Summary
Small conductance Ca2+-activated K+ (SKCa) channels are crucial in regulating vascular tone and blood pressure. The present study tested the hypothesis that SKCa channels play an important role in uterine vascular adaptation in pregnancy, which is inhibited by chronic hypoxia during gestation. Uterine arteries were isolated from nonpregnant and near-term pregnant sheep maintained at sea level (~300 m) or exposed to high-altitude (3801 m) hypoxia for 110 days. Immunohistochemistry revealed the presence of SKCa channels type 2 (SK2) and type 3 (SK3) in both smooth muscle and endothelium of uterine arteries. The expression of SK2 and SK3 channels was significantly increased during pregnancy, which was inhibited by chronic hypoxia. In normoxic animals, both SKCa channel opener NS309 and a large-conductance (BKCa) channel opener NS1619 relaxed norepinephrine-contracted uterine arteries in pregnant but not nonpregnant sheep. These relaxations were inhibited by selective SKCa and BKCa channel blockers, respectively. NS309-induced relaxation was largely endothelium-independent. In high altitude hypoxic animals, neither NS1691 nor NS309 produced significant relaxation of uterine arteries in either nonpregnant or pregnant sheep. Similarly, the role of SKCa channels in regulating myogenic reactivity of uterine arteries in pregnant animals was abrogated by chronic hypoxia. Accordingly, the enhanced SKCa channel activity in uterine arterial myocytes of pregnant animals was ablated by chronic hypoxia. The findings suggest a novel mechanism of SKCa channels in regulating myogenic adaptation of uterine arteries in pregnancy, and in the maladaptation of uteroplacental circulation caused by chronic hypoxia during gestation.
Keywords: Hypoxia, uterine artery, pregnancy, SKCa channel, myogenic tone, relaxation
Introduction
Vascular tone constitutes the major determinant of the resistance of blood vessels, which regulates blood pressure and the distribution of blood flow among and within tissues and organs. Ca2+-activated K+ (KCa) channels contribute significantly to setting the membrane potential, and play a critical role in regulating excitability of vascular smooth muscle cells (VSMCs).1, 2 Based on the conductance, KCa channels are divided into large-conductance (BKCa), intermediate-conductance (IKCa), and small-conductance (SKCa) channels.3 KCa channels have distinct distributions in the vasculature. SKCa channels are believed to be expressed predominantly in endothelial cells,2, 4 and hyperpolarization produced by the activation of SKCa in endothelial cells may be transmitted to VSMCs via the myoendothelial gap junction.5, 6
During pregnancy, uterine blood flow increases substantially to optimize the supply of oxygen and nutrients to the developing fetus via the placenta. Chiefly, this is achieved by adaptive changes such as remodeling of the uterine vasculature,7 reduced pressure-dependent myogenic reactivity,8–10 blunted vasoconstrictor response,11–13 and enhanced vasodilator response and vasodilator production.14–16 Chronic hypoxia during gestation has profound adverse effects on the normal adaptation of uteroplacental circulation to pregnancy,17–21 leading to a 2 to 4-fold increase in the incidence of preeclampsia and fetal intrauterine growth restriction.20–24
Previous studies have demonstrated that BKCa channels participate in the regulation of vascular tone and uterine blood flow during pregnancy.25–27 Upregulated expression of the BKCa channel β1 subunit and enhanced BKCa channel activity contribute to the attenuated myogenic tone of uterine arteries during pregnancy.10 Chronic hypoxia during gestation inhibited pregnancy-induced upregulation of BKCa channel function in uterine arteries by selectively targeting the β1 subunit.19 Although SKCa channels are predominantly expressed in endothelial cells,2 apamin-sensitive K+ currents and positive staining of SKCa channels have been detected in VSMCs of various vascular beds.28–31 Functional roles of SKCa channels in VSMCs remain elusive. SKCa channels are also expressed in the myometrium and are regulated by estrogen during pregnancy.32 Of interest, SKCa channels are subject to regulation by oxygen.33, 34 However, the role of SKCa channels in the regulation of uterine circulation under physiological and pathophysiological conditions such as pregnancy and chronic hypoxia is unclear. In the present study, we tested hypotheses that SKCa channels play an important role in regulating the contractility of uterine arteries during pregnancy; and that chronic hypoxia during gestation impairs this regulation. To test these hypotheses, we first determined whether SKCa channels were expressed in the uterine vasculature and how pregnancy and chronic hypoxia regulated their expression. We then determined SKCa-mediated relaxations of uterine arteries and their regulation by pregnancy and chronic hypoxia. Furthermore, we measured the SKCa channel activity in uterine vascular smooth muscle cells using patch-clamp analysis to see whether pregnancy and chronic hypoxia altered their activities. In addition, we determined the role of SKCa channels in pressure-dependent myogenic tone of uterine arteries and its regulation by pregnancy and chronic hypoxia.
Materials and Methods
An expanded Methods section is available in the online data supplement at http://hyper.ahajournals.org.
Tissue preparation and treatment
Uterine arteries were harvested from nonpregnant and near-term (142–145 days of gestation, the term is about 150 days) pregnant sheep maintained at sea level (~300 m) or exposed to high-altitude (3801 m) hypoxia for 110 days.18 All of the procedures and protocols were approved by the Institutional Animal Care and Use Committee guidelines.
Western immunoblotting
Protein abundance of SKCa type 2 (SK2) and type 3 (SK3) channels was measured in freshly isolated uterine arteries, as described previously.10
Immunohistochemistry
Uterine arteries were fixed in 10% neutral buffered formalin and embedded in paraffin. Immunohistochemical detection of SK2, SK3, and eNOS was performed using the Anti-Ig HRP Detection Kit (BD Biosciences PharMingen, San Diego, CA) as described previously.35, 36
Contraction studies
Fourth-generation branches of the main uterine arteries from nonpregnant and pregnant sheep were separated from the surrounding tissue, and cut into 2-mm ring segments. Isometric tension was measured in the Krebs solution in a tissue bath at 37°C, as described previously.37, 38
Measurement of SKCa channel current
Arterial smooth muscle cells were dissociated enzymatically from resistance-sized uterine arteries, and whole-cell K+ currents were recorded using an EPC 10 patch-clamp amplifier with Patchmaster software (HEKA, Lambrecht/Pfalz, Germany) at room temperature, as previously described.10
Measurement of myogenic tone
Pressure-dependent myogenic tone of resistance-sized uterine arteries was measured as described previously.10, 18, 39
Data analysis
Data are expressed as means ± SEM obtained from the number of experimental animals given. Differences were evaluated for statistical significance (P < 0.05) by ANOVA or t test, where appropriate.
Results
Effect of pregnancy and chronic hypoxia on SKCa channel expression
Protein abundance of both SK2 and SK3 channels was significantly greater in uterine arteries of pregnant sheep than that in nonpregnant animals (Figure 1A). Chronic hypoxia during gestation significantly decreased the expression of SK2 and SK3 channels in uterine arteries of pregnant animals (Figure 1B).
Figure 1. Effect of pregnancy and chronic hypoxia on SKCa channel expression.
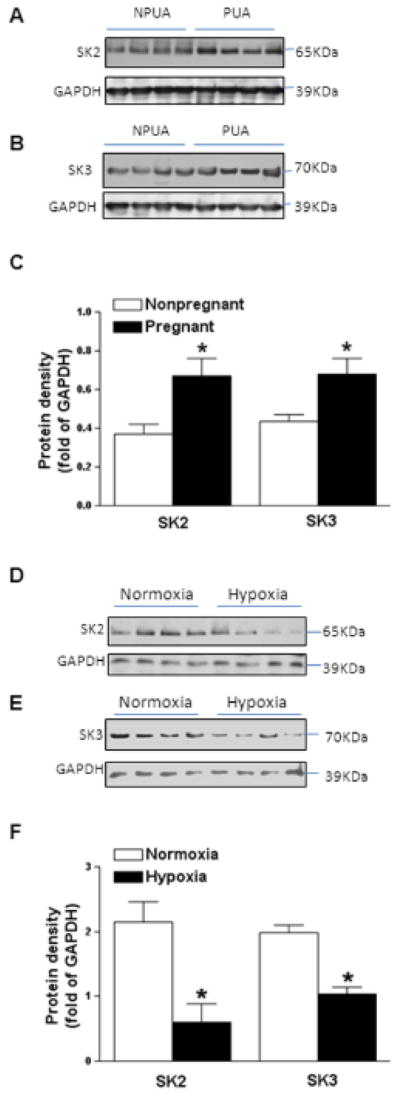
Protein abundance of SK2 and SK3 channels were determined by Western blot analyses in uterine arteries of normoxic nonpregnant (NPUA) and pregnant (PUA) animals (Panels A, B, C), and uterine arteries of normoxic and high altitude hypoxic pregnant animals (Panels D, E, F). Data are means ± SEM of tissues from 4–6 animals of each group, * P < 0.05.
Effect of pregnancy and chronic hypoxia on SKCa channel-mediated relaxation
The effect of SKCa/IKCa channels on contractility of uterine arteries was examined by exposing norepinephrine-contracted arteries to NS309 (Figure 2). In normoxic animals, NS309 had no effect on uterine artery relaxation in nonpregnant sheep, but produced concentration-dependent relaxations of uterine arteries in pregnant animals, with a maximal relaxation of 64.3 ± 7.5% (Figure 2A). As shown in Figure 2B, blocking of BKCa channels with IBTX or blocking of BKCa/IKCa channels with CTX had no significant effect on NS309-induced relaxation, but the SKCa channel blocker apamin significantly inhibited NS309-mediated relaxation, suggesting that NS 309-induced relaxation was conferred chiefly by SKCa channel activation. Long-term, high altitude hypoxia did not change NS309’s effect in nonpregnant sheep, but abrogated NS309-mediated relaxations of uterine arteries in pregnant animals (Figure 2C). Similarly, the BKCa channel activator NS1619-induced relaxations were significantly increased in uterine arteries of pregnant sheep, which was inhibited by chronic hypoxia (Online Figure S1).
Figure 2. Concentration-response curves of NS309-induced relaxation.
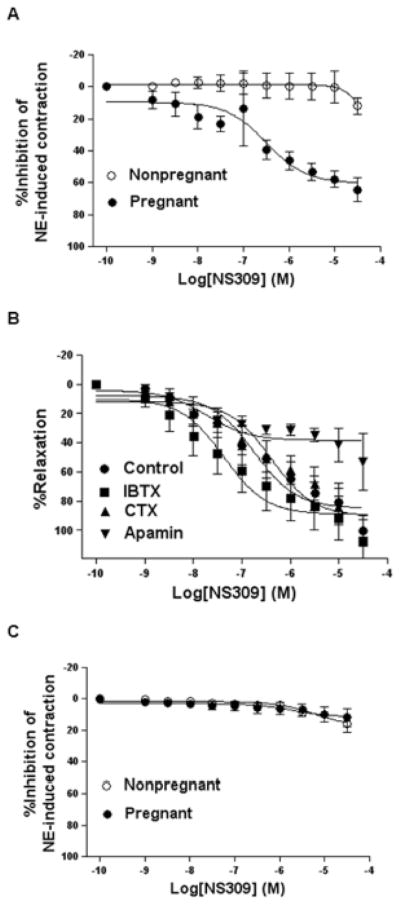
Uterine arteries were contracted with norepinephrine (NE, 3 μmol/L) and followed by additions of NS309. A. Normoxic animals. B. Normoxic pregnant animals in the absence (control) or presence of iberitoxin (IBTX, 100 nmol/L), charybdotoxin (CTX, 70 nmol/L), or apamin (500 nmol/L). C. High altitude hypoxic animals. Data are means ± SEM of tissues from 5–6 animals in each group.
Involvement of smooth muscle cells in SKCa channel-mediated relaxations
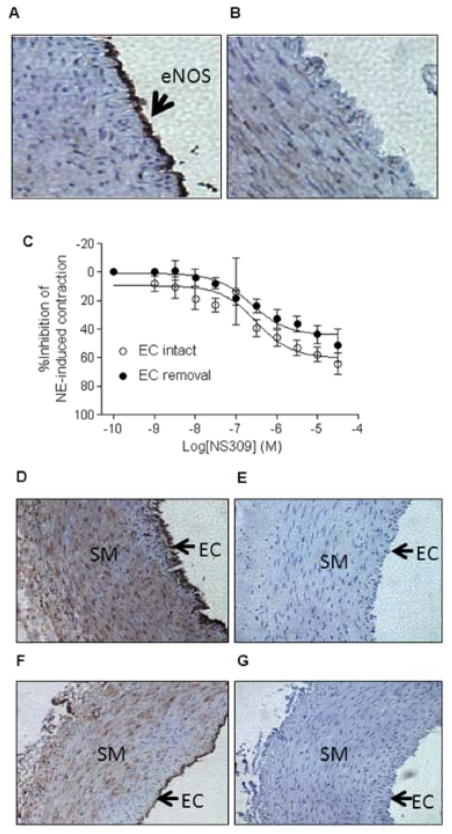
Given that SKCa channels are expressed in endothelial cells, we determined NS309-induced relaxation in endothelium-intact and -denuded uterine arteries. The validity of endothelium removal was confirmed by the absence of eNOS in immunohistochemical staining (Figure 3A). As shown in Figure 3B, endothelial removal did not significantly alter NS309-induced relaxation of uterine arteries (pD2: endothelium-intact: 6.5 ± 0.3; endothelium-denuded: 6.6 ± 0.2; Emax: endothelium-intact: 64.3 ± 7.5%; endothelium-denuded: 51.3 ± 11.4%, P > 0.05). This suggests that NS309-induced relaxation of uterine arteries was mediated mainly by SKCa channels in vascular smooth muscle cells. Immunohistochemical staining revealed that both SK2 and SK3 channels were expressed in endothelial as well as smooth muscle cells in the uterine artery (Figure 3C).
Figure 3. Effect of endothelium on NS309-induced relaxation.
Immunoreactivity of eNOS was present in endothelium-intact arteries (Panel A) but absent in endothelium-denuded arteries (Panel B), demonstrating the effectiveness of endothelium removal. C. NS309-induced relaxation of norepinephrine (NE, 3 μmol/L)-contracted pregnant uterine arteries with (EC intact) or without (EC removal) endothelium. Data are means ± SEM of tissues from 4–6 animals in each group. Immunoreactivity of SK2 (Panel D) and SK3 (Panel F) channels in endothelium (EC) and vascular smooth muscle (SM) of pregnant uterine arteries. Panels E and G show negative controls of SK2 and SK3 staining.
Chronic hypoxia inhibited SKCa channel activity in uterine arteries
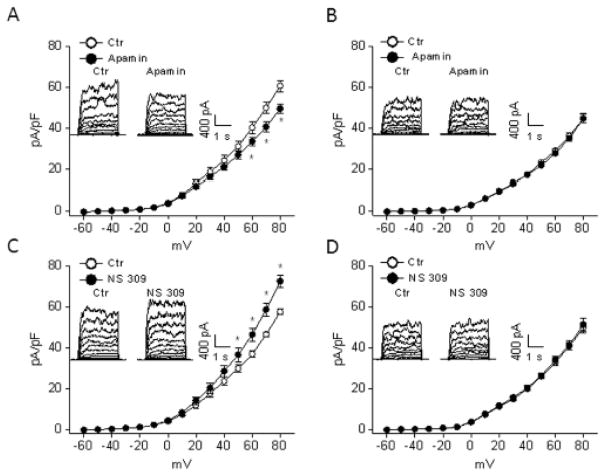
To determine the effect of pregnancy and chronic hypoxia on SKCa channel activity in uterine arterial smooth muscle cells, whole-cell K+ currents were recorded in the absence or presence of apamin or NS309 in myocytes freshly isolated from uterine arteries of normoxic control and hypoxic animals. As shown in normoxic pregnant sheep, apamin significantly reduced whole-cell K+ currents (from 60.7 ± 2.7 pA/pF to 49.6 ± 2.3 pA/pF at +80 mV, P < 0.05) in uterine arterial myocytes (Figure 4A). In contrast, in myocytes of hypoxic animals, apamin was without effect on whole-cell K+ currents (Figure 4B). Similarly, NS309 significantly enhanced whole-cell K+ currents in myocytes of normoxic pregnant animals (from 57.5 ± 1.3 pA/pF to 72.4 ± 2.9 pA/pF at +80 mV, P < 0.05) (Figure 4C) but not in hypoxic animals (Figure 4D). Neither apamin nor NS 309 altered whole-cell K+ currents in uterine arterial myocytes of nonpregnant sheep in either normoxic or hypoxic animals (data not shown).
Figure 4. Effect of chronic hypoxia on SKCa channel currents in uterine arteries of pregnant sheep.
Arterial myocytes were freshly isolated from uterine arteries of pregnant sheep in normoxic and high altitude hypoxic animals. Whole-cell K+ currents were recorded in the absence or presence of apamin (1 μmol/L) or NS309 (1 μmol/L). A, C. Normoxic animals. B, D. High altitude hypoxic animals. Data are means ± SEM of cells from 5–6 animals of each group. * P < 0.05, versus control (Ctr).
Effect of pregnancy and chronic hypoxia on uterine artery SKCa channel-mediated myogenic tone
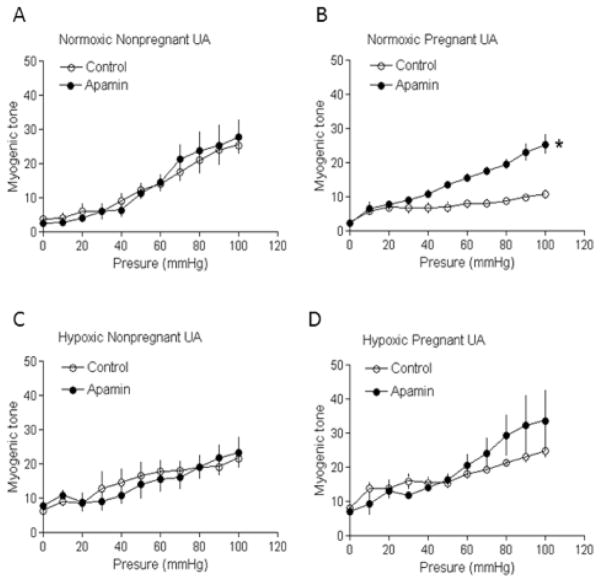
As reported previously, pressure-dependent myogenic tone of uterine arteries was significantly reduced in pregnant sheep in normoxic control animals (Figure 5A and 5B). Blockade of SKCa channels with apamin had no significant effect on pressure-dependent myogenic reactivity in uterine arteries of nonpregnant animals (Figure 5A), but resulted in a significant increase in myogenic tone in uterine arteries of pregnant animals (Figure 5B). In the presence of apamin, there was no significant difference in myogenic tone of uterine arteries between nonpregnant and pregnant animals (Figure 5A and 5B). In hypoxic animals, apamin had no significant effect on pressure-dependent myogenic tone of uterine arteries in either nonpregnant or pregnant animals (Figure 5C and 5D).
Figure 5. Effect of pregnancy and chronic hypoxia on SKCa channel-mediated myogenic tone.
Pressure-dependent myogenic tone was determined in the absence or presence of apamin (500 nmol/L). Data are means ± SEM of tissues from 5–6 animals of each group. * P < 0.05, versus control.
Discussion
In the present study, we have demonstrated for the first time the expression and function of SKCa channels in uterine arteries. The capacity of activation of SKCa channels to relax uterine arteries was markedly increased during pregnancy. Additionally, the SKCa channel blocker apamin significantly increased pressure-dependent myogenic tone in uterine arteries of pregnant sheep and blunted the difference in the myogenic response of uterine arteries between nonpregnant and pregnant animals. These pregnancy-induced changes were accompanied by increased expression of both SK2 and SK3 channels. Consistently, we detected increased activities of SKCa channels in uterine artery smooth muscle cells of pregnant sheep. The concurrence of those findings suggests that pregnancy-induced upregulation of expression and activity of SKCa channels contributes to the reduced myogenic reactivity and vascular contractility of uterine arteries during gestation. Decreased myogenic tone and increased vasorelaxing responses of uterine arteries have been implicated in the increase in uterine blood flow during pregnancy.8–10, 14–16 Hence, our observations provide a novel mechanism of upregulation and heightened activity of SKCa channels in the adaptation of uteroplacental circulation during pregnancy. Furthermore, the up-regulation of SK3 channels appears to have a role in remodeling of the uterine vasculature. A recent study demonstrated that uterine arteries from nonpregnant transgenic SK3T/T mice that overexpress SK3 channels had larger basal diameters and blunted vasoconstrictor response compared to those from wild-type animals,40 although the expression of SK3 channels in uterine arteries was not determined.
At present, the mechanisms responsible for upregulating expression and function of SKCa channels in uterine arteries during pregnancy are not clear. It is conceivable that sex steroid hormones may contribute to this regulation. Activation of estrogen receptors may alter gene transcription, which has a profound impact on cardiovascular function.41 Pregnancy up-regulates the expression of estrogen receptor α and β in uterine arteries.18,42 Moreover, we recently demonstrated the 17β-estradiol-mediated increase in expression and heightened activity of BKCa channels in uterine arteries of pregnant sheep.10 Similarly, SK3 channel expression also was regulated by 17β-estradiol in recombinant expression system,43 hypothalamus,44 and myometrium.32
Although SKCa channels were expressed in uterine arteries of nonpregnant animals, the channel activity was not detected with an electrophysiological approach. Moreover, it appeared that these channels did not participate in regulating myogenic tone and contractility of uterine arteries in nonpregnant animals. One possibility is that in nonpregnant animals SKCa channels are not expressed in the cell membrane of uterine arteries smooth muscle, but rather are retained inside cells. The incapability of those channels to insert into membrane would prevent them from being activated. It is also possible that in nonpregnant animals despite being present in the myocyte membrane, the efficacy of these channels may be too low to be functional. Similar findings have been reported for both IKCa and BKCa channels. Although IKCa channels were stained at both the plasma membrane and within the cytoplasm,30 the selective IKCa channel blocker TRAM-34 was unable to alter vascular tone of cerebral arteries.45 Similarly, BKCa channels in uterine arteries did not participate in the regulation of relaxation (the present study), myogenic tone, vascular resistance and blood flow in nonpregnant animals.10, 46
Initially, SKCa channels were detected in endothelial cells, but not in VSMCs of SK3T/T mice.47 The regulatory role of SKCa channels on vascular function is thought to be mediated exclusively by the endothelium. This notion was supported by the finding that a genetic deficit of SK3 and IK1 channels caused hypertension by abolishing endothelium-derived hyperpolarizing factor-mediated vasodilation.48 In the present study, immunostaining demonstrated the expression of SK2 and SK3 channels in both vascular smooth muscle and endothelial cells in uterine arteries. The functional presence of SKCa channels in uterine arterial smooth muscle cells was confirmed with electrophysiological technique; and a selective SKCa channel blocker apamin decreased whole-cell K+ currents by ~20%. This is in agreement with the previous findings in myocytes of rabbit aorta29 and rat myometrium36 that apamin reduced whole-cell K+ currents by about 20%. Furthermore, previous studies also have shown the presence of SKCa channels in other visceral and vascular smooth muscle cells by immunohistochemistry,31, 49, 50 although the functional roles of these channels are not known. In uterine arteries, NS309-induced relaxation was largely endothelium-independent, suggesting that SKCa channels in vascular smooth muscle mediated mainly NS309-induced vasorelaxation. To our knowledge, the present study is the first to demonstrate that SKCa channels in vascular smooth muscle significantly contribute to the regulation of vascular contractility and tone in this vascular bed. Lines of evidence have also implicated SKCa channels in regulating excitability and contraction of smooth muscle cells from the uterus and urinary bladder, which are highly responsive to sex steroids and in particular estrogen.51–53 Our findings thus provide a novel mechanism of SKCa channels in regulating vascular tone and cardiovascular function.
Previously, chronic hypoxia has been found to abrogate the capacity of BKCa in regulating myogenic reactivity of uterine arteries in pregnant sheep.19 The present findings of diminishment of vasodilator response to NS309 and failure of apamin to alter myogenic tone of uterine arteries in pregnant animals exposed to long-term high altitude hypoxia suggest that chronic hypoxia resulted in a loss of the regulatory role of SKCa channels in vascular smooth muscle excitability and contractility. Hence, the nullification of the regulatory role of KCa channels may attribute to chronic hypoxia-induced reduction in uterine blood flow in pregnancy.20, 21 Our data also suggest that the loss of regulatory role of SKCa channels in uterine arteries of pregnant animals resulted chiefly from reduced channel activities due to suppressed expression of these channels. Similar findings were obtained for BKCa channels in uterine arteries of pregnant sheep 19 and IKCa channels in pulmonary arteries from animals exposed to chronic hypoxia.34 The effect of chronic hypoxia seems to be specific for KCa channels, as voltage-gated K+ (KV) channels were largely unaffected.19 Taken together, experimental evidence suggests that targeted suppression of KCa channels is a major mechanism to alter uterine vascular function by chronic hypoxia and the uterine arteries from chronic hypoxic animals are losing their adaptation to pregnancy. This may account for the increased incidence of preeclampsia and fetal intrauterine growth restriction associated with chronic hypoxia exposure during gestation. Estrogens have been shown to regulate expression of BKCa10, 54 and SKCa32, 43 channels. Ablation of pregnancy-induced upregulation of SKCa and BKCa channels in uterine arteries by chronic hypoxia during gestation likely occurred at the genomic level. Expression of estrogen receptor α in uterine arteries during gestation, but not plasma estrogen levels, was significantly depressed by chronic hypoxia18 due to heightened promoter methylation.55 It is possible that chronic hypoxia-mediated suppression of estrogen receptor α expression led to abrogation of upregulation of KCa channels in uterine arteries during pregnancy. However, Jobe et al has shown there are numerous types of estrogens and estrogen metabolites that are decreased in preeclampsia.56 Therefore, the regulation of KCa channels by estrogens in VSMCs likely has a significant role in physiological and pathophysiological conditions.
Perspectives
The striking increase of uterine blood flow during pregnancy is essential both for optimal growth and survival of the fetus and for cardiovascular wellbeing of the mother. Maladaptation of the uteroplacental circulation during pregnancy is associated with a high incidence of clinical complications including preeclampsia and fetal intrauterine growth restriction. Thus, a comprehensive understanding of regulatory mechanisms of uterine vascular adaptation in pregnancy has long been sought, but has not been achieved. The present study demonstrates a novel mechanism of SKCa channels in uterine arterial smooth muscle, and thus has a major impact in advancing our knowledge in the molecular mechanisms of uterine vascular adaptation to pregnancy. This will help to improve our understanding of the pathophysiological mechanisms underlying maladaptation of uteroplacental circulation and pregnancy complications including preeclampsia and fetal growth restriction associated with chronic hypoxia during gestation.
Supplementary Material
Novelty and Significance.
What Is New?
Expression and activity of SKCa channels in uterine arteries are upregulated during pregnancy.
Chronic hypoxia during gestation inhibits pregnancy-induced upregulation of SKCa channels in uterine arteries.
Blunted SKCa channel function results in an increase in uterine arterial contractility and myogenic reactivity.
What Is Relevant?
The present study identifies a novel mechanism of SKCa in regulating myogenic adaptation of uterine arteries in pregnancy and in the maladaptation of uteroplacental circulation caused by chronic hypoxia during gestation.
Summary
The present study demonstrates a novel mechanism of SKCa channels in uterine arterial smooth muscle, and thus has a major impact in advancing our knowledge in molecular mechanisms of uterine vascular adaptation to pregnancy and in improving our understanding of pathophysiological mechanisms underlying maladaptation of uteroplacental circulation and pregnancy complications including preeclampsia and fetal growth restriction associated with chronic hypoxia during gestation.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health Grants HD031226 (LDL, LZ), HL089012 (LZ), HL110125 (LZ), NSF-DBI 0923559 (SMW), and HD-069746 (SMW).
Footnotes
Disclosures: None.
References
- 1.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 3.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H International union of pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–472. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 4.Hu XQ, Zhang L. Function and regulation of large conductance Ca2+-activated K+ channel in vascular smooth muscle cells. Drug Discov Today. 2012;17:974–987. doi: 10.1016/j.drudis.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets. 2010;14:143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veerareddy S, Cooke CL, Baker PN, Davidge ST. Vascular adaptations to pregnancy in mice: Effects on myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2226–2233. doi: 10.1152/ajpheart.00593.2002. [DOI] [PubMed] [Google Scholar]
- 9.Xiao D, Buchholz JN, Zhang L. Pregnancy attenuates uterine artery pressure-dependent vascular tone: Role of PKC/ERK pathway. Am J Physiol Heart Circ Physiol. 2006;290:H2337–2343. doi: 10.1152/ajpheart.01238.2005. [DOI] [PubMed] [Google Scholar]
- 10.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson S, Zhang L. Pregnancy upregulates large-conductance Ca2+-activated K+ channel activity and attenuates myogenic tone in uterine arteries. Hypertension. 2011;58:1132–1139. doi: 10.1161/HYPERTENSIONAHA.111.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiner C, Liu KZ, Thompson L, Herrig J, Chestnut D. Effect of pregnancy on endothelium and smooth muscle: Their role in reduced adrenergic sensitivity. Am J Physiol. 1991;261:H1275–1283. doi: 10.1152/ajpheart.1991.261.4.H1275. [DOI] [PubMed] [Google Scholar]
- 12.Nelson SH, Steinsland OS, Johnson RL, Suresh MS, Gifford A, Ehardt JS. Pregnancy-induced alterations of neurogenic constriction and dilation of human uterine artery. Am J Physiol. 1995;268:H1694–1701. doi: 10.1152/ajpheart.1995.268.4.H1694. [DOI] [PubMed] [Google Scholar]
- 13.Cooke CL, Davidge ST. Pregnancy-induced alterations of vascular function in mouse mesenteric and uterine arteries. Biol Reprod. 2003;68:1072–1077. doi: 10.1095/biolreprod.102.009886. [DOI] [PubMed] [Google Scholar]
- 14.Ni Y, May V, Braas K, Osol G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Physiol. 1997;273:H938–944. doi: 10.1152/ajpheart.1997.273.2.H938. [DOI] [PubMed] [Google Scholar]
- 15.Gangula PR, Zhao H, Supowit S, Wimalawansa S, DiPette D, Yallampalli C. Pregnancy and steroid hormones enhance the vasodilation responses to CGRP in rats. Am J Physiol. 1999;276:H284–288. doi: 10.1152/ajpheart.1999.276.1.H284. [DOI] [PubMed] [Google Scholar]
- 16.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: Role of NO and intracellular Ca2+ Am J Physiol Heart Circ Physiol. 2001;281:H183–190. doi: 10.1152/ajpheart.2001.281.1.H183. [DOI] [PubMed] [Google Scholar]
- 17.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: Role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1840–1849. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang K, Xiao D, Huang X, Xue Z, Yang S, Longo LD, Zhang L. Chronic hypoxia inhibits sex steroid hormone-mediated attenuation of ovine uterine arterial myogenic tone in pregnancy. Hypertension. 2010;56:750–757. doi: 10.1161/HYPERTENSIONAHA.110.155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu XQ, Xiao D, Zhu R, Huang X, Yang S, Wilson SM, Zhang L. Chronic hypoxia suppresses pregnancy-induced upregulation of large-conductance Ca2+-activated K+ channel activity in uterine arteries. Hypertension. 2012;60:214–222. doi: 10.1161/HYPERTENSIONAHA.112.196097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamudio S, Palmer SK, Dahms TE, Berman JC, Young DA, Moore LG. Alterations in uteroplacental blood flow precede hypertension in preeclampsia at high altitude. J Appl Physiol. 1995;79:15–22. doi: 10.1152/jappl.1995.79.1.15. [DOI] [PubMed] [Google Scholar]
- 21.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295:R906–915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in bolivia. Pediatr Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 23.White MM, Zhang L. Effects of chronic hypoxia on maternal vasodilation and vascular reactivity in guinea pig and ovine pregnancy. High Alt Med Biol. 2003;4:157–169. doi: 10.1089/152702903322022776. [DOI] [PubMed] [Google Scholar]
- 24.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C, Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 1995;79:7–14. doi: 10.1152/jappl.1995.79.1.7. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld CR, Roy T, DeSpain K, Cox BE. Large-conductance Ca2+-dependent K+ channels regulate basal uteroplacental blood flow in ovine pregnancy. J Soc Gynecol Investig. 2005;12:402–408. doi: 10.1016/j.jsgi.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E2b-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281:H422–431. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld CR, Liu XT, DeSpain K. Pregnancy modifies the large conductance Ca2+-activated K+ channel and cGMP-dependent signaling pathway in uterine vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2009;296:H1878–1887. doi: 10.1152/ajpheart.01185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gebremedhin D, Kaldunski M, Jacobs ER, Harder DR, Roman RJ. Coexistence of two types of Ca2+-activated K+ channels in rat renal arterioles. Am J Physiol. 1996;270:F69–81. doi: 10.1152/ajprenal.1996.270.1.F69. [DOI] [PubMed] [Google Scholar]
- 29.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive K+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension. 2004;43:413–419. doi: 10.1161/01.HYP.0000110945.84443.d2. [DOI] [PubMed] [Google Scholar]
- 30.McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen CM, Giese I, Braunstein TH, Holstein-Rathlou NH, Salomonsson M. Closure of multiple types of K+ channels is necessary to induce changes in renal vascular resistance in vivo in rats. Pflugers Arch. 2011;462:655–667. doi: 10.1007/s00424-011-1018-2. [DOI] [PubMed] [Google Scholar]
- 32.Pierce SL, England SK. SK3 channel expression during pregnancy is regulated through estrogen and Sp factor-mediated transcriptional control of the KCNN3 gene. Am J Physiol Endocrinol Metab. 2010;299:E640–646. doi: 10.1152/ajpendo.00063.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keating DJ, Rychkov GY, Roberts ML. Oxygen sensitivity in the sheep adrenal medulla: Role of sk channels. Am J Physiol Cell Physiol. 2001;281:C1434–1441. doi: 10.1152/ajpcell.2001.281.5.C1434. [DOI] [PubMed] [Google Scholar]
- 34.Kroigaard C, Kudryavtseva O, Dalsgaard T, Wandall-Frostholm C, Olesen SP, Simonsen U. KCa3.1 channel downregulation and impaired endothelium-derived hyperpolarization-type relaxation in pulmonary arteries from chronic hypoxic rats. Exp Physiol. 2013;98:957–969. doi: 10.1113/expphysiol.2012.066340. [DOI] [PubMed] [Google Scholar]
- 35.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Neutrophil antimicrobial peptide a-defensin causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2006;43:357–363. doi: 10.1016/j.jvs.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Noble K, Floyd R, Shmygol A, Mobasheri A, Wray S. Distribution, expression and functional effects of small conductance Ca-activated potassium (SK) channels in rat myometrium. Cell Calcium. 2010;47:47–54. doi: 10.1016/j.ceca.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Hu XQ, Zhang L. Chronic hypoxia suppresses pharmacomechanical coupling of the uterine artery in near-term pregnant sheep. J Physiol. 1997;499 (Pt 2):551–559. doi: 10.1113/jphysiol.1997.sp021948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao D, Huang X, Yang S, Longo LD, Zhang L. Pregnancy downregulates actin polymerization and pressure-dependent myogenic tone in ovine uterine arteries. Hypertension. 2010;56:1009–1015. doi: 10.1161/HYPERTENSIONAHA.110.159137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao D, Huang X, Yang S, Zhang L. Direct chronic effect of steroid hormones in attenuating uterine arterial myogenic tone: Role of protein kinase C/extracellular signal-regulated kinase 1/2. Hypertension. 2009;54:352–358. doi: 10.1161/HYPERTENSIONAHA.109.130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rada CC, Pierce SL, Nuno DW, Zimmerman K, Lamping KG, Bowdler NC, Weiss RM, England SK. Overexpression of the SK3 channel alters vascular remodeling during pregnancy, leading to fetal demise. Am J Physiol Endocrinol Metab. 2012;303:E825–831. doi: 10.1152/ajpendo.00165.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by ovine uterine and systemic arteries: ovarian steroid and pregnancy control of ERalpha and ERbeta levels. J Physiol. 2005;565:85–99. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP. Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun. 2003;303:660–668. doi: 10.1016/s0006-291x(03)00408-x. [DOI] [PubMed] [Google Scholar]
- 44.Bosch MA, Kelly MJ, Ronnekleiv OK. Distribution, neuronal colocalization, and 17b-E2 modulation of small conductance calcium-activated K+ channel (SK3) mrna in the guinea pig brain. Endocrinology. 2002;143:1097–1107. doi: 10.1210/endo.143.3.8708. [DOI] [PubMed] [Google Scholar]
- 45.McNeish AJ, Dora KA, Garland CJ. Possible role for K+ in endothelium-derived hyperpolarizing factor-linked dilatation in rat middle cerebral artery. Stroke. 2005;36:1526–1532. doi: 10.1161/01.STR.0000169929.66497.73. [DOI] [PubMed] [Google Scholar]
- 46.Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279:H319–328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- 47.Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- 48.Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, Bond CT, Adelman JP, Wulff H, de Wit C, Hoyer J, Kohler R. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- 49.Chen MX, Gorman SA, Benson B, Singh K, Hieble JP, Michel MC, Tate SN, Trezise DJ. Small and intermediate conductance Ca2+-activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- 50.Potocnik SJ, McSherry I, Ding H, Murphy TV, Kotecha N, Dora KA, Yuill KH, Triggle CR, Hill MA. Endothelium-dependent vasodilation in myogenically active mouse skeletal muscle arterioles: Role of EDH and K+ channels. Microcirculation. 2009;16:377–390. doi: 10.1080/10739680902804042. [DOI] [PubMed] [Google Scholar]
- 51.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown A, Cornwell T, Korniyenko I, Solodushko V, Bond CT, Adelman JP, Taylor MS. Myometrial expression of small conductance Ca2+-activated K+ channels depresses phasic uterine contraction. Am J Physiol Cell Physiol. 2007;292:C832–840. doi: 10.1152/ajpcell.00268.2006. [DOI] [PubMed] [Google Scholar]
- 53.Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1737–1743. doi: 10.1152/ajpregu.00840.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura I, Ui-Tei K, Saigo K, Ishii H, Sakuma Y, Kato M. 17b-estradiol at physiological concentrations augments Ca2+-activated K+ currents via estrogen receptor b in the gonadotropin-releasing hormone neuronal cell line GT1-7. Endocrinology. 2008;149:774–782. doi: 10.1210/en.2007-0759. [DOI] [PubMed] [Google Scholar]
- 55.Dasgupta C, Chen M, Zhang H, Yang S, Zhang L. Chronic hypoxia during gestation causes epigenetic repression of the estrogen receptor-a gene in ovine uterine arteries via heightened promoter methylation. Hypertension. 2012;60:697–704. doi: 10.1161/HYPERTENSIONAHA.112.198242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61:480–487. doi: 10.1161/HYPERTENSIONAHA.111.201624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.