Abstract
Developmental genes are known to regulate cell proliferation, migration, and differentiation; thus, it comes as no surprise that the misregulation of developmental genes plays an important role in the biology of human cancers. One such pathway that has received an increasing amount of attention for its function in carcinogenesis is the Hedgehog (Hh) pathway. Initially the domain of developmental biologists, the Hh pathway and one of its ligands, Sonic Hedgehog (Shh), have been shown to play an important role in body planning and organ development, particularly in the foregut endoderm. Their importance in human disease became known to cancer biologists when germline mutations that resulted in the unregulated activity of the Hh pathway were found to cause basal cell carcinoma and medulloblastoma. Since then, misexpression of the Hh pathway has been shown to play an important role in many other cancers, including those of the pancreas. In many institutions, investigators are targeting misexpression of the Hh pathway in clinical trials, but there is still much fundamental knowledge to be gained about this pathway that can shape its clinical utility. This review will outline the evolution of our understanding of this pathway as it relates to the pancreas, as well as how the Hh pathway came to be a high-priority target for treatment.
THE HEDGEHOG PATHWAY---OVERVIEW
Since the identification of the Hh gene in Drosophila nearly 30 years ago, insights into the molecular pathway and its role in development and tumorigenesis have taken investigation far from the bench to the patient’s bedside. The Hh gene was found initially in Drosophila when a large-scale screening for mutations revealed an altered segmentation pattern of larvae, resulting in a short, fat larva covered in a “lawn” of denticles resembling a hedgehog; thus the name was born.1 Since that time, Hh signaling has been shown to be one of the most important signal transduction pathways in animal development, playing important roles not only in body planning but also in cell fate and stem cell maintenance. Logically, derangement of this pathway can alter profoundly the regulation of normal cell growth, and its misregulation has now been shown to play an important role in human cancers.
Mammals have 3 Hh ligands, Desert Hedgehog (Dhh), Indian Hedgehog (Ihh), and Sonic Hedgehog (Shh). Much of what is known about the Hh pathway has been derived from studies involving Shh, which exerts its effect on pattern formation and control of cell growth through activation of a signaling cascade. Shh is translated as a 45-kDa precursor protein that goes through multiple steps essential for generating active ligands. Shh undergoes autocatalytic processing, which yields a 19-kDa, N-terminal, Shh signaling domain (Shh-N) and a 25-kDa C-terminal domain (Shh-C). Shh-C has no known signaling activity but becomes active when its C terminus is modified by cholesterol and its N terminus by palmitate. When modified Shh-N reaches its target cells, it binds to the 12-pass transmembrane protein Patched (Ptch) with high affinity at the cell surface. Ptch exists as 2 isoforms, Ptch1 and Ptch2. Shh binds equivalently to both of these but is believed to exert its effect mainly through Ptch1. In the absence of Shh ligand, Ptch1 inhibits the activity of Smoothened (Smo), the 7-pass transmembrane protein. When Shh binds to Ptch1, it inhibits Smo repression and results in its activation, which transduces the Hh signal to the cytoplasm. Although the exact mechanism is still unknown, Smo activation results in the formation or release of activating forms of Gli (Gli-1 and Gli-2) while blocking the production of its repressive forms (Gli-3). The balance between the activating and repressing forms of the Gli proteins results in the expression of a variety of target genes (Fig 1).
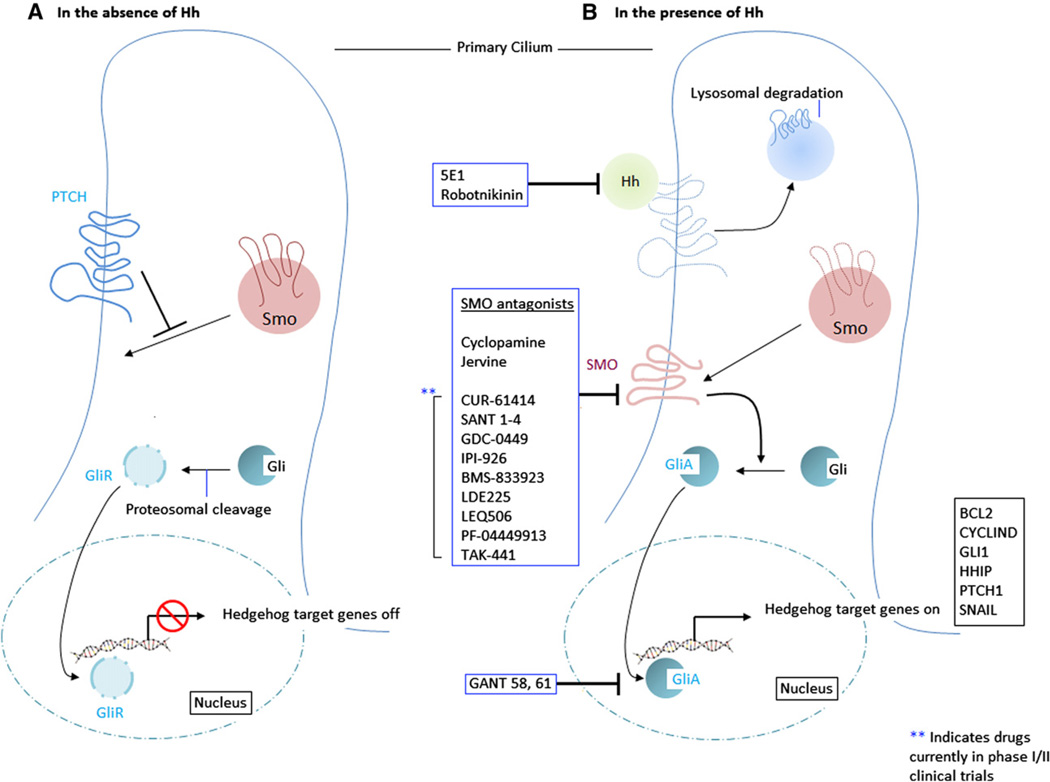
Fig 1.
Diagrammatic representation of the hedgehog (Hh) signaling pathway. In the absence of Hh ligand (A), PATCHED protein (PTCH) prevents smoothened (Smo) from accessing the cilium. The zinc finger protein Gli undergoes proteosomal cleavage and is converted to its repressor form (GliR). Gli R migrates to the nucleus and silences Hh target genes. In the presence of Hh ligand (B), Hh binds to PTCH and inactivates it via endocytosis and lysosomal degradation. This process allows Smo to associate with the cilium, and Smo facilitates the activation of Gli. Activated Gli (GliA) migrates to the nucleus and stimulates Hh target gene transcription. Inhibitors of the Hh signaling pathway have been indicated (blue boxes) and may act primarily on Hh, Smo, or Gli. Of these, Smo antagonists are being evaluated currently in phase 1 and 2 clinical trials.
In addition to this “canonical pathway” of Hh, recent evidence points toward the existence of a noncanonical pathway, where the mechanism of activation deviates from that described previously. To date, noncanonical mechanisms that have been identified include Shh signaling independent of Gli-mediated transcription and interaction or activation of the Hh pathway by other pathways independent of ligand activation. For example, Ptch has been shown to interact directly with cyclin B1 and caspases to inhibit proliferation; this effect is independent of Gli.2 In several other studies, authors further suggest that GLI transcription factors are regulated not solely by Hh signaling but can also be regulated by RAS signaling.3,4 Because of the context- and gradient-dependent manner by which Hh exerts its effects on the cell, spatial and temporal activity of both the canonical and noncanonical Hh pathways must be regulated tightly for normal embryonic development to occur. This type of sophisticated regulation of the Hh pathway is seen during pancreatic development, which relies on temporal and spatial regulation of several Hh ligands.5
Hh SIGNALING AND PANCREATIC DEVELOPMENT
The formation of the mammalian pancreas is a complex process. Development begins with the alignment of endoderm cells along the vertical body axis to form the gut tube, which is then divided into the foregut, midgut, and hindgut. At the boundary between foregut and midgut, the septum transversum generates 2 pancreatic buds called the dorsal and ventral endoderm. Subsequently, these 2 epithelial extensions that branch into the surrounding mesenchyme fuse to form the mature pancreas, which follows the rotation of the stomach.6 The dorsal bud arises first and generates the main part of the pancreas. The ventral bud develops later as part of the hepatic diverticulum; thus, it is associated with the bile duct and forms only part of the head and uncinate process of the pancreas.
All 3 mammalian hedgehog genes, Shh, Ihh, and Dhh, are expressed differentially throughout pancreatogenesis, with Shh showing the widest range of expression.7 Early in development, around embryonic day 8.5 to 9.0 (e8.5–9.0), Shh and Ihh are expressed throughout the endodermal epithelium of the primitive gut but are noticeably absent and down-regulated in the area of the future pancreas. This down-regulation identifies the area of the endoderm that has been programmed specifically to become the future pancreas.8 Ihh and Dhh ligands, including the Ptch1/Smo receptor complex, are expressed later in the development of the pancreas (approximately e13.5).9 It has been reported that epithelial and β-cell expansion are regulated by Hh signaling.10,11
The importance of the regulation of the Hh pathway in pancreatic morphogenesis has been demonstrated by misexpression experiments. Ectopic expression of Shh early in development results in the misdirection of the pancreas toward a duodenal fate,12,13 whereas loss-of-function mutations result in an expansion of the pancreatic domain.14 Loss of Ihh has been associated with annular pancreas, a rare condition characterized by an extension of the pancreatic gland, which then encircles the duodenum.13 To date, complete ablation of Hh signaling in transgenic mice has not been achieved because of the early embryonic lethality associated with Hh depletion.9 These data suggest that misregulation of Shh signaling during embryogenesis results in morphologic and functional defects in the developing pancreas, revealing the important role this pathway plays throughout embryonic pancreatic development.
Interestingly, all Hh ligands are expressed in the adult pancreas, suggesting that Hh may also continue to play an important role in pancreatic maintenance. The Ptch receptor is identified in the mesenchyme and Ptch-2 in the epithelium. The expression of the ligand appears to be restricted in different compartments throughout the pancreas. For example, Ihh is identified in the adult islet, Dhh in the peripheral nerves, and Shh has been found in a discrete epithelial compartment, the set of pancreatic duct glands (PDG).15 Thus, the Hh pathway and its signaling are important not only for pancreatic development but also for homeostasis and regeneration.
Hh SIGNALING IN PANCREATIC REGENERATION
The identification of Hh in the adult pancreas and its up-regulation in response to injury suggest that this pathway plays a central role in pancreatic regeneration. Evidence from studies of pancreatic regeneration after pancreatic parenchymal injury in rodents supports the hypothesis that the global regeneration process occurs via 2 mechanisms. The first mechanism is an expansion of the ductal cell population, followed by differentiation of this expanded pool into mature exocrine, endocrine, or duct cells. The second mechanism occurs via replication of existing beta cells in the remnant pancreas.16,17 The specific signaling pathways required for this regeneration process have been examined by the use of molecular approaches in model systems, including transgenic mice and zebra fish.
To analyze the mechanisms of regenerative repair in the exocrine pancreas after injury, a variety of pharmacologic and surgical approaches have been used to simulate pancreatitis. One of the most common pharmacologic approaches is the use of the cholecystokinin analogue cerulein. In a well-characterized model of acute pancreatitis, exposure to cerulein via intraperitoneal injection in rodents leads to the loss of acinar cell mass followed by regeneration. In the mouse, cerulein leads to a near-complete loss of acinar cells within 72 hours after injection, followed by restoration of the acinar cell compartment within 7 days of injury.18 After acute injury, some acinar cells undergo a process of dedifferentiation with loss of expression of acinar cell markers, expression of pancreatic progenitor cell markers (nestin, Pdx1), and morphologic changes that include the formation of a metaplastic epithelium. These steps involve the rapid proliferation of these cells after re-entering the cell cycle, followed by their redifferentiation into specific cell types.
Evidence for the role of Hh signaling in regeneration of the exocrine compartment comes from studies in which authors have used a combination of pharmacologic and genetic approaches to show that in the absence of an intact Hh pathway, the exocrine compartment is unable to regenerate. During normal regeneration, Hh is up-regulated after injury; indeed, levels of Hh are detected in the regenerating metaplastic epithelium as well as in the acinar cells. After regeneration is complete, Hh levels become undetectable. Inhibition of Hh signaling does not affect the initial steps that lead to the formation of a metaplastic epithelium after acute injury or the severity of injury. The main effect of Hh inhibition is the inability of the dedifferentiated acinar cells to redifferentiate into mature cells after formation of metaplastic lesions and the persistence of these lesions in injured pancreata.19
These results support the hypothesis that exocrine regeneration after pancreatic injury requires the activation of Hh signaling and up-regulation of other embryologic markers, including Pdx1 and nestin. This observation suggests that pancreatic exocrine regeneration recapitulates some aspects of pancreatic development, but, more importantly, that dysregulation of this pathway by chronic inflammation may play an early role in pancreatic metaplasia and cancer.
THE EARLY AND LATE ROLE OF THE Hh PATHWAY IN PANCREATIC CANCER: THE ROLE OF Hh IN CHRONIC INFLAMMATION AND METAPLASIA
Aberrant regulation of developmental pathways has been identified as an important property in many cancers, including pancreatic adenocarcinoma. The same pathways that direct organ patterning and growth during embryonic development also govern tissue homeostasis in adult organs by regulation of stem cell proliferation and differentiation.20 Through inappropriate activation of these pathways, chronic tissue injury may result in misdirection of tissue repair, ultimately resulting in neoplasia. Increasing evidence suggests that the Hh pathway plays a key role in the initiation of pancreatic cancer as well as in its maintenance and progression.
Aberrant expression of members of the Hh pathway in chronic pancreatitis and pancreatic cancer was first noted by Kayed et al21 in 2003, although these authors believed Ihh to be the important ligand in the diseased pancreas. Subsequent research showed that the ligand Shh is expressed aberrantly in pancreatic cancer and its precursor PanIN lesions and that Shh functions as a mediator of cancer initiation and growth.22 Mice with transgenic misexpression of Shh in the pancreatic endoderm developed lesions resembling PanIN, and Hh inhibition by cyclopamine induced apoptosis and blocked proliferation of pancreatic cancer cells in vitro and in vivo. Thus, Hh signaling was identified as an early and late mediator of pancreatic ductal adenocarcinoma (PDAC).22 These findings were supported by a concurrent study in which the authors demonstrated that the up-regulation of Hh ligand is required for the maintenance of several digestive tract cancers, including pancreatic cancer.23 Meanwhile, Hh misexpression has also been described in intraductal papillary mucinous neoplasms (IPMN), the second precursor of invasive pancreatic cancer.24
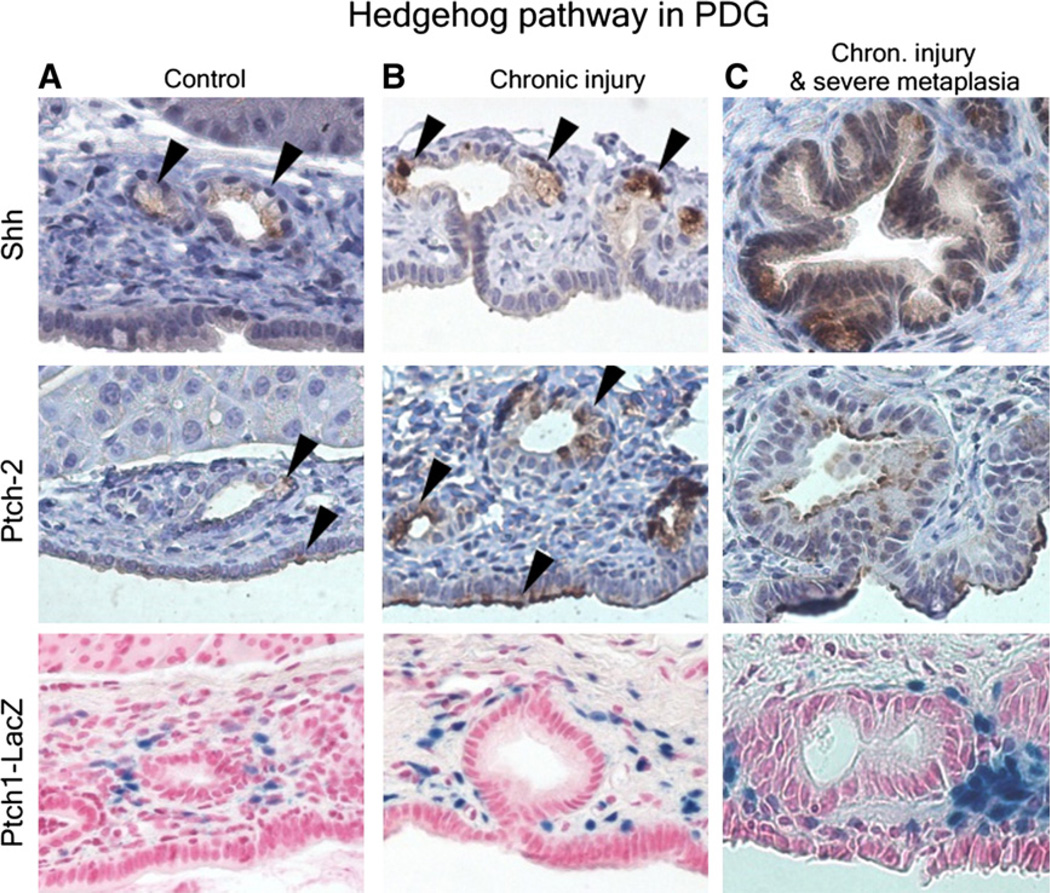
In subsequent studies, investigators focused on the mechanisms of activation of the Hh pathway and how it contributes to carcinogenesis. The special role that aberrant activation of the Hh pathway plays in pancreatic carcinogenesis is explained by its function in pancreatic development. In the embryonic foregut, Shh promotes development of the gastrointestinal tube and represses pancreatic development.25 Shh signaling was thought previously to disappear early in pancreatic development,25 but work from our group identified Shh expression in a specialized epithelial compartment which appears as gland-like outpouches off larger pancreatic ducts; we termed these pancreatic duct glands (ie, PDG).15 In response to chronic injury, PDG proliferate, up-regulate Shh as well as the Hh receptor PTCH (Fig 2), and undergo a gastrointestinal mucinous metaplasia.15
Fig 2.
Pancreatic duct glands (PDG) in the normal pancreas (A), in chronic inflammation (B), and in inflammation-associated severe metaplasia (C). Shh is expressed at a low level in normal PDG and up-regulated in hypertrophied PDG in response to chronic injury (arrowheads). This up-regulation is accompanied by up-regulation of the Hh receptor and downstream pathway member PTCH2 in the epithelium and PTCH1 in the stroma (modified from Strobel et al15).
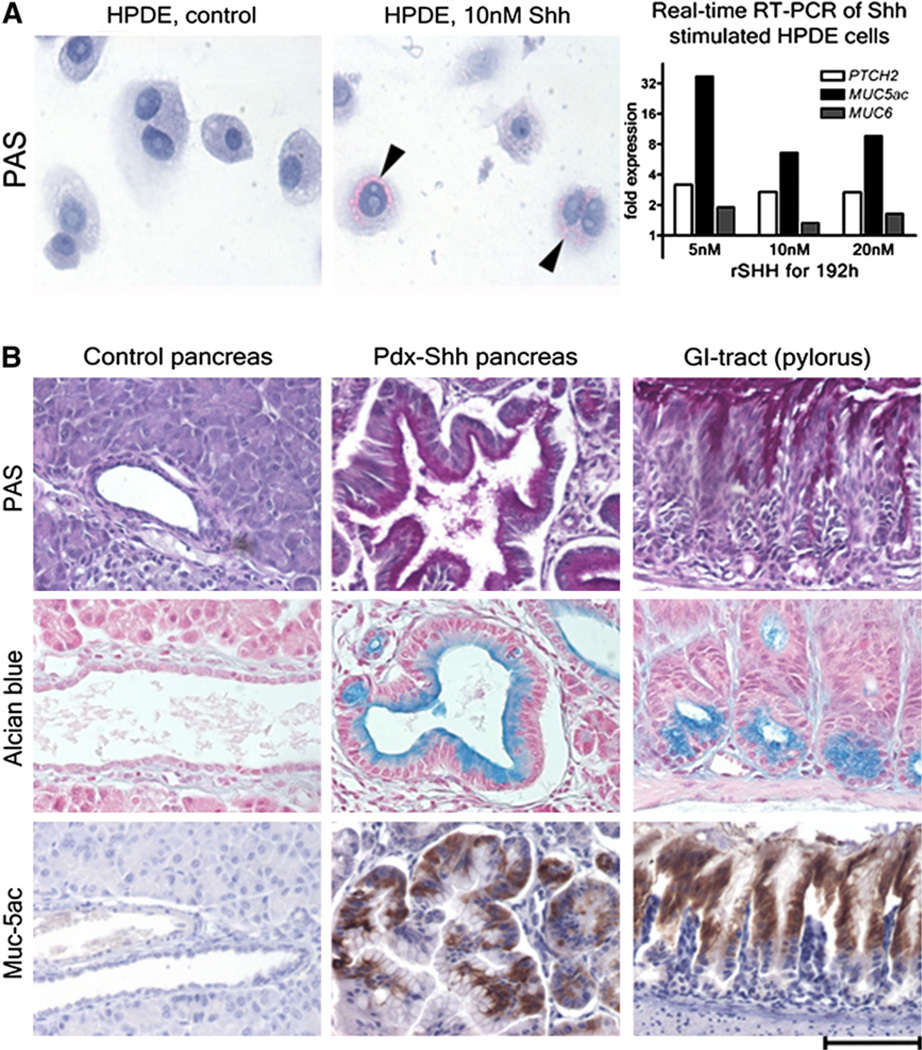
It had been noted previously that pancreatic cancer and its precursor lesions, PanIN, frequently display misexpression of gastrointestinal markers, such as the mucins Muc-5ac and Muc-6,26,27 as well as Shh.22 In vitro, activation of the Hh pathway by transfection with the Hh transcription factor Gli-1 resulted in up-regulation of several foregut markers.28 Strobel et al15 demonstrated that recapitulating its role in development, up-regulation of the ligand Shh is sufficient to misdirect the pancreatic ductal epithelium toward a gastrointestinal metaplastic phenotype both in vitro and in vivo (Fig 3). The underlying up-regulation of Shh in response to chronic injury may be caused by signals involved in tissue repair and expression of other proteins known to mediate inflammatory cues, such as nuclear factor kappa B.29
Fig 3.
Effects of the Shh ligand on pancreatic ducts cells in vitro (A) and in vivo (B). (A) Stimulation of human pancreatic ductal epithelium (HPDE) with recombinant Shh results in a mucinous phenotype with expression of gastrointestinal mucins. (B) Transgenic misexpression of Shh in the pancreas results in misdirection of ductal epithelial differentiation toward a phenotype that resembles closely the gastric pyloric epithelium (modified from Strobel et al15).
Apart from PDG, Shh misexpression can also be identified in mucinous metaplastic lesions that form in response to injury in the pancreatic parenchyma.30 A minority of these lesions have been found by lineage tracing to originate from acinar cells by true transdifferentiation, whereas the majority arise most likely from terminal ductal or centroacinar cells but not from beta cells.30,31
Chronic injury thus appears to result in a Shh-mediated gastrointestinal metaplasia as an early and important step in pancreatic carcinogenesis. This process may induce a common mechanism in the initiation of pancreatic cancer, or perhaps this observation suggests a common compartment of origin that results in both precursor lesions PDAC, PanIN and IPMN. Similar to the role of Barrett’s metaplasia at the gastroesophageal junction, this Shh-mediated gastrointestinal metaplasia in the pancreas may be the basis of further molecular aberrations and mutations that mediate the progression of precursor lesions to invasive cancer.
Other in vitro and in vivo studies demonstrate that Hh contributes to pancreatic carcinogenesis at several stages and through several mechanisms.32 Synergistic molecular crosstalk between the Hh pathway and activated K-RAS, a key mediator of pancreatic carcinogenesis,33 have been clearly demonstrated.3,32,34,35
Hedgehog is not only an early mediator involved in carcinogenesis but is also a late mediator of pancreatic cancer.22 Analyses of global genomic sequencing have recently identified the Hh pathway as one of the few “core” pathways of central importance for the biology of human pancreatic cancer.36 It has meanwhile become evident that Hh signaling contributes to several hallmarks of the maintenance and progression of pancreatic cancer, such as cancer stem cells (CSC), interactions between cancer epithelium and stroma, angiogenesis, and metastases. These different aspects underscore the therapeutic potential of Hh pathway inhibition in pancreatic cancer.
Shh AND CSC AND METASTASIS
Cancer can be defined as a disease of unregulated growth and self-renewal. Many conceptual similarities exist between stem cells and cancer cells, suggesting the existence of CSC that initiate tumorigenesis and possess indefinite potential for self-renewal and multilineage differentiation.37,38 CSC are very resistant to adverse conditions in the tumor microenvironment.39 Interestingly, Shh has been identified recently as being up-regulated in pancreatic CSC, with distinct populations of CSC (CD24+/CD44+/ESA+) shown to have Shh expression 46-fold greater than CD24−/CD44−/ESA−.40 This finding suggests a ubiquitous up-regulation of the Shh ligand in the CSC population compared with the heterogeneous population of the tumor. The role of Shh in pancreatic cancer stem cells remains to be determined, but up-regulation of Shh indicates a role in carcinogenesis driven by CSC.
It is believed widely that CSC are responsible for progression and metastasis of epithelial tumors. Hermann et al41 discovered a population of CD133+/CXCR4+ cells, as compared with CD133+/CXCR4− cells, that is able to set up metastases, which are able to induce tumor growth but do not form distant metastases. The authors suggest the existence of 2 different CSC populations, migratory and stationary CSC. If true, this concept opens a new window for potential treatment to prevent metastatic spread. The Shh expression levels of these distinct cells remain to be determined, but they are likely to be up-regulated because they are up-regulated in their CD24+/CD44+/ESA+ counterparts. The possibility of treating these special CSC to prevent the lethal event of metastatic spread and thus keeping the tumor localized may offer a major insight and advantage for further investigations in the treatment of pancreatic cancer. Only a few cases of CSC have been isolated and purified in pancreatic cancer. The ability to identify and compare those cells of origin that are able to set up metastases would give us the possibility---in PDAC as well as in other epithelial cancers---to finally find a molecular target for potentially promising results in the battle against this dismal disease.
THE Hh PATHWAY AS A POTENTIAL THERAPEUTIC TARGET
With a large body of evidence supporting a role for Shh in PDAC, a number of preclinical studies have been performed that allow us to understand further the role of the Hh pathway in PDAC as well as to evaluate the efficacy of inhibition of the Hh pathway for the treatment of this disease. Using cyclopamine, a naturally occurring Smo inhibitor, our group in collaboration with other authors first reported the efficacy of inhibiting the Hh pathway in decreasing the growth and tumorigenesis of PDAC cell lines.22 In response to cyclopamine, 50% of PDAC cell lines examined showed decreased proliferation and increased apoptosis in vitro relative to control cells. Importantly, the treatment of mice bearing heterotopic xenograft cancers with cyclopamine resulted in a 50% decrease in tumor growth in responsive lines. Two studies in which investigators used an orthotopic model of PDAC demonstrated that inhibition of the Hh pathway with cyclopamine or IPI-269609, an orally bioavailable inhibitor of Smo, prevented distant metastases.42,43 In addition to blocking the activity of Smo, strategies have been used to target Shh directly. The treatment of mice with orthotopic xenograft cancers with a neutralizing antibody against Shh, 5E1, resulted in a greater than 4-fold decrease in tumor volume.44 This treatment also decreased the incidence of metastasis to the lymph nodes, liver, and peritoneum.
Although the use of cells derived from human tumors in these preclinical studies allows one to evaluate the efficacy of inhibiting the Hh pathway in the context of the diverse genetic makeup of PDAC, genetically engineered mouse models of PDAC play an important role in understanding how inhibition of the Hh pathway affects the natural course of tumor development. In one such study, investigators used a mouse model of PDAC induced by the pancreas-specific activation of KRAS and inactivation of Ink4a/Arf.45 Treatment of these mice with cyclopamine resulted in a small increase in survival. In a second study, Olive et al46 used a mouse model of PDAC induced by the activation of KRAS and inactivation of p53. They demonstrated that inhibition of Hh signaling by the Smo inhibitor IPI-926 can enhance the effectiveness of chemotherapeutics. Although the treatment of mice with this compound alone had no effect on survival, combining treatment with gemcitabine resulted in a doubling of the survival time for these mice. Analysis of these tumors revealed that tumors from mice treated with IPI-926 had a decrease in tumor stroma, which resulted in an increased delivery of gemcitabine to the tumor cells.
The results of this study highlight a recent shift in the understanding of Hh signaling in PDAC with a greater focus on its role in the tumor microenvironment. Although early work in the field focused on an autocrine role for Shh in the development of PDAC, recent experiments using cell lines treated with Hh pathway inhibitors have demonstrated that greater levels of inhibitors were required to exert their biologic effect than were needed to inhibit Hh signaling.47 Although this study suggested that the observed biologic effects may be caused by off-target effects, the results of at least one other study suggest that some of the biologic effects are indeed attributable to the inhibition of the Hh pathway.48 Consistent with other studies, the authors demonstrated that the treatment of PDAC cell lines with cyclopamine resulted in decreased proliferation and increased apoptosis. Importantly, when the Smo agonist purmorphamine was added, cell proliferation was restored, but there was no change in apoptosis, indicating that at least some of the biologic effects of cyclopamine are dependent on Hh signaling. Given the wide variation in the sensitivity of PDAC cell lines to Hh inhibitors, the disparity in the amounts of compounds used in various studies, and recent discoveries that Gli can be activated by Smo-independent pathways (such as KRAS and TGF-beta), more rigorous analysis will be needed to define a role for autocrine Shh signaling in PDAC.35
Despite the disparate reports of autocrine signaling in PDAC, a growing body of evidence supports the importance of ligand-dependent activation of Hh signaling in the tumor stroma. In human tumor xenografts, expression of Shh by tumor cells correlated with increased expression of GLI-1 and PTCH1 in the stromal compartment. Pathway inhibition affected only stromal GLI-1 and PTCH1 expression and resulted in decreased tumor growth exclusively in Hh ligand-expressing tumors.47 Consistent with these findings, Tian et al49 demonstrated that the expression of an oncogenic Smoothened (SmoM2) in mouse pancreata neither activates Hh signaling in epithelial cells nor promotes their neoplastic transformation. In murine pancreatic cancer models as well as in human pancreatic cancer specimens, activation of the Hh pathway was observed only in stromal cells surrounding Hh ligand-expressing tumor cells. Not only is activation of the Hh pathway observed in the stroma, but Shh appears to play a role in promoting growth of the stroma. Employing an orthotopic xenograft model of PDAC, it was demonstrated that Capan-2 cells overexpressing Shh induced a more robust desmoplastic reaction than that observed in tumors derived from control cells.50
The aforementioned work by Olive et al46 provided further insight into the mechanism by which inhibition of the Hh pathway affects tumor growth in pancreatic cancer. In a genetically engineered mouse model of PDAC, Olive et al found that inhibition of the Hh pathway resulted in depletion of the stromal compartment together with a transient increase in vascular density. This study is especially important because it was the first to demonstrate the potential importance of the combined treatment of the cancer and stroma, a potentially key therapeutic strategy in treating this disease.39,46
In stark contrast to the findings by Olive et al,46 Hh signaling itself is known to promote angiogenesis both indirectly and directly in nontumor models. In recent studies authors also identified Hh pathway activation as indirectly proangiogenic in tumor models. Several groups have reported an indirect effect of Hh on angiogenesis through the induction of proangiogenic cytokines in mesenchymal cells. Pola et al51 found that the activation of Hh signaling improved blood flow and limb salvage in a mouse model of hind-limb ischemia by inducing expression of vascular endothelial growth factor (VEGF) and angiopoietins-1 and -2 (ANG1, 2) in mesenchymal cells. Asai et al52 identified active Hh signaling during wound repair and found that Hh signaling accelerates healing by increasing vascularity. Topical gene therapy with SHH increased the expression of proangiogenic cytokines in stromal fibroblasts and enhanced the recruitment of endothelial progenitor cells. Using animal models of retinal and choroidal neovascularization, Surace et al53 demonstrated a requirement for Hh signaling during physiologic and pathologic retinal vascularization through increased expression of VEGF in mesenchymal cells. In 2 more recent studies investigators delineate an additional, direct proangiogenic effect of Hh signaling on endothelial cells.54,55 Both found that the Hh ligand does not induce its target genes GLI-1 and PTCH1 in endothelial cells but rather activates the small GTPase RhoA in a SMO-dependent manner. Activation of RhoA leads to cytoskeletal rearrangement and enhanced migration, and ultimately promotes angiogenesis.
More recently, angiogenesis promoted by the activation of Hh signaling was also identified in cancer. Nakamura et al56 reported that inhibition of the Hh pathway results indirectly in a decrease of tumor vasculature by decreased homing of bone marrow-derived cells to PDAC xenografts together with a decrease in expression of stromal Ang-1 and IGF-1 within the tumors. Chen et al57 found that Hh signaling increases indirectly the tumor vascularity in PDAC by the induction of VEGF expression in stromal perivascular cells; however, the tumor cells themselves are commonly thought to secrete the majority of the VEGF found within cancers.58,59
Further studies are, therefore, needed to clarify: (1) how Hh signaling was found to promote vascularization in PDAC in some studies,56,57 whereas other groups found that its inhibition leads to increased tumor vascularity46; (2) why Hh inhibition results in a marked decrease in tumor vascularity,56,57 even though the amount of VEGF contributed by mesenchymal cells (and regulated by Hh signaling) is relatively small compared to the amount secreted by the tumor cells themselves in most cancers58,59; and (3) whether Hh signaling also promotes angiogenesis directly in cancer by acting on endothelial cells. What is clear is that the Hh pathway plays key roles in the maintenance, growth, and migration of PDAC, making it an ideal clinical target.
BEDSIDE: CLINICAL TRIALS USING THE Hh PATHWAY
The development of Hh pathway inhibitors began with cyclopamine, which binds and inactivates Smo.60 In mouse models, cyclopamine and the closely related compound jervine were associated with low affinity, poor bioavailability, chemical instability, and rapid clearance,61 making cyclopamine a poor candidate for clinical use. Hh pathway inhibitors appropriate for the clinics were identified with the use of standard medicinal chemistry and small molecule screens. One of the first drugs developed via the use of small molecule screening, CUR-61414 (Curis),62 induced apoptosis in preclinical mouse models of basal cell carcinoma. This drug was a topical agent, limiting its use in other solid tumors. Since that time, a number of Smo antagonists have been identified. Although most of the current drug development targets Smo, attempts to target members of the Hh pathway both upstream (PTCH) and downstream of Smo (GLI) are under way but are still in preclinical testing.
Seven small-molecule inhibitors of Smo are being evaluated currently in phase 1 and 2 trials. These include GDC-0449 (Genentech), IPI-926 (Infinity Pharmaceuticals), BMS-833923 (BMS/Exelixis), TAK-441 (Millennium), LDE225, LEQ506 (Novartis), and PF-04449913 (Pfizer). Table lists clinical trials in which investigators are using these drugs for pancreatic cancer and other solid tumors. The Smo inhibitors that are furthest along in their development are GDC-0449 and IPI-926. The first of these, GDC-0449, is a synthetic, highly potent small-molecule inhibitor of Smo that is available as an oral formulation.63
Table.
Clinical trials investigating inhibitors of SHH in pancreatic cancer
| Hedgehog inhibitor |
Other interventions |
Study title | Phase | Trial identifier | Status |
|---|---|---|---|---|---|
| GDC-0449 | Gemcitabine and Nab-Paclitaxel | A Phase II Study of Gemcitabine and Nab-Paclitaxel in Combination With GDC-0449 (Hedgehog Inhibitor) in Patients With Previously Untreated Metastatic Adenocarcinoma of the Pancreas | Phase 2 | NCT01088815 | Recruiting |
| GDC-0449 | None | Proof of Mechanism Study of an Oral Hedgehog Inhibitor (GDC-0449) in Patients With Resectable Pancreatic Ductal Adenocarcinoma in the Pre-operative Window Period | Phase 2 | NCT01096732 | Recruiting |
| GDC-0449 | Gemcitabine | Cancer Stem Cells and Inhibition of Hedgehog Pathway Signaling in Advanced Pancreas Cancer: A Pilot Study of GDC-0449 in Combination With Gemcitabine | Phase 2 | NCT01195415 | Recruiting |
| GDC-0449 | Gemcitabine | A Multi-Center, Double Blind, Placebo-Controlled, Randomized Phase II of Gemcitabine Plus GDC-0449 (NSC 747691), a Hh Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer (10052747) | Phase 2 | NCT01064622 | Recruiting |
| GDC-0449 | Erlotinib and Gemcitabine | Phase I Trial of the Combination of GDC-0449 and Erlotinib +/−Gemcitabine | Phase 1 | NCT00878163 | Unknown* |
| IPI-926 | None | A Phase 1 Study of IPI-926 in Patients With Advanced and/or Metastatic Solid Tumor Malignancies | Phase 1 | NCT00761696 | Active, not recruiting |
| IPI-926 | Gemcitabine | A Phase 1b/2 Study Evaluating IPI-926 in Combination With Gemcitabine in Patients With Metastatic Pancreatic Cancer | Phase 1/2 | NCT01130142 | Recruiting |
| IPI-926 | FOLFIRINOX | A Phase I Study of FOLFIRINOX Plus IPI-926 for Advanced Pancreatic Adenocarcinoma | Phase 1 | NCT01383538 | Recruiting |
| LDE-225 | Gemcitabine | Phase 1/2 Safety and Feasibility of Gemcitabine in Combination With LDE-225 as Neoadjuvant Therapy in Patients With Borderline Resectable Pancreatic Adenocarcinoma | Phase 1/2 | NCT01431794 | Not yet recruiting |
| PF-04449913 | None | A Phase 1 Study To Evaluate The Safety, Pharmacokinetics, and Pharmacodynamics of PF-04449913, an Oral Hedgehog Inhibitor, Administered as Single Agent in Select Solid Tumors | Phase 1 | NCT01286467 | Recruiting |
| BMS-833923 | None | Phase 1 Multiple Ascending Dose Study of BMS-833923 (XL139) in Subjects With Solid Tumors | Phase 1 | NCT01413906 | Not yet recruiting |
| TAK- 441 | None | A Multicenter, Open-Label, Dose-Escalation, Phase 1 Study of TAK-441, an Oral Hedgehog Signaling Pathway Inhibitor, in Adult Patients With Advanced Nonhematologic Malignancies | Phase 1 | NCT01204073 | Recruiting |
| LEQ506 | None | A Dose Finding and Safety Study of Oral LEQ506 in Patients With Advanced Solid Tumors | Phase 1 | NCT01106508 | Recruiting |
Indicates status has not been verified in more than 2 years.
ClinicalTrials.gov September 2011.
Results from 2 early-phase clinical trials showed encouraging results with respect to drug safety and tolerance. The first, a phase 1 trial for patients with refractory, locally advanced or metastatic solid tumors, was conducted by LoRusso et al64 with 68 patients (8 of whom had pancreatic cancer). In this trial, the overall response rate for the most common diagnosis, basal cell carcinoma, was 58%; one patient with PDAC experienced stable disease as the best response while being on study for 3 months. This drug was well tolerated. Grade 4 events were observed in 9% of the patients in this study, including hyponatremia, fatigue, and pyelonephritis. On the basis of the relationship between dose increments and the peak observed steady-state plasma concentration, the authors recommended a dose of 150 mg/day for future phase 2 studies. In the second study, Palmer et al65 evaluated interaction between antagonists of the epidermal growth factor receptor and Hh signaling pathways in a phase 1 trial. They compared a combination of erlotinib (epidermal growth factor receptor tyrosine kinase inhibitor) and GDC-0449 as cohort I versus erlotinib + GDC-0449 combined with gemcitabine as cohort II for unresectable solid tumors. Preliminary results from 29 patients indicated that, in cohort I, the only dose-limiting toxicity was rash; in cohort II, the dose-limiting toxicities seen were nausea, visual disturbances, and thrombocytopenia. The authors recommended both erlotinib and GDC-0449 at a dose of 150 mg/day for phase 2 evaluation. In the aforementioned clinical trials GDC-0449 was well tolerated when used alone or in combination with other chemotherapeutic drugs, and both studies deemed GDC-0449 suitable for phase 2 trials.
In addition to GDC-0449, the other small-molecule inhibitor of Smo undergoing phase 1 evaluation currently is IPI-926 (Infinity Pharmaceuticals). Preliminary data from 2 ongoing trials were presented recently at the 2011 meeting of the American Society for Clinical Oncology.66 Rudin et al67 administered IPI-926 in patients with locally advanced or metastatic solid tumors to determine its safety, antitumor activity, and pharmacokinetic properties. In the study population, there were 7 patients with pancreatic cancer. With regard to pharmacodynamic effect, a decrease in Gli-1 expression in normal skin was reported for 72% of the patients. The most common adverse events (grade 3) were fatigue (3%), alanine aminotransferase elevation (8%), and aspartate aminotransferase elevation (4%). There were no grade 4 or 5 events in this study; the maximally tolerated dose was 160 mg/day. In the second trial focusing on PDAC, IPI-926 was combined with gemcitabine and used as first-line treatment for patients with metastatic pancreatic cancer.68 Of the 15 patients enrolled in this trial, 9 underwent postbaseline imaging, and 3 of these showed radiographic partial responses. The most common adverse events included fatigue, nausea (both, overall 40%; grade 3, 0%) and aspartate aminotransferase/alanine aminotransferase elevation (grade 3, 7%). There were no adverse events of grade 4. In addition, there was no adverse interaction between IPI-926 and gemcitabine. On the basis of these encouraging results, the authors have moved this compound into a phase 2 trial.
Mechanisms of resistance also are 4 being identified in these early trials. A patient with metastatic medulloblastoma who responded initially to Hh pathway inhibition subsequently showed a complete loss of therapeutic effect after 3 months of therapy.69 Further investigation revealed a mutation of Smo involving an amino acid substitution of aspartic acid (Asp to His on codon 473) that hampered the ability of GDC-0449 to bind to Smo.70 Further mechanisms of resistance may be those relating to the noncanonical activation of the Hh pathway downstream of Smo. The development of second-generation Smo inhibitors and anti-Gli molecules, such as GANT58, GANT61, and further understanding of key signaling pathways that interact with the Hh pathway, may allow us to anticipate and circumvent these mechanisms of resistance. The overall perception from clinical trials involving GDC-0449 and IPI-926 is that these agents can be used safely in patients. These drugs are well tolerated, have minimal side effects, and can be used without adverse interaction along with other commonly used cytotoxic therapies. Further subset analysis and phase 2 trials are under way to assess the clinical efficacy of Smo inhibitors. The subset of PDAC patients who would derive the maximum benefit from this therapy, and the complete side effect profile, remain unknown.
BACK TO THE BENCH
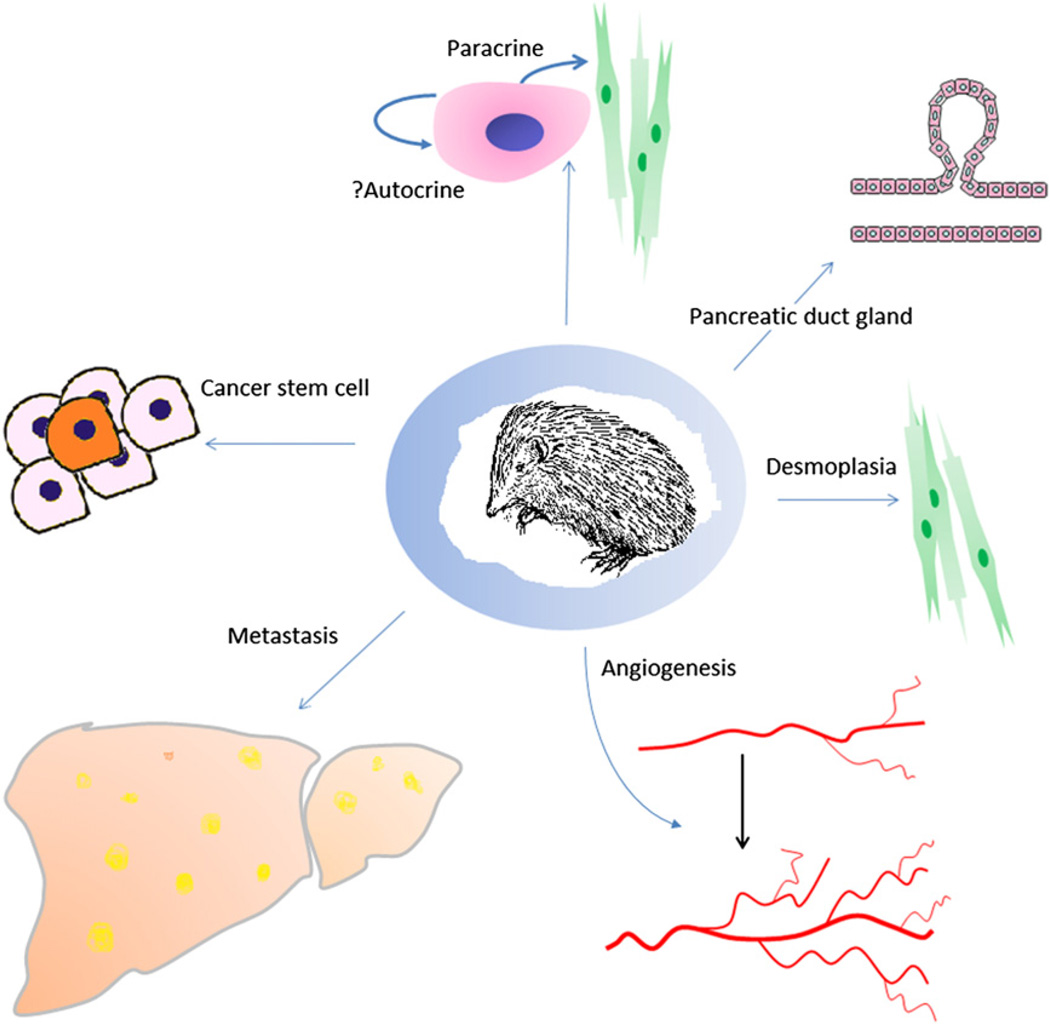
Since its initial discovery as a segment polarity gene in Drosophila and its mammalian orthologs, there has been an explosion of information about the Hh pathway and the wide variety of functions regulated by the Hh pathway during pancreatic embryogenesis and adult pancreatic homeostasis, and more importantly, how aberrations in this pathway contribute to the formation and maintenance of pancreatic cancer. During the past decade, it has become evident that the Hh pathway contributes to the maintenance and progression of pancreatic cancer by maintaining the CSC and the tumor microenvironment, altering angiogenesis, and regulating metastatic spread (Fig 4). Given the vast amount of preclinical studies, academia and industry have been working together to develop novel compounds against this pathway. To date, seven compounds are in clinical trials throughout the United States. Although encouraging interval data have been reported on other solid tumors (basal cell cancers) known to have mutations in the Hh pathway, nothing is yet known about the efficacy of these drugs against pancreatic cancer.
Fig 4.
The functions of Sonic Hedgehog in cancer.
Although Hh research has leaped from the bench to the bedside, many important questions remain unanswered. For example, the molecular interaction between Ptch and Smo, as well as the regulation of activating and repressive forms of Gli, remain to be determined. It is also unclear how Hh regulates the tumor microenvironment and tumor angiogenesis. Most recently, the identification of Shh in an epithelial compartment (PDG) that has progenitor-like function and undergoes a mucinous metaplasia resembling both pancreatic precursor lesions (PanIN and IPMN) suggests that this compartment may be a common compartment of origin for pancreatic cancer. This observation, added to the knowledge that CSC express high levels of Shh, may suggest that cancer may originate from Shh-positive stem cells within the PDG. But much work needs to be done before such speculation is proven to be true, so it’s back to the bench.
SPECIAL ACKNOWLEDGMENT TO DR WARSHAW BY DR SARAH THAYER
History of the pancreatic biology laboratory---the Warshaw laboratory
There was a fair amount of serendipity involved in the establishment of the Pancreatic Biology Laboratory (PBL) by Dr Andrew Warshaw at the Massachusetts General Hospital, beginning with his decision as a surgical resident in 1965 to spend 2 years as a fellow at the National Institutes of Health. He saw this as an alternative to being drafted as a general medical officer just prior to major U.S. involvement in Vietnam, and spending 2 years on an army base. Dr Warshaw joined the National Institute of Arthritis and Metabolic Diseases to work in the Gastroenterology Section and was the first surgeon to join what was essentially a medical hepatology unit. Over time, however, the interests of this group came to include malabsorption and pancreatic insufficiency. By the time he returned to clinical service as a resident in 1967, he was seeing a wide range of pancreatic pathology and publishing his findings.
When Dr Warshaw finished his chief residency and founded the PBL in 1972, rigorous research by American surgeons into pancreatic physiology and biology was still in its relative infancy. The Pancreas Club had just been founded in 1966 and was still an informal gathering of physicians and researchers who discussed primarily acute pancreatitis. The organization even disbanded for a year in 1974, before Dr Warshaw and several other members pushed to restart it. Likewise, the precursors to the American Pancreatic Association began having meetings in 1969, but these too were quite informal, with written minutes beginning only in 1971. It was not until 1979 that the APA was formally incorporated.
In this environment, Dr Warshaw is fond of saying that he chose his ultimate career path because the pancreas was “the last organ that had not been taken.” He had already had 3 articles accepted by the New England Journal of Medicine and despite just finishing his residency, he was already gaining a reputation as an authority on the pancreas. Still, he was given only a single bench and a technician to start out, with no funds or protected time for research.
Research and education
The initial efforts of the PBL were like much pancreatic research done by surgeons at the time---focused primarily on pancreatitis. Dr Warshaw built on his clinical experience with pancreatitis and developed a research interest in amylase isoenzymes, publishing several papers on the subject in the 1970s. Dozens of his articles in this decade offer insight into serum markers, anatomic studies, and clinical observations. For many years, the focus of the PBL was strictly on pancreatic physiology and markers of pancreatitis, but this focus gradually shifted over time to the study of pancreatic tumor biology.
Dr Warshaw was assisted in this transition by the implementation of a fellowship program, beginning in 1986. Given his role as an attending surgeon at Harvard Medical School’s largest and oldest teaching hospital, Dr Warshaw felt a commitment to teaching and training the next generations of surgeons and researchers; indeed, he has trained 29 surgeon-scientists from 4 different continents. Of these fellows, 5 have gone on to become chairs of their respective departments, and many others have developed highly distinguished academic careers.
As one of Dr Warshaw’s surgical protégés, I am honored to have been a small part of his great achievements and hope to continue his legacy of scientific contribution and teaching. In 2001, when Dr Warshaw asked me to become a coinvestigator in the PBL, I began to work on the developmental gene Shh, known to inhibit pancreatic development. Ultimately, this work led to investigation into the Hedgehog pathway as a possible mechanism for understanding pancreatic tumorigenesis and resulted in a seminal report in Nature in 2003. Now, as director of the PBL, I have the pleasure of working with and overseeing great new surgeon–scientists of the future. Many of these talented residents and scientists from this laboratory have contributed greatly to our present-day insights into the Hedgehog pathway. Thus this review was written as a collaborative group effort.
I am indebted to Dr Warshaw’s mentoring, encouragement and promotion, as well as the opportunity to train and work with the gifted fellows of the PBL. ---Sarah Thayer
REFERENCES
- 1.Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins D. Hedgehog signalling: emerging evidence for non-canonical pathways. Cell Signal. 2009;21:1023–1034. doi: 10.1016/j.cellsig.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Zapico ME. Primers on molecular pathways GLI: more than just Hedgehog? Pancreatology. 2008;8:227–229. doi: 10.1159/000134271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayed H, Kleeff J, Osman T, Keleg S, Büchler MW, Friess H. Hedgehog signaling in the normal and diseased pancreas. Pancreas. 2006;32:119–129. doi: 10.1097/01.mpa.0000202937.55460.0c. [DOI] [PubMed] [Google Scholar]
- 6.Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–438. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- 7.Hebrok M. Hedgehog signaling in pancreas development. Mech Dev. 2003;120:45–57. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 8.Aubin J, Déry U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal-epithelial signaling. Development. 2002;129:4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- 9.Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 10.King PJ, Guasti L, Laufer E. Hedgehog signalling in endocrine development and disease. J Endocrinol. 2008;198:439–450. doi: 10.1677/JOE-08-0161. [DOI] [PubMed] [Google Scholar]
- 11.Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes. 2010;59:1211–1221. doi: 10.2337/db09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, Kawahira H, Hebrok M. Hedgehog signaling in pancreas development and disease. Cell Mol Life Sci. 2006;63:642–652. doi: 10.1007/s00018-005-5357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- 15.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138:1166–1177. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner-Weir S, Li W-C, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. β-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 18.Jensen JN, Cameron E, Garay MV, Starkey TW, Gianani R, Jensen J. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2001;411:349–354. doi: 10.1038/35077219. [DOI] [PubMed] [Google Scholar]
- 21.Kayed H, Kleeff J, Keleg S, Buchler MW, Friess H. Distribution of Indian hedgehog and its receptors patched and smoothened in human chronic pancreatitis. J Endocrinol. 2003;178:467–478. doi: 10.1677/joe.0.1780467. [DOI] [PubMed] [Google Scholar]
- 22.Thayer SP, Pasca di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berman DM, Karhadkar SS, Maitra A, Montes DO, Gerstenblith MR, Briggs K, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 24.Ohuchida K, Mizumoto K, Fujita H, Yamaguchi H, Konomi H, Nagai E, et al. Sonic hedgehog is an early developmental marker of intraductal papillary mucinous neoplasms: clinical implications of mRNA levels in pancreatic juice. J Pathol. 2006;210:42–48. doi: 10.1002/path.2019. [DOI] [PubMed] [Google Scholar]
- 25.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim GE, Bae HI, Park HU, Kuan SF, Crawley SC, Ho JJ, et al. Aberrant expression of MUC5AC and MUC6 gastric mucins and sialyl Tn antigen in intraepithelial neoplasms of the pancreas. Gastroenterology. 2002;123:1052–1060. doi: 10.1053/gast.2002.36018. [DOI] [PubMed] [Google Scholar]
- 27.Sessa F, Bonato M, Frigerio B, Capella C, Solcia E, Prat M, et al. Ductal cancers of the pancreas frequently express markers of gastrointestinal epithelial cells. Gastroenterology. 1990;98:1655–1665. doi: 10.1016/0016-5085(90)91104-e. [DOI] [PubMed] [Google Scholar]
- 28.Prasad NB, Biankin AV, Fukushima N, Maitra A, Dhara S, Elkahloun AG, et al. Gene expression profiles in pancreatic intraepithelial neoplasia reflect the effects of Hedgehog signaling on pancreatic ductal epithelial cells. Cancer Res. 2005;65:1619–1626. doi: 10.1158/0008-5472.CAN-04-1413. [DOI] [PubMed] [Google Scholar]
- 29.Nakashima H, Nakamura M, Yamaguchi H, Yamanaka N, Akiyoshi T, Koga K, et al. Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res. 2006;66:7041–7049. doi: 10.1158/0008-5472.CAN-05-4588. [DOI] [PubMed] [Google Scholar]
- 30.Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, et al. In vivo lineage tracing defines the role of acinar-toductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strobel O, Dor Y, Stirman A, Trainor A, Fernández-Del Castillo C, Warshaw AL, et al. Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton JP, Mongeau ME, Klimstra DS, Morris JP, Lee YC, Kawaguchi Y, et al. Sonic hedgehog acts at multiple stages during pancreatic tumorigenesis. Proc Natl Acad Sci USA. 2007;104:5103–5108. doi: 10.1073/pnas.0701158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 34.Lauth M, Bergstrom A, Shimokawa T, Tostar U, Jin Q, Fendrich V, et al. DYRK1B-dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol. 2010;17:718–725. doi: 10.1038/nsmb.1833. [DOI] [PubMed] [Google Scholar]
- 35.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernández-Zapico ME, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 38.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 39.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 41.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Feldmann G, Fendrich V, McGovern K, Bedja D, Bisht S, Alvarez H, et al. An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer. Mol Cancer Ther. 2008;7:2725–2735. doi: 10.1158/1535-7163.MCT-08-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;65:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bailey JM, Mohr AM, Hollingsworth MA. Sonic hedgehog paracrine signaling regulates metastasis and lymphangiogenesis in pancreatic cancer. Oncogene. 2009;28:3513–3525. doi: 10.1038/onc.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldmann G, Habbe N, Dhara S, Bisht S, Alvarez H, Fendrich V, et al. Hedgehog inhibition prolongs survival in a genetically engineered mouse model of pancreatic cancer. Gut. 2008;57:1420–1430. doi: 10.1136/gut.2007.148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 48.Xu XF, Guo CY, Liu J, Yang WJ, Xia YJ, Xu L, et al. Gli1 maintains cell survival by up-regulating IGFBP6 and Bcl-2 through promoter regions in parallel manner in pancreatic cancer cells. J Carcinog. 2009;8:13. doi: 10.4103/1477-3163.55429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, et al. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 52.Asai J, Takenaka H, Kusano KF, Ii M, Luedemann C, Curry C, et al. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413–2424. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 53.Surace EM, Balaggan KS, Tessitore A, Mussolino C, Cotugno G, Bonetti C, et al. Inhibition of ocular neovascularization by hedgehog blockade. Mol Ther. 2006;13:573–579. doi: 10.1016/j.ymthe.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 54.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–579. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 55.Renault MA, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, et al. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol. 2010;49:490–498. doi: 10.1016/j.yjmcc.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura K, Sasajima J, Mizukami Y, Sugiyama Y, Yamazaki M, Fujii R, et al. Hedgehog promotes neovascularization in pancreatic cancers by regulating Ang-1 and IGF-1 expression in bone-marrow derived pro-angiogenic cells. PLoS One. 2010;5:e8824. doi: 10.1371/journal.pone.0008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W, Tang T, Eastham-Anderson J, Dunlap D, Alicke B, Nannini N, et al. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci USA. 2011;108:9589–9594. doi: 10.1073/pnas.1017945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 59.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–1316. [PubMed] [Google Scholar]
- 60.Binns W, James LF, Shupe JL, Everett G. A congenital cyclopian-type malformation in lambs induced by maternal ingestion of a range plant Veratrum californicum. Am J Vet Res. 1963;24:1164–1175. [PubMed] [Google Scholar]
- 61.Lipinski RJ, Hutson PR, Hannam PW, Nydza RJ, Washington IM, Moore RW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the hedgehog signaling antagonist cyclopamine in the mouse. Toxicol Scii. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mas C, Ruiz i Altaba A. Small molecule modulation of HH-GLI signaling: current leads, trials and tribulations. Biochem Pharmacol. 2010;80:712–723. doi: 10.1016/j.bcp.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 64.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palmer SR, Erlichman C, Fernández-Zapico M, Qi Y, Almada L, McCleary-Wheeler A, et al. Phase I trial erlotinib, gemcitabine, and the hedgehog inhibitor, GDC-0449 (abstract) J Clin Oncol. 2011 May 20;29(15) Suppl:3092. [Google Scholar]
- 66.Cheng H, Merika E, Syrigos KN, Saif MW. Novel agents for the treatment of pancreatic adenocarcinoma. JOP; Highlights from the “2011 ASCO Annual Meeting”; June 3–7, 2011; Chicago, IL, USA. 2011. pp. 334–338. (review). [PubMed] [Google Scholar]
- 67.Rudin CM, Jimen A, Miller WH, Eigl BJ, Gettinger SN, Chang ALS, et al. A phase I study of IPI-926, a novel hedgehog pathway inhibitor, in patients (pts) with advanced or metastatic solid tumors. J Clin Oncol. 2011;29(15 Suppl):3014. [Google Scholar]
- 68.Stephenson J, Richards DA, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, et al. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2011;29(15 Suppl):4114. [Google Scholar]
- 69.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yauch RL, Dijkgraaf GJP, Alicke B, Januario T, Ahn CP, Holcomb T, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]