi Tehrani M, Mardi M, Sahebi J, Catalan P, Diaz-Perez A. Genetic diversity and structure among Iranian tall fescue populations based on genomic-SSR and EST-SSR marker analysis. Plant Syst Evol. 2009;282:57–70. [Google Scholar]
- Stockwell CA, Hendry AP, Kinnison MT. Contemporary evolution meets conservation biology. Trends Ecol Evol. 2003;18:94–101. [Google Scholar]
- Tarayre M, Bowman G, Schermann-Legionnet A, Barat M, Atlan A. Flowering phenology of Ulex europaeus: ecological consequences of variation within and among populations. Evol Ecol. 2007;21:395–409. [Google Scholar]
- te Beest M, Le Roux JJ, Richardson DM, Brysting AK, Suda J, Kubesova M, et al. The more the better? The role of polyploidy in facilitating plant invasions. Ann Bot. 2012;109:19–45. doi: 10.1093/aob/mcr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutin TG, Heywood VH, Burges NA, Moore DA, Valentine DH, Walters SM, et al. Rosaceae to Umbelliferae. Cambridge University Press: Cambridge, UK; Flora Europaea. 1968;vol. 2 [Google Scholar]
- van Zeist W. Recherches palynologiques en Bretagne occidentale. Norois. 1963;37:5–19. [Google Scholar]
- van Zeist W. A paleobotanical study of some bogs in western Brittany (Finistère), France. Palaeohist. 1964;10:157–180. [Google Scholar]
- Walker B, Steffen W.1997An overview of the implications of global change for natural and managed terrestrial ecosystems Conservation Ecol[online] 1:2. Available from the internet. URL:http://www.consecol.org/vol1/iss2/art2/ .
- Webb NR. The traditional management of European heathlands. J Appl Ecol. 1998;35:987–990. [Google Scholar]
- Young GFW. Germans in Chile: Immigration and Colonization, 1849–1914. Center for Migration Studies: Staten Island, NY, USA; 1974. [Google Scholar]
- Zabkiewicz JA. The Ecology of Gorse and its Relevance to New Forestry. New Zealand Forest Research Institute Symposium: Rotorua, New Zealand; 1976. [Google Scholar]