Abstract
Introduction
We evaluated the prognostic accuracy of LN variables (N0/N1), numbers of positive lymph nodes (PLN), and lymph node ratio (LNR) in the context of the total number of examined lymph nodes (ELN).
Methods
Patients from SEER and a single institution (MGH) were reviewed and survival analyses performed in subgroups based on numbers of ELN to calculate excess risk of death (hazard ratio, HR).
Results
In SEER and MGH, higher numbers of ELN improved the overall survival for N0 patients. The prognostic significance (N0/N1) and PLN were too variable as the importance of a single PLN depended on the total number of LN dissected. LNR consistently correlated with survival once a certain number of lymph nodes were dissected (≥13 in SEER and ≥17 in the MGH dataset).
Conclusions
Better survival for N0 patients with increasing ELN likely represents improved staging. PLN have some predictive value but the ELN strongly influence their impact on survival, suggesting the need for a ratio-based classification. LNR strongly correlates with outcome provided that a certain number of lymph nodes is evaluated, suggesting that the prognostic accuracy of any LN variable depends on the total number of ELN.
Keywords: Pancreatic cancer, Lymph node ratio, Staging, Survival
Introduction
Pancreatic cancer is the 14th most common cancer in the US, yet it accounts for the fourth highest mortality rate. In 2010, over 43,000 new cases were diagnosed, 39,000 of whom would die of their disease.1 Tumor size, chemotherapy, and curative resection are among the prognostic factors identified in predicting outcome.2–5 The status of nodal metastasis has consistently been evaluated as one of the strongest measures of outcome.6–9 Presently, lymph nodes are classified in the AJCC staging system as “N0: No regional lymph nodes” or “N1: Regional lymph node involvement”3; however, debate continues over whether this, absolute numbers of positive lymph nodes (PLN), or lymph node ratios (LNR) have the best prognostic value. Recent reports have established the role of LNR in the prediction of outcomes after curative intent surgery.10–13 LNR is the ratio between the numbers of positive and examined lymph nodes (ELN). Node ratio has been suggested as a tool to predict outcome in cancers of the esophagus,14 stomach,15 ampulla of Vater,16,17 colon,18 and even non-GI cancers such as breast19 and melanoma.20 However, some authors have suggested that absolute numbers of PLN are an independent21–22 and better predictor23 of survival than LNR. These studies have varied across numbers of patients, numbers of ELN, and setting of study (population-wide or specialized single-institution databases).
The objective of the present study is twofold: (1) to evaluate the prognostic efficiency of LNR against numbers of PLN using a large population-based dataset and then validate our findings in a large single-institution database and (2) to evaluate the effect of the number of ELN on the prognostic capability of these lymph node variables.
Methods
The SEER national dataset of the National Cancer Institute and the tumor registry of a large single institution (MGH), an academic referral center, were queried for data on patients with pancreatic cancer. From SEER, de-identified data on 127,760 patients was retrieved from 1973 to 2009. This data was filtered for patients diagnosed after 1988, when detailed diagnostic codes were introduced with the Extent of Disease (EOD)-10 coding scheme. Similarly, from the MGH tumor registry, data was obtained on 3,496 patients with pancreatic cancer between 1988 and 2009. Of these, patients were included if they underwent a resection for pancreatic adenocarcinoma. The definitions for adenocarcinoma were derived from the third edition of the International Classification of Diseases for Oncology (ICD-O-3). Patients with unspecified neoplasms (ICD 8000-8009), cystic, mucinous or serous tumors (ICD 8440-8449), and mucoepidermoid tumors (ICD 8430-8439) were excluded from analysis.
The clinicopathological data collected included age at diagnosis; sex; year of diagnosis; AJCC T, N and M stages; primary site; grade; regional lymph nodes examined (ELN) and regional lymph nodes positive (PLN); and vital status. The LNR was calculated by dividing the numbers of regional PLN by the number of examined LNs when at least one lymph node was examined. The Social Security Death Index (SSDI), the Massachusetts Registry of Vital Records and Statistics, and the institutional Research Patient Data Registry (RPDR) were used to accurately calculate vital status, cause of death, and survival time for every patient. After applying all inclusion and exclusion criteria, 14,907 patients in SEER and 902 patients in the MGH tumor registry were included in this study.
Continuous Variables were Binned in Order to Analyze Data
Continuous variables such as tumor size, lymph node variables (ELN, PLN, and LNR), and year of diagnosis were binned or categorized. To determine the most appropriate subgroups for these LN variables, cut points were analyzed using log-rank tests of Kaplan–Meier survival curves. Cut point analysis has been utilized previously to identify subgroups for LNR20,24 in large datasets. This analysis was done in order to find the greatest difference in survival and the lowest p value between the resultant subgroups. In the final analysis, lymph node variables (ELN, PLN, and LNR) were analyzed as both continuous and categorical. Using the above method, the SEER database was divided into subgroups with few (≤5), moderate (6–12), and large (≥13) LNs examined. In the MGH dataset, cut point analysis based on examined LN could not identify subgroups with survival differences. Therefore, these patients were divided into three equal groups based on numbers of patients. The corresponding cut points derived were few (≤9), moderate (10–16), and large (≥17) lymph nodes examined. In each dataset, the subgroups that were formed by numbers of ELN were labeled as ‘few,’ ‘moderate,’ and ‘large.’ Age at diagnosis was split into two groups by median age. The subgroups that resulted from this binning are shown in Table 1.
Table 1.
Continuous variables binned into subgroups
| Variable | Bins created |
|---|---|
| Age at diagnosis | <69, ≥69 |
| Year of diagnosis | 1988–1997, 1998–2003, ≥2004 |
| Tumor size (in mm) | 0–19, 20–36, ≥37 mm |
| Regional lymph nodes examined | ‘Few’ (SEER ≤5, SI ≤9), ‘Moderate’ (SEER 6–12, SI 10–16), ‘Large’ (SEER ≥13, SI ≥17) |
| Regional lymph nodes positive | 0, 1, 2, ≥3 |
| Lymph node ratio | 0, <0.2, 0.2–0.3, ≥0.3 |
SEER surveillance, epidemiology, and end results, SI single institution
Statistical Analysis
Means and medians were calculated for age at diagnosis, tumor size, regional LNs examined, regional PLN, and LNR. These were compared by a one-way ANOVA across the LN subgroups. Comparison of distribution of patients across lymph node subgroups for categorical variables such as sex, race/ethnicity, primary site, stage, and grade was done using Pearson’s χ2 test. Survival analysis across both datasets was initially performed using KM survival curves and log-rank tests were used to determine significance. Clinicopathological variables were then factored into the Cox proportional hazards model, and multivariate analyses were performed in subgroups categorized according to numbers of ELN. Hazard ratios (HR) and corresponding 95 % confidence intervals (CI) were calculated in these ELN subsets as previously described.18 The Cox model for SEER and MGH was run in two steps. For each dataset, the model first factored in PLN and then the grouped LNR. We first tested our hypothesis on the SEER patients and then validated it on the MGH institutional dataset. The HR for each lymph node parameter from both patient populations has been summarized indicating relative risk of mortality in relation to that for the reference category. For all statistical tests, p<0.05 was required for significance.
Results
Demographic and Tumor Characteristics of the Study Populations
In the SEER dataset, 14,907 cases were identified for analysis. When this population was divided into LN subgroups, 46 % had few (≤5 LNs), 29 % had moderate (6–12 LNs), and 25 % had large (≥13 LNs) examined LNs (Table 2). The group with more than 13 lymph nodes examined was more likely to have tumors of the head of the pancreas, higher AJCC stage, and more frequently diagnosed after 2004 compared with the fewer than five lymph nodes group. This group was also more likely to have smaller tumors and a lower mean LNR. There were no differences between LN subgroups with respect to the race and gender of patients. In the MGH dataset, 902 patients were identified (Table 3); one-third of these were in each subgroup of analysis. Comparison of these groups revealed that the group of large (≥17) lymph nodes examined were diagnosed in more recent years, had a higher percentage of tumors in the head of pancreas, and had more advanced T stages (T3 and T4) but trended towards smaller tumors, mirroring the patients in the SEER population.
Table 2.
Analysis of SEER patients in subgroups of examined lymph nodes
| ≤5 lymph nodes | 6–12 lymph nodes | ≥13 lymph nodes | p value | |
|---|---|---|---|---|
| n | 6,789 (46 %) | 4,272 (29 %) | 3,846 (25 %) | |
| Primary site | ||||
| Head | 71.1 % | 82.4 % | 85.6 % | <0.01 |
| Body/tail | 28.9 % | 17.6 % | 14.4 % | |
| Mean tumor size (±SD) | 42.6 (±31.5)a | 37.4 (±23.1)b | 38.4 (±24.1)c | pab, ac<0.01, pbc=0.124 |
| AJCC T stage | ||||
| Tis | 3.2 % | 2.1 % | 1.8 % | <0.01 |
| T1 | 9.2 % | 6.3 % | 5.4 % | |
| T2 | 21.4 % | 17.4 % | 15.1 % | |
| T3 | 51.9 % | 68.4 % | 71.7 % | |
| T4 | 14.3 % | 5.8 % | 6.1 % | |
| AJCC N stage | ||||
| N0 | 55.9 % | 41.9 % | 30.8 % | <0.01 |
| N1 | 44.1 % | 58.1 % | 69.2 % | |
| Regional nodes examined (±SD) | 1.66 (±1.78) | 8.8 (±1.97) | 19.86 (±7.44) | <0.01 |
| Regional nodes positive (±SD) | 0.68 (±0.96) | 1.5 (±1.8) | 2.9 (±0.17) | <0.01 |
| Mean lymph node ratio (±SD) | 0.27 (±0.37) | 0.17 (±0.21) | 0.15 (±0.17) | <0.01 |
| Lymph node ratio | ||||
| No positive nodes | 55.9 % | 41.9 % | 30.8 % | <0.01 |
| <0.2 | 4.2 % | 26.9 % | 40.4 % | |
| 0.2–0.3 | 3.9 % | 9.2 % | 11.8 % | |
| ≥0.3 | 36.0 % | 22.0 % | 17.0 % | |
pab is the p value for the difference between a and b. Similarly for pac and pbc
Table 3.
Analysis of single-institution patients in subgroups of examined lymph nodes
| ≤9 LN lymph nodes | 10–16 lymph nodes | ≥17 lymph nodes | p value | |
|---|---|---|---|---|
| n | 301 | 301 | 300 | |
| Primary site | ||||
| Head | 72.9 % | 85.5 % | 82.8 % | <0.01 |
| Body/tail | 27.1 % | 14.5 % | 17.2 % | |
| Mean tumor size (±SD) | 38.2 (±28.5) | 34.6 (±18.7) | 33.6 (±19) | 0.456 |
| AJCC T stage | ||||
| Tis | 0 % | 0.3 % | 0 % | <0.001 |
| T1 | 12.6 % | 8.4 % | 7.1 % | |
| T2 | 40.0 % | 24.6 % | 15.2 % | |
| T3 | 43.9 % | 63.6 % | 74.4 % | |
| T4 | 3.5 % | 3.0 % | 3.3 % | |
| AJCC N stage | ||||
| N0 | 45.6 % | 39.6 % | 27.5 % | <0.01 |
| N1 | 54.4 % | 60.4 % | 72.5 % | |
| Regional nodes examined (±SD) | 5.74 (±2.3) | 13.3 (±2.2) | 23.2 (±4.6) | <0.01 |
| Regional nodes positive (±SD) | 1.1 (±1.4) | 1.8 (±2.2) | 3 (±3.1) | <0.01 |
| Mean lymph node ratio (±SD) | 0.22 (±0.37)a | 0.14 (±0.17)b | 0.13 (±0.14)c | pab, ac<0.01, pbc=0.897 |
| Lymph node ratio | ||||
| No positive nodes | 45.6 % | 39.6 % | 27.5 % | <0.01 |
| <0.2 | 15.6 % | 32.5 % | 45.9 % | |
| 0.2–0.3 | 7.8 % | 12.8 % | 14.0 % | |
| ≥0.3 | 30.9 % | 15.1 % | 12.7 % | |
pab is the p value for the difference between a and b. Similarly for pac and pbc
Node Status and the Lymph Node Ratio are Influenced by the Total Lymph Nodes Harvested
Total number of LNs examined affects N1 status. In SEER, 14,907 patients had complete LN information. The median number of nodes examined was eight. The number of total LNs harvested directly correlated with the percentage of N1 patients. In the group with few (≤5) LNs examined, 44 % were N1, compared to the group with large (≥13) LNs examined, in which 69 % patients were N1 (Table 2). In the MGH dataset, 902 patients had complete LN information, with a median of 13 lymph nodes examined. As in SEER, the number of patients found to be N1 increased stepwise with the number of LNs examined: 54 % were found to be N1 when few (≤9) LNs were examined, 60 % with moderate (10–16) LNs examined, and 73 % in the large subgroup of (≥17) LNs examined (Table 3).
Not too surprisingly, the numbers of LNs evaluated also affected the LNR. In SEER, there was an inverse relationship between examined LNs and LNR. The mean LNR for the subgroup with few (≤5) LNs was 0.27, for moderate (6–12) LNs was 0.17, and in the large subgroup (≥13 LNs) was 0.15 (Table 2). In the MGH patients, the mean LNR showed a similar decrease with increasing numbers of LNs. The LN subgroups few (≤9), moderate (10–16), and large (≥17) had mean LNR of 0.22, 0.14, and 0.13 respectively (Table 3).
Increasing Number of Examined Lymph Nodes Improves Survival in the N0 Cohort and has a Variable Effect on Survival of N1 Patients
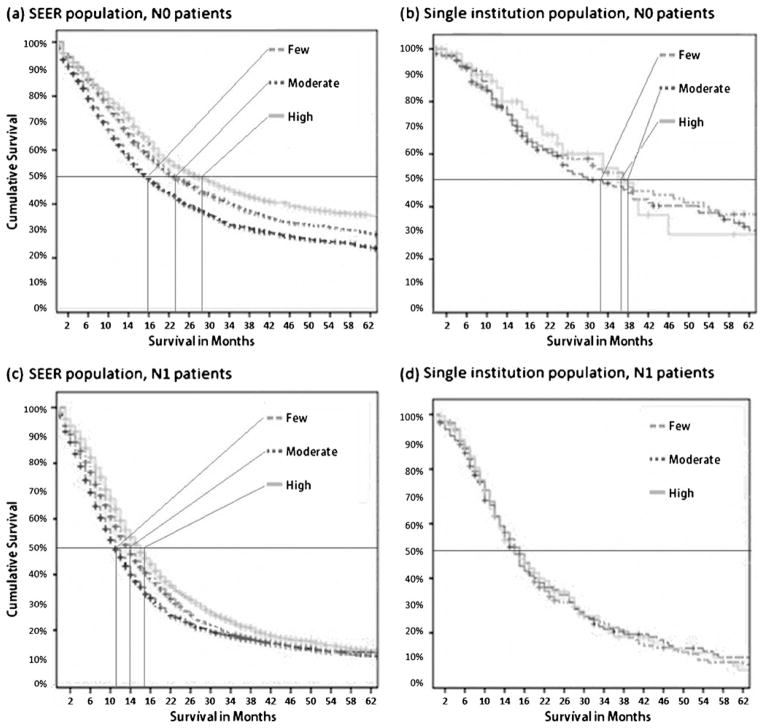
In SEER, there was a significant difference in the median overall survival between N0 and N1 patients (22 months vs. 14 months, p<0.001). When this survival was stratified between different LN subgroups within the N0 patients, significantly better median overall survival was observed when more LNs were examined (Fig. 1a). Median survival was 16 months, 23 months, and 28 months, respectively, when few (≤5), moderate (6–12), or large (≥13) LNs were examined (p<0.001). In a similar analysis of the MGH patients, overall survival for N0 patients was 37 months and for N1 patients, it was 17 months (p<0.001). Within the N0 patient population, there was a trend for better survival in the groups where more LN were evaluated and the median survival was 33 months, 38 months, and 37 months in few (≤9), moderate (10–16), and large (≥17) LN subgroups, respectively. The overall survival for the moderate and large subgroups was significantly longer than the few subgroups of examined LNs, p<0.05. (Fig. 1b).
Fig. 1.
In N0 patients (a and b), the number of examined LNs positively correlated with survival. In SEER (a), patients with ≥13 LNs had better survival compared to those with ≤5 LNs (28 months vs. 18 months). In the MGH (b), patients with ≥17 LNs trended towards a better survival (37 months) than patients with ≤9 LNs (33 months), p=0.63. Among the N1 patients (c and d), the numbers of lymph nodes examined exert a variable effect on overall survival. In the SEER population (c), higher LNs examined improved survival (≥13LNs 16 months vs. ≤5 LNs 11 months, p<0.001) but this effect did not extend to the MGH patients (d)
The total number of LNs can also affect survival in node-positive patients. In the SEER dataset, among patients who were N1, a statistically significant improvement in survival was seen (11 months, 14 months, and 16 months, p<0.001) when progressively higher numbers (≤5, 6–12, and ≥13) of LNs were examined (Fig. 1c). Thus, the absolute number of LNs evaluated affects both nodal status and survival and may also introduce biases that alter the prognostic value of lymph node variables. Therefore these PLN variables were analyzed by subgroups dependent on the total number of LNs evaluated.
Absolute Numbers of PLN Variably Predict Survival in N1 Patients
In the SEER population when PLN were modeled as a continuous variable, every positive node was responsible for a 5 % reduction in overall survival (HR 1.056, CI 1.04–1.06), and in the MGH dataset, every PLN was responsible for an 8 % reduction in overall survival (HR 1.08, CI 1.05–1.12).
PLN were then evaluated within subgroups of the number of LN evaluated. Using the Cox multivariate model, the effect of PLN across LN subgroups was analyzed (Table 4). In the SEER subgroup in which few LNs were evaluated, PLN did not predict a decrease in survival. In the moderate and large LN subgroups, PLN had a limited ability to predict survival. In the moderate LN subgroup, there was no clear association between increasing PLN and decreasing survival. One positive node introduced a statistically significant, 46 % increase risk of death, HR=1.46, p=0.007, while two positive nodes failed to have a statistically significant increased risk with a HR=1.24, p=0.196. However, when large numbers of LN (≥13 LN) were examined, one LN no longer had a significant contribution and there was a linear increase in risk of death (or an increase in HR) with two or more PLN. Two positive nodes increased the risk of death by 44 %, HR=1.44, p=0.03, and three or more positive nodes increased the risk of death by 72 %, HR=1.72 with an increased strength of prediction, p <0.001. Thus in the population-based dataset, the ability of PLN to predict outcome is heterogeneous and depends on the total numbers of LNs evaluated. Furthermore, the contribution of a single LN has decreasing importance in successively higher LN subgroups.
Table 4.
The impact of lymph node parameters (numbers and ratio) on overall survival in a Cox multivariate model
| SEER | ≤5 lymph nodes | p value | 6–12 lymph nodes | p value | ≥13 lymph nodes | p value |
|---|---|---|---|---|---|---|
| Numbers of positive lymph nodes, HR (95 % CI) | ||||||
| No positive node | Reference category | |||||
| One positive node | NS | – | 1.46 (1.11–1.92) | 0.007 | 1.09 (0.77–1.53) | 0.637 |
| Two positive nodes | NS | – | 1.24 (0.9–1.71) | 0.196 | 1.44 (1.03–2.01) | 0.032 |
| Three or more positive nodes | NS | – | 1.62 (1.23–2.14) | <0.001 | 1.72 (1.33–2.24) | <0.001 |
| Lymph node ratio, HR (95 % CI) | ||||||
| LNR=0 | Reference category | |||||
| LNR<0.2 | NS | – | 1.3 (1–1.17) | 0.048 | 1.28 (0.9–1.67) | 0.071 |
| LNR, 0.2–0.3 | NS | – | 1.5 (1.03–2.18) | 0.035 | 1.6 (1.14–2.25) | 0.007 |
| LNR≥0.3 | NS | – | 1.69 (1.29–2.23) | <0.001 | 2.27 (1.67–3.08) | <0.001 |
| Single institution (MGH) | ≤9 lymph nodes | p value | 10–16 lymph nodes | p value | ≥17 lymph nodes | p value |
| Numbers of positive lymph nodes, HR (95 % CI) | ||||||
| No positive node | Reference category | |||||
| One positive node | 1.56 (0.98–2.48) | 0.058 | 1.25 (0.80–1.96) | 0.326 | 1.27 (0.55–2.92) | 0.56 |
| Two positive nodes | 1.72 (1.05–2.79) | 0.028 | 2.47 (1.53–4.00) | <0.001 | 1.38 (0.69–2.76) | 0.36 |
| Three or more positive nodes | 2.54 (1.55–4.18) | <0.001 | 2.03 (1.38–2.99) | <0.001 | 2.36 (1.34–4.18) | 0.002 |
| Lymph node ratio, HR (95 % CI) | ||||||
| LNR=0 | Reference category | |||||
| LNR<0.2 | 1.42 (0.85–2.41) | 0.183 | 1.95 (1.31–2.88) | 0.001 | 2.70 (1.47–4.95) | <0.001 |
| LNR, 0.2–0.3 | 1.29 (0.66–2.53) | 0.461 | 2.10 (1.29–3.43) | 0.003 | 2.18 (1.02–4.65) | 0.044 |
| LNR≥0.3 | 2.07 (1.36–3.15) | 0.001 | 1.85 (1.14–2.99) | 0.012 | 4.95 (2.47–9.92) | <0.001 |
CI confidence interval, HR hazard ratio, NS not significant
Age, gender, race/ethnicity, year of diagnosis, size, grade, and T stage of tumor were used as covariates in separate Cox models for each lymph node variable
In the MGH patients, multivariate analysis revealed that PLN had a variable predictive value. In the subgroup where few numbers of LNs were examined, there was a linear correlation between risk of death and increasing PLN (HR 1.56, 1.72, and 2.54) with increasing statistical significance (one PLN, p=0.058; two PLN, p=0.028; and three PLN, p<0.001), respectively. In the subgroup with moderate numbers of LNs (10–16 LNs) examined, increased risk of death was seen with two or more PLN but this association was not linear. Two PLN increased the risk of death by 147 %, HR: 2.47, p<0.001, while three PLN increased the risk by 103 %, HR: 2.03, p<0.001. In the large examined LN subgroup, three PLN were needed to cause a statistically significant decrease in survival of the N1 population with a HR of 2.36, p=0.002. As seen in the SEER data, the contribution of a PLN to prognosis depends both on the number of PLN and LNs evaluated. Furthermore, the hazard associated with a single LN decreases as the number of LNs evaluated increases. Thus relying on the PLN may introduce a bias of adding too much or too little value to a solitary positive LN (N1) when the total number of LNs harvested is not taken into consideration.
The effect of the total number of LNs on the predictive power of PLN is seen both in the SEER and MGH datasets. Analysis reveals that a minimum number of LNs must be evaluated in order for PLN to have any predictive value. However, the predictive value of a single LN decreases as the evaluated examined LN number increases. Thus, the absolute number of LNs evaluated substantially alters the significance and strength of association of PLN with outcome (Table 4), which speaks to the need of a ratio-based analysis of LN positivity.
Lymph Node Ratios Constitute a Strong Predictive Factor in Survival, Once a Minimum Number of Lymph Nodes Has Been Examined
In both patient populations, increasing LNR was associated with significantly poor survival by univariate analysis. In SEER, for every increment in LNR (<0.2, 0.2–0.3, and ≥0.3), there was a corresponding decrease in overall survival. Using the Cox multivariate model for SEER (Table 4), in the few LN subgroup (≤5 LNs), LNR was not able to predict survival. However, in the moderate (6–12 LNs) and large (≥13LNs) subgroups, LNR consistently predicted survival. For the moderate and large subgroups of LNs examined, the HR increased linearly for LNR <0.20, 0.20–0.30, and ≥0.30, respectively.
The MGH data similarly revealed that LNR were predictive of survival in the moderate (10–16) and large (≥17) groups of examined LNs (Table 4). In the lower (≤9) examined LN group, however, only LNR≥0.3 was associated with a significant risk of death of 107 % (HR 2.07, p=0.001). LNR has a statistically significant predictive value in survival across both datasets, and this risk was linear in the SEER dataset. However, the ability of LNR to predict outcome was absent in the ‘few’ subgroups of examined LN, suggesting that a minimum number of LNs must be evaluated in order for any lymph node variable to contribute to the prognostic equation.
Discussion
LN variables remain one of the most important predictors of survival. However, both LNR and PLN are imperfect as the sole predictor. The numbers of LNs examined are a significant modifier of the effect of PLN and LNR on survival; this study evaluates the impact of PLN, LNR, and changing numbers of LNs evaluated on overall survival in pancreatic cancer over two different populations. The numbers of LNs evaluated are an important determinant of N status and LNR. Additionally, when higher numbers of LNs are evaluated, there is an improvement in survival of N0 patients and, depending on the dataset, a variable effect on N1 patients. PLN have a significant effect on survival but the predictive value and the strength of prediction of PLN are dependent on the number of LNs evaluated. The LNR, which normalizes for the number of evaluated LN, takes away this variability. In this study, LNR were a better predictor of outcome; however, their interpretation is still dependent on evaluated LNs, as they are ineffective below a certain number, and there is an association between higher LNR and increasing risk of death when large numbers of LNs have been evaluated. What remains clear is that the total number of LN affects, albeit differently, the prognostic accuracy of either LN variable (PLN vs. LNR).
In this study, it was noted that increasing examined LNs are associated with an increase in the percentage of N1 patients. This, in conjunction with previous studies,25,26 forms a body of evidence that nodal staging is influenced by the number of LNs evaluated. Pavlidis et al demonstrated in a review of many articles that 70 % of patients with PDAC presented with positive nodes,27 and in our datasets, a similar number was achieved only in the large subgroups of examined LN. This suggests that the accuracy of nodal staging is critically dependent on the number of LNs examined and that examining fewer LNs may result in a misclassification bias where N1 patients are understaged as N0. When looking at the SEER and MGH datasets, 70 % of patients were found to have N1 status when at least 13 LNs (SEER) and 16 LNs (MGH) were evaluated, suggesting the exact number may depend on the dataset used to calculate it. Our analysis suggests that the number of ELN needed for accurate staging likely lies above 13–16 LNs.
In this era of minimally invasive surgery, pancreatic resections for adenocarcinoma are increasingly being performed using laparoscopic pancreaticoduodenectomy and distal pancreatectomy. A major concern with laparoscopic pancreatic resections has been the adequacy of the number of ELN. Recently published studies analyzing laparoscopic pancreatectomy have reported numbers of ELN ranging between 14 and 18.28–30 These are similar to numbers reported from contemporary series of pancreatic resections utilizing the open approach. However, data from larger studies may be needed before any firm conclusions can be established regarding the adequacy of lymph node staging in laparoscopic pancreatic resections.
In addition to staging, examined LNs also play a role in determining survival. The link between improved survival and examining more LNs has been observed in pancreatic cancer11,31,32 and this effect may be linked to more accurate staging. In both datasets that were evaluated in this study, there was an improved survival noted for N0 patients in the successively higher subgroups of examined LNs. Thus, ELN are an important statistical confounder in the analysis of survival, likely resulting from misclassification biases.
There has also been considerable discussion about the influence of harvesting additional lymph nodes on survival of N1 patients in pancreatic cancer. Japanese studies33,34 had previously recommended extensive lymph node removal; however, randomized trials that attempted to validate these studies failed to show a similar benefit to survival.35,36 At least one multicenter, a prospective trial from Padua, Italy, demonstrated a trend towards better survival in node-positive patients with extended rather than standard lymphadenectomy.37 Our study demonstrated equally variable results, and although an improved median survival was seen for N1 patients in the SEER population when more LNs were examined, no such correlation was identified in the MGH dataset. This difference may potentially be explained by the heterogeneity of the SEER tumor registries and by the lack of control of pathologic variables in the SEER dataset. The examination of lymph nodes may indirectly have been linked with standard of care, possibly introducing a confounding bias. The differences in survival and the biases introduced with different numbers of examined LNs underscore the importance of analyzing the positive lymph node variables (PLN and LNR) in subsets of examined LNs.
The effect of PLN on survival can best be characterized as variable. When modeled as a continuous variable, every positive node predicts an added risk to survival. However, when they are compartmentalized by the number of examined LNs, PLN appear to be a very heterogeneous predictor of survival. When very few lymph nodes are examined, PLN are unable to tease out differences in HRs and the number of PLN likely has a weaker correlation with the true extent of nodal disease and hence survival. The fluctuation in hazard exerted by PLN in different LN subgroups further highlights the effect that examined LNs have on the prognostic ability of PLN.
In a retrospective study from Japan, Murakami et al.23 concluded that PLN not only predict survival but are also better at doing so compared to LNR. The median number of examined LNs in their study was 28, significantly higher in relation to contemporary studies from the US and Germany. This study did not find a prognostic value for LNR. It is possible that in their study, a median LNR of 0.10, combined with the cut-off points chosen by them (0, 0.10, and 0.20) may have been too fine to delineate differences in survival. In our own datasets, the prognostic ability of PLN is significantly improved in the large subgroups of ELN. However, we were unable to find it a superior LN variable when compared to LNR.
In comparison to the numbers of PLN, LNR appears to be less influenced by biases of number of nodes examined. In the moderate and large examined LN subgroups, it never loses statistical significance. Further, the greater range in HR compared to PLN within a given dataset indicates that LNR is able to capture populations that PLN do not. Our study, along with previously published single institution analyses by Pawlik,11 Riediger,21 and Bhatti,38 examining the status of LNR in prediction models of pancreatic cancer, concludes that LNR are a more accurate predictor of survival than absolute numbers of positive LNs. In an analysis of a larger population-based dataset, Slidell32 concluded that LNR may predict survival better than the N0/N1 of the AJCC. Our study expands on the inferences drawn in these studies by critically examining the relationship between nodes harvested, PLN and LNR.
A large patient population combined with the internal validation provided by two datasets makes this a useful analysis of LN variables and survival. However, there are certain limitations that this study faces. These limitations stem from the heterogeneity of the SEER population and a lack of pathology-specific covariates such as perineural invasion, vascular invasion, tumor differentiation, margin status, and details of pathologic techniques used to examine LNs. The effect that these variables have on outcome may obfuscate that of the other LN parameters.
Our combined n of 15,809 across two datasets makes it one of the largest series that analyze LN variables and survival. The attempt to compare and contrast our findings in both a larger population and a smaller but more homogeneous and specialized institutional dataset allows us to better understand the biases surrounding LN variables and survival.
Conclusion and Practice Implications
Three LN variables have been proposed to predict outcome in pancreatic cancer, N0/N1, PLN and LNR. The ability of any of these LN variables to accurately predict survival in pancreatic cancer is dependent on the absolute numbers of LN evaluated. N0/N1 prediction is dependent on a minimum number of LNs that must be harvested in order to remove misclassification biases, which in turn hinder the lymph node variables from accurately predicting survival. The ability of positive LNs to accurately predict survival is limited but increases in strength with examined LNs. LNR appears to be a better outcome measure than PLN; however, LNR is also subject to biases introduced by number of LNs evaluated. The prognostic accuracy of any lymph node parameter is dependent first and foremost on a sufficient number of total lymph nodes evaluated.
This study suggests two relevant clinical practice implications. First, a minimum of 13–16 lymph nodes must be examined. The threshold, once crossed, not only allows accurate nodal staging, but also enables any LN variable (PLN and LNR) to be highly predictive of survival. Second, among the LN variables, LNR, rather than PLN, should be used in predicting outcome.
Acknowledgments
We thank Andrew Liss, Ph.D. for his scientific and editorial support. This study was supported by the Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research and grants CA117969 and CA127003 from the National Cancer Institute.
Footnotes
This study was presented, in part, at the 45th Annual Meeting of the Pancreas Club, Chicago, Illinois, May 6, 2011 and the 52nd Annual Meeting of The Society for Surgery of the Alimentary Tract, Chicago, Illinois, May 8, 2011.
Contributor Information
Nakul P. Valsangkar, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Devon M. Bush, Laboratory for Quantitative Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
James S. Michaelson, Laboratory for Quantitative Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Cristina R. Ferrone, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Jennifer A. Wargo, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Keith D. Lillemoe, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Carlos Fernández-del Castillo, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA.
Andrew L. Warshaw, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
Sarah P. Thayer, Email: sthayer@partners.org, Department of Surgery and Andrew L. Warshaw, M.D., Institute for Pancreatic Cancer Research, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA. Pancreatic Biology Laboratory, Department of Surgery, Massachusetts General Hospital, 15 Parkman St., WACC 460, Boston, MA 02114, USA
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA: a cancer journal for clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Moon HJ, An JY, Heo JS, Choi SH, Joh JW, Kim YI. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas. 2006 Jan;32(1):37–43. doi: 10.1097/01.mpa.0000194609.24606.4b. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7. New York: Springer; 2010. Chapters 10 and 24. [Google Scholar]
- 4.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. The British Journal of Surgery. 2004 May;91(5):586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 5.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic Nomogram for Patients Undergoing Resection for Adenocarcinoma of the Pancreas. Annals of Surgery. 2004 Aug;240(2):293–8. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winter JM, Cameron JL, Campbell Ka, Arnold Ma, Chang DC, Coleman J, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. Journal of Gastrointestinal Surgery. 2006 Nov;10(9):1199–210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1. [DOI] [PubMed] [Google Scholar]
- 7.Richter A, Niedergethmann M, Sturm JW, Lorenz D, Post S, Trede M. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World Journal of Surgery. 2003 Mar;27(3):324–9. doi: 10.1007/s00268-002-6659-z. [DOI] [PubMed] [Google Scholar]
- 8.Tseng JF, Raut CP, Lee JE, Pisters PWT, Vauthey J-N, Abdalla EK, et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. Journal of Gastrointestinal Surgery. 2004 Dec;8(8):935–49. doi: 10.1016/j.gassur.2004.09.046. discussion 949–50. [DOI] [PubMed] [Google Scholar]
- 9.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Annals of surgery. 2003 Jan;237(1):74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.House MG, Gönen M, Jarnagin WR, D’Angelica M, DeMatteo RP, Fong Y, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. Journal of Gastrointestinal Surgery. 2007 Nov;11(11):1549–55. doi: 10.1007/s11605-007-0243-7. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007 May;141(5):610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. The American Surgeon. 2004 Mar;70(3):235–40. discussion 240. [PubMed] [Google Scholar]
- 13.Sierzega M, Popiela T, Kulig J, Nowak K. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006 Oct;33(3):240–5. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- 14.Mariette C, Piessen G, Briez N, Triboulet JP. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Annals of surgery. 2008 Feb;247( 2):365–71. doi: 10.1097/SLA.0b013e31815aaadf. [DOI] [PubMed] [Google Scholar]
- 15.Lee S-Y, Hwang I, Park Y-S, Gardner J, Ro JY. Metastatic lymph node ratio in advanced gastric carcinoma: a better prognostic factor than number of metastatic lymph nodes? International journal of oncology. 2010 Jun;36(6):1461–7. doi: 10.3892/ijo_00000632. [DOI] [PubMed] [Google Scholar]
- 16.Falconi M, Crippa S, Domínguez I, Barugola G, Capelli P, Marcucci S, et al. Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Annals of surgical oncology. 2008 Nov;15(11):3178–86. doi: 10.1245/s10434-008-0099-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, et al. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. The American Surgeon. 2011 Mar;77(3):322–9. [PubMed] [Google Scholar]
- 18.Chen SL, Steele SR, Eberhardt J, Zhu K, Bilchik A, Stojadinovic A. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Annals of surgery. 2011 Jan;253(1):82–7. doi: 10.1097/SLA.0b013e3181ffa780. [DOI] [PubMed] [Google Scholar]
- 19.Peparini N, Chirletti P. Lymph node ratio, number of excised nodes and sentinel-node concepts in breast cancer. Breast cancer research and treatment. 2011 Apr;126(3):829–33. doi: 10.1007/s10549-010-1296-y. [DOI] [PubMed] [Google Scholar]
- 20.Spillane AJ, Cheung BLH, Winstanley J, Thompson JF. Lymph node ratio provides prognostic information in addition to American joint committee on cancer N stage in patients with melanoma, even if quality of surgery is standardized. Annals of surgery. 2011 Jan;253(1):109–15. doi: 10.1097/SLA.0b013e3181f9b8b6. [DOI] [PubMed] [Google Scholar]
- 21.Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. Journal of Gastrointestinal Surgery. 2009 Jul;13(7):1337–44. doi: 10.1007/s11605-009-0919-2. [DOI] [PubMed] [Google Scholar]
- 22.Massucco P, Ribero D, Sgotto E, Mellano A, Muratore A, Capussotti L. Prognostic significance of lymph node metastases in pancreatic head cancer treated with extended lymphadenectomy: not just a matter of numbers. Annals of surgical oncology. 2009 Dec;16( 12):3323–32. doi: 10.1245/s10434-009-0672-5. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y, Uemura K, Sudo T, Hayashidani Y, Hashimoto Y, Nakashima A, et al. Number of metastatic lymph nodes, but not lymph node ratio, is an independent prognostic factor after resection of pancreatic carcinoma. Journal of the American College of Surgeons. 2010 Aug;211(2):196–204. doi: 10.1016/j.jamcollsurg.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 24.Xing Y, Badgwell BD, Ross MI, Gershenwald JE, Lee JE, Mansfield PF, et al. Lymph node ratio predicts disease-specific survival in melanoma patients. Cancer. 2009 Jun 1;115(11):2505–13. doi: 10.1002/cncr.24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Archives of surgery. 2007 Aug;142(8):767–723. doi: 10.1001/archsurg.142.8.767. discussion 773–4. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Annals of surgical oncology. 2006 Sep;13(9):1189–200. doi: 10.1245/s10434-006-9016-x. [DOI] [PubMed] [Google Scholar]
- 27.Pavlidis TE, Pavlidis ET, Sakantamis AK. Current opinion on lymphadenectomy in pancreatic cancer surgery. Hepatobiliary & pancreatic diseases international. 2011 Feb;10(1):21–5. doi: 10.1016/s1499-3872(11)60002-7. [DOI] [PubMed] [Google Scholar]
- 28.Kooby Da, Chu CK. Laparoscopic management of pancreatic malignancies. The Surgical clinics of North America. 2010 Apr;90(2):427–46. doi: 10.1016/j.suc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Cruz L, Cosa R, Blanco L, Levi S, López-Boado M-A, Navarro S. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. Journal of Gastrointestinal Surgery. 2007 Dec;11(12):1607–21. doi: 10.1007/s11605-007-0266-0. discussion 1621–2. [DOI] [PubMed] [Google Scholar]
- 30.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surgical endoscopy. 2006 Jul;20(7):1045–50. doi: 10.1007/s00464-005-0474-1. [DOI] [PubMed] [Google Scholar]
- 31.Hellan M, Sun C-L, Artinyan A, Mojica-Manosa P, Bhatia S, Ellenhorn JDI, et al. The impact of lymph node number on survival in patients with lymph node-negative pancreatic cancer. Pancreas. 2008 Jul;37(1):19–24. doi: 10.1097/MPA.0b013e31816074c9. [DOI] [PubMed] [Google Scholar]
- 32.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Annals of surgical oncology. 2008 Jan;15(1):165–74. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa O, Ohhigashi H, Sasaki Y, Kabuto T, Fukuda I, Furukawa H, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Annals of surgery. 1988 Aug;208(2):215–20. doi: 10.1097/00000658-198808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manabe T, Ohshio G, Baba N, Miyashita T, Asano N, Tamura K, et al. Radical pancreatectomy for ductal cell carcinoma of the head of the pancreas. Cancer. 1989 Sep 1;64(5):1132–7. doi: 10.1002/1097-0142(19890901)64:5<1132::aid-cncr2820640528>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Annals of surgery. 2002 Sep;236(3):355–66. doi: 10.1097/00000658-200209000-00012. discussion 366–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: update on 5-year survival. Journal of Gastrointestinal Surgery. 2005 Dec;9(9):1191–204. doi: 10.1016/j.gassur.2005.08.034. discussion 1204–6. [DOI] [PubMed] [Google Scholar]
- 37.Pedrazzoli S, DiCarlo V, Dionigi R, Mosca F, Pederzoli P, Pasquali C, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Annals of surgery. 1998 Oct;228(4):508–17. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatti I, Peacock O, Awan AK, Semeraro D, Larvin M, Hall RI. Lymph node ratio versus number of affected lymph nodes as predictors of survival for resected pancreatic adenocarcinoma. World Journal of Surgery. 2010 Apr;34(4):768–75. doi: 10.1007/s00268-009-0336-4. [DOI] [PubMed] [Google Scholar]