Abstract
Objective
To determine changes in the management strategy of patients with insulinomas and identify critical factors in patient outcome.
Background
Pancreatic insulinomas are rare neoplasms that are present in various ways. The optimal approach to localization, operative management, and follow-up of insulinomas is undetermined.
Methods
Sixty-one patients with a diagnosis of insulinoma requiring surgery at a tertiary care center between 1983 and 2007 were reviewed. Demographic details, mode of presentation, preoperative localization, operative procedures, and pathology data were assessed. The effect of different factors on survival was determined.
Results
Seven of 61 (11%) patients had a diagnosis of multiple endocrine neoplasia-type 1 (MEN-1). Multiple insulinomas were noted in 8% of cases and were more common in MEN-1 patients. The overall rate of malignancy was 8%. Confusion (67%), visual disturbances (42%), and diaphoresis (30%) were the most common presenting symptoms. Weight gain was noted in 44% of patients. The median duration of symptoms before diagnosis was 18 (1–240) months. The sensitivity of preoperative imaging of tumors before 1994 was 75%, compared with 98% after this period, which included use of endoscopic ultrasound scanning (P = 0.012). A combination of palpation and intraoperative ultrasound detected 92% of tumors. Distal pancreatectomy (40%), enucleation (34%), and pancreaticoduodenectomy (16%) were the most common procedures and pancreatic fistula occurred in 18% of patients. Three patients underwent noncurative distal pancreatectomy in the early period. The 10-year disease-specific and disease-free survival was 100% and 90% respectively. There were 5 patients with disease recurrence. Lymph node metastases (P < 0.001), lymphovascular invasion (P < 0.001), and the presence of MEN-1 (P = 0.035) were prognostically significant adverse factors in disease-free survival. Lymphovascular invasion was the only significant factor on multivariate analysis (P = 0.002).
Conclusion
Pancreatic insulinomas can be readily localized preoperatively with modern imaging to avoid unsuccessful blind pancreatic resection. Surgical resection is associated with low morbidity and mortality and achieves long-term disease-free survival in the absence of lymphovascular invasion.
Insulinomas are the most common functioning endocrine neoplasms of the pancreas, with an incidence of 4 cases per million population.1 They have a slight predilection for women and most commonly are sporadic, with less than 10% associated with multiple endocrine neoplasia-type 1 (MEN-1).1,2 Symptoms from insulinoma are related to episodes of hypoglycemia. Vague and subtle complaints, including confusion, behavioral changes, and visual disturbances are common and often result in delayed diagnosis. The severity of symptoms generally has no relationship to the malignant potential of insulinomas.3 Diagnosis is confirmed in the setting of typical symptoms with the demonstration of low serum glucose, inappropriately elevated serum insulin and C-peptide, and exclusion of other causes of hypoglycemia. Surgical resection is considered the treatment of choice in the majority of cases.
There are few contemporary single-institution series that report on the management of insulinomas, and there is controversy as to the best approach for localization of these neoplasms, selection of patients for pancreas sparing surgery and the relationship of various demographic, operative and pathologic factors on survival. Herein, we present the surgical experience of the Massachusetts General Hospital (MGH) in the management of insulinomas over the last 25 years, with particular emphasis on the changes in diagnosis, treatment, and long-term outcomes.
PATIENTS AND METHODS
Patient Population
All patients undergoing surgery for pancreatic insulinoma at the MGH between 1983 and 2007 were included in this study. Those with a diagnosis of “neuroendocrine neo- plasm” were identified from a prospective pancreatic resection database, and cases of insulinoma identified. Crosschecks were made with pathology registers to ensure that all records were identified. Patients were considered to have MEN-1 if they were members of a known MEN-1 kindred or had any other MEN-associated endocrinopathy as defined by Thakker.4 Cases of noninsulinoma pancreatogenous hypoglycemia syndrome were excluded from analysis. Institutional review board approval was obtained before assessment of charts and patient contact.
Preoperative Assessment
Demographic data, symptom duration, type, biochemistry results, and localization studies were obtained from medical records. A more targeted approach for imaging pancreatic neoplasms has been used at MGH since 1994. This includes the inception of arterial protocol helical computed tomography (CT) in 1994, and multidetector CT scanning as well as dynamic gadolinium enhanced magnetic resonance imaging (MRI) from 1999 onwards. Endoscopic ultrasound (EUS), using linear array echo endoscopes, was first used at our institution for assessment of endocrine neoplasms in 1994.5
Differences in preoperative localization of neoplasms between 1983 and 1993 (group 1) and 1994 to 2007 (group 2) were determined. Octreotide scanning using 111Indium-labeled Pentetreotide was performed in a small number of cases, primarily to assess for metastases. Angiography and transhepatic portovenous sampling (THPVS) with calcium stimulation were performed in selected cases.
Operative Procedures and Complications
A pancreatic sparing approach has been used at our institution for the treatment of insulinomas. The majority of operations in this series were performed by the senior surgeon (A.L.W.), with pancreatic resection undertaken when enucleation could not be safely performed without major duct injury, for nonpalpable lesions, and when malignancy was suspected. Spleen-preserving distal pancreatectomy was performed whenever possible, based on the short gastric vessels as previously described.6 Distal pancreatectomy with splenectomy was performed when spleen viability could not be assured and for neoplasms located at the splenic hilum that could not be safely and completely removed without splenic injury. Pancreatic fistula was defined and graded according to the International Study Group on Pancreatic Fistula criteria.7
Pathologic Findings
All specimens were available for analysis and were confirmed as being insulinoma by immunohistochemistry or electron microscopy. The quality of resection was determined according to the R-classification by the International Union against cancer (R0 = no residual disease and Rl = residual microscopic disease). Insulinomas were classified as benign or malignant based on the World Health Organization (WHO) classification of neuroendocrine tumors of the pancreas.8
Follow-up
Follow-up was achieved through review of hospital and office medical records and direct telephone contact. The social security index database was accessed when follow-up was not possible to determine survival status.
Statistical Analysis
Results were expressed as median (range) unless otherwise stated. Comparisons between categorical variables were determined by χ2 and Fisher exact test. Noncategorical variables were assessed by the Mann-Whitney U test or Student t test where appropriate. Survival analysis was done by the Kaplan-Meier limit method and comparison between variables by the log-rank test. Cox-proportional regression analysis was undertaken to determine factors independently associated with disease recurrence after resection. A statistical software package (SPSS Version 11.5, Chicago, IL) was used for statistical analysis.
RESULTS
Patient Population and Symptoms
Sixty-one patients with a diagnosis of insulinoma were surgically treated at the MGH during the study period. Table 1 summarizes the demographic features and presenting symptoms. The median age at presentation was 53 years, with slight women predominance. Seven patients (11%) had MEN-1 and were significantly younger than non-MEN-1 patients, with a median age of 28 years at diagnosis (P = 0.028). Five of these patients had other MEN-1 typical neoplasms.
TABLE 1.
Characteristics of Patients With Insulinoma (n = 61)
| Demographics | |
| Male/female (%) | 26/35 (43/57) |
| Age | 53 (range, 11–82) |
| MEN-1 (%) | 7(11) |
| Ethnicity – white (%) | 48 (79) |
| Body mass index (BMI) | 26 (17–94) |
| Presentation | |
| Symptom duration (mo) | 18 (1–240) |
| Symptoms | |
| Confusion (%) | 41 (67) |
| Visual disturbances (%) | 26 (42) |
| Diaphoresis (%) | 18 (30) |
| Fatigue (%) | 17 (28) |
| Abnormal behavior (%) | 10(16) |
| Seizures (%) | 10(16) |
| Lightheadedness (%) | 10(16) |
| Syncope (%) | 8(13) |
| Anxiety (%) | 7(12) |
| Tremor (%) | 7(12) |
| Amnesia (%) | 5(8) |
| Headache (%) | 4(7) |
| Paresthesias (%) | 4(7) |
| Palpitations (%) | 3(5) |
| Hunger (%) | 2(3) |
| Drowsiness (%) | 1(2) |
| Other | |
| Precipitated by exercise (%) | 17 (28) |
| Weight gain (%) | 27 (44) |
| Weight loss (%) | 1(2) |
The median duration of symptoms before diagnosis was 18 (1–240) months. Confusion (67%), visual disturbances, and diplopia (42%) and diaphoresis (30%) were the most common presenting symptoms. Unintentional weight gain was noted in 44% of cases. There were no patients presenting with jaundice or abdominal pain as initial symptoms.
Diagnosis of insulinoma was based on a positive 72-hour fasting glucose test in 79% of patients and on low blood glucose and high insulin levels in the remainder. The median fasting glucose nadir was 37 (17–50) mg/dL, median insulin 19 (1–184) µIU/mL, and median C-peptide 3.6 (1–23) ng/ mL. Seven patients were placed on diazoxide preoperatively for symptom control. One patient with MEN-1 was diagnosed concurrently with Zollinger-Ellison syndrome, based on symptoms and an elevated serum gastrin level.
Preoperative Imaging
The success of preoperative localization of insulinomas between 1983 and 1993 (group 1) and 1994 and 2007 (group 2) is shown in Table 2.
TABLE 2.
Preoperative Localization of Insulinomas*
| Group 1 (n = 20) 1983–1993 |
Group 2 (n = 41) 1994–2007 |
Total (n = 61) |
|
|---|---|---|---|
| Noninvasive imaging (%) | 5/17 (29) | 30/38 (80) | 34/55 (62) |
| CT scan (%) | 4/17 (24) | 28/35 (80) | 32/52 (62) |
| MRI (%) | 3/7 (43) | 14/20 (70) | 17/27 (63) |
| Abdominal ultrasound (%) | 0/3 (0) | 1/3 (33) | 1/7 (14) |
| Octreotide imaging (%) | NA | 0/4 (0) | 0/4 (0) |
| Invasive imaging (%) | 11/13(85) | 23/23 (100) | 34/36 (94) |
| Angiography (%) | 8/13 (62) | 2/5 (40) | 10/18 (56) |
| THPVS (%) | 3/3 (100) | 4/4 (100) | 7/7 (100) |
| Endoscopic ultrasound (%) | NA | 23/25 (92) | 23/25 (92) |
| EUS - biopsy (%) | NA | 8/14 (57) | 8/14 (57) |
| Overall detection (%) | 15/20 (75) | 40/41 (98) | 55/61 (90) |
Ratios indicate the proportion of patients where the technique was successful.
CT indicates computed tomography; MRI, magnetic resonance imaging; NA, not applicable; THPVS, transhepatic portovenous sampling; EUS, endoscopic ultrasound.
Noninvasive Imaging
Noninvasive imaging identified 29% of insulinomas imaged in group 1, compared with 80% in group 2 (P < 0.001). A major difference was noted in improved sensitivity of CT scanning in the latter group (80% vs. 24%; P < 0.001). Statistically, the sensitivity of MRI in groups 1 and 2 was not significantly different (43% vs. 70% P = 0.365). There was 1 false positive CT result in group 2, with a lesion incorrectly detected in the body of the pancreas.
Invasive Imaging
The detection of insulinomas by invasive imaging was similar in group 1 (85%) and group 2 (100%) (P = 0.124), with a high reliance on angiography from 1983 to 1993 and on EUS from 1994 onwards. There was no difference in the sensitivity of angiography alone in group 1 compared with group 2 (62% vs. 40% P = 0.608).
Twenty-five patients had EUS, with positive localization achieved in 23 cases (92%). No lesions could be detected in 1 patient and lymph nodes were incorrectly interpreted as a neoplasm in another patient. In 4 cases, EUS identified insulinomas after failed CT-MRI detection. The sensitivity of EUS, combined with CT and MRI was 96%. EUS-guided fine needle biopsy identified neoplastic neuroendocrine cells in 8 of 14 (57%) cases sampled.
THPVS with calcium stimulation was used in 7 cases, and localized the tumors in all instances. In 4 cases, tumors were only localized by this method; however only 1 had been previously evaluated by EUS.
Overall
The overall preoperative identification of insulinomas was 90% (55 of 61 cases). The preoperative detection of tumors was significantly higher after 1994 compared with 1983–1993 (98% vs. 75%; P = 0.012), reflecting improved CT scanning and sensitive EUS imaging.
Operative Findings, Procedures, and Complications
Findings
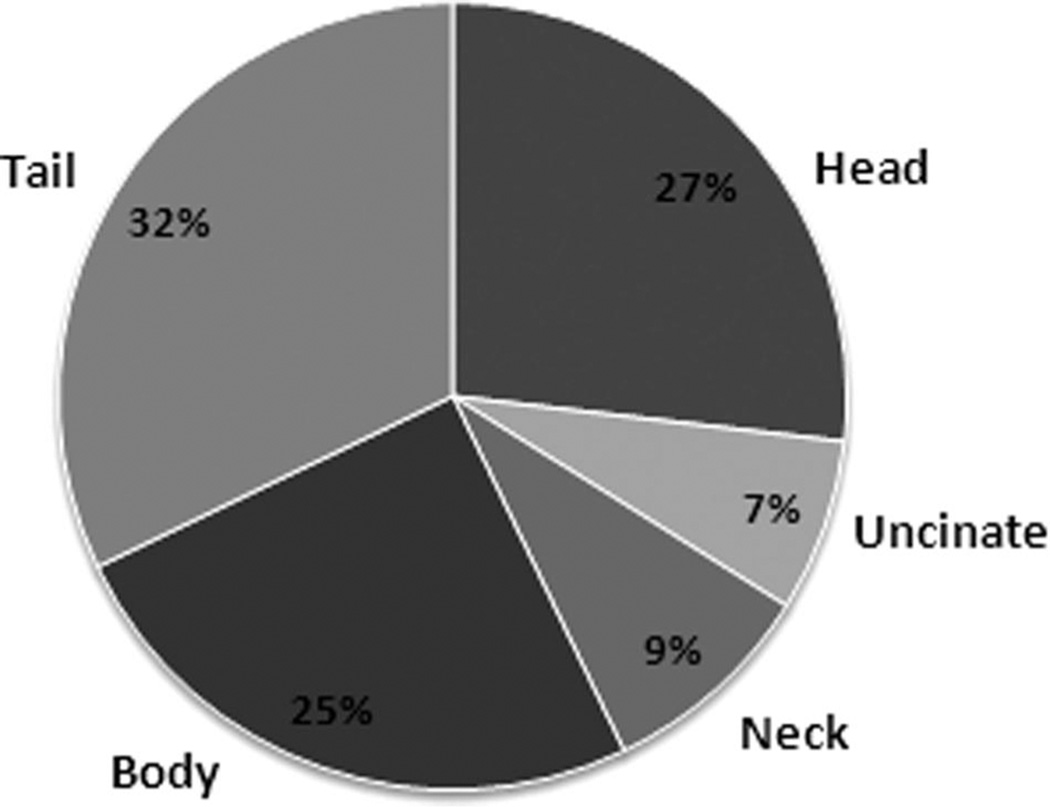
Single neoplasms were detected in 56 of 61 (92%) cases and were distributed relatively evenly within the pancreas as shown in Fig. 1. In 51 of 61 (84%) patients, tumors were palpable at the time of operation. Full mobilization of the pancreas was undertaken to maximize detection when required. Of the 10 nonpalpable lesions, 8 (80%) were located in the head or uncinate region and 2 (20%) within the tail. Intraoperative ultrasound (IOUS) detected lesions in 27 of 33 (82%) patients examined. In 5 cases, insulinomas could only be localized at surgery by IOUS. In 4 patients, insulinomas were neither palpable nor detectable by IOUS. In 1 case, a palpable neoplasm could not be defined by IOUS. Overall detection of tumors intraoperatively in this series by combination of palpation and IOUS was 92% (56 of 61).
FIGURE 1.
Distribution of pancreatic insulinomas in 56 patients with solitary lesions.
Procedures
Enucleation alone was the most common operative procedure, performed in 21 of 61 (34%) cases, one of which was achieved laparoscopically. Two patients had jejunal Roux-en-Y anastomoses to the site of tumor enucleation due to concerns of pancreatic duct leakage. Distal pancreatectomy was performed in 24 (40%) cases, 15 of which were spleen preserving and one of which was laparoscopic. Ten patients (16%) underwent a pancreaticoduodenectomy. Middle pancreatectomy was undertaken in 4 cases, with a jejunal Roux-en-Y anastomoses to the distal pancreatic stump. Total pancreatectomy was required in 1 non-MEN-1 patient with multiple tumors. One patient with MEN-1 and multiple tumors required combined distal pancreatectomy, and enucleation of tumors from the pancreatic head. Frozen section was used to confirm neoplasia in 54 of 61 (89%) cases.
Failed Operations
There were 4 patients who had negative laparotomies before eventual excision of insulinomas. Three of these were before 1994. At surgery, 1 patient was thought to have a midbody neoplasm after full mobilization of the pancreas and underwent distal pancreatectomy. No tumor was identified on histology. Subsequently, THPVS and calcium stimulation demonstrated an insulinoma in the head of the pancreas that was then confirmed by IOUS and enucleated at a second operation.
Another patient was explored and underwent a blind distal resection and splenectomy, with concerns of a small neoplasm near the splenic hilum. No tumor could be identified on histology. Subsequent angiogram showed a lesion in the head of the pancreas. At the time of reoperation, a tumor was identified by palpation and IOUS in the uncinate process and was enucleated.
One patient had undergone a distal pancreatectomy at another institution for presumed insulinoma without symptom relief. THPVS was suggestive of a pancreatic head lesion. This could not be visualized with ultrasound or palpation at surgery and a completion pancreaticoduodenectomy was performed, revealing a 20-mm neoplasm deep within the pancreatic head.
The final patient had a suspected pancreatic body insulinoma on EUS, but with a suspicious lesion in the head of the pancreas on noninvasive imaging. No insulinoma could be located at the time of surgery by palpation or IOUS. The patient was reassessed postoperatively including repeat EUS and THPVS and eventually underwent a successful pancreaticoduodenectomy.
Complications
There was no operative mortality in this study. The median length of stay was 7 (2–49) days. Median operative blood loss was 275 (10 –3000) ml. In total 16 (26%) patients had 1 or more complications. The pancreatic fistula rate was 18%. Of these, 5 were classified as grade A, 1 was grade B, and 5 grade C. The grade C fistulas were manifested as postoperative intra-abdominal collections, which required percutaneous drainage. Five fistulas occurred after enucleations, 3 after distal pancreatectomy, 2 after pancreaticoduodenectomy, and 1 after a middle pancreatic resection. Other complications included 2 major wound infections, 1 case of deep venous thrombosis and pulmonary emboli, 1 case of delayed gastric emptying, 1 case of intra-abdominal abscess, and 1 case of postoperative cholangitis after a pancreaticoduodenectomy.
Four patients developed diabetes mellitus in the postoperative period. One was in an obese patient with a family history of diabetes after enucleation of an insulinoma. Another occurred after a spleen-preserving distal pancreatectomy in a patient with a history of gestational diabetes. Of the remaining 2, 1 had a completion pancreaticoduodenectomy and the other a total pancreatectomy.
Symptom Resolution
Complete symptom resolution was achieved in 55 of 61 (90%) cases after the initial surgery. In 1 patient with a diagnosis of a seizure disorder, symptoms continued, albeit reduced, after resection of a benign insulinoma. Extensive biochemical testing excluded further hypoglycemic episodes as the cause of symptoms. One patient with an insulinoma within the pancreatic tail and unresectable liver metastases had reduced symptoms after distal pancreatectomy, and these were controlled with diazoxide. In the other 4 patients, the insulinoma was not found at the initial operation.
Pathologic Findings
All specimens were confirmed as pancreatic insulinoma based on immunohistochemical or electron microscopy features.
Size and Number
Multiple tumors occurred in 5 (8%) patients with a median of 4 (2–4) lesions per patient. Three of these patients had MEN-1. The median maximum tumor diameter was 15 (5–40) mm, without any significant difference between MEN-1 and non-MEN-1 patients.
Margins
Positive margins (Rl) were noted in 8 (13%) cases. The median follow-up of these patients was 78 (5–150) months. Seven of the 8 patients had insulinomas treated by enucleation. There were no recurrences at the resection site on follow-up.
Malignancy
There were 5 malignant tumors according to the WHO criteria.8 All 5 had lymph node metastases, with 1 having concurrent liver metastases. Three of these 5 patients had recurrence or disease progression. The remaining 2 patients with malignant insulinomas, both treated by distal pancreatectomy, are alive and well 9 years after surgery.
The median size of the 5 malignant tumors was not significantly different from the benign tumors [25 (14–38) mm vs. 15 (5–40) mm; P = 0.120]. Lymphovascular invasion was noted in 3 of 5 malignant insulinomas and in 6 of 61 (10%) patients in the entire series.
Follow-up and Survival
Median follow-up in this study was 77 (0 –291) months. Complete follow-up was possible in 56 of 61 patients. In the remaining patients, social security database was used to confirm survival and they were censored to the point of last follow-up. These patients all had benign tumors without adverse features.
Pancreatic Recurrences and Disease Progression
Pancreatic recurrences were noted in 2 patients and required further resection. One, a 62-year-old woman with known MEN-1, developed presumed new lesions 4 years after initial enucleation of 4 insulinomas, including removal of involved adjacent lymph nodes. She was treated by repeated enucleation of a single tumor in the head of the pancreas and a distal pancreatectomy and splenectomy. Subsequently she was well for another 4 years, when she developed hypoglycemic symptoms and additional tumors were detected. She is currently treated with subcutaneous longacting octreotide and has minimal symptoms.
The second pancreatic recurrence was in a 57-year-old man, without a history of MEN-1, who developed a presumed new primary in the pancreatic neck 3 years after enucleation of a benign lesion from the uncinate process, and required a middle pancreatic resection. He remains disease-free 10 years after the second resection.
Disease progression outside of the pancreas was seen in 3 patients. One was a 33-year-old woman who underwent a distal pancreatectomy and splenectomy for a 25-mm pancreatic tail insulinoma, and was noted to have multiple small liver metastases. These could not be excised due to their number and location. She was given adjuvant chemotherapy and chemoembolization, but had slowly progressive disease. She died as a result of liver metastases 11 years after her initial operation.
The second is a 15-year-old girl, with no history of MEN-1, who developed multiple liver metastases 18 months after excision of a histologically benign neoplasm in the tail of the pancreas by distal pancreatectomy. The liver metastases were unresectable and she was given a brief course of chemotherapy and subsequently underwent liver transplantation. She has been free of disease for 4 years.
The last is a 57-year-old man with MEN-1 and a solitary midbody insulinoma, who underwent a distal pancreatectomy and had confirmed lymph node metastases. Three years later he developed multiple liver metastases, and remains alive with minimal symptoms.
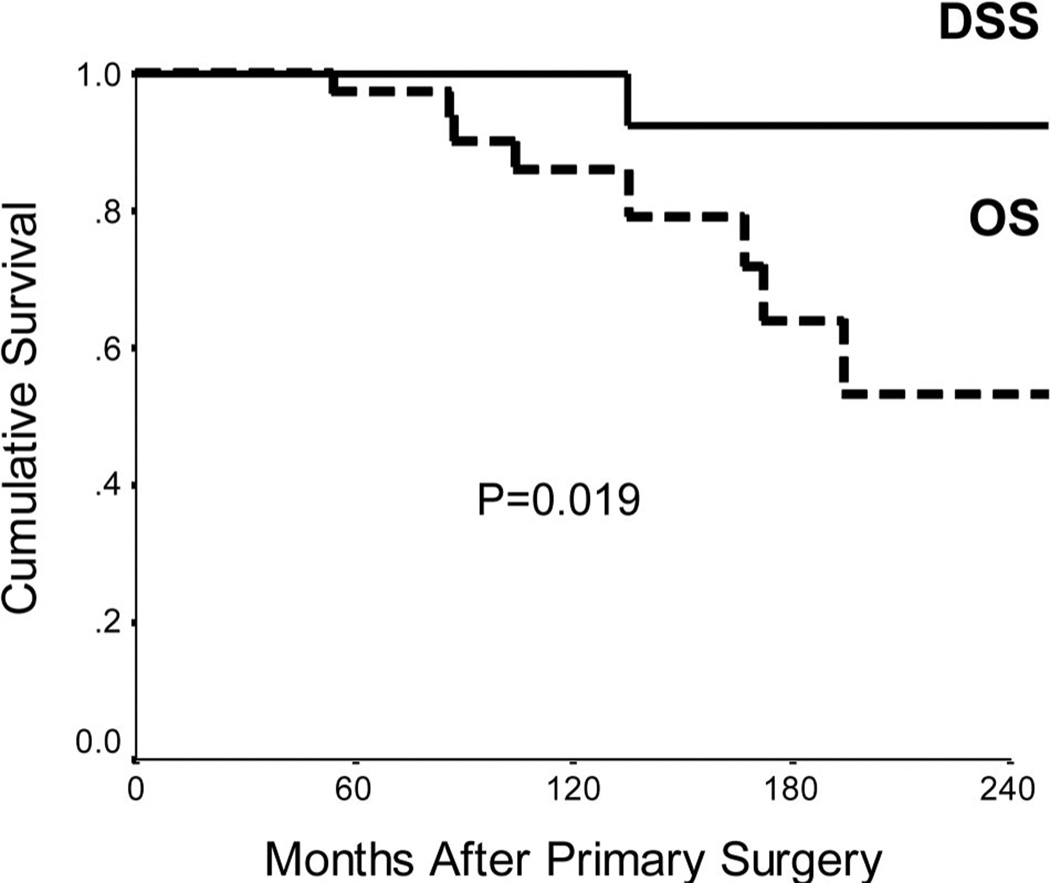
Survival
The overall survival (OS) and disease-specific survival (DSS) is shown in Figure 2. There were 8 deaths during the study period, with only 1 related to disease. The OS at 5, 10, and 20 years was 97%, 86%, and 54% respectively. The DSS at 5, 10, and 20 years was 100%, 100%, and 93% respectively and was significantly better than OS (P = 0.019). During the same period there were 5 patients with disease recurrence. The disease-free survival (DFS) at 5, 10, and 20 years was 90% at all each time point, with all recurrences occurring within 5 years of initial surgery.
FIGURE 2.
Overall survival (OS) and Disease-specific survival (DSS) of patients following surgical resection for insulinomas.
Prognostic Factors
Factors that could potentially influence disease recurrence were analyzed by univariate analysis. Demographic factors (age, sex, body mass index, and MEN-1 status), presenting factors (duration of symptoms, specific symptoms, and weight gain) operative technique (enucleation, pancreatic resection, and complications), and pathologic features (size, involved margins, lymph node status, lymphovascular invasion, and tumor location) were analyzed. Statistically significant factors were assessed by multivariate analysis as shown in Table 3. Although MEN-1 status and lymph node involve- merit were significant factors on univariate analysis, lympho-vascular invasion was the only significant independent factor on multivariate analysis.
TABLE 3.
Predictors of Disease-Free Survival in 61 Patients With Resected Insulinomas
| Variable | Univariate Analysis (Log-Rank Test) (P) |
Multivariate Analysis (Cox-Proportional Hazard Regression) (P) |
Risk Ratio | 95% CI (Confidence Interval) |
|---|---|---|---|---|
| Lymphovascular invasion | <0.001 | 0.002 | 58.324 | 4.212–807.582 |
| Lymph node metastases | <0.001 | 0.095 | 11.424 | 0.654–199.653 |
| MEN-1 | 0.035 | 0.205 | 0.125 | 0.005–3.119 |
MEN-1 indicates multiple neuroendocrine neoplasia type 1.
DISCUSSION
Insulinomas attract clinical attention out of proportion to their low incidence because of their nonspecific and sometimes unusual presenting symptoms and their high cure rate with surgery. The association between insulinoma and hypoglycemia was first recognized in 1927, when extracts from a metastatic insulinoma were injected into a rabbit to produce effects of hypoglycemia.9 Two years later, Roscoe Graham performed the first curative operation for a benign insulinoma in Toronto, Canada.10 Since that time there have been many reports of successful management of insulinoma in both the medical and surgical literature. Most of these reports are based on small numbers of patients or are multi-institutional studies.11 Recommendations regarding investigation of patients with insulinoma before surgical resection, surgical technique and prognostic factors are not clearly defined.12–14
A delay in diagnosis is a common feature of patients with insulinomas. The median duration of symptoms before diagnoses in 224 patients treated at the Mayo Clinic over 60 years was 18 months,1 which is equivalent to our series. Hypoglycaemic events can be nonspecific and patients often self-treat with frequent meals to avoid symptoms. This commonly leads to weight gain, as noted in 44% of our patients. In fact 1 patient, who had symptoms for 3 years before diagnosis, weighed 294 kg (648 pounds) at the time of surgery. The most common presenting symptoms in our series were confusion, visual disturbances, and diaphoresis. Other large series report confusion and behavioral changes as the most common presenting symptoms.2, 15 Seizures were presenting symptoms in 10 patients (16%), 4 of whom had extensive neurologic work-up for presumed primary seizure disorders. Overall, the duration of symptoms and the type of symptoms did not correlate with disease severity and longterm DFS. Biologically more aggressive tumors do not seem to be clinically detected at earlier time points or produce distinct symptoms.
The need for preoperative localization in patients with biochemically proven insulinoma is debated.16–18 Many authors, quoting low preoperative detection rates compared with intraoperative palpation and ultrasound, advocate minimization of preoperative imaging. The rate of intraoperative detection, however, varies between studies, ranging from 83% to 98%.15,16,19,20 In our series, the sensitivity of IOUS and palpation was 92%. This compares very favorably to the 29% sensitivity of preoperative noninvasive imaging between 1983 and 1993. However, since then the sensitivity of noninvasive imaging has improved markedly and was 80% between 1994 and 2007. This included the identification of 4 nonpalpable lesions, including 1 that could not be identified with IOUS. One should also be mindful that most of the recommendations regarding the need for preoperative localization were before the widespread use of EUS. There are several reports quoting sensitivities of 86% to 94% for EUS detection of neuroendocrine neoplasms.21–23 When combined with noninvasive imaging, the sensitivity approaches 100%.21 This is supported by our study in which EUS alone had a sensitivity of 92% and when combined with CT and MRI, increased to 96%. Between 1994 and 2007 our overall preoperative detection rate, utilizing noninvasive and selected invasive modalities, particularly EUS, was 98% (40 of 41). The need for THPVS and calcium stimulation seems unnecessary, since the introduction of EUS. Although there is no doubt that this form of localization is highly accurate, its major role would seem to be when other imaging has failed.24–26 Given that intraoperative palpation and ultrasound does not guarantee tumor detection, we feel strongly that optimized preoperative localization is essential in planning and minimizing unnecessary surgery.
A major dilemma arises at the time of surgery when tumor localization fails. In some series up to 25% of insulinomas go undetected.27,28 In this series, 3 patients (5%) had failed blind distal pancreatic resections and were eventually found to have tumors located in the uncinate process or deep within the pancreatic head. Of interest, 8 of 10 (80%) nonpalpable insulinomas in our study were located in the pancreatic head. There are reports that up to 67% of pancreatic head insulinomas are nonpalpable.20 In an international multicenter review of 396 resected insulinomas, there were 53 reoperations, with 46 (87%) of them being for pancreatic head lesions.11 Based on these reports and our own experience, historical arguments for blind distal pancreatic resection should be abolished.29 In fact, more concerted efforts should be placed on fully displaying and imaging the pancreatic head and uncinate in situations when initial tumor localization fails. If there is any diagnostic doubt, reassessment, including THPVS and calcium stimulation, is indicated.24–26
The appropriate surgical policy for insulinomas is unclear. A pancreatic sparing approach is however advocated by most authors.1,11,30 Enucleation (34%) and distal pancreatectomy (40%) were the preferred surgical techniques in our series.
Compared with other large reviews, enucleation was performed less commonly in our series.1,11 In fact, in the review by Rothmund et al, enucleation was performed in 208 of 383 (54%) cases, and pancreaticoduodenal resection was performed in only 19 (5%).11 Comparatively, in our series pancreaticoduodenal resection was performed in 10 of 61 cases (16%). These differences may reflect our greater reluctance to perform enucleation, with its risk of pancreatic duct injury, when lesions are deep within the pancreatic head. Pancreaticoduodenal resection in our institution is safe, with acceptable morbidity and very low mortality.31 The overall median hospital stay of our patients was 7 days and the pancreatic fistula rate 18%, with most fistulas having minimal impact on postoperative stay.
Our study shows that enucleation can be performed with acceptable morbidity in selected patients with insulinoma, and long-term DFS can be achieved even in the presence of positive resection margins. We limited enulceation to superficially located insulinomas less than 25 mm (median of 15 mm) in maximum diameter. Two cases were complemented with a Roux-en Y onlay to the pancreatic defect, when pancreatic duct injury was suspected. Nonetheless, pancreatic fistulas were detected in 5 of 21 (24%) patients after enucleation, 4 of which had minimal clinical impact (Grade A). This compares favorably to other series that report pancreatic fistula rates as high as 57% after enucleation3,32 Of note, 7 of 21 (33%) patients treated by enucleation had positive margins on histology. None had recurrence at the site of enucleation on long-term follow-up. One may infer that “enucleation” implies positive margins. However, the aim of enucleation is to “minimize” pancreatic parenchymal excision by using the tumor pseudo-capsule to guide the limits of resection, including excision of the layer of compressed tissue that surrounds the specimen. To our knowledge, no earlier reports have examined the implication of a positive margin after enucleation of insulinomas.
The best surgical approach for patients with insulinomas in the setting of MEN-1 is controversial. Because these patients are more likely to have multiple and malignant neoplasms, some advocate an 85% subtotal pancreatectomy to the level of the portal vein along with enucleation of lesions from the pancreatic head.33,34 The recurrence rate at 20 years after resection in MEN-1 patients is quoted as 21%.33 However, whether more extensive pancreatic resection has an impact on the OS of these patients is unknown. Not all patients with MEN-1 have aggressive disease, and such an approach cannot be definitively advocated unless one can identify the subset of patients with specific MEN-1 mutations or pathologic features that are most likely to benefit from radical surgery. Subtotal pancreatectomy and enucleation of a neoplasm in the pancreatic head was performed as initial surgery in 1 patient in our series with multiple lesions. In another MEN-1 patient with multiple lesions, development of new metachronous islet-cell tumors required reoperation 4 years after initial enucleations. Given that redo pancreatic surgery in large volume centers has low morbidity,35 a reoperation policy for patients with MEN-1 and recurrent insulinoma remains an alternative option to initial subtotal pancreatectomy.
The pathologic features of insulinomas observed in our series were consistent with other large reports and collective reviews.1,15,16 In patients with sporadic insulinomas, multiple tumors were noted in only 4% of cases, and the rate of malignancy was only 6%. The overall median tumor size was 15 mm. We found no association between size, malignancy, and symptoms. This contrasts with a multi-institutional review of 62 patients with malignant insulinomas, which showed a relationship between tumor size and malignancy.36 The median size of malignant tumors in that report was 47 (10 –90) mm, and was greater than the median size of 25 (14 –38) mm of malignant insulinomas in our study. The actual definition of pancreatic neuroendocrine malignancy per se is controversial. To be classified as malignant by the WHO classification, a neuroendocrine neoplasm must show either local invasion into surrounding soft tissue or organs, or display lymph node or distal metastases.8 There are growing reports that vascular invasion, perineural invasion, mitotic rate, proliferation, necrosis, and the presence of a pancreatic ductal phenotype are all features of more aggressive biologic behaviors and should be considered in the classification or definition of malignacy.37,38 Tumor lymphovascular invasion was noted in 10% of patients in our series and proved to be an important prognostic factor, but given the low number of recurrences (5 cases), this finding may not be conclusive. Whether these patients would benefit from a more extensive pancreatic resection and whether such tumor features could be detected by tumor frozen section to guide surgery is unknown.
The 5, 10, and 20-year DSS of patients with insulinoma in this study was 100%, 100%, and 93% and is consistent with other larger series.13,15,39 This was significantly better than the overall survival, indicating that most patients with resected insulinomas eventually die of other causes. The 5-year DFS in this series was 90%, with no further recurrences thereafter. Danforth et al reported a 63% recurrence rate in 62 patients with malignant insulinomas at a median interval to recurrence of 2.8 years.36 Based on this study and our series, one can infer that most recurrences after resection of insulinomas occur within 5 years. On univariate analysis, only the presence of lymph node metastases, tumor lymphovascular invasion, and MEN-1 affected DFS and on multivariate analysis, the only predictor was lymphovascular invasion. These patients may be the ones to potentially benefit most from novel adjuvant therapies.
In summary, this 25-year single-institution series highlights the contemporary management of insulinomas, allowing formulation of a useful treatment algorithm. It is clear that a delay in diagnosis continues to be a feature of insulinomas, although this does not seem to have major prognostic implications. Diagnosis must be confirmed with appropriate laboratory testing in the setting of hypoglycemic symptoms. Preoperative localization is essential to minimize unsuccessful blind surgical resection, and currently arterial enhanced high-resolution CT or MRI scanning is the initial imaging of choice. If these fail to identify the lesion, EUS will achieve detection of tumors in the majority of cases. THPVS should be reserved for exceptional cases that are not identified by less-invasive imaging. At the time of surgery, most lesions are detectable by a combination of palpation and IOUS. Those that remain occult are most likely to be located in the pancreatic head. In patients without MEN-1, enucleation, or a conservative pancreatic resection is the preferred surgical option. A positive margin after enucleation is common but seems not to increase local recurrence. Although most insulinomas are benign and long-term survival is expected after surgery, a few do recur. Lymphovascular invasion is an independent predictor of recurrence and may potentially guide the need for adjuvant therapies or perhaps more extensive resection if discovered by intraoperative frozen section.
ACKNOWLEDGMENTS
The authors thank Deborah McGrath, RN, for her assistance in obtaining follow-up data.
REFERENCES
- 1.Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinomaincidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc. 1991;66:711–719. doi: 10.1016/s0025-6196(12)62083-7. [DOI] [PubMed] [Google Scholar]
- 2.Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol. 2005;19:783–798. doi: 10.1016/j.bpg.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Stefanini P, Carboni M, Patrassi N, et al. Beta-islet cell tumors of the pancreas: results of a study on 1,067 cases. Surgery. 1974;75:597–609. [PubMed] [Google Scholar]
- 4.Thakker RV. Multiple endocrine neoplasia type 1. Endocrinol Metab Clin North Am. 2000;29:541–567. doi: 10.1016/s0889-8529(05)70150-x. [DOI] [PubMed] [Google Scholar]
- 5.Mallery JS, Centeno BA, Hahn PF, et al. Pancreatic tissue sampling guided by EUS, CT/US, and surgery: a comparison of sensitivity and specificity. Gastrointest Endosc. 2002;56:218–224. doi: 10.1016/s0016-5107(02)70181-8. [DOI] [PubMed] [Google Scholar]
- 6.Warshaw AL. Conservation of the spleen with distal pancreatectomy. Arch Surg. 1988;123:550–553. doi: 10.1001/archsurg.1988.01400290032004. [DOI] [PubMed] [Google Scholar]
- 7.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Kloppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004;1014:13–27. doi: 10.1196/annals.1294.002. [DOI] [PubMed] [Google Scholar]
- 9.Wilder RM, Allan FM, Power MH, et al. Carcinoma of the islands of the pancreas: hyperinsulinism and hypoglycemia. JAMA. 1927;89:348–355. [Google Scholar]
- 10.Howland G, Campbell WR, Maltby EJ, et al. Dysinsulinism: convulsions and coma due to islet cell tumor of the pancreas with operation and cure. JAMA. 1929;93:647. [Google Scholar]
- 11.Rothmund M, Angelini L, Brunt LM, et al. Surgery for benign insulinoma: an international review. World J Surg. 1990;14:393–398. doi: 10.1007/BF01658536. discussion 398–399. [DOI] [PubMed] [Google Scholar]
- 12.Boukhman MP, Karam JM, Shaver J, et al. Localization of insulinomas. Arch Surg. 1999;134:818–822. doi: 10.1001/archsurg.134.8.818. discussion 822–813. [DOI] [PubMed] [Google Scholar]
- 13.Lo CY, Lam KY, Kung AW, et al. Pancreatic insulinomas. A 15-year experience. Arch Surg. 1997;132:926–930. doi: 10.1001/archsurg.1997.01430320128023. [DOI] [PubMed] [Google Scholar]
- 14.Grant CS. Gastrointestinal endocrine tumours. Insulinoma. Baillieres Clin Gastroenterol. 1996;10:645–671. doi: 10.1016/s0950-3528(96)90017-2. [DOI] [PubMed] [Google Scholar]
- 15.Boukhman MP, Karam JH, Shaver J, et al. Insulinoma—experience from 1950 to 1995. West J Med. 1998;169:98–104. [PMC free article] [PubMed] [Google Scholar]
- 16.Proye C, Boissel P. Preoperative imaging versus intraoperative localization of tumors in adult surgical patients with hyperinsulinemia: a multicenter study of 338 patients. World J Surg. 1988;12:685–690. doi: 10.1007/BF01655886. [DOI] [PubMed] [Google Scholar]
- 17.Falconi M, Molinari E, Carbognin G, et al. What preoperative assessment is necessary for insulinomas? Calculating the degree of waste: analysis of 29 cases. Chir Ital. 2002;54:597–604. [PubMed] [Google Scholar]
- 18.Hashimoto LA, Walsh RM. Preoperative localization of insulinomas is not necessary. J Am Coll Surg. 1999;189:368–373. doi: 10.1016/s1072-7515(99)00163-5. [DOI] [PubMed] [Google Scholar]
- 19.Norton JA, Shawker TH, Doppman JL, et al. Localization and surgical treatment of occult insulinomas. Ann Surg. 1990;212:615–620. doi: 10.1097/00000658-199011000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton JA. Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann Oncol. 1999;10(Suppl 4):182–184. [PubMed] [Google Scholar]
- 21.McLean AM, Fairclough PD. Endoscopic ultrasound in the localisation of pancreatic islet cell tumours. Best Pract Res Clin Endocrinol Metab. 2005;19:177–193. doi: 10.1016/j.beem.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271–2277. doi: 10.1111/j.1572-0241.2000.02480.x. [DOI] [PubMed] [Google Scholar]
- 23.Zimmer T, Stolzel U, Bader M, et al. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996;39:562–568. doi: 10.1136/gut.39.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiesli P, Brandle M, Schmid C, et al. Selective arterial calcium stimulation and hepatic venous sampling in the evaluation of hyperinsulinemic hypoglycemia: potential and limitations. J Vasc Interv Radiol. 2004;15:1251–1256. doi: 10.1097/01.RVI.0000140638.55375.1E. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds LR, Park AE, Miller RE, et al. Combined use of calcium infusion localization and a minimally invasive surgical procedure in the management of insulinoma. Endocr Pract. 2002;8:329–334. doi: 10.4158/EP.8.5.329. [DOI] [PubMed] [Google Scholar]
- 26.Pasieka JL, McLeod MK, Thompson NW, et al. Surgical approach to insulinomas. Assessing the need for preoperative localization. Arch Surg. 1992;127:442–447. doi: 10.1001/archsurg.1992.01420040088015. [DOI] [PubMed] [Google Scholar]
- 27.Finlayson E, Clark OH. Surgical treatment of insulinomas. Surg Clin North Am. 2004;84:775–785. doi: 10.1016/j.suc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Angelini L, Bezzi M, Tucci G, et al. The ultrasonic detection of insulinomas during surgical exploration of the pancreas. World J Surg. 1987;11:642–647. doi: 10.1007/BF01655841. [DOI] [PubMed] [Google Scholar]
- 29.Hirshberg B, Libutti SK, Alexander HR, et al. Blind distal pancreatectomy for occult insulinoma, an inadvisable procedure. J Am Coll Surg. 2002;194:761–764. doi: 10.1016/s1072-7515(02)01177-8. [DOI] [PubMed] [Google Scholar]
- 30.Galbut DL, Markowitz AM. Insulinoma: diagnosis, surgical management and long-term follow-up. Review of 41 cases. Am J Surg. 1980;139:682–690. doi: 10.1016/0002-9610(80)90363-3. [DOI] [PubMed] [Google Scholar]
- 31.Balcom JHt, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 32.Menegaux F, Schmitt G, Mercadier M, et al. Pancreatic insulinomas. Am J Surg. 1993;165:243–248. doi: 10.1016/s0002-9610(05)80519-7. [DOI] [PubMed] [Google Scholar]
- 33.O’Riordain DS, O’Brien T, van Heerden JA, et al. Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg. 1994;18:488–493. doi: 10.1007/BF00353743. discussion 493–484. [DOI] [PubMed] [Google Scholar]
- 34.Demeure MJ, Klonoff DC, Karam JH, et al. Insulinomas associated with multiple endocrine neoplasia type I: the need for a different surgical approach. Surgery. 1991;110:998–1004. discussion 1004–1005. [PubMed] [Google Scholar]
- 35.Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–866. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 36.Danforth DN, Jr, Gorden P, Brennan MF. Metastatic insulin-secreting carcinoma of the pancreas: clinical course and the role of surgery. Surgery. 1984;96:1027–1037. [PubMed] [Google Scholar]
- 37.Deshpande V, Fernandez-del Castillo C, Muzikansky A, et al. Cytokeratin 19 is a powerful predictor of survival in pancreatic endocrine tumors. Am J Surg Pathol. 2004;28:1145–1153. doi: 10.1097/01.pas.0000135525.11566.b4. [DOI] [PubMed] [Google Scholar]
- 38.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Service FJ, Dale AJ, Elveback LR, et al. Insulinoma: clinical and diagnostic features of 60 consecutive cases. Mayo Clin Proc. 1976;51:417–429. [PubMed] [Google Scholar]