Abstract
Oncolytic viruses can be neutralized in the bloodstream by antiviral antibodies whose titers increase progressively with each exposure, resulting in faster virus inactivation and further reductions in efficacy with each successive dose. A single dose of cyclophosphamide (CPA) at 370 mg m−2 was not sufficient to control the primary antiviral immune responses in mice, squirrel monkeys and humans. We therefore tested clinically approved multidose CPA regimens, which are known to kill proliferating lymphocytes, to determine if more intensive CPA therapy can more effectively suppress antiviral antibody responses during virotherapy. In virus-susceptible mice, primary antibody responses to intravenously (i.v.) administered oncolytic measles virus (MV) or vesicular stomatitis virus (VSV) were partially or completely suppressed, respectively, by oral (1 mg × 8 days) or systemic (3 mg × 4 days) CPA regimens initiated 1 day before virus. When MV- or VSV-immune mice were re-challenged with the respective viruses and concurrently treated with four daily systemic doses of CPA, their anamnestic antibody responses were completely suppressed and antiviral antibody titers fell significantly below pre-booster levels. We conclude that the CPA regimen of four daily doses at 370 mg m−2 should be evaluated clinically with i.v. virotherapy to control the antiviral antibody response and facilitate effective repeat dosing.
Keywords: cyclophosphamide, vesicular stomatitis virus, measles virus, oncolytic, antibodies
INTRODUCTION
Tumor-selective oncolytic viruses hold great promise as the next new modality for cancer therapy.1,2 Viruses from diverse families have demonstrated potent antitumor activity against a variety of cancer types in animal models and modest activity with an excellent safety record in clinical trials.3 Although many of the clinical trials conducted to date have used an intratumoral route of administration, it is clear that oncolytic viruses will have to be delivered by the intravenous (i.v.) route for successful eradication of metastatic cancer.
The two key steps to successful oncolytic virotherapy are first the delivery of the virus to sites of tumor growth, and second the spread of the virus through each infected tumor. Adaptive antiviral immune responses can have a major negative impact on both of these processes, with antiviral antibodies primarily blocking vascular delivery and cytotoxic T cells primarily blocking intratumoral spread. Circulating antibodies can efficiently neutralize and sequester blood-borne viruses and their titers increase progressively with each exposure to the virus until they reach a very high level at which administered viruses are neutralized so rapidly that there is no further boosting. Several studies have been conducted to demonstrate that the antitumor efficacy of i.v. administered oncolytic viruses is greatly diminished or completely negated by even relatively low titers of circulating antiviral antibodies.4–7
Cyclophosphamide (CPA) is an inexpensive alkylating agent commonly used both as an anticancer chemotherapeutic and as an immunosuppressive agent for the treatment of several autoimmune diseases, to protect solid organ transplants from rejection by the host and to suppress graft versus host disease.8–10 The immunosuppressive activity of CPA derives from its ability to kill proliferating lymphocytes, including natural killer, T and B cells, all of which are exquisitely sensitive to the drug.8 Previous studies have shown that CPA can be exploited to enhance the intratumoral spread of oncolytic herpes simplex viruses in immune-deficient mice by killing stromal macrophages.11–13 Other virotherapy agents such as reovirus, vaccinia virus, measles virus and adenovirus also benefit from combination therapy with CPA.14–17 CPA has also been shown to suppress the antiadenoviral immune response in Syrian hamsters, thereby enhancing intratumoral propagation and antitumor efficacy.18
Little has been published on the optimization of CPA regimens to suppress humoral immunity to oncolytic viruses. Thomas et al.18 used a very aggressive twice-weekly CPA regimen (with addition of an antibiotic, Baytril, in the drinking water to prevent opportunistic infections) to fully suppress the primary antibody response to an oncolytic adenovirus. On the other hand, our preliminary studies revealed that a single intermediate dose of CPA could only partially suppress the primary antibody response to an oncolytic measles virus. Here we sought to determine whether and to what extent the well-established CPA regimens that are routinely used in clinical practice can suppress the primary and anamnestic antiviral antibody responses to two different i.v. administered oncolytic viruses in mice. Our results show that antiviral antibody responses can be efficiently controlled by using well-established clinically acceptable oral or parenteral CPA regimens in combination with oncolytic virotherapy, pointing to the possibility of a convenient solution for the repeat dosing problem.
RESULTS
Low-intensity CPA could not suppress induction of the primary anti-measles virus (MV) humoral response
CPA is potently cytotoxic to proliferating B lymphocytes and cancer cells and has long been used for both immune modulation and cancer therapy.8,10 The drug can be administered orally or parenterally over a wide dose range that varies in the setting of neoplastic disease from 1 to 100 mg kg−1. The four standard CPA regimens that are most widely used in human cancer patients with a broad spectrum of neoplastic diseases are shown in Table 1 alongside the equivalent dosing regimens for mouse and hamster.19,20 When the dose of CPA is expressed in mg m−2, the area under the serum concentration-time curve for CPA has been demonstrated to be essentially the same in all species tested, including mouse, hamster, dog, rat, monkey and man.21
Table 1.
Dose level and schedule of cyclophosphamide used in humans and the corresponding doses in mice and hamsters
| Human (mg kg −1) |
Route | Intensity | Mouse (mg kg −1) |
Hamster (mg kg −1) |
|---|---|---|---|---|
| 3–5 | Intravenous | Twice weekly | 37–62 | 22–37 |
| 10–15 | Intravenous | Every 7–10 days | 123–185 | 74–111 |
| 40–50a | Intravenous | Over 2–5 days | 492–615 | 296–370 |
| 1–5a | Oral | Daily | 12–62 | 7–37 |
Human equivalent schedules used in this murine study. To convert human doses expressed in mg kg−1 to animal doses, the human dose was multiplied by a factor of 12.3 for mouse and 7.4 for hamster. Clinical dose schedules were obtained from the Thomson Reuters (Healthcare) (Greenwood Village, CO, USA) DRUGDEX database.19 Human-to-animal conversion factors are based on recommendation by the US Food and Drug Administration for estimating the maximum safe starting dose in clinical trials.20 Mice were dosed intraperitoneally at 120 mg kg−1 daily for 4 days (human 10 mg kg−1 × 4 doses) or by oral gavage at 40 mg kg−1 daily for 8 days (human 3 mg kg−1 × 8).
Initially, we performed studies to determine whether a low single dose of CPA could significantly impact the primary antiviral antibody response. The anti-measles antibody response was not affected by a single i.v. dose of 2.5 mg CPA (125 mg kg−1) given to CD46 transgenic mice 2 days before a single i.v. dose of 106 TCID50 (50% tissue culture infective dose) MV-NIS (MV expressing human thyroidal sodium iodide symporter). The reciprocal of anti-MV antibody titers in mice that received MV only or CPA/MV at day 22 post administration were 1080 ± 603 (n = 6, mean ± s.d.) and 1350 ± 763 (n = 6), respectively. At day 91, the titers were 1350 ± 763 (n = 6, MV group) and 870 ± 754 (n = 6, CPA/MV group). Analysis (t-test) indicated that there is no significant difference in anti-MV antibody titers between the two groups at day 22 (P = 0.3) or at day 91 (P = 0.2). Subsequent studies in nonhuman primates and humans indicate that an equivalent dose of CPA (31 mg kg−1) administered 4 h before i.v. MV-NIS administration in squirrel monkeys22 and myeloma patients (10 mg kg−1) was also not effective at suppressing the antiviral humoral response (A Dispenzieri, unpublished data). These data show that a single dose of CPA (10 mg kg−1 in humans or 370 mg m−2) is not sufficient to control the primary antiviral immune responses in mice, squirrel monkeys and humans.
Comparing CPA with external beam irradiation or dexamethasone (Dex) regimens for immune suppression
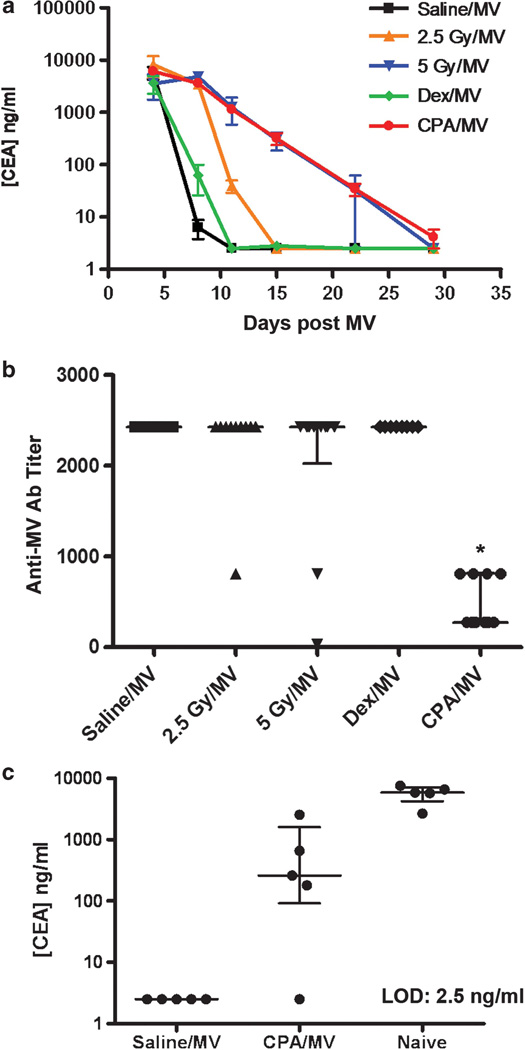
We next explored using slightly higher doses of CPA and alternative methods of immune suppression. Measles-susceptible CD46 transgenic mice were given whole-body external beam irradiation at 2.5 or 5 Gy 1 day before, or Dex (1.5 mg kg−1) or CPA (250 mg kg−1) 4 h before intraperitoneal (i.p.) administration of 4 × 106 TCID50 MV-CEA, a recombinant measles virus encoding the soluble domain of human carcinoembryonic antigen (CEA) as a marker protein. Analysis of CEA levels in the mouse serum allows accurate quantitation of the overall levels of viral gene expression.23 Viral gene expression, as indicated by serum CEA levels, was comparable in all groups at day 2 post virus administration but started to decline over time because of presumed cellular-mediated immune clearance of virally infected cells in these animals (Figure 1a). By day 8, viral gene expression was <1% of the respective day 2 levels in the saline/MV- and Dex/MV-treated groups (no difference between these two groups). In contrast, the irradiated and CPA-treated mice maintained significantly higher (P<0.05, t-test) levels of MV-CEA gene expression (between 50 and 100% of their respective day 2 values) than the saline/MV group. Serum CEA was not detectable in saline/MV-, Dex/MV- and 2.5 Gy/MV-treated groups by day 22. Similarly, four out of five mice in the 5 Gy/MV-treated group had undetectable serum CEA levels (<2.5 ng ml−1), and only one mouse was positive for CEA (151 ng ml−1). In contrast, viral gene expression was prolonged and CEA remained detectable at day 22 in all mice from the CPA/ MV group (34 ± 20, mean ± s.d., range 16–57 ng ml−1).
Figure 1.
Comparing CPA, whole-body irradiation and Dex for suppression of host immune response against measles virus. Measles-naive CD46 transgenic mice were given MV-CEA in combination with saline, whole-body irradiation (2.5 or 5 Gy), Dex or CPA. (a) Serum levels of virally encoded CEA in mice from different treatment groups (mean ± s.e.m., n = 5). (b) Anti-MV antibody (Ab) titers in the serum of mice at day 36 after virus administration. Each point represents data from one mouse (n = 10). The median with interquartile range is indicated. *Significantly lower than each of the other treatment groups (P<0.005, nonparametric Wilcoxon test). (c) Serum CEA levels after re-dosing with MV-CEA in measles-immunized mice from the saline/MV and CPA/MV groups or in measles-naive mice. The median with interquartile range is indicated.
Anti-MV antibody titers were measured at day 36 (Figure 1b). All mice (n = 10 per group) developed robust anti-MV antibody with comparable titers, except for mice that received CPA/MV. Their median anti-MV titer was significantly lower (P<0.005, nonparametric Wilcoxon test) than the respective median in the other treatment groups (n = 10 per group).
To evaluate if prior CPA treatment permits effective repeat dosing of MV-CEA, two cohorts (saline/MV and CPA/MV) of mice received a second dose of MV-CEA (4 × 106 TCID50) i.p. 53 days after the initial challenge. A new control group of measles-naive mice was given MV-CEA. At 3 days post virus administration, there was no detectable MV-CEA gene expression in any of the mice previously given saline/MV-CEA (Figure 1c). In contrast, four out of five CPA/MV mice showed significantly higher levels of CEA compared with the saline/MV group (P = 0.025, Wilcoxon nonparametric test) after the second dose of MV-CEA (Figure 1c). However, the median of 260 ng ml−1 in the CPA/MV group (range 2.5–2540 ng ml−1, n = 5) was significantly lower (P = 0.012, Wilcoxon nonparametric test) than in naive mice (median 5880 ng ml−1, range 2660–7580 ng ml−1, n = 5).
Tolerability of standard intensity, clinically approved CPA regimens in mice
As a single dose of CPA (5 mg per mouse) was only partially effective at suppressing the primary antibody response to measles virus, we next performed exploratory studies to establish tolerability of CPA regimens equivalent to standard human CPA protocols in which the drug is administered either parenterally or orally for several consecutive days (Table 1).19 When 120 mg kg−1 CPA (~3 mg per 25 g mouse, human equivalent dose (HED) 10 mg kg−1) was administered daily by i.p. injection on four consecutive days, the treatment was well tolerated. Some mice became dehydrated on this regimen and were given supportive care, including subcutaneous fluids or caloric supplements (Nutrical, Vetroquinol USA Inc., Fort Worth, TX, USA) as needed, and they typically recovered by day 14 with stabilization of weight loss. However, with an increase in the daily dose to 5 mg per mouse, the treatment was poorly tolerated and mice became weak, lethargic, dehydrated and lost >30% of body weight, meeting euthanasia criteria within a week of initiating the treatment. However, it is also important to point out that the CPA toxicity seen in mice (dehydration and cystitis) can be easily managed in humans with supportive care and by ensuring hydration of the patients. In addition to the above parenteral regimen, an oral regimen equivalent to the clinically approved continuous oral CPA protocol in Table 1 was tested. Mice were administered 40 mg kg−1 CPA daily by oral gavage daily for eight consecutive days (1 mg per mouse per dose, HED 3 mg kg−1) and the treatment was well tolerated. An increase in the dose intensity of this oral regimen to 52 mg/kg−1 CPA (~1.3 mg per mouse, HED4 mg kg−1) resulted in a higher frequency of lethal toxicity. These two protocols were therefore adopted in subsequent studies (Table 1).
CPA suppression of the primary antiviral humoral response
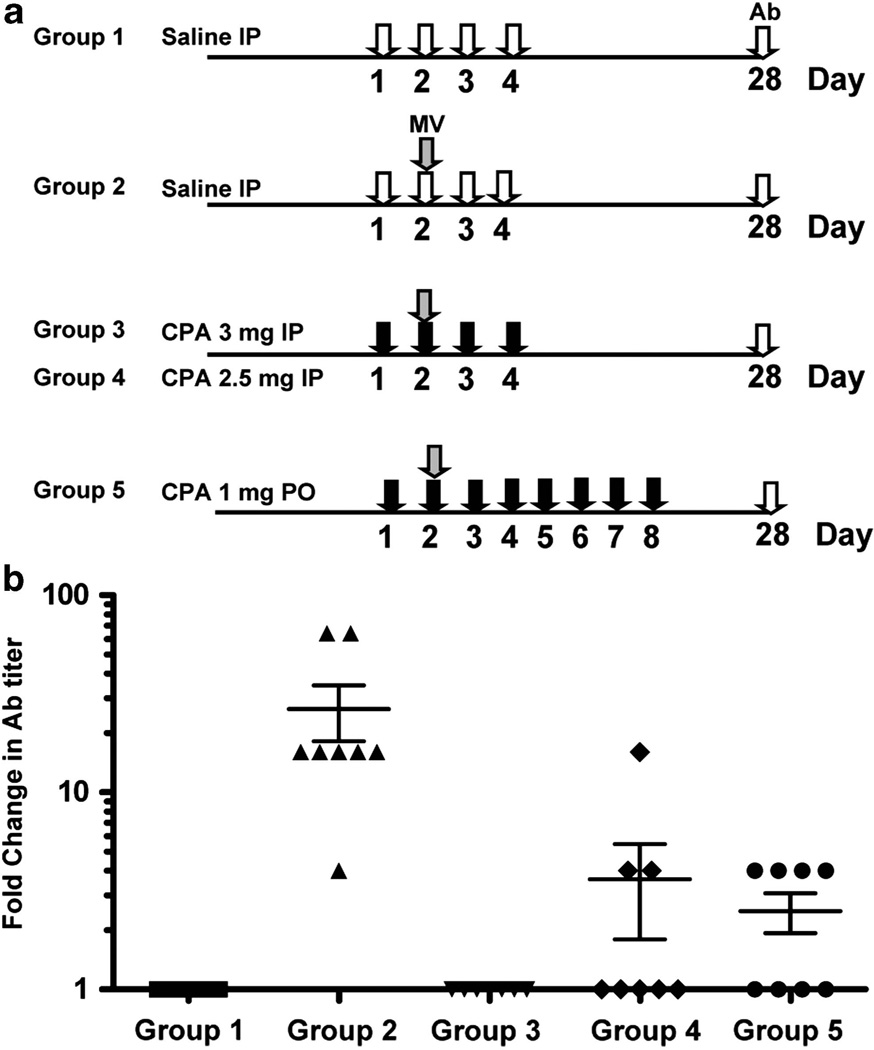
CD46 transgenic mice were randomized into 5 treatment groups (n = 8 per group) and given saline i.p., MV-NIS i.p. (5 × 106 TCID50), 4 daily i.p. doses of CPA (3 2.5 mg/per mouse per dose) starting 1 day before virus or 8 daily doses of CPA given by oral gavage (1 mg per mouse per dose) starting 1 day before virus. At 1 month after treatment, there was robust induction of anti-MV antibody in mice given MV alone (Figure 2). In contrast, mice that received CPA in conjunction with virus dosing had significantly lower anti-MV antibody titers (P<0.005, t-test) compared with the MV-NIS-treated group 2. In all mice, 4 days of parenteral CPA at 3 mg per dose was most effective at completely suppressing the induction of anti-MV antibody. Also, 8 days of oral CPA at 1 mg per dose completely suppressed anti-MV antibody induction in 50% of mice and partially suppressed it in the remainder.
Figure 2.
CPA suppresses the primary immune response to MV. (a) Measles-susceptible CD46 transgenic mice were given saline i.p., or 4 i.p. doses of CPA at 2.5 or 3 mg per dose or 8 oral doses of CPA at 1 mg per dose as shown in the schematic diagram. MV-NIS was given i.p. to groups 2 to 5 at 1 day post saline or CPA. (b) Anti-MV antibody (Ab) titers were measured 1 month later and the fold change in anti-MV Ab titers compared with saline control group 1 was plotted (n = 8 per group, mean ± s.e.m.). The respective fold increase in anti-MV Ab titer in groups 3, 4 and 5 were significantly (P<0.005, t-test) less than group 2.
We also tested if the regimen of four daily parenteral CPA (3 mg per mouse) was effective at suppressing the primary humoral response against another oncolytic virus, vesicular stomatitis virus (VSV), in immunocompetent C57BL/6 mice. Mice were given 108 TCID50 of a recombinant VSV expressing murine interferon-β i.v. with or without concurrent CPA treatment (4 daily doses i.p. 3 mg per mouse, starting 1 day before VSV). In the absence of CPA, the mice mounted a robust anti-VSV humoral response by day 5 post virus administration. In contrast, there was no induction of anti-VSV antibody if mice received concurrent CPA treatment (data not shown).
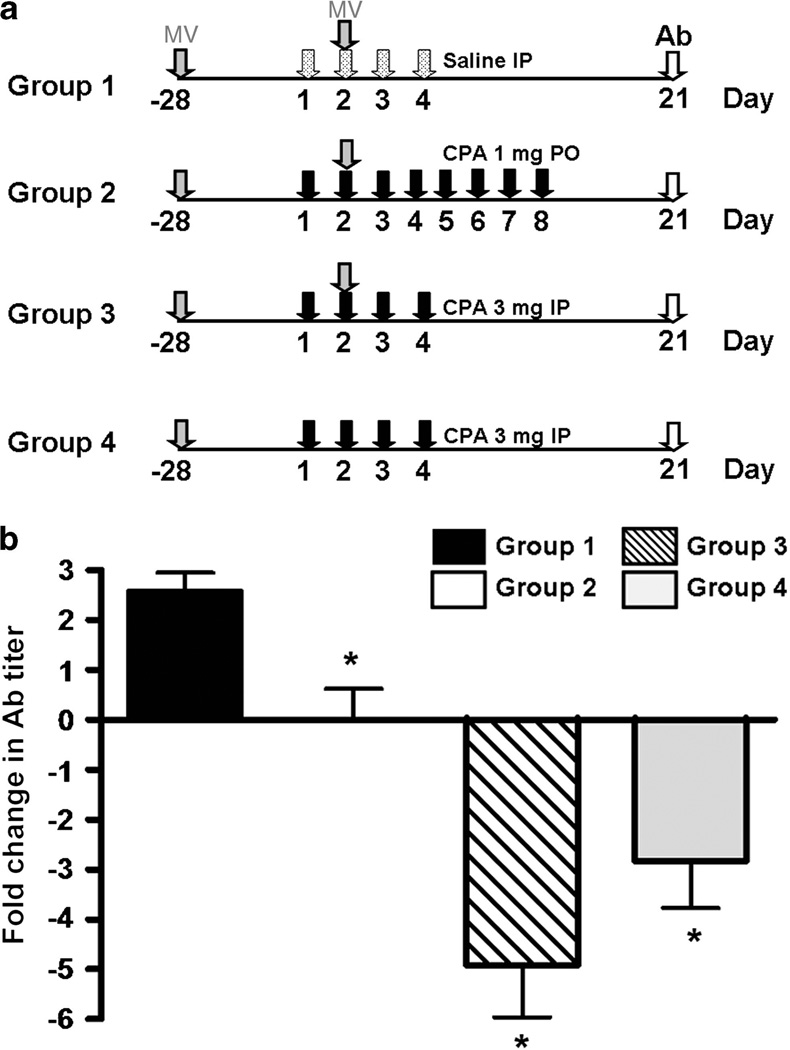
CPA suppresses the anamnestic response to MV
One of the major limiting factors to delivery of MV is the presence of pre-existing antibodies. CD46 transgenic mice were immunized with i.p. injections of MV-NIS and were randomized 28 days later into four groups based on their anti-MV antibody titers. Three groups of mice (groups 1–3) were re-immunized with booster doses of MV-NIS administered by i.p. injection with or without concurrent CPA (see schema in Figure 3). Mice in group 4 received CPA without the MV boost. After 21 days, mice were bled and the fold changes in anti-MV antibody titers were plotted (Figure 3). As expected, MV-NIS re-administration induced a robust anamnestic antibody response in MV/saline-treated (group 1) mice. In contrast, both the oral (group 2) and the parenteral (group 3) CPA regimens prevented the anamnestic response (Figure 3). Compared with the oral regimen, delivery of MV in conjunction with 3 mg × 4 i.p. doses of CPA had the advantage of decreasing the anti-MV antibody titer five fold. The 4-day parenteral CPA regimen with no MV boost also resulted in a significantly greater drop (P = 0.02, t-test) in anti-MV titers compared with the 8-day oral regimen. The decrease in anti-MV antibody titers of fivefold in group 3 (MV/CPA) and threefold in group 4 (saline/CPA) was not statistically different (P = 0.17, t-test).
Figure 3.
CPA suppresses host anamnestic immune response to MV in measles-immune mice. (a) Measles-immune CD46 transgenic mice were given saline or CPA according to the treatment regimens as shown in schematic diagram before re-challenge with MV-NIS i.p. (b) Anti-MV antibody (Ab) titers were measured 21 days later and plotted as fold change from starting titer (mean ± s.e.m., n = 8–26 mice per group). *Statistically different from group 1 (P<0.01, t-test).
Effect of CPA on decay of antibody titers
The brisk anamnestic antibody response after MV booster injection is because of activation and proliferation of antigen-specific memory B cells in previously immunized subjects. Previous studies have shown that the decay of antibody titers to sheep or human red blood cells can be accelerated by giving a booster red blood cell vaccination with CPA.21,24–26 The idea is that the booster vaccine provokes proliferation of red blood cell-specific memory B cells that are promptly killed by the CPA (proliferating lymphocytes are exquisitely sensitive to CPA). We therefore hypothesized that a booster dose of virus in combination with CPA could result in accelerated decrease of antiviral immunoglobulin G (IgG) titers because of preferential destruction of actively proliferating MV-specific memory B cells. Results in Figure 3 suggested that a booster MV dose did not significantly accelerate decay of anti-MV titers. To further investigate the hypothesis, we performed a crossover experiment with two different viruses, where the CD46 transgenic mice were immunized with MV-NIS or replication-defective VSV-ΔG expressing GFP (VSV-ΔG-GFP).
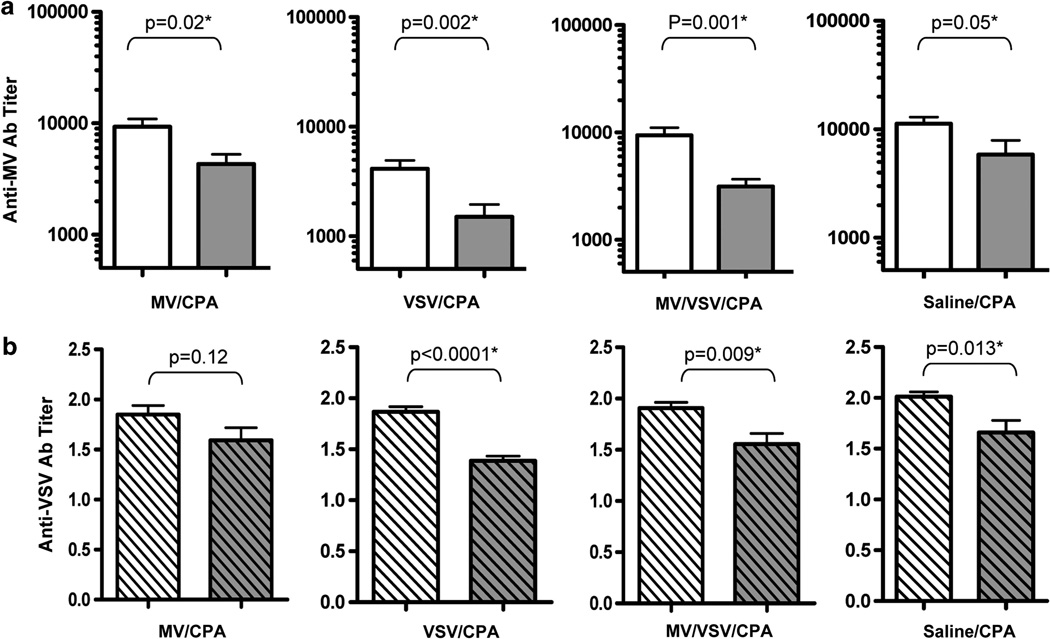
A total of 80 CD46 transgenic mice were immunized i.p. with a mixture of 105 TCID50 MV-NIS and 106 TCID50 VSV-ΔG-GFP. After 4 weeks, antiviral antibody titers were determined using measles-specific or VSV-specific enzyme-linked immunoassays. Mice immune to both MV and VSV were then randomized into four groups (n = 20 per group) and were re-challenged with virus (106 TCID50 MV-NIS and/or 107 TCID50 VSV-ΔG-GFP) or saline on day 1. The 4-day parenteral CPA regimen was administered to all of the mice and the first dose of CPA was given 1 day before virus boost. Mice either received MV-NIS/CPA, VSV-ΔG-GFP/CPA and MV-NIS/VSV-ΔG-GFP/CPA or CPA only. After 1 month, mice were bled for anti-MV antibody titers.
The reciprocal of anti-MV antibody titers for the respective groups are shown in Figure 4. Co-administration of CPA with MV or VSV or the MV/VSV mixture significantly suppressed the induction of anti-MV antibody in these virus-immune mice (Figure 4). Importantly, the anti-MV antibody titers were significantly lower than the starting values. Suppression of anti-MV titers did not appear to be antigen specific; that is, antibody titers decreased relative to pre-CPA treatment levels in all groups regardless of the identity of the virus used for antigen boost. Indeed, CPA alone was sufficient to induce a decrease in anti-MV antibody titers compared with baseline (P = 0.05, t-test).
Figure 4.
Antigen boost in combination with CPA did not accelerate decay of anti-MV or anti-VSV antibodies in immune mice. CD46 transgenic mice that were previously immunized with VSV-ΔG-GFP and MV were given MV/CPA, VSV-ΔG-GFP/CPA, MV/VSV-ΔG-GFP/CPA or saline/CPA. CPA was given 1 day before virus boost (four daily doses of 3 mg CPA per mouse). (a) Anti-MV antibody (Ab) and (b) anti-VSV Ab titers at the start (empty histograms) before CPA or 3 weeks post CPA treatment (gray histograms) are shown for each of the groups. N = 10 mice per group (mean ± s.e.m.). P-values (t-test) are shown. The asterisks indicate that the two groups are statistically different.
DISCUSSION
Here we have shown that CPA regimens in routine use for cancer therapy can be used to modulate counterproductive antiviral antibody responses during oncolytic virotherapy. Primary antibody responses to i.v. administered oncolytic MV or VSV were completely suppressed by parenteral or oral CPA regimens initiated 1 day before virus administration. The same CPA regimens also inhibited anamnestic antibody responses when administered at the time of second and subsequent virus exposures, and caused accelerated decay of antiviral antibody titers below pre-booster levels. Although there are many available immunosuppressive drugs, the alkylating agent CPA is an appealing choice for use in combination with oncolytic viruses because it has for many years been routinely used in combination with other chemotherapy drugs to treat a broad array of human malignancies including Hodgkin’s and non-Hodgkin’s lymphoma, acute and chronic leukemias, multiple myeloma, malignant histiocytoma, mycosis fungoides, neuroblastoma, osteosarcoma, soft tissue sarcomas and breast, testicular, prostate, endometrial, cervical, ovarian and lung cancers.19,27
Previous studies have shown that CPA can enhance the delivery and intratumoral propagation of oncolytic viruses, both by inhibiting innate antiviral defenses and blocking antiviral CTL responses.11,12,18,28,29 Extensive work by Chiocca and colleagues30 characterized the important role of macrophages in limiting viral spread and demonstrated the benefit of incorporating CPA in the oncolytic HSV treatment regimen. One dose of CPA (80 mg kg−1) given 2 days after HSV injection into orthotopic gliomas in the brains of immunocompetent Fischer 344 rats enhanced both intratumoral propagation and therapeutic outcome. CPA increased virus yield in the tumor by killing of macrophages that otherwise would have cleared the virus-infected cells. This rat CPA regimen corresponds to a HED of 13 mg kg−1. In contrast, we have shown here that a single HED dose of 10 mg kg−1 is not sufficient to prevent the induction of antiviral antibodies. Thus, it appears that CPA could affect the innate cellular antiviral defenses at doses much lower than those required to affect the antiviral antibody response. On the other hand, Wold and colleagues18 demonstrated profound suppression of both cellular and humoral arm antiadenoviral immune response by CPA in immunocompetent Syrian hamsters bearing HaK renal cell cancers, which significantly slowed the clearance of the virus from infected tumors and substantially improved suppression of tumor growth. The CPA regimen that they used for those studies was aggressive, comprising 140 mg kg−1 followed by 100 mg kg−1 and thereafter twice weekly for each hamster for the duration of the study.18,29 These immune-suppressed animals were also given Baytril, an antibiotic, in their drinking water as a precaution to protect them from opportunistic infections. Comparing this dose with the standard twice-weekly parenteral regimen used in human cancer patients (Table 1), it is apparent that the hamsters were receiving approximately three times the recommended human dose. Our finding in the current study that clinically approved CPA dosing regimens can efficiently suppress the humoral responses to two different oncolytic viruses is therefore of considerable translational significance.
Oncolytic viruses can be broadly classified into (1) those to which human exposure is common and widespread and in which pre-existing immunity is prevalent and (2) those for which human exposure is infrequent and pre-existing immunity is therefore rare. Measles virus typifies the first category and VSV the second. For viruses such as oncolytic VSV, where most cancer patients do not have pre-existing immunity, CPA could be incorporated into the oncolytic virotherapy regimen to prevent induction of antiviral antibodies to allow repeat systemic virus administration. However, there is also reason for caution as the suppression of adaptive T- and B-cell responses can contribute to viral toxicities resulting from destructive infection of normal tissues. Several previous studies have shown that CPA therapy can increase viral pathogenicity in a variety of experimental animal models,14,31 and hence it will be important to ensure that oncolytic viruses used in combination with CPA are highly specific for their targeted cancer.
In the case of viruses where patients have pre-existing antiviral antibodies (for example, adenovirus, measles, HSV, vaccinia), addition of CPA stops the antibody titers from increasing after re-exposure to the previously sampled virus by efficiently suppressing the anamnestic B-cell response, and by an unknown mechanism also accelerates the decay of antiviral antibody titers.21,24,25 Our initial hypothesis was that memory B cells would be stimulated to proliferate after antigen boosting and would therefore become exquisitely sensitive to CPA therapy as previously demonstrated for mice immunized with sheep red blood cells.21,24–26 However, our crossover experiments in measles and VSV immune mice demonstrated clearly that the accelerated decay of antibody titers after CPA therapy did not require antigen boosting. Repeated exposure to antineoplastic drug cocktails with potent antiplasma cell activity (often incorporating CPA) may help to explain why the majority of heavily pretreated myeloma patients do not have protective titers of antimeasles antibodies in their blood.32 Successful measles desensitization of patients with high-titer antimeasles antibodies would be expected to take several months because IgG has a circulating half-life of >20 days and it will therefore take a long time for pre-preformed measles antibodies to decay even after halting the production of new anti-measles IgG.
In summary, we have demonstrated that oral and parenteral CPA regimens that are widely used in oncology practice are potently suppressive to the adaptive antiviral immune responses leading to the production of antiviral antibodies. Higher doses of CPA are toxic and unnecessary, whereas lower doses of CPA are less effective. In addition, CPA has also been shown to improve the delivery of herpes simplex virus to tumor by reducing virus inactivation by natural immunoglobulins and complement.28,33 As such, clinical testing of CPA regimens in combination with oncolytic virotherapy protocols is warranted. Based on this study, we recommend using the CPA protocol of four daily i.v. doses of 10 mg kg−1 CPA, starting 1 day before virus in human clinical trials using oncolytic measles viruses.
MATERIALS AND METHODS
Viruses
MV-CEA or MV-NIS or replication-incompetent VSV-ΔG-GFP and replication-competent VSV expressing murine interferon-β (a kind gift of Dr G Barber, University of Miami, Miami, FL, USA) were propagated as described previously.23,34–36
Animal experiments
All procedures involving animals were reviewed and approved by the institutional animal care and use committee of Mayo Foundation. C57BL/6 mice (4–5 weeks old) were purchased from Jackson Labs (Bar Harbor, ME, USA). Measles-susceptible CD46 transgenic mice defective for the type I interferon receptor (IFNRko) and expressing the human CD46 with humanlike tissue specificity37 were bred in-house and both sexes (6–8 weeks old) were used in these experiments.
CD46 transgenic mice were immunized by i.p. administration of 0.25 ml of MV-NIS (105 TCID50) and/or VSV-ΔG-GFP (106 TCID50). Typically, mice were bled via the retroorbital plexus 21–28 days post immunization for analysis of anti-MV antibody titers in the serum. Mice were exposed to different CPA regimens per protocol with or without a booster dose of MVNIS (1 × 106 TCID50). The parenteral CPA regimen is 3 mg per mouse for four daily i.p. doses and the oral regimen of CPA is by oral gavage at 1 mg per mouse for eight daily doses.
Anti-MV antibody titers
BION measles virus antigen substrate slides (Bion Enterprises, Des Plaines, IL, USA) were used to assess titers for anti-MV antibody in mice per instructions from the BION manufacturer. Serial dilutions of serum in phosphate-buffered saline (PBS) were made. A total of 20 µl of each serum dilution was applied to a well on a BION slide and incubated at room temperature for 30 min in a moist chamber. Serum dilutions were removed and washed twice with PBS for 5 min. Then, 20 µl of a 1:2000 dilution of ALEXA 488 goat anti-mouse IgG (Invitrogen, Eugene, OR, USA) was added to each well and incubated at room temperature for 30 min in a dark moist chamber. Secondary antibody was removed, slides washed and were read with a Nikon Eclipse E4000 fluorescence microscope (Nikon Instruments Inc., Melville, NY, USA). A grading scale of 4, 3, 2, 1 and 0 was used to score the fluorescence intensity of measles antigen-positive syncytia in each well. The anti-MV antibody titer of the sample is the reciprocal of the final dilution at which syncytia were visibly fluorescent.
Anti-VSV antibody titers
An enzyme-linked immunosorbent assay was developed to determine the titer of anti-VSV antibodies. Carbonate bicarbonate buffer (Sigma, St Louis, MO, USA) was used as coating buffer. Nunc PolySorp plates (Nalge Nunc, Rochester, NY, USA) were coated with 107 TCID50 VSV-hIFNβ36 diluted in carbonate bicarbonate coating buffer (Sigma) at 100 µl per well and placed at 4 °C overnight. The plates were washed with PBS with 0.1% Tween and casein (Vector Labs, Burlingame, CA, USA) was added to plates to block at 100 µl per well for 2 h at room temperature. Serum samples were diluted 1:500 in PBS and added in duplicate at 100 µl per well. The plates were incubated at room temperature for 1 h and washed 3×. Biotin goat anti-mouse (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted in PBS to 1:1000 and added at 100 µl per well for incubation at room temperature for 1 h. The plates were washed as before with PBS with 0.1% Tween. Peroxidase-labeled streptavidin (KPL, Inc., Gaithersburg, MD, USA) was diluted in PBS at 1:1000 and added at 100 µl per well at room temperature for 1 h. The plates were washed as previously described. TMB substrate (KPL) was prepared immediately before use and 100 µl of TMB was added to each well. The plates were incubated at room temperature for 5 min while color developed. To each well, 1N HCl was added at 100 µl to stop the reaction. The plates were read at 450 nm on a spectrometer (Molecular Devices, Sunnyvale, CA, USA).
Immunosuppressive regimens with MV-CEA re-dosing
CD46 transgenic mice, 6 to 8 weeks old, were divided into five treatment groups (n = 10 mice per group) and were given either whole-body irradiation at 2.5 or 5 Gy 1 day before virus, or saline, dex (1.5 mg kg−1) or CPA (250 mg kg−1) 4 h before i.p. administration of MV-CEA (4 × 106 TCID50). Mice in each treatment group were divided into two cohorts and bled alternately on days 4, 8, 11, 16 and 22 to obtain serum CEA levels, and all mice were bled at day 36 to obtain serum anti-MV titers. Subsequently, a new group of measles-naive mice and measles-immune mice in the saline/MV or CPA/MV groups were re-challenged with a second dose of MV-CEA (4 × 106 TCID50). All mice were bled at day 4 to obtain serum CEA levels.
CEA analysis
Analysis for CEA levels was performed by the Mayo Central Clinical Laboratory using a CEA-specific immunoassay on the Beckman Coulter UniCel DXI 800 machine (Brea, CA, USA).
Statistical analysis
Paired t-test (two-tailed) or Wilcoxon nonparametric test were used to compare statistical differences between the study groups. A P-value of <0.05 is considered to be statistically significant.
ACKNOWLEDGEMENTS
We are grateful to the Mayo Foundation and NIH/NCI for funding support (CA100634, CA129966, CA136547 and the Mayo Clinic Ovarian SPORE CA136393). We thank the Mayo Clinic Center for Translational Science Activities (CTSA) Biostatistics service for help with data analysis (NIH/NCRR UL1 RR024150).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent design: combination therapy with oncolytic viruses. Mol Ther. 2010;18:251–263. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell SJ, Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 4.Raki M, Sarkioja M, Escutenaire S, Kangasniemi L, Haavisto E, Kanerva A, et al. Switching the fiber knob of oncolytic adenoviruses to avoid neutralizing antibodies in human cancer patients. J Gene Med. 2011;13:253–261. doi: 10.1002/jgm.1565. [DOI] [PubMed] [Google Scholar]
- 5.Shikano T, Kasuya H, Sahin TT, Nomura N, Kanzaki A, Misawa M, et al. High therapeutic potential for systemic delivery of a liposome-conjugated herpes simplex virus. Curr Cancer Drug Targets. 2011;11:111–122. doi: 10.2174/156800911793743673. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Russell SJ, Peng KW. Systemic therapy of disseminated myeloma in passively immunized mice using measles virus-infected cell carriers. Mol Ther. 2010;18:1155–1164. doi: 10.1038/mt.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Therapy. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- 8.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33:369–383. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 9.Bayraktar UD, Champlin RE, Ciurea SO. Progress in haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2011;18:372–380. doi: 10.1016/j.bbmt.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palumbo A, Rajkumar SV. Multiple myeloma: chemotherapy or transplantation in the era of new drugs. Eur J Haematol. 2010;84:379–390. doi: 10.1111/j.1600-0609.2010.01431.x. [DOI] [PubMed] [Google Scholar]
- 11.Wakimoto H, Johnson PR, Knipe DM, Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Therapy. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 12.Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 16.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19:1737–1746. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungerechts G, Frenzke ME, Yaiw KC, Miest T, Johnston PB, Cattaneo R. Mantle cell lymphoma salvage regimen: synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Therapy. 2010;17:1506–1516. doi: 10.1038/gt.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas MA, Spencer JF, Toth K, Sagartz JE, Phillips NJ, Wold WS. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DRUGDEX System [Internet database] Greenwood village, Colo.: Thomson Reuters (Healthcare Inc.); Updated periodically. [Google Scholar]
- 20.US Food and Drug Administration. Guidance for Industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. http://www.fda.gov/downloads/Drugs/./Guidances/UCM078932.pdf.
- 21.Hill DL, editor. A Review of Cyclophosphamide. Springfield, IL: Charles C Thomas Publisher; 1975. pp. 60–85. [Google Scholar]
- 22.Myers RM, Greiner SM, Harvey ME, Griesmann G, Kuffel MJ, Buhrow SA, et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin Pharmacol Ther. 2007;82:700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng KW, Facteau S, Wegman T, O’Kane D, Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- 24.Gordon RO, Santanelli JP, Wade ME. The production of tolerance to human erythrocytes in the rat with cytosine arabinoside or cyclophosphamide. II. Previously immunized animals. J Immunol. 1971;106:865–867. [PubMed] [Google Scholar]
- 25.Frisch AW, Davies GH. Inhibition of hemagglutinin synthesis by cytoxan. Cancer Res. 1965;25:745–751. [PubMed] [Google Scholar]
- 26.Aisenberg AC. Studies on cyclophosphamide-induced tolerance to sheep erythrocytes. J Exp Med. 1967;125:833–845. doi: 10.1084/jem.125.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des. 1999;5:555–560. [PubMed] [Google Scholar]
- 28.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 29.Dhar D, Spencer JF, Toth K, Wold WS. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Therapy. 2004;11:214–223. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastrukoff LF, Lau AS, Leung GY, Thomas EE. Contrasting effects of immunosuppression on herpes simplex virus type I (HSV I) induced central nervous system (CNS) demyelination in mice. J Neurol Sci. 1993;117:148–158. doi: 10.1016/0022-510X(93)90167-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dingli D, Peng KW, Harvey ME, Vongpunsawad S, Bergert ER, Kyle RA, et al. Interaction of measles virus vectors with auger electron emitting radioisotopes. Biochem Biophys Res Commun. 2005;337:22–29. doi: 10.1016/j.bbrc.2005.08.261. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda K, Wakimoto H, Ichikawa T, Jhung S, Hochberg FH, Louis DN, et al. Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol. 2000;74:4765–4775. doi: 10.1128/jvi.74.10.4765-4775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dingli D, Peng KW, Harvey ME, Greipp PR, O’Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 35.Majid AM, Ezelle H, Shah S, Barber GN. Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle. J Virol. 2006;80:6993–7008. doi: 10.1128/JVI.00365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrkic B, Odermatt B, Klein MA, Billeter MA, Pavlovic J, Cattaneo R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J Virol. 2000;74:1364–1372. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]