Abstract
For many years the function of the sebaceous gland (SG) was underestimated and suggested by Albert M. Kligman as a remnant of human development, a ‘living fossil with a past but no future’. However, the last two decades of studies and the discovery of neuro-endocrine pathways in skin have determined the importance of the SG in cutaneous biology and homeostasis. SGs play their role in cutaneous homeostasis by contribution to local steroidogenic pathways, antimicrobial activity, and display of immune (both pro- and anti-inflammatory) properties. Despite several important manuscripts and reviews regarding SG biology and function, there was an urgent need for a high quality methodological guide through SG identification and quantitative evaluation. In this issue of Experimental Dermatology, Hinde et al. present a practical guide to SG research- outlining methods, defining immunohistochemial markers, and providing guidance to both novice and more experienced SG researchers.
Keywords: Sebaceus gland, stress, neuroendocrine, immune, markers
Commentary
Long the bane of pimple-faced teenagers, sebaceous glands were previously understood to be superfluous, occasionally annoying, evolutionary remnants (1–3). Past research focused on the production of sebum, which in animals covers the fur and assists in hydrophobic protection and thermoregulation (2, 3). However, our understanding of the sebaceous gland has changed dramatically in the past two decades. Recent findings have shown that the functions of sebocytes go far beyond the production of sebum and the formation of the passive cutaneous barrier (2, 4, 5). The sebaceous gland is a neuro-immuno-endocrine mini-organ which has multiple complex functions (4, 5). These functions include the regulation of cutaneous steroidogenesis, local androgen synthesis, interaction with neuropeptides, synthesis of specific lipids with antimicrobial activity, and exhibition of pro- and anti-inflammatory properties (2, 3, 6, 7).
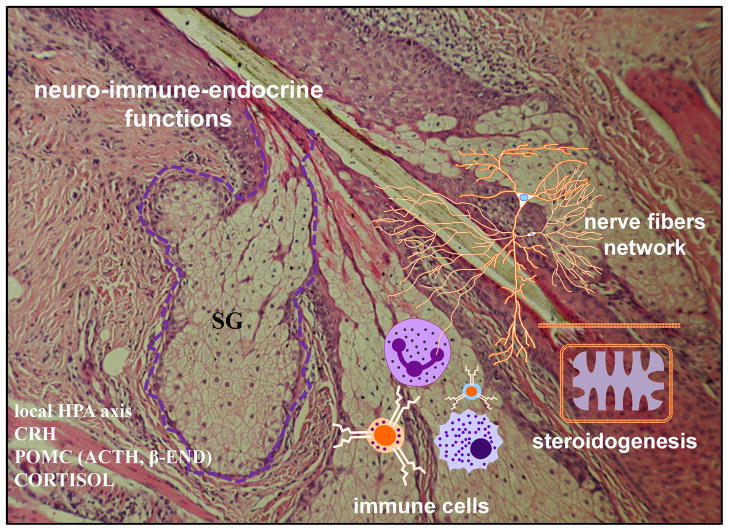
Of particular interest is the role of the sebaceous gland in the proposed cutaneous equivalent of the hypothalamic-pituitary-adrenal (HPA) axis, a hypothesis first introduced in 1996 (8, 9) and further substantiated over the last two decades of research (10, 11). SGs have been shown to both produce and have receptors for neurotransmitters and mediators of HPA axis, including CRH, urocortin, proopiomelanocortin (POMC)-derived β-endorphin, α-MSH and ACTH (4, 12–18) and have a potential to produce glucocorticoids and metabolize (13, 19, 20). Therefore, SGs are thought to be involved in functions of cutaneous stress responses system (7, 10, 18) with CRH playing a central role in this regulatory mechanism (11). Additionally, sebocytes have fascinating immune-like functions, releasing proinflammatory cytokines which could interact with receptors localized on sensory nerve endings, such as substance P (SP) (2–5, 7). Supporting the unique role of the SG as a place where neuro-immuno-endocrine systems meet and cooperate, there is evidence of a rich innervation and presence of Langerhans cells combined with high steroidogenic activity (Fig. 1) (2–5, 7, 19).
Figure 1.
Sebaceous gland (SG), a place where neuro-immuno-endocrine systems meet and cooperate.
Sebaceous glands have historically constituted a technical challenge to researchers. Because of their rapid differentiation, primary isolated sebaceous glands and sebocytes could not be grown in culture for extended periods (21, 22). Additionally, diseases such as acne vulgaris are exclusively human diseases and were a challenge to study in animal models (2, 3, 6, 23).
Fortunately, solutions have been found. Imperfect animal models have largely been replaced by human sebaceous-gland derived cell lines which have improved our insight into sebaceous gland biology and molecular regulation (3, 24, 25).
As increasing numbers of researchers begin working in this field, there was a need for a review of SG research methodology. In this issue of Experimental Dermatology, Hinde et al. (5) present a practical guide to SG research- outlining methods, indicating immunohistochemical markers, and providing guidance to both novice and more experienced SG researchers. The authors provide practical considerations for tissue processing for any analysis of the SG. They discuss SG histochemical stains and their possible clinical uses such as oil red staining, which is useful in the diagnosis of sebaceous gland carcinoma and their metastatic lesions. They also recommend confocal or three-dimensional microscopy for quantitative morphometry of SG. This review provides inclusive information on available markers of SGs. Authors provide nicely summarized information in succinct subchapters and tables for reader’s convenience.
In summary, the intriguing findings of studies on the sebaceous gland have raised many other questions. Possible targeted regulation of cutaneous HPA axis elements for therapy and/or immunomodulation would be a fascinating approach to treat sebaceous gland related diseases. Looking to the future, it is our hope studies using these methods will lead to discoveries that decrease suffering of patients with SG diseases.
Acknowledgments
Writing of this commentary was supported in part by grants from National Science Foundation (# IOS-0918934) and National Institutes of Health (# 1R01AR056666-01A2) to AS. Reza Nejati, Cezary Skobowiat and Andrzej Slominski analyzed the data and wrote the paper
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kligman AM. The Uses of Sebum. The British journal of dermatology. 1963;75:307–319. doi: 10.1111/j.1365-2133.1963.tb13567.x. [DOI] [PubMed] [Google Scholar]
- 2.Zouboulis CC, Baron JM, Bohm M, et al. Frontiers in sebaceous gland biology and pathology. Experimental dermatology. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 3.Toth BI, Olah A, Szollosi AG, Czifra G, Biro T. “Sebocytes’ makeup”: novel mechanisms and concepts in the physiology of the human sebaceous glands. Pflugers Archiv : European journal of physiology. 2011;461:593–606. doi: 10.1007/s00424-011-0941-6. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes -- a pathogenetic link between stress and acne. Experimental dermatology. 2004;13 (Suppl 4):31–35. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 5.Hinde E, Haslam IS, Schneider MR, et al. A practical guide for the study of human and murine sebaceous glands in situ. Experimental dermatology. 2013:n/a–n/a. doi: 10.1111/exd.12207. [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis CC. Acne and sebaceous gland function. Clinics in dermatology. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Advances in anatomy, embryology, and cell biology. 2012;212:v, vii, 1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slominski A, Mihm MC. Potential mechanism of skin response to stress. International journal of dermatology. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, Ermak G, Mihm M. ACTH receptor, CYP11A1, CYP17 and CYP21A2 genes are expressed in skin. J Clin Endocrinol Metab. 1996;81:2746–2749. doi: 10.1210/jcem.81.7.8675607. [DOI] [PubMed] [Google Scholar]
- 10.Slominski A, Wortsman J, Tuckey RC, Paus R. Differential expression of HPA axis homolog in the skin. Molecular and cellular endocrinology. 2007;265–266:143–149. doi: 10.1016/j.mce.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slominski AT, Zmijewski MA, Zbytej B, DJT, Theoharides CT, Rivier J. Skin stress response system: central role of CRF Endocrine Reviews. 2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slominski A, Paus R, Mazurkiewicz J. Proopiomelanocortin expression in the skin during induced hair growth in mice. Experientia. 1992;48:50–54. doi: 10.1007/BF01923606. [DOI] [PubMed] [Google Scholar]
- 13.Ito N, Ito T, Kromminga A, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Seltmann H, Hiroi N, et al. Corticotropin-releasing hormone: an autocrine hormone that promotes lipogenesis in human sebocytes. Proc Natl Acad Sci U S A. 2002;99:7148–7153. doi: 10.1073/pnas.102180999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slominski A, Roloff B, Curry J, Dahiya M, Szczesniewski A, Wortsman J. The skin produces urocortin. Journal of Clinical Endocrinology and Metabolism. 2000;85:815–823. doi: 10.1210/jcem.85.2.6381. [DOI] [PubMed] [Google Scholar]
- 16.Slominski A, Pisarchik A, Tobin DJ, Mazurkiewicz JE, Wortsman J. Differential expression of a cutaneous corticotropin-releasing hormone system. Endocrinology. 2004;145:941–950. doi: 10.1210/en.2003-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito N, Ito T, Betterman A, Paus R. The human hair bulb is a source and target of CRH. The Journal of investigative dermatology. 2004;122:235–237. doi: 10.1046/j.1523-1747.2003.22145.x. [DOI] [PubMed] [Google Scholar]
- 18.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 19.Slominski A, Zbytek B, Nikolakis G, et al. Steroidogenesis in the skin: Implications for local immune functions. The Journal of steroid biochemistry and molecular biology. 2013 doi: 10.1016/j.jsbmb.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slominski AT, Zmijewski MA, Semak I, et al. Cytochromes P450 and Skin Cancer: Role of Local Endocrine Pathways. Anti-cancer agents in medicinal chemistry. 2013 doi: 10.2174/18715206113139990308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujie T, Shikiji T, Uchida N, Urano Y, Nagae H, Arase S. Culture of cells derived from the human sebaceous gland under serum-free conditions without a biological feeder layer or specific matrices. Archives of dermatological research. 1996;288:703–708. doi: 10.1007/BF02505281. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfield RL. Relationship of sebaceous cell stage to growth in culture. The Journal of investigative dermatology. 1989;92:751–754. doi: 10.1111/1523-1747.ep12722448. [DOI] [PubMed] [Google Scholar]
- 23.Wheatley VR, Potter JE, Lew G. Sebaceous gland differentiation: II. The isolation, separation and characterization of cells from the mouse preputial gland. The Journal of investigative dermatology. 1979;73:291–296. doi: 10.1111/1523-1747.ep12531708. [DOI] [PubMed] [Google Scholar]
- 24.Thiboutot D, Jabara S, McAllister JM, et al. Human skin is a steroidogenic tissue: steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1) The Journal of investigative dermatology. 2003;120:905–914. doi: 10.1046/j.1523-1747.2003.12244.x. [DOI] [PubMed] [Google Scholar]
- 25.Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) The Journal of investigative dermatology. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]