Abstract
We have developed a superfusion method utilizing an open-volume microfluidic device for administration of pharmacologically active substances to selected areas in brain slices with high spatio-temporal resolution. The method consists of a hydrodynamically confined flow of the active chemical compound, which locally stimulates neurons in brain slices, applied in conjunction with electrophysiological recording techniques to analyze the response. The microfluidic device, which is a novel free-standing multifunctional pipette, allows diverse superfusion experiments, such as testing the effects of different concentrations of drugs or drug candidates on neurons in different cell layers with high positional accuracy, affecting only a small number of cells. We demonstrate herein the use of the method with electrophysiological recordings of pyramidal cells in hippocampal and prefrontal cortex brain slices from rats, determine the dependence of electric responses on the distance of the superfusion device from the recording site, document a multifold gain in solution exchange time as compared to whole slice perfusion, and show that the device is able to store and deliver up to four solutions in a series. Localized solution delivery by means of open-volume microfluidic technology also reduces reagent consumption and tissue culture expenses significantly, while allowing more data to be collected from a single tissue slice, thus reducing the number of laboratory animals to be sacrificed for a study.
Keywords: Microfluidic technology, Perfusion, Brain slices, Prefrontal cortex, Hippocampus, Glutamate, Electrophysiological techniques
Introduction
In vitro brain slices constitute a valuable experimental model system for studying communication between, or the effects of drugs on, neurons and astrocytes in an environment which preserves the cellular network, i.e. the organization in cell layers, processes, and synapses. Unlike in vivo studies on brain using live anesthetized animals, experiments on brain slice preparations have the advantage that the influence of anesthetics on cellular functions is eliminated. Single cells are more easily accessible by probes and imaging techniques, which enables precise physiological and pharmacological studies of the functions and properties of the neuronal networks present in these slices.
There are several examples of micro-perfusion devices designed for extracellular delivery of biologically active substances to brain slices. For example, glass micropipettes, with the capability of applying substances via a laminar flow through a micro scale aperture, have been widely used (for review see Huang et al., 2012). However, control of flow dynamics is quite limited due to diffusion, limiting solution confinement in addition to challenging device fabrication and complex construction.
Microfluidic devices are emerging as powerful tools for neuroscientists (Huang et al., 2012), allowing spatiotemporal control over solution delivery to the extracellular environment around neurons and astrocytes in brain tissue preparations. The major ambitions of microfluidics in the context of brain tissue slices are to provide efficient nutrient and oxygen delivery, in addition to waste removal, while allowing spatio-temporal control over the local chemical environment, and enabling diverse imaging and probing techniques. Solutions to one or more of these problems have emerged, utilizing microfluidic flow chambers which support interstitial flow for better gas penetration of thick tissue slices (Rambani et al., 2009), or focal perfusion within conventional slice chambers for improved control over the solution environment in a selected slice region (Blake et al., 2007). A new promising approach is the use of hydrodynamically confined flow (HCF) technology for localized microperfusion (Queval et al., 2010). By continuous uptake of the delivered solution, HCF devices produce a confined liquid volume outside the device, i.e., a virtual flow chamber which merely touches a selected area of the tissue slice. This principle is an extension of the many variants of the push-pull arrangement of glass capillaries (Veselovski et al., 1996). The use of microfabricated devices eliminates the disadvantages associated with glass needles, and adds additional benefits such as facilitated interfacing and a broader choice of materials.
A distinct problem with the initially introduced HCF devices is the difficulty to use conventional light microscopes, as the vertical architecture with perpendicular apertures blocks the optical path. Moreover, it is impossible to use auxiliary probes, such as electrophysiological recording electrodes, in conjunction with this superfusion architecture. We have recently developed a HCF based free-standing multifunctional pipette in polydimethylsiloxane (PDMS) which largely overcomes these disadvantages (Ainla et al., 2010; Ainla et al., 2012). Briefly, the test solution is selected from a set of local reservoirs by an internal fast-acting liquid switch zone, and fed to a delivery channel at the tip of the pipette, close to the biological specimen. Vacuum channels located on either side of the delivery channel enable the formation of a virtual flow chamber of the test solution. The device is applied at an angle to the microscope table, allowing additional probes to be used in tandem, addressing the same environment at the tip of the pipette.
Here, we demonstrate the capabilities and benefits of the multifunctional pipette to administer the glutamate receptor agonist α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and its antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to electrophysiologically recorded pyramidal cells within rat hippocampal and cortical brain slices.
2. Materials and methods
2.1 Preparation of brain slices
2.1.1 Hippocampus
Hippocampal slices were prepared (see Abbas et al., 2011) from 2- to 4-week-old Sprague-Dawley rats (Scanbur BK, Sollentuna, Sweden). The procedures conformed to the guidelines of the Swedish Council for Laboratory Animals and were approved by the Gothenburg Ethical Committee for Animal Experimentation. The animals were sacrificed by decapitation after initial isoflurane anesthesia, and the hippocampi were quickly dissected. Transverse slices (400 µm thick) were prepared by an in-house designed McIlwain-type tissue chopper and placed in a holding chamber containing an oxygenated Ringer’s solution at room temperature, composed of (in mM): NaCl 119, KCl 2.5, CaCl2 2.0, MgCl2 2.0, NaHCO3 26, NaH2PO4 1.0, and D-glucose 10, gassed with 95% O2/ 5% CO2. After storage for at least 90 min, the slices were transferred as needed to a recording chamber where they were submerged by a continuously superfusing solution saturated with 95% O2 and 5% CO2. The composition of the perfusion solution was the same as in the holding chamber except for using 2.5 mM CaCl2 and 1.3 mM MgCl2, with the pH balanced to 7.4. A peristaltic pump (Ismatec, Labinett Lab AB, Sweden) was used to recirculate the solution (1.5–2.0 mL/min), keeping the flow rate constant and avoiding any flow artifacts. Experiments were performed at 30–31°C.
2.1.2 Medial prefrontal cortex
Male Sprague-Dawley rats (Charles River, Germany) were used (80–250 g) for all the experiments. The animals were housed under standard laboratory conditions and maintained on a 12 hour light/dark cycle (lights on at 06:00) with ad libitum access to food and water. All experiments at Karolinska Institutet were approved by, and conducted in accordance with, the Stockholm North Committee on Ethics of Animal Experimentation. Procedures for the preparation of rat medial prefrontal cortex (mPFC) slices have been described previously (Arvanov et al., 1997; Arvanov and Wang, 1998; Konradsson et al., 2006). Briefly, the rats were decapitated under halothane anaesthesia. The brains were then rapidly removed and cooled in ice-cold Ringer’s solution (pH 7.4) consisting of (in mM): NaCl 126, KCl 2.5, CaCl2 2.4, MgCl2 1.3, NaH2PO4 1.2, D-glucose 10, NaHCO3 18, and oxygenated with 95% O2/ 5% CO2. The brains were then cut coronally, using a Vibroslice (Campden model MA 752, World Precision Instruments, Sarasota, FL, USA) instrument, in order to produce 450 µm slices. The brain slices were removed from the instrument and kept submerged in oxygenated Ringer’s solution at room temperature for at least 1 hour to allow for recovery.
2.2 Electrophysiological recordings
2.2.1 Extracellular recording of field excitatory postsynaptic potentials (fEPSPs) in the hippocampus
The commissural–Schaffer collateral pathway of the hippocampal CA1 area was stimulated using an in-house developed programmable pulse generator. Stimuli consisted of 100 µs, 20–50 µA, negative constant-current pulses, delivered via an insulated sharpened tungsten wire (type TM33B01, World Precision Instr., FL, USA) at a rate of 0.1–0.2 Hz. Extracellular field potentials were recorded in the middle of the pyramidal cell dendritic layer (stratum radiatum), 200–500 µm away from the stimulating electrode, via a glass micropipette filled with 1 M NaCl (resistance 3–5 MΩ; made from Kwik-Fil borosilicate glass capillaries, World Precision Instr., FL, USA; pulled by a P-97, Sutter Instruments, Novato, CA, USA). Signals were amplified, filtered, digitized and transferred to a computer for analysis, using electronic equipment based on an Eagle Technology (RSA) multifunction board. Off-line data analysis was performed using pCLAMP-Clampfit software (Molecular Devices, CA, USA). The size of the recorded fEPSP, which was dominated by the contribution from AMPA-type glutamate receptors, was estimated as the negative peak amplitude relative to the pre-stimulus baseline. This measurement provides an index of the efficacy of AMPA receptor-mediated synaptic transmission (Muller et al., 1988; Muller et al., 1989; Shahi and Baudry, 1992).
2.2.2 Intracellular recording of AMPA-induced currents in medial prefrontal cortex
A single slice containing the mPFC was transferred to a recording chamber (32°C) and was held submerged between two nylon nets. The chamber was continuously perfused with oxygenated Ringer’s solution at a flow rate of 1–2 mL/min. A standard intracellular single-electrode technique was used to record from pyramidal cells in layers V and VI of the mPFC slices, as described previously (Arvanov et al., 1997; Konradsson et al., 2006). Briefly, electrodes were pulled from borosilicate glass capillaries (Warner Instruments, Hamden, CT, USA) using a horizontal electrode puller (Model P-87, Sutter Instruments, Novato, CA, USA). The electrodes were filled with 2 M potassium acetate and used for intracellular recordings of pyramidal cells in the mPFC, with an Axoclamp 2A amplifier (Axon Instruments, Foster City, CA, USA). Voltage-clamp (holding potential: −60 mV) recordings were performed in the discontinuous mode with a sampling rate of 5–6.2 kHz, and acquired using digital/analogue sampling and data acquisition software (Clampex 9, Axon Instruments, Foster City, CA, USA). Tetrodotoxin (TTX; 0.5 µM), glycine (1 µM), and bicuculline (5 µM) were routinely included in the perfusion solution during the recordings.
2.3 Drugs and Buffers
Potassium chloride, sodium dihydrogen phosphate, calcium chloride dihydrate, sodium hydrogen carbonate and potassium acetate were all obtained from Merck (Darmstadt, Germany). Magnesium chloride hexahydrate, AMPA, glycine, and bicuculline methiodide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Glucose was purchased from VWR international (Leicestershire, UK) and sodium chloride from Riedel deHaen (Seelze, Germany). CNQX was obtained from Tocris Bioscience (Bristol, UK) or Ascent Scientific Ltd (UK). TTX (citrate buffer) was obtained from Tocris Bioscience (Bristol, UK) and halothane (fluothane) was purchased from Astra Zeneca (Stanhope Gate, London, UK).
2.4 Multifunctional pipette
The multifunctional pipette has been described previously (Ainla et al., 2012), from which operation parameters, such as air pressure and vacuum and inflow/outflow ratio were extracted. Briefly, a flow rate of ~ 10nl/s was applied forming a recirculation zone at the tip of the pipette. The pipette tip was positioned so that a distance of 10 µm was maintained from the tissue surface. Prior to loading into the pipette, all solutions were filtered through a 0.2 µm syringe filter, to prevent blockages within the microfluidic device.
In some of the experiments, the multifunctional pipette was compared with a simple “Picospritzer type” pressure ejector, i.e. a glass pipette (ca 5 MΩ resistance) connected to a source of compressed air (1 atm) via a computer controlled electromagnetic valve. The glass pipette was filled with CNQX solution and superficially inserted into the slice tissue towards the recording pipette, keeping the distance between them within 200 µm. Ejection of CNQX was conducted by a series of brief pressure pulses (50 ms each, delivered at 1–2 Hz) applied for 1–2 min.
3. Results and Discussion
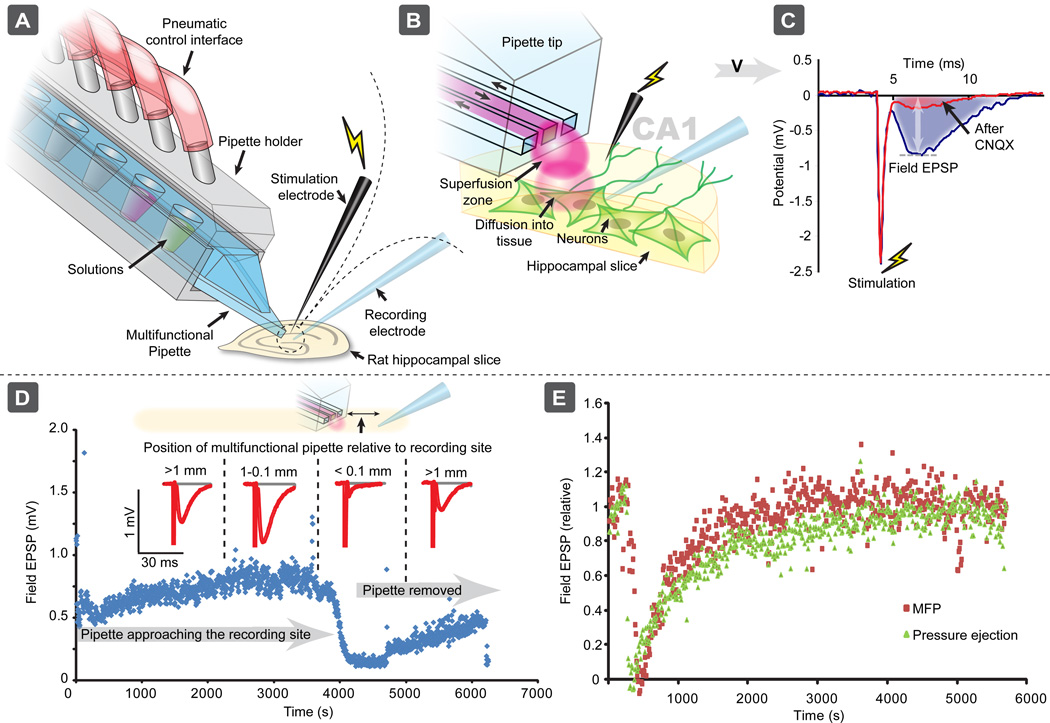
The present experimental applications demonstrate the ability of the multifunctional pipette to administer biologically active substances, to specific parts or regions of brain slices, with high spatial and temporal resolution. To characterize the spatial resolution of the pipette, electrically stimulated fEPSPs were recorded extracellularly in vitro, in the CA1 region of rat hippocampal slices (Fig. 1a). The fEPSPs were blocked by the AMPA receptor-antagonist CNQX which was locally delivered to the recording site by the multifunctional pipette (Figs. 1b and 1c). Figure 1d illustrates the spatial resolution of CNQX exposure, by the pipette. The pipette was gradually translated closer to the recording site, until the distance to the pipette tip was < 0.1 mm, the approximate diameter of the superfusion zone. Inhibition of the fEPSP was observed, implying that the targeted cells were being exposed to CNQX. The electrically stimulated fEPSPs immediately started to recover after the pipette was moved > 0.1 mm from the site of recording in the slice. Thus, by using the multifunctional pipette, biologically active substance can be delivered to a very small extracellular volume, where it can control the pharmacology of the brain slice in a strictly localized manner.
Fig. 1.
The multifunctional pipette for local administration of biologically active substances to cells in brain slices. a) and b) The pipette was used to locally deliver CNQX for blocking electrically stimulated fEPSPs in the CA1 region of a hippocampal brain slice, by exposing the surface to the recirculation region at the tip of the pipette (red volume). c) and d) The electrically stimulated fEPSP was reduced by CNQX with the multifunctional pipette at the CA1 region of rat hippocampal slice. When the multifunctional pipette was positioned within 100 µm from the recording site, i.e. a distance which was the diameter of the recirculating area of the pipette, the fEPSP was immediately reduced by administered CNQX. The fEPSPs began to recover when the pipette was moved away from the recording electrode. e) Temporal comparison of the multifunctional pipette with pressure ejection in delivering CNQX to pyramidal cells in the CA1 region of hippocampal brain slices.
We also compared the multifunctional pipette with a conventional pressure ejection system. Here, a glass pipette was inserted into the hippocampal tissue at a distance less than 200 µm from the recording pipette. Fig. 1e shows that the temporal resolution of delivering CNQX by the multifunctional pipette and by the pressure ejection system is comparable. However, the multifunctional pipette system offers additional possibilities, such as delivery of a series of concentrations of a biologically active substance or variable positioning of the pipette on different spots of the tissue without “contaminating” the entire slice preparation. Thus, the multifunctional pipette may offer several advantages compared to conventional perfusion techniques (e.g. pressure ejection).
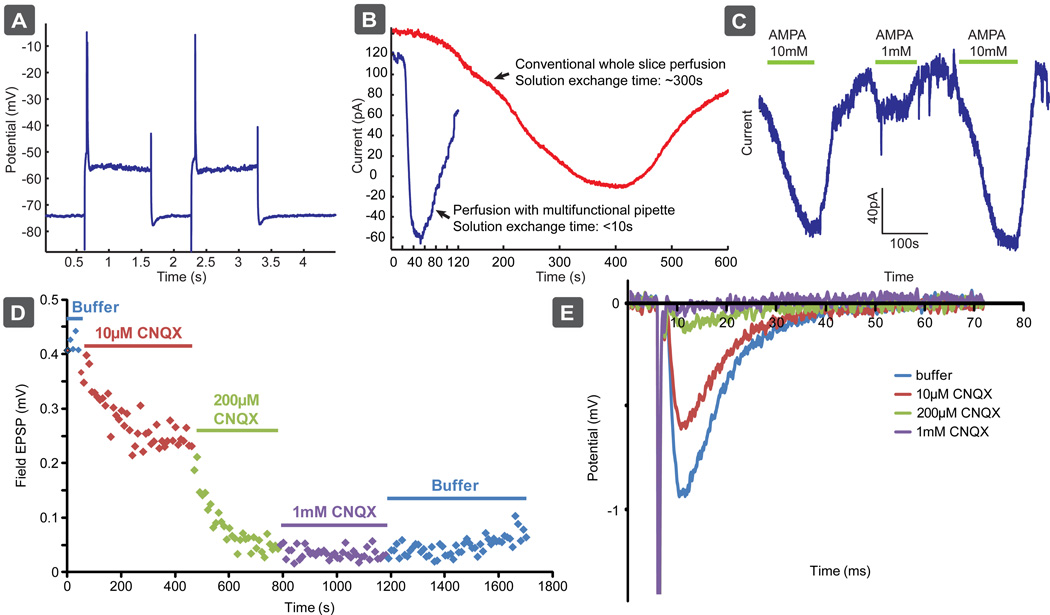
In another test of the temporal resolution of the multifunctional pipette, we examined the ability of the pipette to administer the glutamate receptor agonist AMPA to intracellularly recorded pyramidal cells of the rat mPFC. The electrophysiological criteria for distinguishing pyramidal from non-pyramidal cells in layers V and VI of the mPFC have been described previously (Arvanov et al., 1997; Jardemark et al., 2005; Konradsson et al., 2006). Briefly, the presumed pyramidal cells of the mPFC have relatively a long spike duration (1–3 ms at half-maximum spike amplitude) and show a pronounced spike-frequency adaptation in response to constant-current depolarization pulses (see Fig. 2a, illustrating that only single spikes were induced by long pulses). As seen in Figure 2b, the multifunctional pipette leads to a substantially faster response time (seconds) than conventional whole chamber perfusion (minutes). Since the drug is delivered along with the circulating buffer in a conventional scheme, the drug concentration in the bath will gradually increase before it reaches a steady state, and gradual decrease at the end of drug application. Although the response time was faster than with conventional perfusion it was slower than can be achieved with local perfusion of isolated cells, often <0.2 s (see Huang et al., 2012; Ainla et al., 2012) due to the depth of the cells under investigation. As the cells in tissue experiments are embedded in surrounded tissue, a significant diffusion barrier is present, slowing down the localized delivery.
Fig. 2.
Local administration of AMPA and CNQX at different concentrations by the microfluidic pipette to recorded pyramidal cells of the rat mPFC under intracellular recording, and to hippocampal slices under field potential recording. a) Electrophysiological characterization of the pyramidal cells in the mPFC. b) The temporal resolution of AMPA administration to intracellularly recorded pyramidal cells in the mPFC was significantly improved when using the microfluidic pipette compared to when conventional whole slice perfusion was used. c), d), and e) The microfluidic pipette contains multiple reservoirs which enables local administration of different concentrations of agonists (e.g. AMPA) and antagonists (e.g. CNQX) to pyramidal cells of the mPFC (c) and hippocampus (d, e), respectively.
An additional benefit of the pipette is its ability to house multiple solutions within integrated solution reservoirs enabling markedly reduced reagent use, compared to conventional perfusion systems. Moreover, these reservoirs can be preloaded with different drugs, or different drug concentrations, the latter allowing easy determination of concentration-response profiles, examples of which can be seen in Figs. 2c and 2d–e, for varying concentrations of AMPA and CNQX, respectively.
4. Conclusions
We demonstrate high spatial and temporal resolution, for delivery of drugs or drug candidates, at different concentrations to brain slices, using the multifunctional pipette. Application in combination with electrophysiological probes was shown, demonstrating the flexibility with other advanced experimental setups. Through localized administration of AMPA to pyramidal cells, a substantial decrease in response time was observed when using the microfluidic pipette compared to conventional perfusion, allowing kinetics to be probed in greater detail. As the multifunctional pipette operates in open volumes, multiple sites of the tissue slice can be addressed without contamination, enabling greater numbers of experiments to be performed on each slice, minimizing the amount of tissue required.
The multifunctional pipette offers a significant advantage over conventional schemes, both temporally and spatially, while lowering reagent use and tissue required. The spatial flexibility of the pipette may also allow advanced studies of drug effects on specific cells or layers of cells in tissue slices constituting models related to symptoms of diseases, such as psychiatric and neurological diseases.
Highlights.
-
-
A microfluidic device for perfusion of drugs to brain slices is presented.
-
-
This device administers drugs to the slices with high spatio-temporal resolution.
-
-
The tool delivers solution to cells 20× faster than conventional perfusion systems.
-
-
The device is able to store and deliver up to 4 solutions in series.
-
-
The tool is free standing and can be used in combination with other probes.
Acknowledgements
We acknowledge the European Research Council, the Swedish Research Council (VR), the Royal Society of Arts and Sciences in Gothenburg (KVVS), the Wallenberg Foundation, the Åhlén foundation, and the National Institutes of Health (NIH) through grant GM R01-066018.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None of the authors have any conflicts of interest to disclose.
References
- Abbas AK, Huang FS, Li R, Ekström J, Wigström H. Emetine treatment masks initial LTP without affecting long-term stability. Brain Res. 2011;1426:18–29. doi: 10.1016/j.brainres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Ainla A, Jansson ET, Stepanyants N, Orwar O, Jesorka A. A microfluidic pipette for single-cell pharmacology. Anal. Chem. 2010;82:4529–4536. doi: 10.1021/ac100480f. [DOI] [PubMed] [Google Scholar]
- Ainla A, Jeffries GDM, Brune R, Orwar O, Jesorka A. A multifunctional pipette. Lab. Chip. 2012;12:1255–1261. doi: 10.1039/c2lc20906c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-D-aspartate- and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J. Pharmacol. Exp. Ther. 1997;283:226–234. [PubMed] [Google Scholar]
- Arvanov VL, Wang RY. M100907, a selective 5-HT2A receptor antagonist and a potential antipsychotic drug, facilitates N-methyl-D-aspartate-receptor mediated neurotransmission in the rat medial prefrontal cortical neurons in vitro. Neuropsychopharmacology. 1998;18:197–209. doi: 10.1016/S0893-133X(97)00126-7. [DOI] [PubMed] [Google Scholar]
- Blake AJ, Pearce TM, Rao NS, Johnson SM, Williams JC. Multilayer PDMS microfluidic chamber for controlling brain slice microenvironment. Lab. Chip. 2007;7:842–849. doi: 10.1039/b704754a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Williams JC, Johnson SM. Brain slice on a chip: opportunities and challenges of applying microfluidic technology to intact tissues. Lab. Chip. 2012;12:2103–2117. doi: 10.1039/c2lc21142d. [DOI] [PubMed] [Google Scholar]
- Jardemark K, Marcus MM, Konradsson A, Svensson TH. The combination of nicotine with the D2 antagonist raclopride or the weak D4 antagonist L-745,870 generates a clozapine-like facilitation of NMDA receptor-mediated neurotransmission in pyramidal cells of the rat medial prefrontal cortex. Int. J. Neuropsychopharmacol. 2005;8:157–162. doi: 10.1017/S1461145704004742. [DOI] [PubMed] [Google Scholar]
- Konradsson A, Marcus MM, Hertel P, Svensson TH, Jardemark KE. Inhibition of the glycine transporter GlyT-1 potentiates the effect of risperidone, but not clozapine, on glutamatergic transmission in the rat medial prefrontal cortex. Synapse. 2006;60:102–108. doi: 10.1002/syn.20286. [DOI] [PubMed] [Google Scholar]
- Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Muller D, Larson J, Lynch G. The NMDA receptor-mediated components of responses evoked by patterned stimulation are not increased by long-term potentiation. Brain Res. 1989;477:396–399. doi: 10.1016/0006-8993(89)91435-2. [DOI] [PubMed] [Google Scholar]
- Queval A, Ghattamaneni NR, Perrault CM, Gill R, Mirzaei M, McKinney RA, Juncker D. Chamber and microfluidic probe for microperfusion of organotypic brain slices. Lab. Chip. 2010;10:326–334. doi: 10.1039/b916669f. [DOI] [PubMed] [Google Scholar]
- Rambani K, Vukasinovic J, Glezer A, Potter SM. Culturing thick brain slices: An interstitial 3D microperfusion system for enhanced viability. J. Neurosci. Methods. 2009;180:243–254. doi: 10.1016/j.jneumeth.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahi K, Baudry M. Increasing binding-affinity of agonists to glutamate receptors increases synaptic responses at glutamatergic synapses. Proc. Natl. Acad. Sci. U S A. 1992;89:6881–6885. doi: 10.1073/pnas.89.15.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselovsky NS, Engert F, Lux HD. Fast local superfusion technique. Pflugers Arch. 1996;432:351–354. doi: 10.1007/s004240050143. [DOI] [PubMed] [Google Scholar]