Abstract
Chondroitin sulfate proteoglycans (CSPGs) play a pivotal role in many neuronal growth mechanisms including axon guidance and the modulation of repair processes following injury to the spinal cord or brain. Many actions of CSPGs in the central nervous system (CNS) are governed by the specific sulfation pattern on the glycosaminoglycan (GAG) chains attached to CSPG core proteins. To elucidate the role of CSPGs and sulfated GAG chains following traumatic brain injury (TBI), controlled cortical impact injury of mild to moderate severity was performed over the left sensory motor cortex in mice. Using immunoblotting and immunostaining, we found that TBI resulted in an increase in the CSPGs neurocan and NG2 expression in a tight band surrounding the injury core, which overlapped with the presence of 4-sulfated CS GAGs but not with 6-sulfated GAGs. This increase was observed as early as 7 days post injury (dpi), and persisted up to 28 dpi. Labeling with markers against microglia/macrophages, NG2+ cells, fibroblasts and astrocytes showed that these cells were all localized in the area, suggesting multiple origins of chondroitin-4-sultate increase. TBI also caused a decrease in the expression of aggrecan and phosphacan in the pericontusional cortex with a concomitant reduction in the number of perineuronal nets. In summary, we describe a dual response in CSPGs where they may be actively involved in complex repair processes following TBI.
INDEXING TERMS: Chondroitin sulfate proteoglycan, traumatic brain injury, extracellular matrix, perineuronal nets
INTRODUCTION
Traumatic brain injury (TBI) is a major health concern to civilian and military populations. Every year, 1.4 million people in the USA alone suffer a TBI, of which 50,000 die from head injury. Subsequent to immediate neuronal loss from the initial injury, TBI leads to further neuronal death in the pericontusion region meditated by a secondary cascade of events that causes a gradual atrophy (Yi and Hazell, 2006). We have yet to fully understand the patho-physiological sequalae following TBI and lack successful ways to treat them.
TBI results in the formation of a glial scar, likely in response to the release of cytokines and growth factors, such as TGF-β, from resident brain cells and invading macrophages. This scar is proposed to act as a major barrier to inflammatory processes and axonal regeneration (Myer et al., 2006). The glial scar is rich in chondroitin sulfate proteoglycans (CSPGs), which are thought to serve as inhibitors of axon growth and regeneration. CSPGs are composed of a protein core with associated chondroitin sulfate glycosaminoglycan (CS-GAG) side chains. In vitro, CS-GAG chains inhibit neurite outgrowth and repel axons (Asher et al., 2000; Properzi et al., 2005; Silver and Miller, 2004; Wang et al., 2008), as well as bind various growth factors and cytokines (Hirose et al., 2001). In vivo, removal of GAG chains with chondroitinase ABC following injury permits axonal sprouting (Bradbury et al., 2002), suggesting a primary functional role for GAG chains.
CS-GAGs are comprised of a series of disaccharides of glucuronic acid (GlcA) and N-acetyl-galatosamine (GalNAc) decorated with sulfation at various positions of the sugars. It has been proposed that the sulfation pattern on the GAGs confers specific biological activity on axonal growth, either inhibiting (Gilbert et al., 2005; Properzi et al., 2005; Wang et al., 2008) or promoting (Gama et al., 2006; Lin et al., 2011) growth. The majority of CS-GAG sulfation in the mammalian central nervous system is found as disaccharides with a sulfate group on the 4 or 6 position of GalNAc (Ishii and Maeda, 2008; Kitagawa et al., 1997). Following spinal cord injury, 4-sulfated CS-GAGs are rapidly increased (Wang et al., 2008), while 6-sulfated GAGs are increased following cortical stab wound injury (Properzi et al., 2005). Understanding spatiotemporal changes of sulfated GAGs following cortical injury will be crucial in determining the clear role of the CS-GAGs following TBI. Despite this importance, there are no publications demonstrating changes in specific sulfation of GAGs following a contusion injury to the head.
In this report, we have investigated the spatiotemporal change in CSPGs following a controlled cortical impact to the mouse forebrain with a special focus to the sulfated GAGs. We find that there are specific changes in the level and localization of CSPGs and CS-GAGs in response to injury, with the predominant increase being in 4-sulfated GAG chains around the injury core, as well as a decrease in the CSPGs in perineuronal nets in the pericontusional area. In addition, we have used antibodies against both GAG chains and CSPG core proteins to evaluate the cellular sources of CSPGs following TBI.
MATERIALS AND METHODS
Animals
All animal studies were approved by the Uniformed Services University Health Science (USUHS) Institutional Animal Care and Use Committee and were conducted in accordance with the NRC guide to the Care and Use of Laboratory Animals. A total of 66 mice (36 for GAG enrichment, western blot and ELISA, 30 for immunofluorescence) were used for this study. Male mice (C57BL/6) of age 8–10 weeks (NCI, MD, USA) were kept under 12:12 light and dark cycle with access to food and water ad libitum. Typically, surgery was done after several days of recovery from transportation-related stress.
Controlled Cortical Impact Injury
Mice were anesthetized with isoflurane (4% for induction, 2~3% for maintainance) and their heads were securely placed in a mouse stereotaxic frame (Digital Lab Standard, Stoelting Co, IL, USA). Body temperature was kept constant using an isothermal heating pad (Stoelting, Co, IL, USA) throughout surgery. Respiration rate was visually monitored during the period when mice were under anesthesia. Following cleansing with alcohol and iodine, an incision was made over the forehead, and the scalp was reflected to expose the skull. A 4 mm diameter craniotomy was made over the left hemisphere with a surgical drill (Stoelting, Co, IL, USA), and the bone flap was carefully removed with fine forceps. Mice were injured over the left somatosensory cortex (0 bregma, 2 mm lateral to the suture line) at an impact depth of 1 mm with a 2 mm diameter round impact tip (speed 3.6 m/sec, dwell time 100 msec) using an electromagnetically driven controlled cortical impact injury device (Brody et al., 2007), (Impact One™ stereotaxic impactor CCI, Leica Microsystems Gmbh, Wetzlar, Germany). The angle of the impactor tip was set so the bottom was aligned flat with the dura, typically 10~15°. The dura remained intact following craniotomy. Impact caused tearing of the dura with occasional subdural hemorrhages and mild edema. Following injury, the bone flap was replaced, and the scalp was sutured closed. An antiseptic ointment (Neosporin) was applied over the sutured area. Mice were given a saline bolus (i.p., 100 μl) and transferred to a thermal heating pad until fully recovered from anesthesia. Mice were under isoflurane for no longer than 15 minutes. Sham-injured animals received the same surgery without the impact injury. Because sham-injured tissue showed increased levels of CSPGs, a group of naïve animals were added as an additional control (uninjured).
Antibody characterization
Please refer to Table 1 for the list of antibodies used in this study.
Table 1.
List of primary antibodies and their dilutions used for immunofluorescence.
| Antigen | Immunogen | Manufacturer | Dilution |
|---|---|---|---|
| IBA1 | C-terminus of IBA1 (N′-PTGPPAKKAISELP-C) | Wako chemicals, Cat. No. 016-20001, rabbit IgG, polyclonal | 1:200 |
| ER-TR7 | Mouse thymic reticulum | Santa Cruz, Cat. No. sc-73355, rat IgG, monoclonal | 1:200 |
| β-III-tubulin | Rat brain derived microtubules | Covance, Cat. No. PRB-435p, rabbit IgG, monoclonal | 1:1000 |
| CSPG | Ventral membranes of chicken gizzard fibroblasts | Sigma-Aldrich, Cat. No. C-8035, mouse IgM, monoclonal | 1:200 |
| Chondroitin-4S | Proteoglycan from 10-day-old rat brain | Seikagaku, Clone no. 2H6, Cat. No. 370710, mouse IgM, monoclonal | 1:200 |
| Chondroitin-6S | Adult rat bone protein | Seikagaku, Clone No. MC21C, Cat. No. 270423, mouse IgM, monoclonal | 1:100 |
| Chondroitin-2,6S | Proteoglycan from chick embryo limb bud | Seikagaku, Clone No. Mo225, Cat. No. 370615, mouse IgM, monoclonal | 1:200 |
| Neurocan | CHO cell-derived Neurocan (aa23~637) | R&D systems, Cat. No. BAF5800, sheep IgG, biotinylated | 1:200 |
| Aggrecan | GST fusion protein containing amino acids 1177~1326 of mouse aggrecan | EMD Millipore, Cat. No. AB1031, rabbit IgG, polyclonal | 1:200 |
| Phosphacan | Chondroitin sulfate proteoglycans purified from 10-day-old rat brain | Seikagaku, Clone No. 6B4, Cat. No. 370725-1, mouse IgM, monoclonal | 1:200 |
| Versican GAGα | Amino acids 535~598 of mouse versican | EMD Millipore, Cat. No. AB1032, rabbit IgG, polyclonal | 1:200 |
| Stub 4S | Chondroitinase digested 4-sulfated mouse proteoglycan | EMD Millipore, Cat. No. MAB2030, mouse IgG, monoclonal, clone BE-123 | 1:3000 |
| Stub 6S | Chondroitinase digested 6-sulfated mouse proteoglycan | EMD Millipore, Cat. No. MAB2035, mouse IgG, monoclonal, clone MK-302 | 1:3000 |
| GFAP | GFAP isolated from cow spinal cord | Dako, Cat. No. Z033429, rabbit IgG, polyclonal | 1:3000 |
| NG2 | Immunoaffinity purified NG2 chondroitin sulfate proteoglycan from rat | EMD Millipore, Cat. No. AB5320, rabbit IgG, polyclonal | 1:200 |
| NeuN | Purified cell nuclei from mouse brain | EMD Millipore, Cat. No. MAB377x, mouse IgG, monoclonal, FITC conjugated | 1:200 |
Antibody CS-56 is reported to be specific for the GAG chains of CSPGs. The immunogen was the extracellular matrix proteins produced by cultured chicken gizzard fibroblasts (Avnur and Geiger, 1984). This antibody recognizes the chondroitin sugar moiety on proteoglycans of different molecular weight. On western blots of mouse nervous system tissue, the antibody recognizes high-molecular weight bands representing CSPGs (Shearer et al., 2003); reactivity is abolished by treatment of CSPGs with chondroitinase ABC (cABC), which digests the GAG chains, leaving protein cores intact, demonstrating that CS-56 does not bind to proteins. Binding of CS-56 to brain sections is similarly abolished after treatment of sections with cABC (Bao et al., 2005). Although the exact immunogen structure is lacking, the binding of CS-56 is inhibited by addition of GAG chains of chondroitin sulfate A (4-sulfated on GalNAc, CS-A), chondroitin sulfate C (6-sulfated on GalNAc, CS-C), and CS-D (2-sulfated on GlcA and 4-sulfated on GalNAc) but not by CS-B (dermatan sulfate, 4-sulfated on GalNAc with iduronic acid instead of GlcA) nor CS-E (4,6-sulfated on GalNAc) (Avnur and Geiger, 1984; Yamamoto et al., 1995), demonstrating selectivity in the recognition of different sulfated GAGs.
Anti-chondroitin 4S (2H6) is a mouse monoclonal antibody (IgM) raised against soluble CSPG purified from 10-day-old rat brains (Oohira et al., 1994). The competitive immunohistochemical studies using this antibody and different CS-GAGs showed 4-monosulfated CS-A being much more effective in preventing the antibody binding than CS-C, CS-D, and CS-E (Yamamoto et al., 1995). The antibody intensely labels postnatal mouse brain; immunoreactivity declines as the animal approaches adulthood (Maeda et al., 2003). Owing to the varying length of sugar chains and the high molecular weight nature of proteoglycans, the antibody detects diffuse bands at a high molecular weight when western blot was performed using P12 rat cortex samples (Shimazaki et al., 2005). This band was effectively removed when the samples were treated with chondroitinase, demonstrating that this antibody does no react with proteins (Shimazaki et al., 2005).
Anti-Chondroitin 6S (MC21C) is a mouse monoclonal antibody (IgM) that recognizes intact chondroitin sulfate sugars and not hyaluronan or collagen, because tissue digesting with cABC (and not hyaluronidase or collagenase) prior to incubation with this antibody effectively abolishes the binding (Mark et al., 1989). The immunogen for this antibody is a partially purified preparation of adult rat bone proteins which contains chondroitin sugar. The Ouchterlony diffusion assay shows higher preference for MC21C to detect CS-C than A (Mark et al., 1989). In mouse cerebral cortex, MC21C labeling was shown to coincide with area demarcated by astrocytes (Horii-Hayashi et al., 2010). On western blots of rat spinal cord tissue, MC21C also detects diffuse high molecular weight bands, characteristic of proteoglycan bands that are abolished by cABC treatment (Imagama et al., 2011). This suggests specificity towards sugar moiety and not to the core protein.
The immunogen for monoclonal antibody (IgM) anti-Chondroitin 2,6S (Mo225) antibody is proteoglycans isolated from stage 22–23 chick embryo limb bud proteoglycan (Yamagata et al., 1987). The binding of Mo225 to proteoglycans is strongly inhibited by chondroitin sugars containing chondroitin sulfate D (GlcA-2-SO4 →GalNAc-6-SO4) units (Yamagata et al., 1987). The immunoreactivity of Mo225 to proteoglycans is abolished by cABC and cACII, but not keratinase (Yamagata et al., 1987), showing its specificity towards condroitin sugars. In postnatal mouse brain, Mo225 intensely stains midbrain and cerebellum (Maeda et al., 2003). On western blots of postnatal day 12 rat cerebral cortical tissue, Mo225 detects diffuse bands of high molecular weight that are abolished by cABC treatment of the sample (Shimazaki et al., 2005).
Anti-stub 4S antibody (MAB2030, clone BE-123) is a mouse monoclonal antibody (IgG1) that detects neo-epitopes on CS sugars only after chondroitin 4S digested with cABC. The immunogen was a partially purified preparation of human articular cartilage treated with cABC (Poole et al., 1991). This antibody has been used to verify chondroitinase activity (Bukhari et al., 2011). MAB2030 does not detect native CS-GAGs. The epitope recognized by this antibody is unsaturataed uronic acid and N-acetylgalactosamine-4-sulfate formed by digestion of chondroitin or dermatan sugars with cABC or cACII (Poole et al., 1991). On western blots of human cartilage extracts this antibody recognizes chondroitin chains attached to proteoglycan core proteins only after digestion with cABC (Cs-Szabo et al., 1995). In our hands, this antibody detects a high molecular weight smear above 200kDa and a strong band at around 100kDa with cABC digested mouse brain samples enriched with a DEAE column (Fig. 1L).
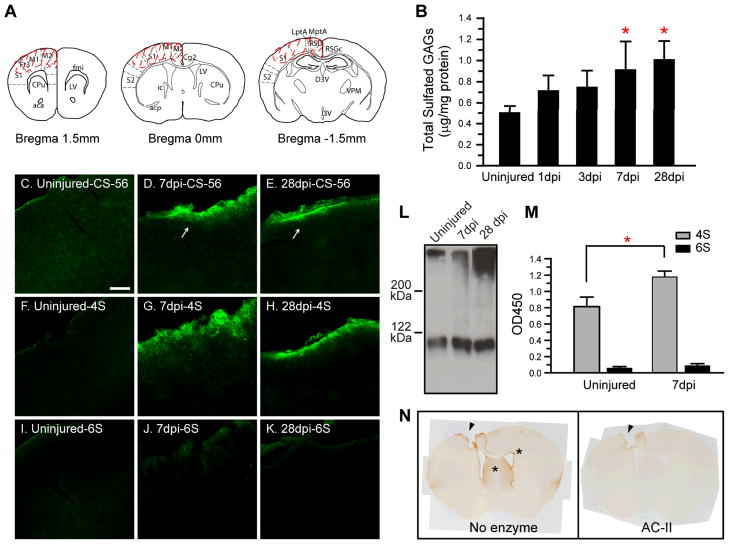
Fig. 1.
4-sulafted glycosaminoglycan (4S-GAG) content increases following traumatic brain injury (TBI) in the injured cerebral cortex. (A) Coronal maps showing the area collected for GAG analysis (dotted line, see methods). (B) TBI induces a gradual increase in total sulfated GAG content. At 7 and 28 dpi, total sulfated GAG content was significantly increased compared to uninjured levels. Values shown are mean ± S.D. of 6 mice. *Significantly different compared to uninjured, p<0.05. (C~E) An increase in chondroitin sulfate-GAG immunoreactivity is detected at 7 and 28 dpi in the pericontusion area by CS-56 (arrows). (F~K) Immunoreactivity for 4S-GAG chains (F~H), but not 6S-GAG chains (I~K), overlaps with CS-56. Bar = 100 μm. (L) Representative immunoblot showing increased 4S-GAGs (MAB2030) at 7 and 28 dpi compared to the uninjured area. (M) ELISA results showing a significant increase in immunoreactivity for 4S, but not 6S, GAGs at 7 dpi. Values shown are mean ± S.D. of 6 mice. *Significantly different compared to uninjured, p<0.05. (N) The observed pericontusional 4S increase is eliminated when the brain section is digested with chondroitinase ACII prior to the staining (arrow heads). Brain sections from 7dpi were used. * depicts area where high 4S staining is observed (thin layer of cells surrounding Subventricular zone, as well as lateral septal nuclei).
Anti-stub 6S (MAB2035, aka MK-302) is a mouse monoclonal antibody (IgG1) that detects a neo-epitope created by CS digested with cABC. Similar to the MAB2030, the immunogen was a partially purified preparation of articular cartilage treated with cABC (Poole et al., 1991). MAB2035 does not react with native proteoglycans, isolated-4-sulfate, or chon-droitin-6-sulfate chains, or with tetrasaccharides or disaccharides of chondroitin-4-sulfate (Poole et al., 1991). This antibody has also been used to detect the activity of cABC expressed in adult bone marrow mononuclear cells transplanted into CNS injury site (Coulson-Thomas et al., 2008). The epitope for this antibody is both saturated and unsaturated uronic acid residue bound to N-acetylgalactosamine-6-sulfate attached to the core protein through the linkage tetrasaccharide (Poole et al., 1991). On western blots of bovine aggrecan digested with cABC, this antibody detects multiple proteoglycan bands of differing molecular weight (Pratta et al., 2000), owing to the presence of multiple proteins with 6-sulfated CS-GAGs as GAG chains.
Anti-aggrecan antibody (AB1031) is a rabbit polyclonal antibody is specific for mouse aggrecan. The immunogen for this antibody is a GST fusion protein containing amino acids 1177-1326 of mouse aggrecan core protein. This antibody does not recognize sugars attached to the core protein; the GAG chains actually hinder the binding because digestion with cABC enhances the binding of this antibody to aggrecan on the western blot. This antibody labels aggrecan in the perineuronal nets formed from wild type mouse and not from cartilage matrix deficiency (cmd) mice which lack aggrecan (Giamanco et al., 2010). On western blot, this antibody detects a diffuse high molecular weight band which is characteristic of proteoglycan bands (Velasco et al., 2011). After removal of CS GAG chains with cABC, this antibody detects band above 200kDa which is close to the expected molecular weight of the aggrecan core protein (210~250 kDa).
Anti-versican GAGα antibody (AB1032) is a rabbit polyclonal antibody that detects mouse versican. The immunogen for anti-versican GAGα antibody is a GST-fusion peptide from amino acids 535~598 of mouse versican. This antibody particularly detects versican GAG α (versican V2 and V0) domain of mouse versican (manufacturer’s webpage) and not GAGβ domain (V1, V3). On the gel, the antibody detects a high molecular weight band at around 400kDa from cerebral cortex homogenate (Horii-Hayashi et al., 2008). Immunofluorescence staining demonstrated that this antibody labels cortical neurons (Horii-Hayashi et al., 2008).
Anti-phosphacan antibody (6B4) is a mouse monoclonal antibody (IgM) that detects phosphacans located at the perineuronal nets in the barrel fields in mouse brain (Nakamura et al., 2009). This monoclonal antibody was originally shown to recognize large chondroitin sulfate proteoglycan of 400~1000kDa with 250kDa core protein that stained perineuronal nets (Maeda et al., 1992), which was later found to be phosphacan. The immunogen for this antibody is chondroitin sulfate proteoglycans purified from 10-day-old rat brain, but has been reported to cross-react with mouse and chicken phosphacan. It recognizes a thick band above 250kDa from brain tissues on western blot (Miyata et al., 2004) which is the expected size of rat phosphacan core protein. In our hands, this antibody detects a diffuse high molecular weight band above 200kDa marker (Fig. 6O).
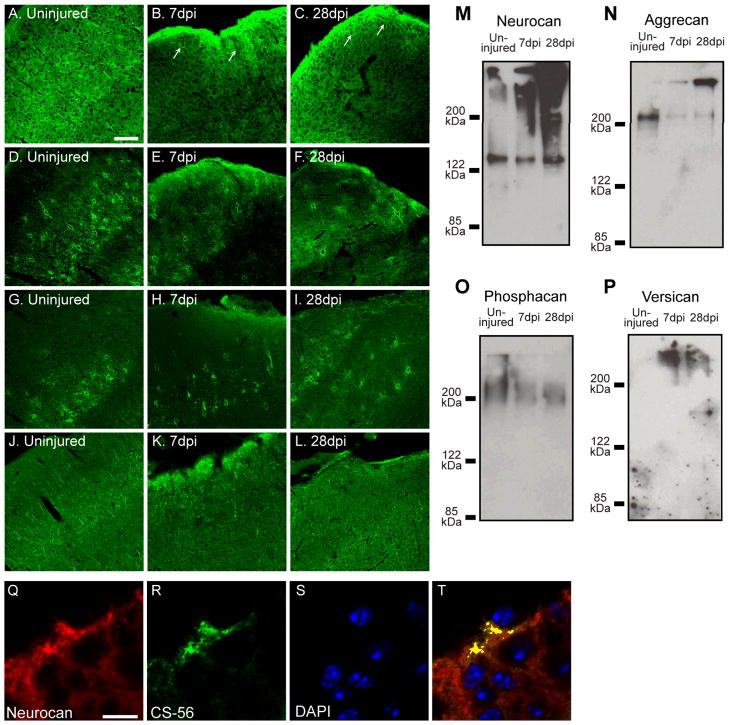
Fig. 6.
Chondroitin sulfate proteoglycan (CSPG) core proteins are differentially regulated in the injured cortex following TBI. (A~C) Immunofluorescence staining shows an increase in neurocan in the tight band around the injury core (arrows, B and C). (D~I) Aggrecan (D~F) and phosphacan (G~I) labeling showed a decreased number of PNNs in the pericontusion area after TBI. (J~L) Similar to neurocan, versican immunoreactivity is also increased at the tight band around the injury core at 7 and 28 dpi. Bar, 100 μm. (M~P) Immunoblot analysis shows an increase in neurocan and versican and a decrease in aggrecan and phosphacan at 7 and 28 dpi compared to the uninjured area. (Q~T) Neurocan and CS-GAG immunoreactivity spatially over-lap in the pericontusion area at 7 dpi. Bar, 10 μm.
Anti-neurocan antibody is a biotinylated polyclonal antibody raised in sheep that detects mouse neurocan. The immunogen for this antibody is CHO cell-derived mouse neurocan (aa23~637). In our hands (Fig. 6M), this antibody detects on the western blot both intact neurocan and neurocan-130 corresponding to the n-terminal fragment of neurocan (Asher et al., 2000). This antibody recognizes the extracellular matrix and the perineuronal nets on mouse brain sections when utilized for immunofluorescence labeling.
Anti-β-III-tubulin antibody is a rabbit polyclonal antibody that detects a microtubule element expressed by neurons and is a popular neuronal marker (Brennand et al., 2011) that stains the soma, axons and dendrites of neurons. The immunogen for this antibody is rat brain derived microtubules. In our hands, this antibody detects a strong band around 50kDa on the western blot, and labels neuronal axons and soma when examined by western blot and immunostaining respectively.
Anti-GFAP antibody is a rabbit polyclonal antibody and will detect GFAP intermediate filament expressed in the brain astrocytes and does not detect other cell types (Lyck et al., 2008). The immunogen for this antibody is GFAP derived from cow spinal cord. On western blot, this antibody detects a single band around 50kDa (Shimada et al., 2011). Staining of mouse brain sections with this antibody shows positive labeling of cells typical of astrocytes.
Anti-NG2 antibody is a polyclonal antibody raised in rabbits that detect NG2 proteoglycan presented in the surface of oligodendrocyte progenitor cells (O2A) and pericytes (Dore-Duffy et al., 2006; Levine and Stallcup, 1987). The immunogen for this antibody is immunoaffinity purified NG2 chondroitin sulfate proteoglycan from rat. On the gel, this antibody detects a high molecular weight band around 300kDa (Shearer et al., 2003). Staining with this antibody shows oligodendrocyte progenitor cells and pericytes in close proximity of blood vessels in the mouse brain. Following brain injury, other cell types such as macrophages and blood cells do express NG2 proteoglycan and often seen in the injured site.
Anti-NeuN antibody is a mouse monoclonal antibody (IgG) whcih labels neuronal nuclei and soma. The immunogen for this antibody is purified cell nuclei from mouse brain, but later has been shown to detects RNA spicing factor Fox-3 (Kim et al., 2009). In our hands, not all neuronal nuclei are labeled with this antibody in the brain. This antibody may cross-react with other protein that labels axonal structures in mouse brain section if section is not well permeated with triton. On the gel, it gives rise to 2 bands at around 38 and 50kDa (Kim et al., 2009).
Anti-IBA1 (ionized calcium binding adapter molecule 1) is a rabbit polyclonal antibody that detects a 17 kDa EF hand protein that is expressed by microglia and macrophages (Kanazawa et al., 2002; Ohsawa et al., 2000). The immunogen for this antibody is the c-terminus of IBA1 (N′-PTGPPAKKAISELP-C). In the adult mouse brain, we detect resident mi-croglia and macrophages and do not cross-react with neurons or astrocytes.
Anti-ER-TR7 antibody is a rat monoclonal antibody (IgG) that recognizes an uncharacterized antigen present in reticular fibroblasts. The immunogen for this antibody is the mouse thymic reticulum. It has been shown to label meningeal fibroblasts in the brain (Hu et al., 2011; Takahashi et al., 2011). In our hands, the antibody detects primarily meninges in the brain and very low expression in the other parts of the brain.
GAG proteoglycan enrichment
At 1, 3, 7 and 28 days post injury (dpi), mice were deeply anesthetized and euthanized by decapitation. A total of 6 brains for each time point were rapidly removed and snap-frozen in 2-methylbutane solution on dry ice. The ipsilateral portion of cerebral cortex spanning the lesion (+1.5~−1.5 bregma, Fig. 1A) was coronally dissected on dry ice and transferred to a microcentrifuge tube; the same area was collected from naïve animals as well (Fig. 1A). Samples were carefully dissected to avoid including the white matter. Tissue was homogenized in Tris-based lysis solution (25 mM Tris, pH 7.5, 4 M Urea, 1 % Triton X100, 20 mM EDTA, and 1x Protease Inhibitor Cocktail (PIC) Set I (Calbiochem, CA, USA)). Following determination of protein content using the Bradford assay (Biorad, USA), 2.5 mg protein was loaded into syringes packed with DEAE Sephacel™ (1 ml packing volume, GE healthcare, Uppsala, Sweden). After elution with a high salt elution buffer, the resulting samples were concentrated using Amicon Ultra (10 kDa molecular cut-off, EMD Millipore, CA, USA). After concentration, buffer was exchanged to PBS with PIC by adding 20 volumes of PBS containing PIC and repeating the concentration step. Final concentrates were made to equal volume with PBS.
Total GAG measurement
Total GAG measurement was performed using the Blyscan™ kit according to the manufacturer’s protocol (Biocolor, UK). Briefly, enriched GAGs from brain samples, along with known standard GAG (CS-A, provided by manufacturer), were complexed with 1,9-dimethylmethylene blue (DMMB) and allowed to form a GAG-dye complex, which was precipitated, redissolved, and detected by UV absorbance at 656 nm.
Enzyme-link immunosorbent assay (ELISA)
The conditions for ELISA were adopted and modified from Wang et al. (2008) and optimized for mouse brain samples. Enriched GAG proteoglycan samples, along with CSPG standard (EMD Millipore, CA, USA) were coated on a 96-well Immulon flat-bottomed microplates overnight (Thermo Scientific, MA, USA) and digested with 100 mU/mL protease-free chondroitinase ABC (Seikagaku, Chiyoda, Japan) for 2 hours at 37°C. Wells were blocked with 5 % BSA (Sigma Aldrich, St-Louis, MO, USA) in PBS containing 0.1 % Tween 20. Subsequent to washes, wells were incubated with monoclonal antibody to stub 4S (MAB2030) or 6S (MAB2035) for 2 hours at RT. Wells were washed again, and incubated with Goat anti-mouse IgG F(ab)′2-HRP (Abcam, UK). The peroxidase reaction was terminated by adding equal volume of 1N HCl and absorbance was measured using SpectraMax190 microplate reader (Molecular Device, CA, USA).
Immunofluorescence and confocal microscopy
At varying time points after injury, mice were deeply anesthetized using ketamine/xylazine (90 mg·kg−1/10 mg·kg−1) and transcardially perfused with 4 % buffered formalin solution. Sham injured animals were perfuse-fixed at 7 days post operation. Brains were carefully removed and post fixed in 4 % buffered formalin solution overnight at 4°C, then immersed in 30 % sucrose solution until they sank. Coronal sections of 30 μm thickness were made using a Leica SM2000 R sliding microtome with an attached freezing stage. Sections were kept in a PBS-based antifreeze solution containing ethylene glycol at −20°C until used. For immunofluorescence staining, free-floating sections were washed in PBS, blocked for 1 hour in Tris buffered saline (TBS) with 10% goat serum (Invitrogen, CA, USA) and 0.1~1% Triton X-100 (Sigma Aldrich, MO, USA) followed by primary antibody incubation in the same solution using the primary antibodies listed in Table 1. Following washes, sections were incubated with appropriate secondary antibodies: goat anti-rabbit IgG-488 or 568 (Molecular Probes, Invitrogen, CA, USA); goat anti-mouse IgM F(ab)′2-488 (Abcam, Cambridge, UK); streptavidin-488 or 568 (Jackson Labs, ME, USA); goat anti-rat IgG F(ab)′2-594 (Molecular Probes, Invitrogen, CA, USA). Sections were mounted on silanated slides (KD medicals, MD, USA) and coverslipped using Fluoromount™ (Sigma Aldrich, MO, USA) with or without DAPI. Confocal images were taken using a Zeiss LSM 510 UV microscope.
For counting the number of WFA+, aggrecan+ or phosphacan+ perineuronal nets (PNNs), 2 representative sections (30 μm thickness, at 0 bregma) from uninjured, 7 or 28 dpi group (n = 5) were immunolabelled with either biotinylated Wisteria Floribunda Agglutinin (WFA, Sigma Aldrich, MO, USA), anti-aggrecan or anti-phosphacan antibodies and subsequently with strep-tavidin-488 or 568 (Jackson ImmunoResearch, PA, USA) or appropriate secondary antibodies as mentioned above. Low magnification images spanning entire sections were taken using a Leica microscope using a 10X objective. A montage of the entire section was created using Photoshop and a person blinded to the identity of the animal counted the number of PNNs stained positive for WFA, aggrecan or phosphacan from the entire cerebral cortex region, ipsi- or contralateral from the injury using the cell counter plug-in of ImageJ (Version 1.45r, available at http://rsbweb.nih.gov/ij/).
Immunoblotting
Samples prepared as described in the GAG proteoglycan enrichment section were digested with chondrotinase ABC (cABC, 100 mU/ml) for 2 hours at 37°C in PBS containing PIC. Equal volume of samples were mixed with 2 x SDS loading buffer (0.1 M Tris, 2 mM EDTA, 4 % SDS, 20 % glycerol), loaded onto a 7.5 % SDS-PAGE gel, ran under constant current for 2 h and transferred to PVDF membrane (EMD Millipore, CA, USA) using a semi-dry transfer system (Atto corporation, Taito, Japan). Following overnight blocking with either 5 % milk or 5 % BSA (Sigma-Aldrich, MO, USA) in PBS with 1 % Tween 20 (Invitrogen, CA, USA), membranes were incubated for 2 hours with antibodies against 4S, stub-4S or 6S, neurocan, aggrecan, phosphacan, or versican GAGα, and subsequently incubated with appropriate secondary antibodies after thorough washing. Primary antibodies used for this study are listed in Table 1. Membranes were incubated with either LumiGlo (KPL, MD, USA) or Amersham ECL Plus™ and chemiluminescence (GE healthcare, Uppsala, Sweden) chemiluminescence reagent. Bands were detected using Kodak Biomax Light film (Kodak USA, NY, USA).
Statistical Analysis
Statistical analysis for the ELISA and Blyscan™ results were performed using one way Analysis of Variance (ANOVA) with post-hoc Tukey test (*p < 0.05; **p < 0.01). Statistical analysis for the number of PNNs from cerebral cortex ipsilateral versus contralateral to the injury were performed using one way ANOVA with a paired t-test.
RESULTS
Changes in GAGs following TBI
GAG chains have been implicated in modulating many processes in the extracellular milieu such as plasticity and axon sprouting (Corvetti and Rossi, 2005; Pizzorusso et al., 2002). GAG chains are upregulated after several types of injuries to the CNS and are thought to reduce the permissiveness of the extracellular matrix, potentially influencing repair (Bradbury et al., 2002). We hypothesized that increases in GAGs following TBI, similar to that found in SCI (Wang et al., 2008), may be involved in the injury response. We therefore measured GAG levels in DEAE-enriched brain proteoglycan fractions to determine the change in total sulfated GAG content in the cerebral cortex following TBI. Total sulfated GAG in the uninjured cerebral cortex was 0.51 ± 0.06 μg/mg protein (Fig. 1B). There was a gradual increase in the total amount of sulfated GAGs following TBI as compared to the uninjured naïve brain (Fig. 1B). By 7 dpi, the content of total sulfated GAG in the injured cortex was 0.92 ± 0.26 μg/mg, significantly higher than in the uninjured cortex. Total sulfated GAG remained increased up to 28 dpi (1.0 ± 0.17 μg/mg).
Proteoglycan GAG chains can be of several different types: heparin sulfate GAG chains are thought to be permissive for neuronal growth, while CS GAG chains and keratin sulfate are major growth inhibitory molecules in the glial scar (Bartus et al., 2011; Zhang et al., 2006). Since measurement of total sulfated GAGs detects multiple forms of GAGs, we performed immunofluorescence staining using the anti-CS antibody CS-56 on brain sections to determine localized changes in CS-GAG following TBI. We detected an increase in CSPG immunoreactivity confined to the tight band around the injury core at 7 and 28 dpi (Fig. 1C~E, white arrows). Staining at earlier time points yielded high background and was inconclusive (not shown).
CS GAG chains are comprised of a series of polymerized disaccharide units of GlcA and GalNAc, with various patterns of sulfation (Sato and Oohira, 2009). In brain, sulfation is predominantly found on the 4- or 6-position of GalNAc (termed CS-A unit and CS-C unit, respectively) (Kitagawa et al., 1997). Previous reports have shown an increase in 6-sulfated CS-GAG and its synthesizing enzymes after a stab wound to the rat cerebral cortex (Properzi et al., 2005) whereas a specific increase in 4-sulfated CS-GAG was found after a spinal cord injury in the mouse (Wang et al., 2008). Because 4-sulfated CS-GAG is axon-inhibitory (Wang et al., 2008) whereas 6-sulfated CS-GAG may have a positive influence on axon regeneration (Lin et al., 2011), we evaluated whether the increased CS-GAG in our mouse model of TBI is 4- or 6-sulfated CS-GAG or both. Using antibodies that discriminate between 4-sulfated CS (described as 4S hence forth) from 6-sulfated CS (described as 6S hence forth) we found that the upregulation in CS-GAG is mainly 4S, but not 6S (Fig. 1F~H). We also detected an increase in 2,6S immunoreactivity in the same location of the injured cortex (data not shown). The injury-induced 4S increase was also corroborated by immunoblot of brain extracts using an antibody which recognizes 4S in cABC–digested GAGs (Fig. 1L) as well as ELISA assay which showed a significant (47%) increase in 4S immunoreactivity from 7 dpi samples when compared to that found in the uninjured samples (Fig. 1M). However, there was no significant change in 6 sulfated immunoreactivity after injury (Fig. 1M)
It is not uncommon to find non-specific immunoreactivity associated with mouse Igs near the contusion core when using mouse antibodies on mouse TBI brain sections. Since our antibodies directed against 4S or 6S are mouse monoclonal antibodies, we wanted to confirm that the 4S staining was specific for CS-GAG and not due to artifact staining arising from using anti-mouse antibodies on mouse tissue. To this aim, we digested TBI brain sections (7 dpi) with chondroitinase ACII (cACII), an enzyme which digests CS-GAGs, prior to staining; GAG labeling, including the increased staining at the tight band around the injury core, was completely eliminated by cACII (Fig. 1N). The identical result was obtained when chondroitinase ABC (cABC) was used (data not shown). The 4S labeling in uninjured brain was detected in the following areas: meninges, cerebral cortex (diffuse staining), and the junction between corpus callosum, putamen and lateral ventricle (Fig 1N, star). In the injured brain, the tight band around the core of injury was intensely stained (Fig. 1N, no enzyme).
Multiple origins of 4S expression in the cerebral cortex
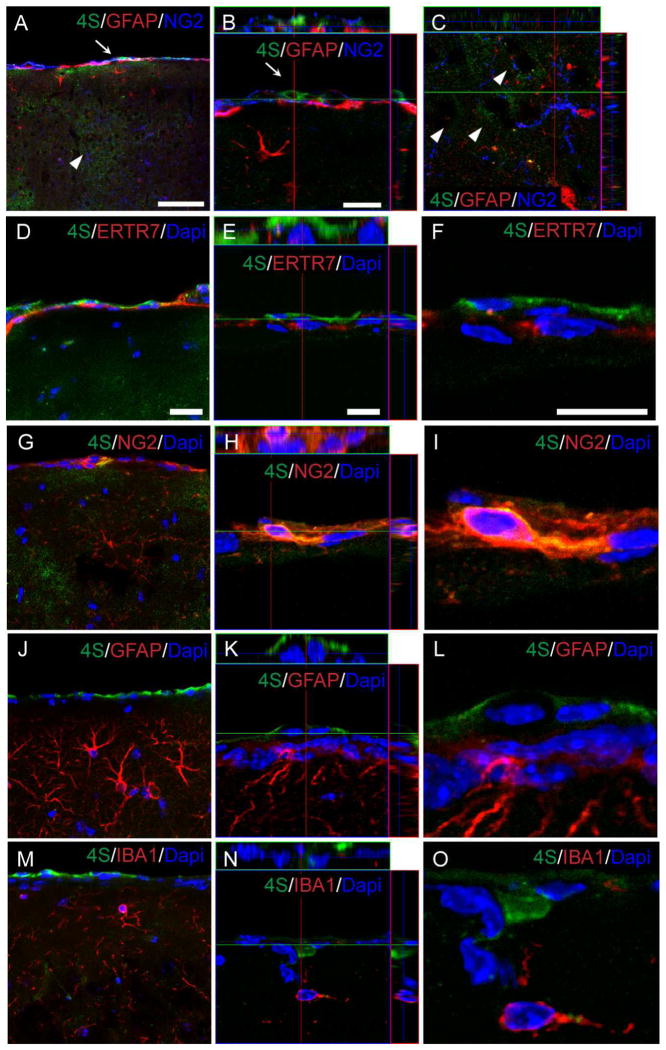
There are many potential cellular sources of CSPGs in the injured brain, including astrocytes, oligodendrocyte progenitors, meningeal fibroblasts as well as neurons. We first investigated the cellular sources of 4S expression in the uninjured brain by performing triple-labeling experiments using cell-type specific markers along with 4S specific antiserum (Fig. 2). 4S was found localized in two particular locations in the uninjured cerebral cortex: the meninges and the cortical parenchyma (Fig. 2A). 4S staining was found colocalized with NG2 (Fig. 2B, G, H, I) in meningeal cells expressing the fibroblast marker ER-TR7 (Fig. 2D~F); 4S did not colocalize with GFAP (Fig. 2A, J~L), nor IBA1 (Fig. 2M~O). The expression of 4S and NG2 in fibroblasts was also reported in vitro using meningeal fibroblast cultures (Morgenstern et al., 2003). In the inner layer of the cerebral cortex, 4S staining was generally very weak (Fig. 2C). Owing to the fact that the 4S expression was very low and diffuse (extracellular), it was not possible to confirm the cellular origin of 4S in the parenchyma of the uninjured cerebral cortex.
Fig. 2.
Cellular localization of 4S expression in the uninjured cerebral cortex. (A~C) 4S and NG2 colocalize in the meninges (A &B, arrow). 4S immunoreactivity is highest in the meningeal layer with patchy staining (A & C, arrowhead) throughout the cortical parehncyma. Panel b shows a confocal section near the meninges showing a fibroblast expressing 4S and NG2, but not GFAP. Panel c shows a confocal section of patchy 4S expression in the inner layer of the cerebral cortex (arrowheads). Note that no overlap is observed between 4S, NG2 and GFAP in this region. (D~O) Meningeal fibroblasts stained for ER-TR7 (D–F) and NG2 (G–I) also show 4S immunoreactivity. No 4S co-localization is observed with either GFAP (J–L) or IBA1 (M–O). E, H, K, and N show sections through a z-stack and F, I, L, O show higher magnification from the boxed areas in E, H, K and N. Bars, 20 μm (A, D, G, J, M); 10 μm (E, F, H, I, K, L, N, O).
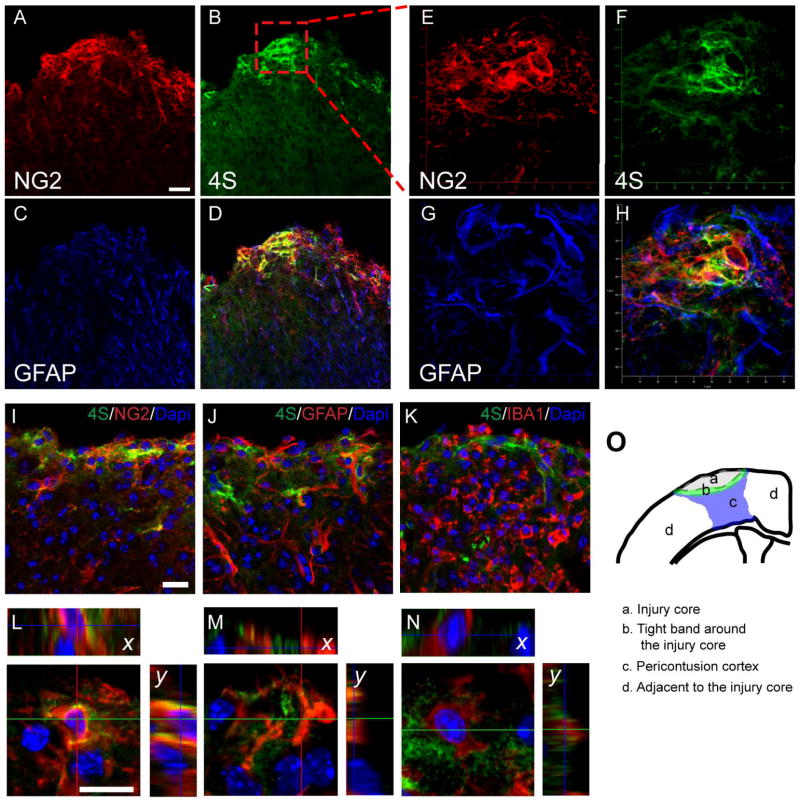
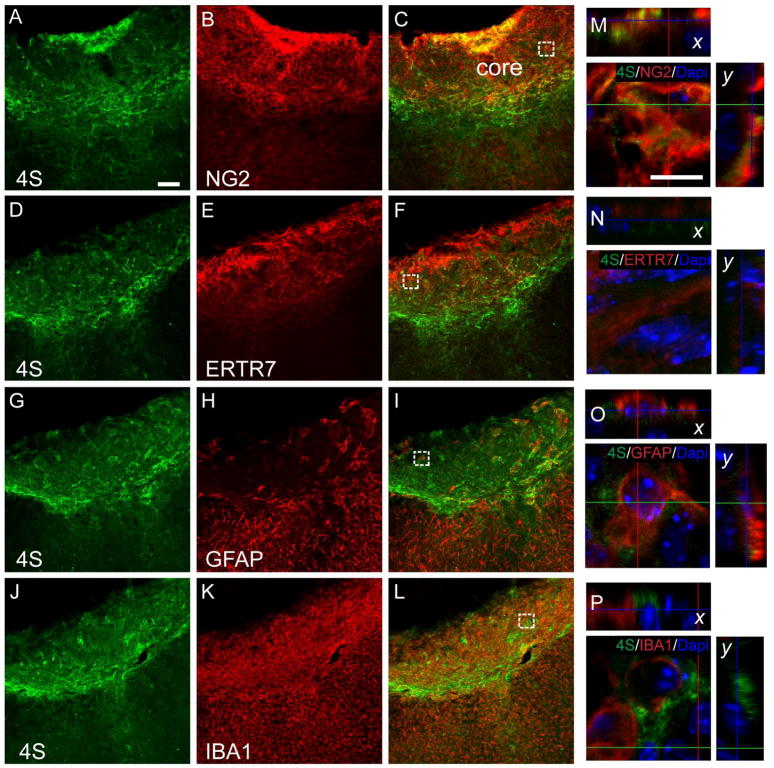
We next determined the cellular origin of 4S expression in 7 dpi brain sections using the panel of cell-type specific markers (Fig. 3). 4S labeling was prominent in the tight band around the impact core where many glial cells were also present. Triple labeling with 4S, anti-GFAP and NG2 showed a spatial overlap between NG2 and 4S in this area (Fig. 3A~D). Z-stack images revealed more overlap between 4S and NG2 than 4S and GFAP (Fig. 3E~H). At high magnification, 4S labeling overlapped with NG2 (Fig. 3I, L), and was found in proximity to GFAP (Fig. 3J, M) and IBA1 (Fig. 3K, N) suggesting that 4S may also be expressed by astrocytes and macrophages.
Fig. 3.
Cellular localization of 4S expression in the tight band around the injury core following TBI (7 dpi). (A~D) Triple labeling of NG2, 4S, and GFAP shows 4S expression overlapping with NG2 expression in the tight band near the injury core and the pericontusion region depicted in (O). (E~H) z-projection from the boxed area in (B). Note that 4S labeling shows a higher degree of overlap with NG2 than with GFAP. 4S double labeled with NG2 (I, L), GFAP (J, M), or IBA1 (K, N). L, M and N show sections through a Z-stack. Bars, 100 μm (A); 20 μm (I, J, K); 10 μm (L, M, N). (O) Schematic diagram depicting different regions of injured cortex (coronal, at 0 bregma).
TBI typically results in pan-necrosis and subsequent cavity formation where the injury core is usually lost while processing the tissue for histochemical analysis. To investigate whether 4S is expressed within the core as well, we took TBI mice where the impact core was intact after the tissue processing and performed triple labeling using the cell-type specific markers (Fig. 4, Supplementary Fig. 1). Seven days following TBI, a large number of NG2+ cells and ER-TR7+ fibroblasts were readily seen in the injury core (Fig. 4A~C and D~F, Supplementary Fig. 4A~C and D~F). Interestingly, only a few GFAP+ astrocytes were detected inside the core (Fig. 4G~I, Supplementary Fig. 1G~I). The injury core also contained particularly high number of IBA1+ macrophages (Fig. 4J~L, Supplementary Fig. 1J~L). Higher magnification Z-stack images showed 4S overlapping with NG2+ cells within the core (Fig. 4M, Supplementary Fig. 1M). Although ER-TR7+ cells were found in the vicinity of 4S labeling, co-labeling was not confirmed using Z-stacks (Fig. 4N, Supplementary Fig. 1N). Despite the fact that there were only few GFAP+ astrocytes present within the injury core, they were often found associated with 4S staining as well (Fig. 4O, Supplementary Fig. 1O). This was same with IBA+ macrophages within the core as well (Fig. 4P, Supplementary Fig. 1P). Altogether, these results suggest multiple origins of 4S following injury.
Fig. 4.
Expression of 4-sulfated CS in respect to NG2, ER-TR7, GFAP and IBA1 within the injury core at 7 days following TBI. (A~L) 4S expression in the lesion core (A, D, G, J) is compared to NG2 (B, C), ER-TR7 (E, F), GFAP (H, I) and IBA1 (K, L). Intense labeling for 4S, NG2, ER-TR7 and IBA1 is observed in the lesion core whereas comparatively little GFAP immunoreactivity is found. Bar, 100 μm. (M~P) Z-stacked images of 4S, DAPI and NG2 (M), ER-TR7 (N), GFAP (O), and IBA1(P) within the core of injury. Many NG2 positive cells are found colocalized with 4S within the core. Some GFAP or IBA1 positive cells were found colabeled with 4S whereas little or no colabeling was observed between ER-TR7 and 4S. Bar, 10 μm.
CSPGs are differentially localized in the cerebral cortex
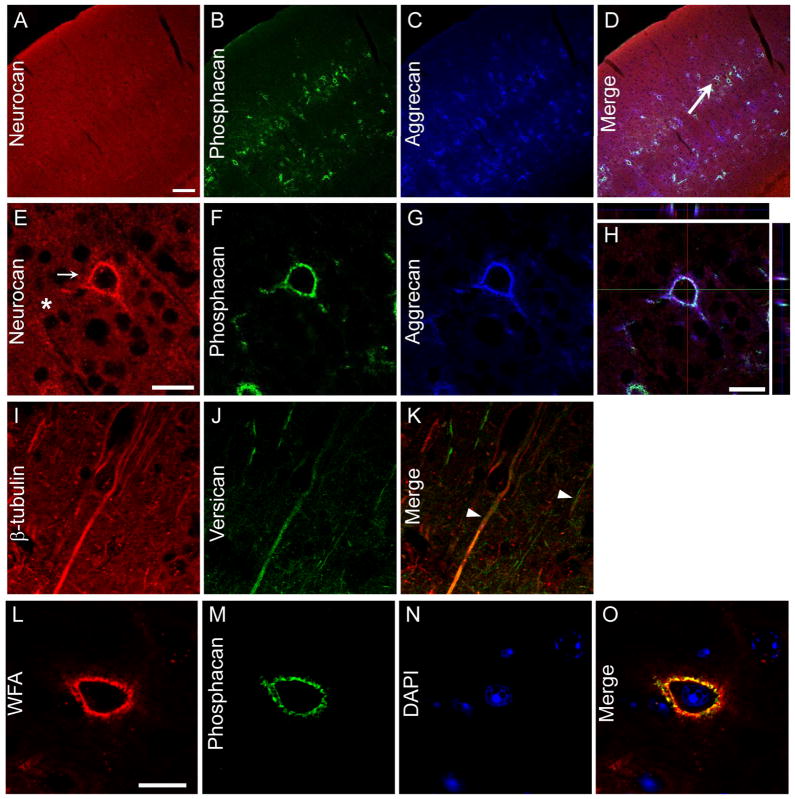
CSPGs are comprised of CS GAG chains attached to one or more sites on proteoglycan core proteins. The major core proteins in the brain are aggrecan, neurocan, phosphacan and versican. In the uninjured cortex, the major sites of CSPG expression are PNNs (Carulli et al., 2006). It has been reported that PNN CSPG staining is decreased following TBI in the rat (Harris et al., 2010). To better understand the spatial expression of these proteoglycans in the mouse cerebral cortex, we investigated their localization by double-labeling experiments using antibodies against the CSPGs aggrecan, neurocan, phosphacan and versican along with the lectin WFA which recognizes terminal GalNAc and is a marker for PNNs (Brauer et al., 1993). As expected, in the uninjured cerebral cortex, the expression of aggrecan and phosphacan were found in PNNs which colabeled with WFA (Fig. 5, Supplementary Fig. 2). When coronal sections of the cerebral cortex were stained with anti-neurocan, anti-aggrecan and anti-phosphacan antibodies, PNNs were labeled by all three antibodies (Fig. 5E~H, Supplementary Fig. 2E~H). For neurocan, a high level of expression was also found in the parenchyma (Fig. 5E, Supplementary Fig. 2E). In accordance with the findings reported previously (Horii-Hayashi et al., 2008), we found versican GAGα labeling to be expressed on neurons, as staining often overlapped with β-III-tubulin (Fig. 5K, Supplementary Fig. 2K).
Fig. 5.
Proteoglycans expression in the cerebral cortex. (A~H) Expression of aggrecan, neurocan and phosphacan in the uninjured cerebral cortex. Higher magnification of perineuronal net (PNN, D, arrow) shows all 3 core proteins labeled the PNN. Cell indicated by arrow in (D) is shown magnified in (E). Neurocan shows parenchymal staining as well (E, star). H is a z-stack image showing overlap between neurocan, aggrecan and phosphacan. (I~K) Versican expression in the brain shows overlap with β-III-tubulin (arrowheads). (L~O) Phosphacan expression is observed in the wisteria floribunda agglutinin (WFA)-positive PNNs. Bar, 100 μm for A~D, 50 μm for E~H, 20 μm for L~O.
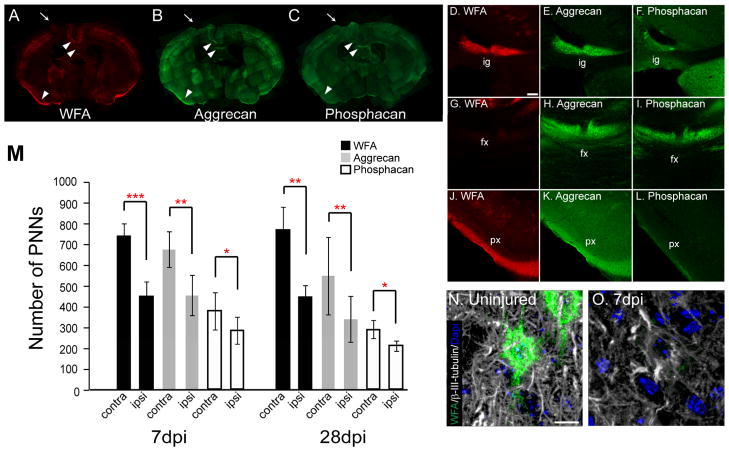
To verify if aggrecan and phosphacan labeling spatially overlap with WFA in the area of brain other than the cerebral cortex, we took brain sections from uninjured animals and triple labeled with WFA and antibodies against aggrecan and phosphacan (Fig. 7D~L). There were anatomical structures where WFA, aggrecan and phosphacan did not overlap. For example, the diffuse staining observed in the fornix area in the triangular septal nuclei in aggrecan- and phosphacan-labeled section was absent in WFA-stained sections (Fig. 7G~I). On the other hand, phosphacan labeling was weak or absent in indusium griseum (supracallossal giri, Fig. 7D~F). The fact that some structures show differential proteoglycan expression may indicate different roles played by these proteoglycans in the region.
Fig. 7.
PNN labeling decreases in the pericontusion cortex following TBI. (A~C) Representative brain sections at 7 dpi showing a reduced number of PNNs labeled with WFA, aggrecan or phosphacan in the injured cortex. Core of the lesion is depicted by arrows. (D~L) Higher magnification images of areas shown in A, B and C (arrowheads). Strong aggrecan labeling is observed in the induseum grissum (ig), fornix (fx, G~I) and piriform cortex (px) whereas phosphacan labeling is absent in ig and px. On the other hand, WFA labeling is not observed in the fx compared to aggrecan or phosphacan. (M) The number of WFA, aggrecan, and phosphacan-positive PNNs is significantly decreased in the injured cortex compared to the contralateral cortex at 7 and 28 dpi. Values shown are mean number of PNNs ± S.D. in cerebral cortex ipsi-or contralateral to the injured cortex. Two representative coronal sections at 0 bregma per mouse were used for counting (n = 5)(ANOVA with paired t test. *p<0.05, **p<0.005, ***p<0.0001). (N, O) Z-projection of a WFA-positive PNN and the surrounding axons labeled with β-III-tubulin in the uninjured cerebral cortex (N) and in the injured cerebral cortex (O) at 7 dpi (right). Bars: 50 μm (D~L); 10 μm (N, O).
Changes in CSPGs following TBI
A previous study demonstrated a decrease in neurocan, versican, aggrecan and phosphacan in the rat cerebral cortex following TBI (Harris et al., 2009). To address if this is true for mouse CCI as well, we performed immunofluorescence and immunoblotting (Fig. 6). To our surprise, we found increased neurocan protein expression in the tight band around the core at 7 and 28 dpi (Fig. 6B, C, white arrows). This increase was found at the location where an increased level of CS-GAG is observed (Figs. 1 & 6). Indeed, there was an overlap between neurocan and CS-56 in the tight band surrounding the injury core when 7 dpi sections were double-labeled with antibodies to neurocan and CS (Fig. 6Q~T). In contrast, aggrecan and phosphacan levels decreased in the injured cerebral cortex (Fig. 6D~F, G~I) while TBI caused an increase in versican levels in the tight band around the injury core (Fig. 6J~L). We confirmed the observed changes in CSPGs labeling by western blot on DEAE-enriched, cABC-digested brain extracts (Fig. 6M~P). Representative bands for neurocan and versican showed increases at 7 and 28 dpi compared to that of the naïve control, whereas bands for aggrecan and phosphacan showed a decreased level at 7 and 28 dpi compared to the naïve animal (Fig. 6M~O).
The number of PNNs decreases in the injured cerebral cortex following TBI
Perineuronal nets have been shown to be implicated in the restriction of synaptic plasticity (Pizzorusso et al., 2002). A decreased number of PNNs and a concomitant increase in GAP-43 at the pericontusion area have been reported following TBI in the rat, suggesting an increased regional plasticity (Harris et al., 2010). To investigate if there was any change in the number of PNNs following TBI, we counted the number of WFA-, aggrecan-, or phosphacan- positive PNNs in the entire injured cortex and compared it to the number of PNNs found in the contralateral cortex from the same section. Consistent with the previous report in the rat (Harris et al., 2010), there was a significant decrease in the total number of PNNs in the injured mouse cortex (Fig. 7M). The number of WFA-positive PNNs found in the cerebral cortex contralateral to the injury was 752 ± 63 (mean ± SD). TBI resulted in a decreased number of WFA+ PNNs to 456 ± 74, 61 % of the contralateral value at 7 dpi (Fig. 7M). The number of WFA+ PNNs was 58 % of the contralateral value at 28 dpi. The number of aggrecan positive PNNs were 689 ± 89 and 560 ± 191 in the contralateral cortex of 7 and 28 dpi brain section, respectively. Similar to the number of WFA-positive PNNs, the number of aggrecan-positive PNNs dropped to 67 % and 61 % of the contralateral values at 7and 28 dpi respectively. Fewer phosphacan-positive PNNs were detected when compared to the WFA-positive PNNS in the contralateral cortex of 7dpi (386 ± 91) and 28dpi (295 ± 45) brain sections. Nevertheless, TBI resulted in a significant 25 % and 28 % decrease in the number of phosphacan-positive PNNs compared to the contralateral side by 7 and 28 dpi, respectively. The loss of aggrecan and phosphacan was mostly within the pericontusional area and correlated with the loss of WFA-positive PNNs. Because of the possibility of changes in the contralateral cortex following injury, we also counted PNNs in the cortex of uninjured control animals, and found no difference between the contralateral cortex and uninjured controls (Data not shown).
Animals were also evaluated for changes in phospho-MAP1B, a marker for increased axon sprouting. However, we were not able to find changes in phospho-MAP1B labeled axons (data not shown). At 7 dpi, the total number of axons labeled with β-III-tubulin has substantially diminished around partially degraded PNNs, when compared to the intact the axons around an intact PNN from contralateral cortex (Fig. 7N, O).
The increased 4S forms barrier for axons following TBI
In vitro, 4S repels axons of cerebellar granule neurons (Wang et al., 2008). We hypothesized that the increase in 4S after TBI is acting as a major barrier to nearby axons. To address if the spatial localization of 4S increase is in accordance with this hypothesis, we took 7dpi brain sections and stained with 4S and the neuronal markers NeuN and β-III-tubulin (Fig. 8, Supplementary Fig. 3). As expected, the injury core is devoid of neurons and neuronal processes (Fig. 8A~C, Supplementary Fig. 3A~C). Double-labeling with 4S and β-III-tubulin shows the 4S labeling layering between the spared neuronal processes of the pericontusional area and the injury core (Fig. 8D~F, Supplementary Fig. 3D~F). A projection of Z-stack images (Fig. 8F, Supplementary Fig. 3F) at higher magnification shows β-III-tubulin-stained processes localized away from the 4S and subsequently from the injury core (Fig. 8G, Supplementary Fig. 3G). The localization suggests that 4S GAG expression in the tight band surrounding injury core serves to separate the core from the axons of spared neurons.
Fig. 8.
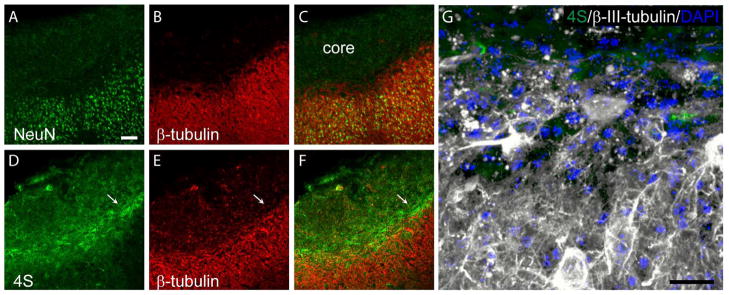
4S expression in the tight band around the injury core forms a wall to protect axons from crossing over to the core of injury. NeuN (A) and β-III-tubulin (B and E) labeling at 7 days post TBI showing the injury core is devoid of neurons (C). 4S (D) and β-III-tubulin labeling showing that 4S forms a boundary that separates the injury core and the axons of spared neurons in the pericontusional cortex (F). Bar, 100 μm. (G) Z-projection showing 4S, β-III tubulin, and DAPI in the region indicated by the arrow in F. Bar, 50 μm.
DISCUSSION
In this report we describe an increase in sulfated CS-GAG expression in the injury core and in a tight band surrounding it, and a decrease in the number of PNNs and the level of aggrecan and phosphacan in PNNs in the pericontusion region (summarized in Fig. 9). Immunolabeling analysis with antibodies specific for GAG chain sulfation demonstrated that the increased GAG observed in the injury core and the tight band around the core is primarily the 4-sulfated form of CS-GAG. Similarly, double labeling with antibodies directed against core proteins show that neurocan and NG2 are likely accountable for the increased 4S expression following TBI. On the other hand, TBI resulted in a decreased number of PNNs and the decreased level of aggrecan and phosphacan CSPGs in the pericontusional cortex. These changes were detected as early as 7 dpi and up to 28 dpi. Altogether, the dual changes in the expression of CSPGs observed in this study following TBI may reflect an endogenous process of repair after an insult to the head where increased CS-GAG in the vicinity of the injury core acts as a barrier, limiting the entry of axons from surviving neurons near the contusion area into the damaged tissue, while the decreased CSPGs in the PNN in the pericontusion cortex may permit a localized increase in plasticity to facilitate regrowth of injured processes.
Fig. 9.
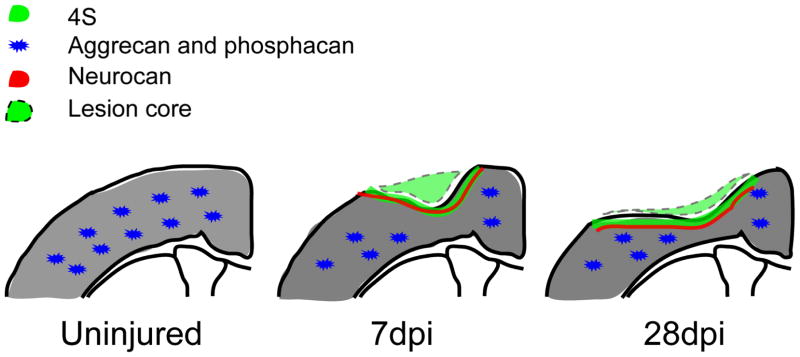
Dual response in the CSPGs following TBI. Following injury, there is an increase in 4S in the tight band around the injury core and a decrease in PNNs and CSPGs (aggrecan and phosphacan) in the pericontusion area at 7 and 28dpi.
Several lines of evidence support the importance of differentially sulfated CS-GAGs in controlling axon regeneration following CNS injury, with 4S being inhibitory while 6S is either inhibitory or permissive (Gilbert et al., 2005; Lin et al., 2011; Properzi et al., 2005; Wang et al., 2008). Using antibodies specific for sulfation patterns on GAG chains, we detected an increased level of 4S, but not 6S, in a tight band around the injury core, overlapping with the neurocan staining. Judging from the spatial location and considering its potent inhibitory action in axon outgrowth in cell culture (Laabs et al., 2007; Wang et al., 2008), a 4S increase may serve as a barrier for axons of surviving neurons around the impact core, as suggested by others (Davies et al., 1999; Lemons et al., 1999; Wang et al., 2008). It was surprising to us that CCI did not cause any increase in 6S, which is in contrast to the results following a cortical stab wound injury, where 6S, along with the enzymes involved in the synthesis for 6S, are increased (Properzi et al., 2005). On the other hand, GAG chains decorated with 6S may serve to promote axon regeneration, as a C6ST-1 KO animal exhibited poorer axon regeneration as compared to the wild type animal following a nigrostriatal lesion (Lin et al 2011). In our model, these results show that injury produces a contusion region with a boundary that is axon inhibitory.
The tight band of 4S CSPG staining around the injury core following CCI is associated with the core proteins neurocan and NG2. Increased neurocan expression is consistent with other reports showing induction of neurocan levels following different types of insults to the mammalian central nervous system, including a knife wound injury to the cortex (Asher et al., 2000; Asher et al., 2002), a contusion injury to the spinal cord(Andrews et al., 2012; Massey et al., 2008) as well as inflammatory insults (Sajad et al., 2011) and chronic glial scars (McKeon et al., 1999). In cell culture, neurocan is anti-adhesive and can effectively repel axons, and these actions were effectively abrogated after treatment of neurocan with chondroitinase ABC, supporting a primary role of GAG chains in actions of neurocan (Friedlander et al., 1994). Neurocan GAG chains are primarily 4-sulfated, which is consistent with the overall increase in 4-sulfation we found following CCI (Rauch et al., 2001). Neurocan mRNA and protein is colocalized in brain astrocytes (Jones et al, 2003) and is produced in astrocytes and O2A cells in culture (Asher et al., 2000). While we did observe 4S overlap with neurocan which was found in close proximity to the cells expressing GFAP in the tight band around the injury core, there was a low degree of overlap between GFAP+ astrocytes with 4S staining in the brain parenchyma following injury, especially within the injury core. The absence of GFAP+ staining in the injury core suggests that the major source of 4S in this region to be other than astrocytes.
TBI also resulted in the increased expression of NG2, a part-time proteoglycan and also a well-established marker for OPCs and pericytes, which were found often overlapped with increased 4S expression in the lesion core and in the tight band around the core. Others have also found NG2 increased after CNS injury: NG2 is increased following a needle injury to the head (Levine, 1994) and a knife wound injury to the mouse brain (Wang et al., 2007) or rat spinal cord (Jones et al., 2002). In our model, we observed cells expressing a high level of NG2 as early as 1 dpi in the injured cortex and these cells appear to congregate into injury core following CCI. By 7 dpi, high numbers of NG2+ cells were detected within and around the core. NG2 was first described as melanoma chondroitin sulfate proteoglycan (MSCP) and is thought to play a role in the cancer cell migration and metastasis (Burg et al., 1998). The GAG chain in NG2 may be important for targeting the NG2 to specific microdomains of the cell membrane (Stallcup and Dahlin-Huppe, 2001) and its interaction with MT3-MMP (Iida et al., 2001), together which could be crucial in the migratory capability of the cell expressing it. Although the exact role of NG2 or the cells expressing it are not certain, NG2+ cells may migrate into the lesion site and differentiate into oligodendrocytes (Komitova et al., 2011) as part of the repair process.
Our results showed a decrease in the total number of PNNs as well as the number of aggrecan or phosphacan positive PNNs in the pericontusional cortex following TBI, in aggrement with a previous finding (Harris et al., 2010). PNNs are comprised of CSPGs, along with link protein, hyaluronan and tenascin-R, which come from different cellular sources (Kwok et al., 2011). The decrease in PNNs could then be due to a reduction in the synthesis of any of the components, or an increase in PNN turnover. Two lines of evidence support the latter: 1) PNNs have a slow turnover rate and 2) there is a reported increase in matrix metalloproteases after TBI (Grossetete et al., 2009). Disruption of PNNs with chondroitinase ABC leads to an increase in plasticity in the adult brain (Pizzorusso et al., 2002). It is plausible that the decreased number of aggrecan- and phosphacan-positive PNNs support an increased axon regenerative capacity. However, we found no change in phosphorylated microtubule associated protein 1B (MAP1B), an indicator of sprouting (Sato-Yoshitake et al., 1989) in the pericontusion area (data not shown). Moreover, we found fewer axons labeled with β-III-tubulin around the partially degraded PNNs compared to those in the intact PNN, showing that there are fewer axons despite lack of PNNs. This indicates that collateral sprouting is not readily observed and the functional recovery may not entirely involve the anatomical reorganization of the lesioned area but rather a gross functional compensation by the unaffected cortex. This is because the lesion is not a complete cortico-ectomy and the contusion at this level does not cause complete destruction of the entire motor cortex. These data also suggest that treatments targeted to promote neural network formation will likely be beneficial in our injury model. Indeed, it has been suggested that chondroitinase treatment in conjunction with rehabilitation and training is a promising therapeutic treatment in spinal cord injury (Garcia-Alias et al., 2009; Wang et al., 2011).
In summary, we have described a dual response of CSPGs in a mouse model of TBI. The increased CSPG expressions in the injury core and in a tight band surrounding it were found to be neurocan and NG2 with 4 sulfated CS GAG chains. The decreased number of PNNs and the decreased expression of CSPGs aggrecan and phosphacan in PNNs in the pericontusion region are likely to represent an opportunistic window for treatment promoting reconnection. Further investigation into the role of 4S increase in the tight band around the injury core will help us understand its significance in TBI. In addition, understanding the impact of the decrease of PNNs and aggrecan/phosphacan may lead to a potential therapeutic strategy in TBI.
Supplementary Material
Supplementary Fig. 1. Figure 4 in magenta.
Supplementary Fig. 2. Figure 5 in magenta.
Supplementary Fig. 3. Figure 8 in magenta.
Acknowledgments
Grant sponsor: Center for Neuroscience and Regenerative Medicine to Drs Aviva J Symes and Herbert M Geller (300604-11.0-60855). Center for Neuroscience and Regenerative Medicine postdoctoral fellowship to Dr Jae-Hyuk Yi (G1709B).
We are grateful for the technical assistance of Panpan Yu, Claire Liepmann, and Ashley Campbell. We also acknowledge the assistance of the NHLBI DIR light microscopy core and the members of CNRM mouse animal facility. This work has been supported by the CNRM grant to Drs. Herbert M Geller and Aviva J Symes (G175PD). Jae-Hyuk Yi is supported by a CNRM post doctoral fellowship (G1709B).
Footnotes
To be submitted to : Dr. Jeffrey H. Kordower, Rush University Medical Center: Neurodegeneration and CNS Repair, Basal Forebrain, Cerebral Cortex.
References
- Andrews EM, Richards RJ, Yin FQ, Viapiano MS, Jakeman LB. Alterations in chondroitin sulfate proteoglycan expression occur both at and far from the site of spinal contusion injury. Exp Neurol. 2012;235:174–187. doi: 10.1016/j.expneurol.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J Neurosci. 2000;20:2427–2438. doi: 10.1523/JNEUROSCI.20-07-02427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J Neurosci. 2002;22:2225–2236. doi: 10.1523/JNEUROSCI.22-06-02225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnur Z, Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell. 1984;38:811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- Bao X, Pavao MS, Dos Santos JC, Sugahara K. A functional dermatan sulfate epitope containing iduronate(2-O-sulfate)alpha1-3GalNAc(6-O-sulfate) disaccharide in the mouse brain: demonstration using a novel monoclonal antibody raised against dermatan sulfate of ascidian Ascidia nigra. J Biol Chem. 2005;280:23184–23193. doi: 10.1074/jbc.M503036200. [DOI] [PubMed] [Google Scholar]
- Bartus K, James ND, Bosch KD, Bradbury EJ. Chondroitin sulphate proteoglycans: Key modulators of spinal cord and brain plasticity. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Brauer K, Hartig W, Bigl V, Bruckner G. Distribution of parvalbumin-containing neurons and lectin-binding perineuronal nets in the rat basal forebrain. Brain Res. 1993;631:167–170. doi: 10.1016/0006-8993(93)91205-7. [DOI] [PubMed] [Google Scholar]
- Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, McCarthy S, Sebat J, Gage FH. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Mac Donald C, Kessens CC, Yuede C, Parsadanian M, Spinner M, Kim E, Schwetye KE, Holtzman DM, Bayly PV. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari N, Torres L, Robinson JK, Tsirka SE. Axonal regrowth after spinal cord injury via chondroitinase and the tissue plasminogen activator (tPA)/plasmin system. J Neurosci. 2011;31:14931–14943. doi: 10.1523/JNEUROSCI.3339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MA, Grako KA, Stallcup WB. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J Cell Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K, Strata P, Fawcett JW. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- Corvetti L, Rossi F. Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat. J Neurosci. 2005;25:7150–7158. doi: 10.1523/JNEUROSCI.0683-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson-Thomas YM, Coulson-Thomas VJ, Filippo TR, Mortara RA, da Silveira RB, Nader HB, Porcionatto MA. Adult bone marrow-derived mononuclear cells expressing chondroitinase AC transplanted into CNS injury sites promote local brain chondroitin sulphate degradation. J Neurosci Methods. 2008;171:19–29. doi: 10.1016/j.jneumeth.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Cs-Szabo G, Roughley PJ, Plaas AH, Glant TT. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995;38:660–668. doi: 10.1002/art.1780380514. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–624. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol. 1994;125:669–680. doi: 10.1083/jcb.125.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, 3rd, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW. Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci. 2009;12:1145–1151. doi: 10.1038/nn.2377. [DOI] [PubMed] [Google Scholar]
- Giamanco KA, Morawski M, Matthews RT. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Gilbert RJ, McKeon RJ, Darr A, Calabro A, Hascall VC, Bellamkonda RV. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Grossetete M, Phelps J, Arko L, Yonas H, Rosenberg GA. Elevation of matrix metalloproteinases 3 and 9 in cerebrospinal fluid and blood in patients with severe traumatic brain injury. Neurosurgery. 2009;65:702–708. doi: 10.1227/01.NEU.0000351768.11363.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Carmichael ST, Hovda DA, Sutton RL. Traumatic brain injury results in disparate regions of chondroitin sulfate proteoglycan expression that are temporally limited. J Neurosci Res. 2009;87:2937–2950. doi: 10.1002/jnr.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Mironova YA, Hovda DA, Sutton RL. Pericontusion axon sprouting is spatially and temporally consistent with a growth-permissive environment after traumatic brain injury. J Neuropathol Exp Neurol. 2010;69:139–154. doi: 10.1097/NEN.0b013e3181cb5bee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–5234. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- Horii-Hayashi N, Okuda H, Tatsumi K, Ishizaka S, Yoshikawa M, Wanaka A. Localization of chondroitin sulfate proteoglycan versican in adult brain with special reference to large projection neurons. Cell Tissue Res. 2008;334:163–177. doi: 10.1007/s00441-008-0698-1. [DOI] [PubMed] [Google Scholar]
- Horii-Hayashi N, Tatsumi K, Matsusue Y, Okuda H, Okuda A, Hayashi M, Yano H, Tsuboi A, Nishi M, Yoshikawa M, Wanaka A. Chondroitin sulfate demarcates astrocytic territories in the mammalian cerebral cortex. Neurosci Lett. 2010;483:67–72. doi: 10.1016/j.neulet.2010.07.064. [DOI] [PubMed] [Google Scholar]
- Hu H, Li J, Gagen CS, Gray NW, Zhang Z, Qi Y, Zhang P. Conditional knockout of protein O-mannosyltransferase 2 reveals tissue-specific roles of O-mannosyl glycosylation in brain development. J Comp Neurol. 2011;519:1320–1337. doi: 10.1002/cne.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J, Pei D, Kang T, Simpson MA, Herlyn M, Furcht LT, McCarthy JB. Melanoma chondroitin sulfate proteoglycan regulates matrix metalloproteinase-dependent human melanoma invasion into type I collagen. J Biol Chem. 2001;276:18786–18794. doi: 10.1074/jbc.M010053200. [DOI] [PubMed] [Google Scholar]
- Imagama S, Sakamoto K, Tauchi R, Shinjo R, Ohgomori T, Ito Z, Zhang H, Nishida Y, Asami N, Takeshita S, Sugiura N, Watanabe H, Yamashita T, Ishiguro N, Matsuyama Y, Kadomatsu K. Keratan sulfate restricts neural plasticity after spinal cord injury. J Neurosci. 2011;31:17091–17102. doi: 10.1523/JNEUROSCI.5120-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Maeda N. Spatiotemporal expression of chondroitin sulfate sulfotransferases in the postnatal developing mouse cerebellum. Glycobiology. 2008;18:602–614. doi: 10.1093/glycob/cwn040. [DOI] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H, Ohsawa K, Sasaki Y, Kohsaka S, Imai Y. Macrophage/microglia-specific protein Iba1 enhances membrane ruffling and Rac activation via phospholipase C-gamma -dependent pathway. J Biol Chem. 2002;277:20026–20032. doi: 10.1074/jbc.M109218200. [DOI] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Tsutsumi K, Tone Y, Sugahara K. Developmental regulation of the sulfation profile of chondroitin sulfate chains in the chicken embryo brain. J Biol Chem. 1997;272:31377–31381. doi: 10.1074/jbc.272.50.31377. [DOI] [PubMed] [Google Scholar]
- Komitova M, Serwanski DR, Lu QR, Nishiyama A. NG2 cells are not a major source of reactive astrocytes after neocortical stab wound injury. Glia. 2011;59:800–809. doi: 10.1002/glia.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JC, Dick G, Wang D, Fawcett JW. Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol. 2011;71:1073–1089. doi: 10.1002/dneu.20974. [DOI] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J Neurosci. 2007;27:14494–14501. doi: 10.1523/JNEUROSCI.2807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999;160:51–65. doi: 10.1006/exnr.1999.7184. [DOI] [PubMed] [Google Scholar]
- Levine JM. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J Neurosci. 1994;14:4716–4730. doi: 10.1523/JNEUROSCI.14-08-04716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB. Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci. 1987;7:2721–2731. doi: 10.1523/JNEUROSCI.07-09-02721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok JC. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One. 2011;6:e21499. doi: 10.1371/journal.pone.0021499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyck L, Dalmau I, Chemnitz J, Finsen B, Schroder HD. Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex. J Histochem Cytochem. 2008;56:201–221. doi: 10.1369/jhc.7A7187.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N, He J, Yajima Y, Mikami T, Sugahara K, Yabe T. Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule. J Biol Chem. 2003;278:35805–35811. doi: 10.1074/jbc.M305530200. [DOI] [PubMed] [Google Scholar]
- Maeda N, Matsui F, Oohira A. A chondroitin sulfate proteoglycan that is developmentally regulated in the cerebellar mossy fiber system. Dev Biol. 1992;151:564–574. doi: 10.1016/0012-1606(92)90194-l. [DOI] [PubMed] [Google Scholar]
- Mark MP, Butler WT, Ruch JV. Transient expression of a chondroitin sulfate-related epitope during cartilage histomorphogenesis in the axial skeleton of fetal rats. Dev Biol. 1989;133:475–488. doi: 10.1016/0012-1606(89)90051-1. [DOI] [PubMed] [Google Scholar]
- Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Akagi A, Hayashi N, Watanabe K, Oohira A. Activity-dependent regulation of a chondroitin sulfate proteoglycan 6B4 phosphacan/RPTPbeta in the hypothalamic supraoptic nucleus. Brain Res. 2004;1017:163–171. doi: 10.1016/j.brainres.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Naidu M, Carlstedt T, Levine JM, Fawcett JW. Expression and glycanation of the NG2 proteoglycan in developing, adult, and damaged peripheral nerve. Mol Cell Neurosci. 2003;24:787–802. doi: 10.1016/s1044-7431(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nakano K, Morita S, Nakashima T, Oohira A, Miyata S. Expression of chondroitin sulfate proteoglycans in barrel field of mouse and rat somatosensory cortex. Brain Res. 2009;1252:117–129. doi: 10.1016/j.brainres.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113 ( Pt 17):3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- Oohira A, Matsui F, Watanabe E, Kushima Y, Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody IG2 in the rat cerebrum. Neuroscience. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Poole CA, Glant TT, Schofield JR. Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J Histochem Cytochem. 1991;39:1175–1187. doi: 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- Pratta MA, Tortorella MD, Arner EC. Age-related changes in aggrecan glycosylation affect cleavage by aggrecanase. J Biol Chem. 2000;275:39096–39102. doi: 10.1074/jbc.M006201200. [DOI] [PubMed] [Google Scholar]
- Properzi F, Carulli D, Asher RA, Muir E, Camargo LM, van Kuppevelt TH, ten Dam GB, Furukawa Y, Mikami T, Sugahara K, Toida T, Geller HM, Fawcett JW. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur J Neurosci. 2005;21:378–390. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]
- Sajad M, Zargan J, Chawla R, Umar S, Khan HA. Upregulation of CSPG3 accompanies neuronal progenitor proliferation and migration in EAE. J Mol Neurosci. 2011;43:531–540. doi: 10.1007/s12031-010-9476-0. [DOI] [PubMed] [Google Scholar]
- Sato-Yoshitake R, Shiomura Y, Miyasaka H, Hirokawa N. Microtubule-associated protein 1B: molecular structure, localization, and phosphorylation-dependent expression in developing neurons. Neuron. 1989;3:229–238. doi: 10.1016/0896-6273(89)90036-6. [DOI] [PubMed] [Google Scholar]
- Sato Y, Oohira A. Chondroitin sulfate, a major niche substance of neural stem cells, and cell transplantation therapy of neurodegeneration combined with niche modification. Curr Stem Cell Res Ther. 2009;4:200–209. doi: 10.2174/157488809789057419. [DOI] [PubMed] [Google Scholar]
- Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, Verhaagen J, Fawcett JW. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci. 2003;24:913–925. doi: 10.1016/j.mcn.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Shimada IS, Borders A, Aronshtam A, Spees JL. Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke. 2011;42:3231–3237. doi: 10.1161/STROKEAHA.111.623280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki Y, Nagata I, Ishii M, Tanaka M, Marunouchi T, Hata T, Maeda N. Developmental change and function of chondroitin sulfate deposited around cerebellar Purkinje cells. J Neurosci Res. 2005;82:172–183. doi: 10.1002/jnr.20639. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kanesaki H, Igarashi T, Kameya S, Yamaki K, Mizota A, Kudo A, Miyagoe-Suzuki Y, Takeda S. Reactive gliosis of astrocytes and Muller glial cells in retina of POMGnT1-deficient mice. Mol Cell Neurosci. 2011;47:119–130. doi: 10.1016/j.mcn.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, Plaas A. Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. J Biol Chem. 2011;286:26016–26027. doi: 10.1074/jbc.M110.208694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J Cell Sci. 2008;121:3083–3091. doi: 10.1242/jcs.032649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Moges H, Bharucha Y, Symes A. Smad3 null mice display more rapid wound closure and reduced scar formation after a stab wound to the cerebral cortex. Exp Neurol. 2007;203:168–184. doi: 10.1016/j.expneurol.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Kimata K, Oike Y, Tani K, Maeda N, Yoshida K, Shimomura Y, Yoneda M, Suzuki S. A monoclonal antibody that specifically recognizes a glucuronic acid 2-sulfate-containing determinant in intact chondroitin sulfate chain. J Biol Chem. 1987;262:4146–4152. [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Oohira A, Suzuki Y. Immunohistochemical localization of chondroitin sulfate in the forestomach of the sheep. Eur J Histochem. 1995;39:265–272. [PubMed] [Google Scholar]
- Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K. N-Acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology. 2006;16:702–710. doi: 10.1093/glycob/cwj115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Figure 4 in magenta.
Supplementary Fig. 2. Figure 5 in magenta.
Supplementary Fig. 3. Figure 8 in magenta.