Abstract
Background and Purpose
There is considerable debate regarding the efficacy of amphetamine to facilitate motor recovery after stroke or experimental brain injury. Different drug dosing and timing schedules and differing physical rehabilitation strategies may contribute to outcome variability. The present study was designed to ascertain (1) whether short-term amphetamine could induce long-term functional motor recovery in rats after an ischemic lesion modeling stroke in humans; (2) how different levels of physical rehabilitation interact with amphetamine to enhance forelimb-related functional outcome; and (3) whether motor improvement was associated with axonal sprouting from intact corticoefferent pathways originating in the contralesional forelimb motor cortex.
Methods
After permanent middle cerebral artery occlusion, rats received vehicle or amphetamine during the first postoperative week (2 mg/kg, subcutaneously on Postoperative Days 2, 5, and 8). In both treatment groups, separate cohorts of rats were exposed to different levels of “physical rehabilitation” represented by a control environment, enriched environment, or enriched environment with additional sessions of focused activity. Skilled forelimb performance was assessed using the forelimb reaching task and ladder rung walk test. Anterograde tracing with biotinylated dextran amine was used to assess new fiber outgrowth to denervated motor areas.
Results
All treatment groups showed significant motor improvement as compared with control-housed, vehicle-treated animals. However, animals housed in an enriched environment that received amphetamine paired with focused activity sessions performed significantly better than any other treatment group and was the only group to achieve complete motor recovery (ie, reached preoperative performance) by 8 weeks. This recovery was associated with axonal sprouting into deafferentated subcortical areas from contralesional projection neurons.
Conclusions
This study suggests that, after stroke, short-term pairing of amphetamine with sufficiently focused activity is an effective means of inducing long-term improvement in forelimb motor function. The anatomic data suggests that corticoefferent plasticity in the form of axonal sprouting contributes to the maintenance of motor recovery.
Keywords: forelimb reaching task, ladder rung walk test, MCAO, physical therapy, rat
Ischemic stroke often leads to chronic functional limitations that adversely affect activities of daily living making it a leading cause of serious, long-term disability in the United States.1 Physical therapy is the mainstay of rehabilitation strategies to reduce chronic impairment of sensory and motor function. However, there is a need for improved treatment.
A variety of pharmacological adjuncts and physical therapy strategies have been tested in an attempt to improve rehabilitation outcomes. Of particular interest is the pharmacological agent amphetamine, a potent psychomotor stimulant that induces neuronal release of the monoamines norepinephrine, dopamine, and, to a lesser extent, serotonin.2 Numerous experimental studies have demonstrated that amphetamine improves forelimb function after brain injury.3–10 By comparison, clinical studies of the effects of amphetamine on motor function have been more variable.11–15 It has been widely acknowledged that differences in experimental design (physical therapy regimes, drug dosing and timing, outcome measures) may contribute to outcome variability.15,16 Of considerable significance is the variability in the duration of amphetamine administration because the majority of clinical studies demonstrated little or no long-term motor improvement (eg, lasting weeks to months) after short-term amphetamine administration of ≤1 week.17 Understanding whether amphetamine can be efficacious under short-term use is important considering the reported trend toward increased mortality from cardiovascular side effects with continued use.15
Mechanistically, amphetamine is reported to induce alterations in gene transcription, protein synthesis, and dendritic structure.18,19 This neural plasticity is similar to what is seen in animals exposed to an enriched environment after ischemic infarction and which appears to be associated with improved functional outcome.20–23 Although amphetamine has been shown to increase the expression of proteins related to axonal growth and synaptogenesis after experimental stroke,4 direct evidence of amphetamine-induced axonal growth has not been shown. However, our previous work has shown that amphetamine induces axonal growth from the contralesional cortex after unilateral cortical aspiration lesions.3
The present study was designed to answer 3 questions. Is short-term amphetamine (3 doses on Days 2, 5, and 8 postinjury) sufficient to achieve and maintain recovery of skilled forelimb function after stroke? Is the level of environmental enrichment and “physical therapy” an important determinant of outcome using amphetamine? Is amphetamine-induced functional improvement after ischemic infarct correlated with axonal outgrowth from corticoefferent motor pathways of the unlesioned cortex? The results are discussed with respect to the use of amphetamine as a rehabilitative strategy in patients after stroke.
Materials and Methods
Animals
Adult male Long Evans, black-hooded rats (250 to 300 g) were maintained in a temperature- and humidity-controlled room under a 12:12-hour light/dark cycle. Food intake was moderately restricted throughout the study to maintain body weight at 95% of ad libitum weight. Water was available ad libitum. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Housing/Rehabilitation/Drugs
Immediately after surgery, rats were allocated to different experimental groups with differing housing conditions as described subsequently. Control housing conditions (CON) consisted of singly housed animals in a standard Plexiglas cage (24 cm × 36 cm × 15 cm) with no additions. Enriched environment (ENR) consisted of group-housed animals (3 per cage) in larger Plexiglas cages (81.5 cm × 61 cm × 45 cm) furnished with inclined ladders, hanging toys, chewable material, and tunnels. Once a week the objects were replaced with novel items. A subset of animals in enriched housing also received focused activity sessions (ENR/FA).
Focused activity (FA) sessions began 2 days after middle cerebral artery occlusion (MCAO) and continued twice daily for the first 3 weeks. For the remaining 5 weeks, FA sessions were conducted once daily. Individual sessions were 20 minutes in length and began after completion of daily behavioral testing and 10 minutes after drug injection to coincide with the peak pharmacological actions of amphetamine.24 Focused activity consisted of daily regimented activities that relied heavily on the use of forelimbs but were distinct from the specific tasks being assessed. During sessions, cage mates (3 animals/session) were placed simultaneously in a 5′ × 5′ enclosed space containing a 45o inclined ladder (200 cm × 5 cm), vertical rope (100 cm), and a vertical cylindrical grid (100 cm × 10 cm; 1-cm2 mesh). Participation among the rats was equalized by having 2 investigators follow the rats and physically place each animal on a different piece of exercise equipment at approximately 1-minute intervals during the 20-minute sessions.
D-amphetamine sulfate powder (Sigma Chemical Co, St Louis, Mo) was dissolved in 0.9% sterile saline at a concentration of 2 mg/mL (based on salt weight). All animals received either D-amphetamine sulfate (AMPH) injection (2 mg/kg subcutaneously) or vehicle (0.9% sterile saline) at 2, 5, and 8 days after MCAO. Animals receiving focused activity began the session 10 minutes after injection.
Training/Behavioral Testing
All animals were trained to criterion on the skilled forelimb reaching task and assessed for their performance on the ladder rung walk test. Animals then underwent stroke surgery after which they were allocated to different experimental groups (see the Table). Each animal was tested for performance on the skilled forelimb reaching task beginning the first day postoperatively and then daily (Monday through Friday) for 8 weeks. Additionally, each animal was tested for performance on the ladder rung walk test on the second postoperative day and then once weekly for 8 weeks. On days when amphetamine or vehicle was administered (ie, Days 2, 5, and 8 postoperatively), all behavior testing was performed before drug injection. Investigators performing behavioral testing were blinded to the treatment groups.
Table 1.
Lesion Analysis Among Treatment Groups
| Group (n) | Drug/Housing+Activity Conditions | Stroke Volume (% of Contralesional Hemisphere Volume) |
|---|---|---|
| VEH/CON (9) | Vehicle-treated animals singly housed under control conditions; no activity sessions | 8.75±1.46 |
| AMPH/CON (7) | AMPH-treated animals singly housed under control conditions; no activity sessions | 8.68±2.06 |
| VEH/ENR (6) | Vehicle-treated animals group-housed in enriched environment; no activity sessions | 10.29±1.98 |
| AMPH/ENR (8) | AMPH-treated animals group-housed in enriched environment; no activity sessions | 12.36±1.68 |
| VEH/ENR+FA (11) | Vehicle-treated animals group-housed in enriched environment; plus focused activity sessions | 11.64±1.82 |
| AMPH/ENR+FA (11) | AMPH-treated animals group-housed in enriched environment; plus focused activity sessions | 9.21±1.15 |
After training, animals were subjected to MCAO and distributed among the different treatment groups depicted. Details of drug treatment, housing, and activity conditions are described in more detail in “Methods.” After 8 weeks of behavioral testing, animals were microinjected with BDA and euthanized 2 weeks later for histological analysis. No significant difference in lesion size among groups was observed (F5,46=0.872, P=0.5). Data represent the mean ± SEM of the indicated number of animals per group.
Skilled forelimb reaching was tested as previously described.25,26 Animals were placed in a transparent Plexiglas chamber (30 cm × 36 cm × 30 cm) and trained to reach through a window (1.5 cm × 3 cm) to retrieve small sucrose pellets (45 mg; Bilaney Consultants, Frenchtown, NJ) placed on a platform at a distance of 1 cm. During the initial days of training, limb preference was determined and placement of pellets was adjusted to favor the use of the preferred forelimb. Before surgery, baseline performance (defined as the average of the last 3 testing sessions of the preoperative testing) was established. Success was defined as an animal grasping the pellet on the first attempt and placing it into the mouth (ie, “first reach success”). Each testing session consisted of 20 reaching opportunities using the preferred forelimb. Attempts using the nonpreferred forelimb were not included in analyses. The preoperative criterion was at least 16 successes in 20 attempts for 3 consecutive days. A maximum time limit of 5 minutes/testing session was given.
The ladder rung walk was used to assess deficits in accurate forelimb placement as previously described.26 In this test, animals are scored for their ability to cross a 1-m long horizontal metal-rung runway with varying gaps of 1 to 2 cm between the rungs.27 All animals underwent 3 familiarization sessions with the apparatus before preoperative baseline testing. Baseline, 2-day postoperative, and then weekly sessions for 8 weeks were videotaped and evaluated. A forelimb foot error was defined as a complete miss or slip from the rung. The mean preoperative scores for all experimental groups were fewer than one foot error per 10 steps. Baseline and postoperative testing sessions consisted of 3 runway crossings. The total number of errors and steps by the preferred forelimb in each session was counted and an error frequency was calculated.
Stroke Surgery
All animals underwent MCAO as described previously.25 Animals were anesthetized with sodium pentobarbital (50 mg/kg intraperito-neally). Bilateral common carotid arteries were isolated, a vertical 2-cm long incision was made between the eye and ear, and the temporalis muscle was retracted. A burr hole was made to expose the middle cerebral artery and it was permanently occluded with a 10 – 0 suture. The common carotid artery ipsilateral to the MCAO was permanently occluded with a 4 – 0 suture and the contralateral common carotid artery was temporarily occluded for 45 minutes. The wounds were then closed and animals warmed under a heating lamp until they awoke.
Neuroanatomical Tracing
After 8 weeks of behavioral testing, animals were anesthetized with sodium pentobarbital (50 mg/kg intraperitoneally). The sensorimotor cortex opposite to the stroke lesion site was exposed, and 2 injections of 1 μL each of a 10% biotinylated dextran amine (BDA) solution (Molecular Probes) were placed stereotaxically into the forelimb sensorimotor cortex (0.5 mm anterior, 2.5 mm lateral, 1.5-mm depth, relative to bregma) as defined by Neafsey et al.28 Two weeks after BDA injection, animals were overdosed with sodium pentobarbital (100 mg/kg intraperitoneally) and perfused transcardially with 4% paraformaldehyde. Brains and spinal cords were removed, placed in 30% sucrose for 1 to 2 days, embedded in OCT freezing compound (Miles, Inc), frozen, and stored at −80°C. Alternate coronal cryo-sections (50 μm thick) were reacted for BDA-positive fibers or processed with Nissl stain and analyzed for lesion location and extent.
Neuroanatomical Analysis
Anatomic structures were identified with the atlas of Paxinos and Watson. The corticoefferent projections to the red nucleus and cervical spinal cord were quantitatively analyzed ipsi- and contralaterally to the BDA injection site as previously described using computer-aided image analysis with National Institutes of Health Image software.25 The number of labeled corticoefferent fibers in the cerebral peduncle ipsilateral to the BDA injection site was determined and used to correct for interanimal variances in BDA tracing as described previously.25 Quantification of sprouting corticorubral fibers from the contralesional side to the deafferentated (ipsilesional) red nucleus was performed by counting all BDA-positive fibers crossing the midline at the level of the red nucleus. Interanimal differences in the number of sections containing the red nucleus were normalized to that of the animal with the least amount of sections (6 sections/animal). To correct for tracing differences, the number of the BDA-positive, midline crossing fibers was divided by the total number of labeled corticoefferent fibers. The quantification of sprouting corticospinal tract fibers from the contralesional hemisphere to the deafferentated spinal cord was performed at levels C5 to C8 by counting BDA-positive fibers crossing a vertical line positioned at the midline (25 sections per animal). The sum of all the values of midline crossing fibers was then normalized to labeled corticoefferent fibers to account for interanimal tracing variability. For all analyses, the slides were coded and investigators were blind to the treatment group.
Stroke Size Analysis and Exclusion Criteria
Stroke volume was quantitatively analyzed on Nissl-stained sections (+4.7 to −5.2 mm from bregma according to Paxinos and Watson) using National Institutes of Health Image as described previously.25 Stroke size was expressed as a percentage of the contralesional hemispheric volume. Stroke size data were omitted for 2 animals in the VEH/CON group and one animal in the VEH/ENR group due to poor histology. Animals were excluded from all statistical analyses if the lesion was found not to impinge on the forelimb region of the sensorimotor cortex and/or if subcortical damage was observed.
Statistics
Data were analyzed using SigmaStat (Systat Software Inc). Stroke size was analyzed by one-way analysis of variance (ANOVA). Skilled forelimb reaching and ladder rung walking were analyzed independently using a repeated-measures one-way ANOVA for overall treatment effect. Specific post hoc comparisons were made using Student-Newman-Keuls multiple comparison test. A 2-way repeated measures ANOVA was used to compare the mean values among all groups at end point (ie, 8 weeks postoperatively) versus baseline (preoperative) performance with post hoc comparisons using Student-Newman-Keuls test to compare performance among treatment groups at 8 weeks.
Analysis of fiber-count data (midline-crossing fibers) was performed using a one-way ANOVA followed by Student-Newman-Keuls test in case of paired comparisons. Correlations of fiber-count results and behavioral results were analyzed for significance with paired analysis (midline-crossing fiber counts at the level of the red nucleus or cervical spinal cord paired with contralesional forelimb reaching or ladder rung performance for each animal) by Pearson product moment correlation. In all cases, a probability value ≤0.05 was considered significant. All data are presented as mean values ± SEM.
Results
Lesion Analysis Among Treatment Groups
The topographical location of all lesions included the fore-limb area of the sensorimotor cortex ipsilateral to the occluded middle cerebral artery with no obvious evidence of any gross damage to underlying subcortical matter. As shown in the Table, no significant difference was found in infarct volume among the 6 different treatment groups (F5,46=0.872, P=0.5).
Skilled Forelimb Function Analysis
The 6 experimental groups described in the Table were tested on the skilled forelimb reaching test and ladder rung walk test as described in “Methods.” It should be stressed that for both behavioral tests, the data reflect performance of the preferred (ie, affected) forelimb only.
Skilled Forelimb Reaching
On Postoperative Day 2, before administering any drug or focused activity sessions, reaching performance in all groups showed a marked deficit resulting in a mean success of 2 to 4 pellets/20 attempts with no significant difference among groups (F5,49=0.684, P=0.64). Figure 1A shows that reaching performance improved to some extent in all groups over the course of 8 weeks. A one-way repeated-measures ANOVA across groups demonstrated a treatment effect (F5,391=10.64, P=0.001). Post hoc analysis showed that overall reaching performance in rats receiving short-term AMPH combined with enriched housing plus focused activity sessions (AMPH/ ENR+FA) was significantly better than all other groups (P<0.05). The only other significant difference was that overall reaching performance in vehicle-treated animals receiving either enriched housing plus focused activity (VEH/ ENR+FA) or just enriched housing (VEH/ENR) was significantly better than vehicle-treated animals in control housing (VEH/CON; P<0.05).
Figure 1.
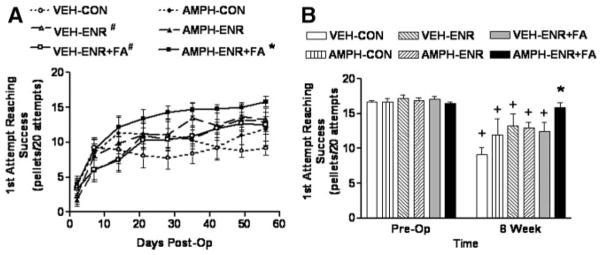
Skilled forelimb reaching performance after MCAO recovers to preoperative performance after AMPH/ENR+FA. A, Time course—all animals enrolled in the study achieved the preoperative criteria of an average of 16 successes in 20 attempts for 3 days before surgery. At Day 2 postoperatively, before any treatment, the mean deficit in reaching was not significantly different among groups (F5,49=0.684, P=0.64). AMPH/ENR+FA produced a significant overall enhancement in reaching performance compared with all other groups (*P<0.05, one-way repeated-measures ANOVA followed by Student-Newman-Keuls). VEH/ENR and VEH/ENR+FA were also significantly better than VEH/CON (#P<0.05, Student-Newman-Keuls). B, End point analysis—at 8 weeks after MCAO, reaching performance in AMPH/ENR+FA was significantly better than all other groups (*P<0.05, 2-way ANOVA followed by Student-Newman-Keuls). A 2-way repeated-measures ANOVA indicated that all groups except AMPH/ENR+FA-treated animals still displayed significant deficits in reaching when compared with preoperative performance (+P<0.001, Student-Newman-Keuls), which demonstrates that only AMPH/ENR+FA induced a recovery to baseline performance in the forelimb reaching task. Data represent the mean ± SEM for the indicated number of animals/group.
To determine whether full recovery had occurred, forelimb performance at 8 weeks was statistically compared with preoperative performance (Figure 1B). Two-way ANOVA revealed a significant treatment effect (F5,49=2.95, P=0.021), time effect (F1,49=80.69, P<0.001), and treatment × time interaction (F5,49=4.07, P=0.004). Post hoc comparison revealed that performance at experimental end point (8 weeks postoperatively) in all groups except AMPH/ENR+FA was significantly lower than preoperative performance (P<0.001). At end point, the AMPH/ENR+FA group achieved a score of 15.9 ± 0.9 pellets that was not significantly different from preoperative performance (P=0.57). Post hoc comparison of treatment effect at end point revealed that performance in the AMPH/ENR+FA group was significantly better than all other groups (P<0.05). As would be expected based on previous literature, the end point performance of animals receiving vehicle and enriched environment plus focused activity (VEH/ENR+FA) was significantly better than their controls (VEH/CON) despite the fact that improvement did not reach the level of AMPH/ENR+FA.
Ladder Rung Walk
On Postoperative Day 2, all groups showed a marked deficit in accurate placement of the contralesional forelimb, resulting in a mean of approximately 4 errors/10 steps across all groups with no significant difference (F5,49=1.15, P=0.35). Figure 2A shows that, similar to reaching performance, ladder walk performance improved to some extent in all groups over 8 weeks. One-way repeated-measures ANOVA demonstrated a treatment effect (F5,389=40.78, P<0.001) with post hoc analysis revealing a number of statistical differences among groups. Importantly, overall performance in the AMPH/ ENR+FA group was significantly better than any other group (P<0.001 for all comparisons). Unlike the results for the reaching task, overall ladder walk performance of animals housed in an enriched environment alone without focused activity was significantly better than their respective housing controls (ie, VEH/ENR+VEH/CON, P=0.015 and AMPH/ ENR>AMPH/CON, P<0.001). However, the addition of focused activity to vehicle-treated animals in enriched housing (VEH/ENR+FA) did not further improve ladder walk performance as compared with VEH/ENR (P=0.23).
Figure 2.
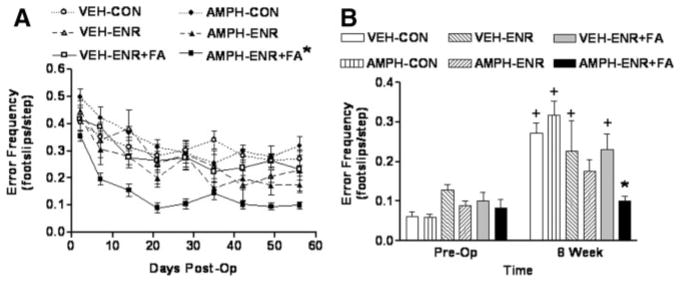
Ladder rung walking performance after MCAO recovers to preoperative performance after AMPH/ENR+FA. A, Time course—At Day 2 postoperatively, before any treatment, the mean deficit in skilled forelimb placement was not significantly different among groups (F5,49=1.15, P=0.35). AMPH/ENR+FA produced a significant overall enhancement in ladder walk performance compared with all other groups (*P<0.001, one-way repeated-measures ANOVA followed by Student-Newman-Keuls). B, End point analysis—at 8 weeks after MCAO, ladder walk performance in AMPH/ENR+FA was significantly better than all other groups (*P<0.05, 2-way ANOVA followed by Student-Newman-Keuls) with the exception of AMPH/ENR (P<0.05). A 2-way repeated-measures ANOVA indicated that only animals receiving AMPH/ENR or AMPH/ENR+FA achieved end point performance that was not different from preoperative levels, whereas all other groups still displayed significant deficits when compared with preoperative performance (+P<0.05, Student-Newman-Keuls). Data represent the mean ± SEM for the indicated number of animals/group.
A 2-way ANOVA comparing end point (8-week) performance versus preoperative performance revealed a significant treatment effect (F5,49=3.74, P=0.006), time effect (F1,49=67.86, P<0.001), and treatment × time interaction (F5,49=5.47, P<0.001). Post hoc analysis revealed that by 8 weeks, only the AMPH/ENR and AMPH/ENR+FA groups returned to ladder walk performance that was not significantly different from preoperative performance (P=0.087 and 0.640, respectively), whereas performance in all other groups remained significantly lower (P<0.001). Post hoc comparison of treatment effect at end point revealed that performance in the AMPH/ENR+FA group was significantly better than all other groups (P<0.01) with the exception of AMPH/ENR, which bordered on significance(P=0.05). Unlike results in forelimb reaching, the end point performance of animals receiving vehicle and enriched environment plus focused activity (VEH/ENR+FA) was not significantly better than their controls (VEH/CON; P=0.245).
Neuroanatomical Plasticity After Middle Cerebral Artery Occlusion and Treatment
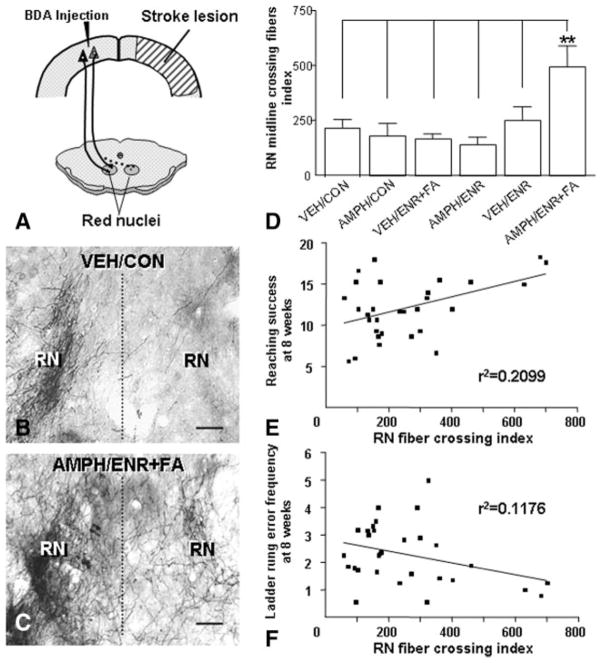
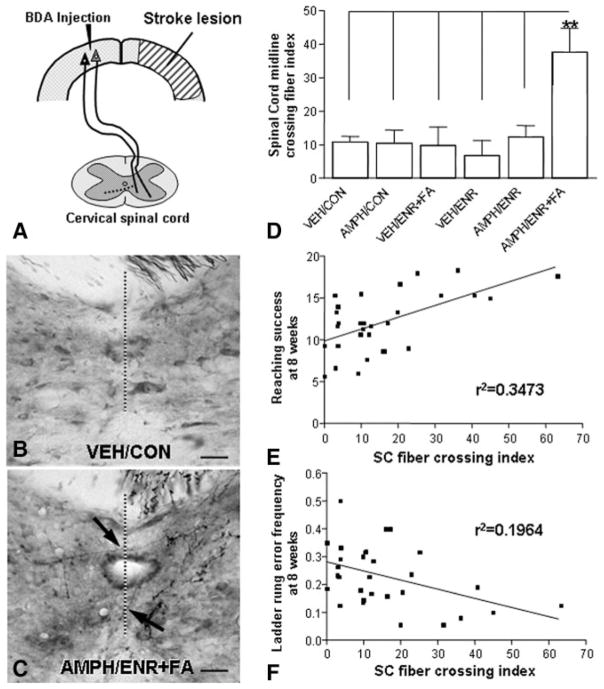
The significant forelimb functional recovery observed in the AMPH/ENR+FA group led us to investigate neuroanatomical changes of corticoefferent pathways originating in the contralesional forelimb sensorimotor cortex to the red nucleus and cervical spinal cord as depicted schematically in Figures 3A and 4A, respectively. Cortical projections to the ipsilateral red nucleus (Figure 3B) and contralateral cervical spinal cord (Figure 4B) in animals receiving MCAO followed by vehicle and control condition showed the typical topographical innervation pattern with only a few fibers crossing the midline and terminating within the deafferentated red nucleus and spinal cord. After AMPH/ENR+FA, an increase of midline crossing fibers was seen at the level of the red nucleus (Figure 3C) and cervical spinal cord (Figure 4C). Midline crossing at the level of the red nucleus increased significantly by 2-fold (P<0.01; Figure 3D) and at the cervical spinal cord midline-crossing fibers increased by 4-fold (P<0.01; Figure 4D). The degree of fiber crossing at the red nucleus (Figure 3E–F) and the cervical spinal cord (Figure 4E–F) correlated significantly with improved reaching and ladder rung walk performance 8 weeks after MCAO (Pearson r, P<0.05).
Figure 3.
AMPH/ENR+FA treatment after MCAO enhances corticorubral plasticity that correlates with impaired forelimb function. A, Scheme of the new projection from the intact primary motor cortex to the contralateral (dashed lines) red nucleus. The antero-grade tracer BDA was injected into the contralesional sensorimotor cortex. B, Corticorubral projection after MCAO in a VEH/CON animal shows scarce BDA-positive fibers crossing the midline (dashed line) to the denervated side (right). C, AMPH/ENR+FA induced many midline BDA-positive crossing fibers and an increased innervation pattern to the side denervated by the lesion (right). D, Quantification of midline-crossing fibers in the area of the red nucleus normalized to the total labeled cerebral peduncle fibers (to correct for differences in the tracing). **P<0.01; one-way ANOVA. Midline-crossing fibers at the level of the red nucleus was plotted against performance in the forelimb reaching task (E) and ladder rung walk (F) at 8 weeks. Linear regression analysis showed a significant correlation of corticorubral fiber crossing and forelimb function. RN, red nucleus. Scale bars in (B) and (C)=50 μm. Error bars indicate SE.
Figure 4.
AMPH/ENR+FA treatment after MCAO enhances corticospinal plasticity that correlates with improved forelimb function. A, Scheme of the new projection from the intact corticospinal tract to the contralateral (dashed lines) spinal cord at the level of the cervical enlargement. All fibers crossing the midline were counted and normalized to the cerebral peduncle. B, Corticospinal projection after MCAO in a VEH/CON animal shows scarce BDA-positive fibers crossing the midline to the denervated spinal cord. C, AMPH/ ENR+FA treatment induced many midline BDA-positive crossing fibers (arrows). D, Quantification of BDA-positive midline-crossing fibers shows a significant difference between the AMPH/ENR+FA treated group compared with the control groups. **P<0.01; one-way ANOVA. Midline-crossing fibers at the level of the cervical enlargement was plotted against performance in the forelimb reaching task (E) and ladder rung walk (F). Linear regression analysis showed a significant correlation of corticospinal fiber crossing and forelimb function. Scale bars in (B) and (C)=100 μm. Error bars indicate SE.
Discussion
In the present study, we demonstrated that the level of physical activity profoundly affects the efficacy of short-term AMPH in improving motor outcome and for inducing axonal growth after stroke. The latter observation is significant because direct evidence of amphetamine-induced axonal growth and reinnervation of motor areas suggests that lasting anatomical changes can be achieved with short-term treatment. Only when paired with focused activity and an enriched environment did AMPH induce motor recovery to preoperative performance and elicit measurable axonal outgrowth. By comparison, AMPH alone or various rehabilitation strategies alone induced a significant but limited level of improvement over control animals.
Despite numerous preclinical studies that demonstrate the motor recovery-enhancing effects of AMPH after brain damage, the rehabilitation efficacy of AMPH in treating stroke in humans remains unclear.15 The duration of AMPH administration appears to be an important variable in that limited AMPH treatment is likely to yield negative results, but the reported trend toward cardiovascular side effects caused by AMPH seen in clinical studies suggest the need for caution.15 For these reasons, we chose to focus on using a restricted AMPH regimen (Postoperative Days 2, 5, and 8) to determine how different levels of “rehabilitation” (ie, enriched environment alone or enriched environment with focused activity sessions) might modify the effects AMPH. An enriched environment in an experimental setting is defined as “a combination of complex inanimate and social stimulation.”29 In this context, we provided toys and group-housed animals in larger cages, but animals were left to interact spontaneously. Focused activity sessions took the form of climbing activities that had similar forelimb motor-specific requirements as the motor tasks being assessed (ie, grasping, grip strength, placement, and coordination). Superimposing focused activity sessions on this environment was designed to provide more intensive motor practice but in a manner distinct from the actual motor testing procedure. To avoid the potential confounds of AMPH influencing performance in the behavioral motor assessment tasks, we administered AMPH after behavioral test sessions but paired closely in time (ie, 10 minutes before) to the focused activity sessions. Under these conditions, AMPH paired with focused activity led to a full recovery to preoperative baseline performance in both motor tests. Because our analysis only included data from the preferred or “affected” forelimb, this recovery cannot be attributed to increased use of the nonaffected (ie, ipsilesional) forelimb. However, it should be noted that compensatory behavioral adaptations have been shown to contribute to improvement in motor performance after stroke.30 Moreover, studies have shown that ipsilesional forelimb function can either be enhanced or diminished, possibly depending on lesion size, in a manner that can affect motor performance.31,32 It would be interesting to determine how AMPH affects neurobiological mechanisms related to the nonaffected hemisphere.
The administration of AMPH to animals housed in the enriched environment alone (ie, not receiving additional focused activity) had no effect on motor performance as compared with vehicle-treated animals housed under enriched conditions. This latter finding is consistent with the recent study of Alaverdashvili et al33 in which amphetamines administered to rats in an enriched environment had no effect on skilled reaching performance after ischemic cortical damage. However, it must be considered that the D-methamphetamine used in the latter study is a somewhat less potent releaser of norepinephrine as compared with D-amphetamine sulfate.24 This difference may be important given that noradrenergic mechanisms have been implicated in facilitating motor improvement after brain damage (discussed subsequently).
It could be argued that our form of enriched environment was sufficiently different from what has been used in previous studies and therefore failed to engage the necessary mechanisms to allow for improved motor function. This seems unlikely because we observed a partial improvement in skilled reaching (but not ladder rung walk) in vehicle-treated animals housed in an enriched environment that was similar in extent to what has been previously reported (approximately 70%).20,22,23 Interestingly, adding focused activity sessions to animals in an enriched environment did not result in further motor improvement. Taken together, these data emphasize the importance of the physical rehabilitation strategy used in our study and suggest that focused physical therapy is needed to realize the therapeutic benefits of AMPH.
The observation that only 3 pairings of AMPH and focused activity were required to retain long-term benefits suggests that clinical rehabilitation strategies can restrict the use of AMPH and thereby reduce the propensity for cardiovascular side effects. It is difficult to directly compare the dose of D-amphetamine sulfate used in the present study (2 mg/kg based on salt weight) with what has been used in humans because of the rapid distribution and elimination of AMPH in the rat (T1/2=45 minutes)34 compared with that seen in patients with stroke (T1/2=14 hour).35 However, the current dose is representative of what has been used in previous experimental studies.3 The finding of long-term benefit after short-term exposure is consistent with the knowledge that short-term administration of AMPH induces profound and persistent changes in intracellular signaling, gene transcription as well as altered synaptic morphology.18,19,36 The mechanisms involved in this plasticity may include an up-regulation in growth factors such as basic fibroblast growth factor. Basic fibroblast growth factor is a potent neurotrophic factor that has been shown to enhance recovery of motor function after ischemic brain damage, presumably by contributing to synaptogenesis.37–42 After brain damage, basic fibro-blast growth factor expression shows a persistent increase that can last for weeks43,44 and which can be localized to both neuronal and astrocyte populations.45,46 In addition, antibody neutralization of basic fibroblast growth factor leads to impaired motor recovery after brain damage.42 Basic fibro-blast growth factor has also been implicated in mediating AMPH-induced neural and behavioral plasticity.47,48 Interestingly, AMPH induces a persistent increase in basic fibroblast growth factor that lasts for at least 1 month after a similar protocol of 3 injections.47 The histological processing of tissue necessary for the current study precluded a quantitative analysis of cortical basic fibroblast growth factor levels by immunoblotting or immunoassay. Although the neurobiological mechanisms underlying AMPH-induced recovery are unclear, the pharmacological mechanisms appear to involve noradrenergic and/or dopaminergic transmission. Goldstein and Bullman49 demonstrated that 6-hydroxydopamine-induced lesion of the medial forebrain bundle in the contralesional hemisphere before an aspiration lesion blocked AMPH-enhanced recovery on beam walking. Additional experimental studies have demonstrated that intracerebral administration of norepinephrine improves, and noradrenergic antagonists impair, motor recovery after brain damage.50,51 Studies are currently underway to further delineate the pharmacological and neurobiological mechanisms involved in mediating the effects of AMPH on neurite outgrowth in vitro and axonal growth in vivo.
Although a causative relationship was not established, the present study demonstrated that increased corticoefferent sprouting from the contralesional cortex to denervated subcortical motor areas is associated with motor improvement. Despite the inherent complexities in relating neural plasticity to skilled motor behavior addressed by Whishaw et al,52 numerous studies have shown that axonal outgrowth from the opposite cortex can occur and contribute to motor recovery. In neonatal rats incurring unilateral sensorimotor cortical lesions, the contralesional sensorimotor cortex sends new corticoefferent projections to subcortical motor areas, including the striatum,53 thalamus,54 red nucleus,55,56 basilar pontine nuclei,57,58 and spinal cord.59,60 Further preclinical studies suggest that these new neuronal connections are responsible for improved functional recovery after neonatal brain injury.61–63 Likewise, our past work has shown that suppression of the actions of neurite growth inhibitors can markedly enhance functional recovery and axonal outgrowth from the contralesional hemisphere after experimental stroke or cortical aspiration lesions.25,26,64 More recently, we have shown corticoefferent sprouting from the contralesional cortex to the denervated pontine nuclei that was associated with motor recovery after AMPH treatment combined with rehabilitation after an aspiration lesion.3 In the adult brain, neuronal growth and reorganization after injury also occurs and rehabilitation is believed to contribute to this process.65–67 However, spontaneous redirection of major corticoefferent pathways has not been seen.
Taken together, the present findings suggest that short-term administration of amphetamine, when paired with sufficiently focused physical therapy, is a valuable treatment strategy for long-term motor recovery after ischemic stroke. However, care should be taken when extrapolating our results in animals to the design of patient clinical trials. Our data also extend previous biochemical findings by implicating axonal outgrowth from the contralesional cortex as a mechanism for recovery. The recovery of function after AMPH treatment likely relies on the ability of AMPH to activate mechanisms associated with the formation of new motor pathways and support the hypothesis that the addition of focused activity “targets” these new connections toward improvement of motor function.68 Studies are underway to delineate the pharmacological and neurobiological mechanisms involved.
Acknowledgments
Sources of Funding
This work was supported by the Department of Veterans Affairs and PHS Grants HD 44772 (to W.A.W.) and NS 40960 (to G.L.K.).
Footnotes
Disclosures
None.
References
- 1.American Heart Association. Heart Disease and Stroke Statistics—2008 Update. Dallas: American Heart Association; [Google Scholar]
- 2.Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- 3.Ramic M, Emerick AJ, Bollnow MR, O’Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 4.Stroemer RP, Kent TA, Hulsebosch CE. Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke. 1998;29:2381–2393. doi: 10.1161/01.str.29.11.2381. [DOI] [PubMed] [Google Scholar]
- 5.Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- 6.Hovda DA, Fenney DM. Amphetamine with experience promotes recovery of locomotor function after unilateral frontal cortex injury in the cat. Brain Res. 1984;298:358–361. doi: 10.1016/0006-8993(84)91437-9. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein LB, Davis JN. Influence of lesion size and location on amphetamine-facilitated recovery of beam-walking in rats. Behav Neurosci. 1990;104:320–327. doi: 10.1037//0735-7044.104.2.320. [DOI] [PubMed] [Google Scholar]
- 8.Schmanke TD, Avery RA, Barth TM. The effects of amphetamine on recovery of function after cortical damage in the rat depend on the behavioral requirements of the task. J Neurotrauma. 1996;13:293–307. doi: 10.1089/neu.1996.13.293. [DOI] [PubMed] [Google Scholar]
- 9.Adkins DL, Jones TA. D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci Lett. 2005;380:214–218. doi: 10.1016/j.neulet.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 10.Gilmour G, Iversen SD, O’Neill MF, O’Neill MJ, Ward MA, Bannerman DM. Amphetamine promotes task-dependent recovery following focal cortical ischaemic lesions in the rat. Behav Brain Res. 2005;165:98–109. doi: 10.1016/j.bbr.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 11.Crisostomo EA, Duncan PW, Propst M, Dawson DV, Davis JN. Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol. 1988;23:94–97. doi: 10.1002/ana.410230117. [DOI] [PubMed] [Google Scholar]
- 12.Walker-Batson D, Smith P, Curtis S, Unwin H, Greenlee R. Amphetamine paired with physical therapy accelerates motor recovery after stroke. Further evidence. Stroke. 1995;26:2254–2259. doi: 10.1161/01.str.26.12.2254. [DOI] [PubMed] [Google Scholar]
- 13.Treig T, Werner C, Sachse M, Hesse S. No benefit from D-amphetamine when added to physiotherapy after stroke: a randomized, placebo-controlled study. Clin Rehabil. 2003;17:590–599. doi: 10.1191/0269215503cr653oa. [DOI] [PubMed] [Google Scholar]
- 14.Martinsson L, Hardemark HG, Eksborg S. Should amphetamines be given to improve recovery after stroke? Stroke. 2007;38:2400–2401. [Google Scholar]
- 15.Martinsson L, Hardemark H, Eksborg S. Amphetamines for improving recovery after stroke. Cochrane Database Syst Rev. 2007;1:CD002090. doi: 10.1002/14651858.CD002090.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB. Amphetamines and related drugs in motor recovery after stroke. Phys Med Rehabil Clin N Am. 2003;14:S125–134. doi: 10.1016/s1047-9651(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 17.Long D, Young J. Dexamphetamine treatment in stroke. QJM. 2003;96:673–685. doi: 10.1093/qjmed/hcg113. [DOI] [PubMed] [Google Scholar]
- 18.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 19.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 20.Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–5280. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson BB, Ohlsson AL. Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol. 1996;139:322–327. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- 22.Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. J Cereb Blood Flow Metab. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Keyvani K, Sachser N, Witte OW, Paulus W. Gene expression profiling in the intact and injured brain following environmental enrichment. J Neuropathol Exp Neurol. 2004;63:598–609. doi: 10.1093/jnen/63.6.598. [DOI] [PubMed] [Google Scholar]
- 24.Kuczenski R, Segal DS, Cho AK, Melega W. Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine. J Neurosci. 1995;15:1308–1317. doi: 10.1523/JNEUROSCI.15-02-01308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadopoulos CM, Tsai SY, Alsbiei T, O’Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 26.Emerick AJ, Kartje GL. Behavioral recovery and anatomical plasticity in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. Behav Brain Res. 2004;152:315–325. doi: 10.1016/j.bbr.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- 28.Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–576. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- 30.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 31.Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–199. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- 32.Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006;201:479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Alaverdashvili M, Lim DH, Whishaw IQ. No improvement by amphetamine on learned non-use, attempts, success or movement in skilled reaching by the rat after motor cortex stroke. Eur J Neurosci. 2007;25:3442–3452. doi: 10.1111/j.1460-9568.2007.05594.x. [DOI] [PubMed] [Google Scholar]
- 34.Melega WP, Williams AE, Schmitz DA, DiStefano EW, Cho AK. Pharmacokinetic and pharmacodynamic analysis of the actions of D-amphetamine and D-methamphetamine on the dopamine terminal. J Pharmacol Exp Ther. 1995;274:90–96. [PubMed] [Google Scholar]
- 35.Martinsson L, Yang X, Beck O, Wahlgren NG, Eksborg S. Pharmacokinetics of dexamphetamine in acute stroke. Clin Neuropharmacol. 2003;26:270–276. doi: 10.1097/00002826-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Park YH, Kantor L, Wang KK, Gnegy ME. Repeated, intermittent treatment with amphetamine induces neurite outgrowth in rat pheochromocytoma cells (PC12 cells) Brain Res. 2002;951:43–52. doi: 10.1016/s0006-8993(02)03103-7. [DOI] [PubMed] [Google Scholar]
- 37.Baird A. Potential mechanisms regulating the extracellular activities of basic fibroblast growth factor (FGF-2) Mol Reprod Dev. 1994;39:43–48. doi: 10.1002/mrd.1080390108. [DOI] [PubMed] [Google Scholar]
- 38.Patel MN, McNamara JO. Selective enhancement of axonal branching of cultured dentate gyrus neurons by neurotrophic factors. Neuroscience. 1995;69:763–770. doi: 10.1016/0306-4522(95)00281-m. [DOI] [PubMed] [Google Scholar]
- 39.Kawamata T, Dietrich WD, Schallert T, Gotts JE, Cocke RR, Benowitz LI, Finklestein SP. Intracisternal basic fibroblast growth factor enhances functional recovery and up-regulates the expression of a molecular marker of neuronal sprouting following focal cerebral infarction. Proc Natl Acad Sci U S A. 1997;94:8179–8184. doi: 10.1073/pnas.94.15.8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawamata T, Speliotes EK, Finklestein SP. The role of polypeptide growth factors in recovery from stroke. Adv Neurol. 1997;73:377–382. [PubMed] [Google Scholar]
- 41.Kawamoto Y, Nakamura S, Kawamata T, Akiguchi I, Kimura J. Cellular localization of brain-derived neurotrophic factor-like immunoreactivity in adult monkey brain. Brain Res. 1999;821:341–349. doi: 10.1016/s0006-8993(99)01082-3. [DOI] [PubMed] [Google Scholar]
- 42.Rowntree S, Kolb B. Blockade of basic fibroblast growth factor retards recovery from motor cortex injury in rats. Eur J Neurosci. 1997;9:2432–2441. doi: 10.1111/j.1460-9568.1997.tb01660.x. [DOI] [PubMed] [Google Scholar]
- 43.Takami K, Iwane M, Kiyota Y, Miyamoto M, Tsukuda R, Shiosaka S. Increase of basic fibroblast growth factor immunoreactivity and its mRNA level in rat brain following transient forebrain ischemia. Exp Brain Res. 1992;90:1–10. doi: 10.1007/BF00229250. [DOI] [PubMed] [Google Scholar]
- 44.Reilly JF, Kumari VG. Alterations in fibroblast growth factor receptor expression following brain injury. Exp Neurol. 1996;140:139–150. doi: 10.1006/exnr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 45.Chadi G, Tinner B, Agnati LF, Fuxe K. Basic fibroblast growth factor (bFGF, FGF-2) immunoreactivity exists in the noradrenaline, adrenaline and 5-HT nerve cells of the rat brain. Neurosci Lett. 1993;160:171–176. doi: 10.1016/0304-3940(93)90406-b. [DOI] [PubMed] [Google Scholar]
- 46.Wei OY, Huang YL, Da CD, Cheng JS. Alteration of basic fibroblast growth factor expression in rat during cerebral ischemia. Acta Pharmacol Sin. 2000;21:296–300. [PubMed] [Google Scholar]
- 47.Flores C, Rodaros D, Stewart J. Long-lasting induction of astrocytic basic fibroblast growth factor by repeated injections of amphetamine: blockade by concurrent treatment with a glutamate antagonist. J Neurosci. 1998;18:9547–9555. doi: 10.1523/JNEUROSCI.18-22-09547.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flores C, Samaha AN, Stewart J. Requirement of endogenous basic fibroblast growth factor for sensitization to amphetamine. J Neurosci. 2000;20:RC55. doi: 10.1523/JNEUROSCI.20-02-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldstein LB, Bullman S. Effects of dorsal noradrenergic bundle lesions on recovery after sensorimotor cortex injury. Pharmacol Biochem Behav. 1997;58:1151–1157. doi: 10.1016/s0091-3057(97)00324-9. [DOI] [PubMed] [Google Scholar]
- 50.Feeney DM, De Smet AM, Rai S. Noradrenergic modulation of hemiplegia: facilitation and maintenance of recovery. Restor Neurol Neurosci. 2004;22:175–190. [PubMed] [Google Scholar]
- 51.Goldstein LB. Neurotransmitters and motor activity: effects on functional recovery after brain injury. NeuroRx. 2006;3:451–457. doi: 10.1016/j.nurx.2006.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. 2008;192:124–136. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 53.Kolb B, Gibb R, van der KD. Cortical and striatal structure and connectivity are altered by neonatal hemidecortication in rats. J Comp Neurol. 1992;322:311–324. doi: 10.1002/cne.903220303. [DOI] [PubMed] [Google Scholar]
- 54.Yu XH, Moret V, Rouiller EM. Re-examination of the plasticity of the corticothalamic projection after unilateral neonatal lesion of the sensorimotor cortex in the rat: a phaseolus vulgaris-leucoagglutinin tracing study. J Hirnforsch. 1995;36:123–133. [PubMed] [Google Scholar]
- 55.Nah SH, Leong SK. Bilateral corticofugal projection to the red nucleus after neonatal lesions in the albino rat. Brain Res. 1976;107:433–436. doi: 10.1016/0006-8993(76)90242-0. [DOI] [PubMed] [Google Scholar]
- 56.Naus C, Flumerfelt BA, Hrycyshyn AW. An anterograde HRP-WGA study of aberrant corticorubral projections following neonatal lesions of the rat sensorimotor cortex. Exp Brain Res. 1985;59:365–371. doi: 10.1007/BF00230916. [DOI] [PubMed] [Google Scholar]
- 57.Castro AJ, Mihailoff GA. Corticopontine remodelling after cortical and/or cerebellar lesions in newborn rats. J Comp Neurol. 1983;219:112–123. doi: 10.1002/cne.902190111. [DOI] [PubMed] [Google Scholar]
- 58.Kartje-Tillotson G, Neafsey EJ, Castro AJ. Topography of corticopontine remodelling after cortical lesions in newborn rats. J Comp Neurol. 1986;250:206–214. doi: 10.1002/cne.902500207. [DOI] [PubMed] [Google Scholar]
- 59.Kartje-Tillotson G, Neafsey EJ, Castro AJ. Electrophysiological analysis of motor cortical plasticity after cortical lesions in newborn rats. Brain Res. 1985;332:103–111. doi: 10.1016/0006-8993(85)90393-2. [DOI] [PubMed] [Google Scholar]
- 60.Rouiller EM, Liang FY, Moret V, Wiesendanger M. Trajectory of redirected corticospinal axons after unilateral lesion of the sensorimotor cortex in neonatal rat; a phaseolus vulgaris-leucoagglutinin (PHA-L) tracing study. Exp Neurol. 1991;114:53–65. doi: 10.1016/0014-4886(91)90084-p. [DOI] [PubMed] [Google Scholar]
- 61.Goldberger ME. Mechanisms contributing to sparing of function following neonatal damage to spinal pathways. Neurochem Pathol. 1986;5:289–307. doi: 10.1007/BF02842940. [DOI] [PubMed] [Google Scholar]
- 62.Kartje-Tillotson G, O’Donoghue DL, Dauzvardis MF, Castro AJ. Pyramidotomy abolishes the abnormal movements evoked by intracortical microstimulation in adult rats that sustained neonatal cortical lesions. Brain Res. 1987;415:172–177. doi: 10.1016/0006-8993(87)90283-6. [DOI] [PubMed] [Google Scholar]
- 63.Kolb B, Whishaw IQ. Plasticity in the neocortex: mechanisms underlying recovery from early brain damage. Prog Neurobiol. 1989;32:235–276. doi: 10.1016/0301-0082(89)90023-3. [DOI] [PubMed] [Google Scholar]
- 64.Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O’Brien TE, Castro AJ, Schwab ME, Kartje GL. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 65.Schaechter JD. Motor rehabilitation and brain plasticity after hemiparetic stroke. Prog Neurobiol. 2004;73:61–72. doi: 10.1016/j.pneurobio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–1647. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 68.Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–1528. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]