Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is prevalent in swine farms worldwide and is a major source of economic loss and animal suffering. Rapid genetic variation of PRRSV makes it difficult for current vaccines to confer protection against newly emerging strains. We recently demonstrated that a novel peptide nanofiber hydrogel (H9e) could act as a potent adjuvant for killed H1N1 vaccines. Therefore, the objective of this study was to evaluate H9e as an adjuvant for PRRSV modified live virus (MLV) vaccines. Pigs were vaccinated with Ingelvac PRRSV MLV with or without H9e adjuvant before being challenged with the VR-2332 (parental vaccine strain) or MN184A (genetically diverse strain) PRRSV. Pigs vaccinated with MLV+H9e had higher levels of circulating vaccine virus. More importantly, pigs vaccinated with MLV+H9e had improved protection against challenge by both PRRSV strains, as demonstrated by reduced challenge-induced viremia compared with pigs vaccinated with MLV alone. Pigs vaccinated with MLV+H9e had lower frequency of T-regulatory cells and IL-10 production but higher frequency of Th/memory cells and IFN-γ secretion than that in pigs vaccinated with MLV alone. Taken together, our studies suggest that the peptide nanofiber hydrogel H9e, when combined with the PRRSV MLV vaccine, can enhance vaccine efficacy against two different PRRSV strains by modulating both host humoral and cellular immune responses.
Keywords: Adjuvant, Hydrogel, Modified live vaccine, PRRSV
1. Introduction
Pork is one of the most widely consumed meats in the world, accounting for more than a third of meat production worldwide. Infectious diseases remain the biggest threat to the pork industry, resulting in billions of dollars in economic losses [1]. One particularly devastating disease known to lead to the dramatic decline of swine herds and increased pork prices is porcine reproductive and respiratory syndrome (PRRS) [1]. Clinical features of PRRS include massive abortion in sows and weight loss, respiratory disease, and mortality in young pigs. PRRS outbreaks continue to emerge rapidly and with increased virulence; if left untreated, outbreaks will endanger swine industries worldwide.
PRRS is caused by the PRRS virus (PRRSV). Field isolates often differ significantly in the degree of virulence and pathogenicity, presumably due to a high degree of genetic variation among strains [2]. PRRSV can be broadly divided into two distinct genotypes, Type 1 (European) and Type 2 (North American). Each genotype also contains several subtypes, which are also genetically diverse and lead to immunity limited to the initial infecting genotype, with only partial or no protection from reinfection by other subtypes [3]. Due to genetic diversity and the rapid evolution rate of PRRSV, development of a broadly protective PRRSV vaccine is challenging, but vaccination remains the most effective way to control PRRS. Several types of commercial vaccines, including killed or modified live vaccines, have been widely used [4]. Current killed vaccines are largely ineffective in preventing both PRRSV infection and disease, so most farms vaccinate their herds with modified live vaccines to control PRRS outbreaks. Modified live vaccines are shown to reduce disease caused by genetically similar strains, but they provide very limited or no protection against genetically unrelated field isolates [5]. Therefore, broad cross-protection against genetically dissimilar PRRSV strains should be the main consideration for the design of improved PRRSV vaccines.
Adjuvants including oil-in-water emulsions, polymers, and bacterial antigens have been tested in combination with modified live vaccines in an effort to reduce the antigenic load and improve vaccine efficacy [6, 7]. Results from these studies suggest that addition of adjuvant to MLV PRRSV vaccines can lead to increased protection to PRRSV challenge. Peptide hydrogels also might be a promising delivery system for vaccines due to their high water content, polymer network and reversible sol-gel (solution to gel) formation. Peptide hydrogels have been well studied as drug delivery systems, for tissue engineering applications, and in 3-D cell culture and show promising results [8, 9]. We recently developed a novel peptide that can form a flexible nanofiber hydrogel (H9e) and functions as a potent adjuvant for killed H1N1 influenza vaccines [10]. To further characterize the capabilities of the H9e hydrogel, we evaluated H9e as an adjuvant for PRRSV MLV vaccines. Results show that the addition of H9e to MLV enhanced protection of pigs to both homologous and heterologous strains of PRRSV. Compared with pigs vaccinated with MLV alone, animals vaccinated with MLV+H9e developed earlier and more robust PRRSV-specific neutralizing antibodies as well as increased PRRSV-specific Th1 cytokine IFN-γ and reduced immunosuppressive cytokine IL-10. Together, these results suggest that PRRS MLV vaccine formulated with H9e adjuvant may increase vaccine efficacy against genetically diverse PRRS viruses.
2. Materials and Methods
2.1. Cells, virus and adjuvant preparation
MARC-145 cells were maintained in modified Eagle’s medium (MEM) supplemented with 7% fetal bovine serum (FBS) containing penicillin (100U/ml) and streptomycin (100 µg/ml) at 37 °C with 5% CO2. For virus infection and titration, MEM supplemented with 2% FBS was used. Ingelvac PRRS® modified live virus vaccine (MLV) was purchased from Boehringer Ingelheim Vetmedica Inc (St. Joseph, MO). PRRSV MN184A was a kind gift from Dr. Kay Faaberg (National Animal Disease Center, USDA-ARS, Ames, IA). PRRSV strains VR-2332 (ATCC, Manassas, VA) and MN184A were prepared and titered in MARC-145 cells and stored in aliquots at −80 °C until use. H9e peptide was prepared as previously described with a final concentration of 17.5 mg/ml [10]. PRRS MLV vaccine was resuspended in 50 ml vaccine diluent, provided by the manufacturer, to yield a 2-fold concentrate of vaccine virus. MLV-alone vaccine was then mixed 1:1 with vaccine diluent. A solution of 6 mg H9e with 1.2% porcine serum in diluent/MEM medium was added 1:1 with 2× MLV to prepare MLV+H9e vaccine.
2.2. Pigs, vaccination and PRRSV challenge
Thirty-five female/unvaccinated (3 weeks old) Large White-Duroc crossbred PRRSV-free pigs were divided into 7 groups (n = 5) and housed in separate pens within the Large Animal Research Center (LARC) at Kansas State University. These piglets were confirmed sera-negative for antibodies to PRRSV by ELISA and PRRSV-free in serum by RT-PCR. Pigs were immunized intramuscularly on day 0 with placebo, PRRS-MLV (1×106 TCID50/ pig), or PRRS-MLV+H9e (1×106 TCID50 + 6 mg H9e/ pig). Twenty-eight days post vaccination (DPV), the pigs were challenged with either homologous PRRSV VR-2332 (1×106 TCID50) or heterologous MN184A (5×105 TCID50). Body weight measurements and blood samples were collected weekly (0, 7, 14, 21, 28 DPV and 7, 14 DPC). Pigs were also monitored daily for rectal temperature and clinical signs after challenge. All pigs were humanly euthanized 15 days post challenge (DPC). All animal experiments were approved by the Institutional Animal Care and Use Committee at Kansas State University.
2.3. Analysis of serum virus titer
Total RNA was extracted from serum and one-step SyBR Green real-time PCR (Bio-Rad, Hercules, CA) was performed to evaluate the PRRSV ORF7 expression level as previously described [11]. For quantification, total RNA of a known TCID50 of virus was 10-fold serially diluted and were used to generate a standard curve. The virus quantities of unknown samples were determined by linear extrapolation of the Ct value plotted against the standard curve.
2.4. PRRSV-specific and virus neutralizing antibody titration
PRRSV-specific ELISA antibody titers were measured using the Herdcheck Porcine Reproductive and Respiratory Syndrome X3 Antibody Test (IDEXX Laboratories, Westbrook, ME) as described by the manufacturer. Virus neutralizing antibody titer in the serum was analyzed as previously described [11]. Briefly, serum samples were heat-inactivated and serial dilutions were mixed with PRRSV VR-2332 or MN184A viruses. After incubation, the mixtures were transferred to MARC-145 cells and incubated for 72 hours. Cytopathic effect (CPE) was used to determine the end-point titers that were calculated as the reciprocal of the highest serum dilution to neutralize >90% CPE induced by 200 TCID50 of PRRSV in duplicate wells per sample.
2.5. Analysis of cytokine responses
Pig sera were collected at 7 DPC to evaluate IL-4, IL-8, IL-10, IFN-γ, and TNF-α cytokine secretion profiles by ELISA. Procedures were performed as per the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Additionally, at necropsy (15 DPC), 106 tracheobronchial lymph node (TBLN) mononuclear cells (MNCs) and lung MNCs were restimulated with 200 TCID50 of the respective challenge PRRSV similar to that described in Ferrari et al. [12]. Cell culture supernatants were analyzed by ELISA for IL-10 cytokine secretion (Invitrogen).
2.6. Flow cytometry analysis
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples by Ficoll-Hypaque gradient centrifugation using Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO). Flow cytometry analysis was performed to determine different lymphocyte populations based on the cell surface marker phenotype: T-helper cells (CD3+CD4+CD8−), cytotoxic T lymphocyte (CD3+CD4−CD8+), Th/memory cells (CD3+CD4+CD8+), T-regulatory cells (CD4+FoxP3+CD25+) and γδ T cells (CD8+ TcR1N4+). The mouse anti-pig TcR1N4 antibody was purchased from VMRD (Pullman, WA), and all other antibodies were purchased from BD Biosciences (San Jose, CA). Immuno-stained cells were acquired using a FACS Caliber (BD Biosciences) flow cytometer. Frequencies of individual lymphocytes were analyzed by 100,000 events using FlowJo software (Tree Star, Inc., Ashland, OR).
2.7. Statistical analysis
All data were expressed as the mean value of five pigs ± SEM. The differences in the level of humoral response, cytokine production and viremia among each group were determined by the one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s test using Sigmaplot 11 software (Systat Software Inc., San Jose, CA). Differences were considered statistically significant when p<0.05.
3. Results
3.1. H9e adjuvant enhances cross-protection efficacy of MLV to heterologous PRRSV infection in pigs
Our previous studies showed that H9e hydrogel can be a safe, efficacious adjuvant for the killed H1N1 swine influenza vaccines, resulting in significantly higher hemagglutination inhibition titers and antibody titers to swine influenza than immunization with antigen alone [10]. Since H9e acts as a potent adjuvant for killed subunit vaccines, we hypothesized that H9e hydrogels could also work as an adjuvant for a modified live PRRS vaccine.
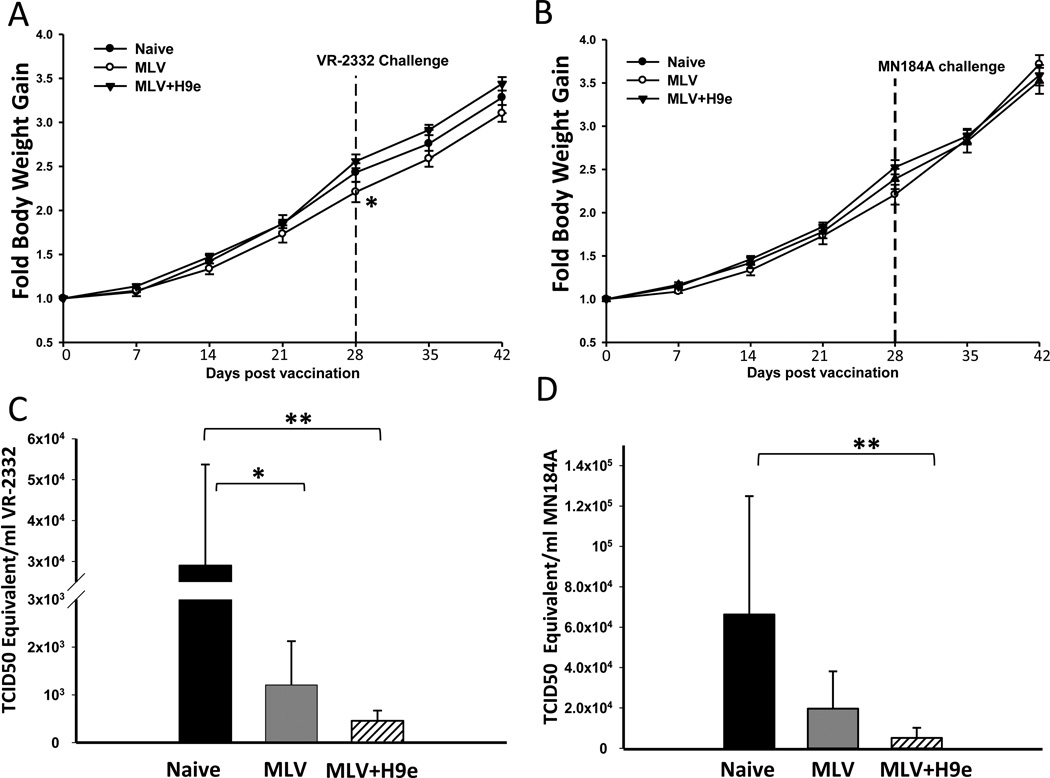
H9e solution is a free-flowing solution at ambient temperature, and forms an injectable hydrogel at physiological conditions. H9e was easily mixed with MLV and showed no virucidal effects on the vaccine virus (data not shown). Pigs were vaccinated with Ingelvac PRRS MLV vaccine alone (MLV), Ingelvac PRRS MLV vaccine adjuvanted with hydrogel H9e (MLV+H9e), or PBS (mock). Twenty-eight days post vaccination (DPV), pigs were subjected to virus challenge with low virulence homologous VR-2332 (1 × 106 TCID50/pig) or moderately virulent heterologous MN184A (5 × 105 TCID50/pig) strains of PRRSV. The mean body temperature of unvaccinated pigs challenged with VR-2332 or MN184A was 0.3 or 1.0 °C higher than vaccinated pigs, respectively, with no difference between vaccinated groups (data not shown). Interestingly, pigs vaccinated with MLV gained significantly less weight than unvaccinated and MLV+H9e vaccinated pigs at 28 DPV (Fig. 1a, b), suggesting that the un-adjuvanted MLV vaccine virus may cause sub-clinical disease in pigs.
Figure 1. H9e adjuvant enhances protection efficacy of MLV to homologous and heterologous PRRSV infection in pigs.
Pigs (3-week-old) were vaccinated with MLV or MVL+H9e and challenged with the VR-2332 or MN184A strain of PRRSV 28 days post vaccination. (A, B) Fold body weight gain during the duration of the experiment was determined. (C, D) Viral RNA in the serum (TCID50 equivalent /mL) was measured on 7 days post challenge (DPC) by RT-PCR. Viremia data are shown as means ± SEM (n=5). Bracketed groups were compared and * denotes p <0.05, ** denotes p <0.01.
To determine if vaccinated pigs were protected from homologous or heterologous virus challenge, titers of circulating virus were measured 7 days post challenge (DPC). We found that the pigs vaccinated with MLV+H9e were able to significantly clear both the VR-2332 and MN184A strains circulating in the blood 7 days post challenge (7 DPC). Pigs vaccinated with MLV alone were able to significantly clear the homologous VR-2332 virus strain (Fig.1c). The pigs vaccinated with MLV alone had reduced viral load in the blood after MN184A challenge; however, it was not statistically significant from that in the unvaccinated-challenged group of pigs (Fig. 1d). Taken together, these results suggest that the addition of H9e adjuvant to PRRSV MLV vaccines can enhance protection against genetically distinct stains of PRRSV.
3.2. Pigs vaccinated with MLV+H9e have increased vaccine virus circulation in the blood after vaccination
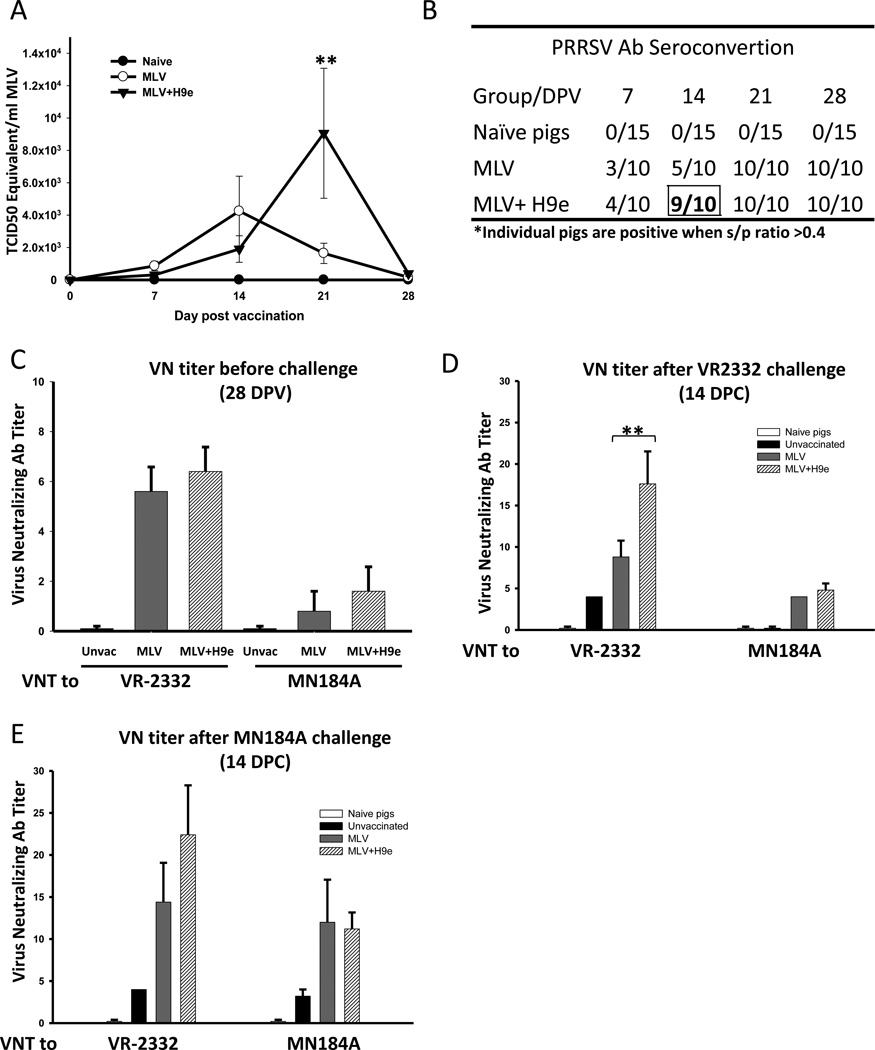
H9e rapidly forms a gel once it is formulated with MLV. Therefore, we suspect that the H9e nanofiber network may act as a scaffold to prolong vaccine virus entry into the blood or enhance its replication within the host to enhance the vaccine’s protective effects. To determine if H9e adjuvant affects the viral load of the vaccine strain of PRRSV in vaccinated pigs, we measured circulating viral load in the serum of all pigs weekly. Interestingly, pigs vaccinated with MLV+H9e started to develop significantly higher levels of circulating virus two weeks after vaccination and reached an average maximum of 5.5-fold (9,057±402 TCID50 equivalent/ml) higher virus titers than that in pigs vaccinated with MLV alone (1,638± 625 TCID50 equivalent/ml) on 21 DPV (Fig. 2a). By 28 DPV, the MLV virus was eliminated from the blood of pigs in all groups. Therefore, our results suggest that H9e may act to stabilize the MLV virus within the host and thus to increase the exposure of antigen to the host immune system.
Figure 2. Pigs vaccinated with MLV+H9e have increased vaccine virus circulation and produce an earlier on-set of PRRSV-specific antibodies.
(A) Viral RNA of MLV vaccine virus in the serum (TCID50 equivalent /mL) was determined by RT-PCR weekly after vaccination with MLV, or MLV+H9e. Data were shown as mean ± SEM (n=5) ** p <0.01. (B) Serum from vaccinated pigs was assayed for PRRSV-specific antibodies with IDEXX HerdCheck ELISA kit. The threshold for seroconvertion was set at a sample-to-positive (s/p) ratio of 0.4 according to manufacturers’ instructions. (C–E) Serum samples were titrated individually in MARC-145 cells for the levels of anti-PRRSV neutralizing antibodies 28 days post vaccination (DPV) or 14 days post challenge (DPC) determined as the highest dilution that inhibited CPE. Data are shown as mean ± SEM (n= 5). Bracketed groups were compared and ** denotes p <0.01.
3.3. H9e-MLV vaccinated pigs show enhanced PRRSV-specific antibodies and PRRSV neutralizing antibodies
To determine whether increased antigen exposure might lead to enhanced humoral and cellular immune responses in vaccinated pigs, we first evaluated antibody responses of pigs vaccinated with PRRSV MLV in the presence or absence of H9e. Serum samples were analyzed by commercial IDEXX PRRSV-specific antibody ELISA. By 14 DPV, 9 out of 10 pigs vaccinated with MLV+H9e were positive for PRRSV-specific antibodies (as defined by manufacturer at S/P ratio ≥ 0.4), compared with only 5 out of 10 pigs in MLV alone groups (Fig. 2b and Supplemental Figure 1). Therefore, these results suggest that addition of H9e adjuvant results in the earlier onset of PRRSV antibodies than MLV alone. By 21 DPV, all vaccinated pigs had seroconverted to anti-PRRSV antibody positive.
PRRSV MLV vaccination is characterized by generation of early non-protective antibodies specific to the nucleocapsid protein (as measured by IDEXX ELISA) and delayed generation of protective virus neutralizing antibodies. To determine if the H9e-mediated prolonged viremia affects the production of neutralizing antibodies as well, the PRRSV neutralizing antibody titers (VN titers) were analyzed. On 28 DPV, pigs vaccinated with MLV+H9e had similar VN titers to both VR-2332 and MN184A as that in pigs vaccinated with MLV-alone (Fig.2c). After homologous VR-2332 viral challenge, pigs vaccinated with MLV+H9e had significantly higher VN titer to VR-2332 and comparable VN titer to MN184A compared with pigs vaccinated with MLV alone (Fig. 2d). However, all vaccinated pigs developed similar levels of VN titers after heterologous MN184A viral challenge (Fig. 2e). Therefore, our results show that the addition of H9e hydrogel adjuvant can induce early on-set and enhanced antibody production over vaccinating pigs with MLV alone.
3.4. Pigs vaccinated with MLV+H9e hydrogel have increased pro-inflammatory cytokines and reduced immunosuppressive cytokine secretion profiles
Because we found that H9e can improve the humoral immune responses of pigs to the PRRS MLV vaccine, we next assayed the effects of H9e adjuvant on MLV-elicited cytokine profiles. In doing so, sera at 7 DPC were analyzed for the presence of IL-4, IL-8, IL-10, IFN-γ and TNF-α. As shown in Fig. 3, the levels of IFN-γ, but not TNF-α, in the sera from MLV+H9e vaccinated pigs was significantly higher than that in pigs vaccinated with MLV-alone after challenge (Fig3a,b). The levels of IL-4 and IL-8 in sera from pigs vaccinated with MLV+H9e were significantly higher than that from pigs vaccinated with MLV-alone when the pigs were challenged with VR-2332 PRRSV (Fig. 3c, d). Conversely, the secretion of immunosuppressive cytokine IL-10 in sera of MLV+H9e vaccinated pigs was reduced compared with that in the MLV-alone vaccinated pigs after challenge with both VR-2332 and MN184A (Fig. 3e). IL-10 expression levels of lung and TBLN MNCs also were analyzed at necropsy (15 DPC). As shown in Fig. 3e, after these cells were re-stimulated with either VR-2332 or MN184A in vitro, reduced IL-10 cytokine levels were observed in the supernatant of lung and lymph node MNCs of the pigs vaccinated with MLV+H9e. Therefore, our results suggest that addition of H9e to MLV vaccine alters cytokine expression profiles.
Figure 3. Pigs vaccinated with MLV+H9e have increased PRRSV-specific IFN-γ, IL-4, IL-8 and reduced IL-10 cytokine secretion.
Cytokine expression profiles in the sera of pigs 7 days post challenge (DPC) were examined by quantitative ELISA, (A) IFN-γ (B) TNF-α (C) IL-4 and (D) IL-8. (E) IL-10 concentrations in serum samples and supernatants of PBMCs and lung MNCs which were collected at necropsy (15 DPC) and restimulated with corresponding PRRSV strains. Bracketed groups were compared and * denotes p <0.05, ** denotes p <0.01, and NS=no difference.
3.5. Pigs vaccinated with MLV+H9e display decreased T-regulatory and increased Th/memory lymphocyte subpopulations
To verify if the change in cytokine expression patterns was associated with changes in lymphocytes population, the frequencies of T-helper cells, cytotoxic T lymphocyte, Th/memory cells, T-regulatory cells and γδ T cells in blood, lung, and lymph nodes were evaluated using flow cytometry analysis. As shown in Fig. 4, a significant decrease of the T-regulatory (Treg) lymphocyte population (Fig. 4a) and increase of the Th/memory lymphocyte population (Fig. 4b) was observed in the blood of pigs vaccinated with MLV+H9e than that in pigs vaccinated with MLV alone 4 weeks after vaccination. The decrease of Treg lymphocyte population and increase of Th/memory lymphocyte population were also observed 14 DPC in pigs challenged with homologous VR-2332 or heterologous MN184A PRRSV in blood, TBLN, and lung MNC samples (Fig. 4c, d). Additionally, we examined Th cells, CTL, γδ T cells and NK cell population before and after challenge and found no significant changes in any groups (data not shown).
Figure 4. Pigs vaccinated with MLV+H9e have decreased T-regulatory and increased Th/memory lymphocyte subpopulations.
Whole blood was collected and stained for CD4, CD8, FoxP3, and CD25. (A) Shown are the percentages of T-reg cells that were triple-positive for CD4/FoxP3/CD25 28 days post vaccination (DPV) and (C) 14 days post challenge (DPC). (B) Also shown are the percentages of Th/memory cells that were double-positive for CD4/CD8 on 28 DPV and (D) on 14 DPC. Data is shown as mean ± SEM (n=5). Bracketed groups were compared and * denotes p <0.05, ** denotes p <0.01, and NS=no difference.
4. Discussion
Current commercial vaccines, both killed virus and modified live, are deficient in protecting swine herds from the consistently evolving field isolates of PRRSV [13]. One approach to improving PRRSV vaccine efficacy is the addition of immunomodulatory adjuvants including water-oil emulsions, aluminum, bacterial components, and polymers to killed or live modified PRRSV vaccines [14]. Interestingly, a new class of adjuvants, nanoparticles, has been shown to increase the cross-protection efficacy of killed PRRSV vaccines. In a recent study by Dwivedi et al, PLGA nanoparticle-entrapped killed PRRSV vaccine induces a cross-protective immune response against heterologous PRRSV challenge via enhanced innate and PRRSV-specific adaptive responses [15]. However, further studies are needed to reduce the cost and complexity of nanoparticle production before nanoparticle-based vaccines can be widely used as commercial products. Some more cost-effect commercial water-in-oil emulsion and polymers adjuvants, such as MontanideTM ISA 15A and Gel01 ST, have also been utilized in live modified PRRSV vaccines [6]. Deville et al. showed that pigs vaccinated with adjuvanted MLV vaccine containing 50% of the antigen load had equivalent protection as pigs vaccinated with full dose of vaccine without the adjuvant.
We recently reported that a biodegradable hydrogel could act as an adjuvant for killed swine influenza vaccines [10]. These previous results show that when H9e hydrogel was used in place of the supplied adjuvant of commercially available FluSure XP (Zoetis Inc), the H9e-adjuvanted vaccine led to significantly higher HAI titers and equivalent IgG antibody responses than the standard formulation of FluSure. Based on these results, we explored the ability of H9e hydrogel to act as an adjuvant for PRRS modified live virus vaccine and here we demonstrated that H9e hydrogels enhanced the vaccine’s protective effects for both homologous and heterologous PRRSV infection.
H9e hydrogel forms a biodegradable nanofiber network under physiological conditions [16]. Peptide-based nanofiber networks have been shown to aid in the controlled release of growth factors, therapeutics, and viruses [17–19]. Therefore, we hypothesized that this nanofiber network could create pockets that the vaccine virus could occupy and thus act as an antigen depot such that PRRS virus is presented slowly to the host immune system. We show here that the H9e had no virucidal effects and was able to facilitate increased PRRS vaccine virus presentation to the host, as shown by enhanced vaccine virus circulation in the blood (Fig. 2a). These results suggest high vaccine virus titers in the blood induced by MLV+H9e vaccination may facilitate the generation of an early and robust PRRSV immune response and provide better protection against genetically diverse strains of PRRSV.
In addition to high circulating vaccine virus, pigs vaccinated with MLV+H9e had earlier on-set of PRRS-specific ELISA antibodies and enhanced neutralizing antibody titers to homologous virus. Previous reports have shown that PRRSV-specific antibodies can appear as soon as one week post-vaccination or challenge, however seroconversion is often observed between 14–21 days post exposure [6, 20]. Our results are consistent with these reports and we found that addition of H9e adjuvant reduced the time that most pigs became positive for PRRSV-specific antibodies (Fig.2b and Supplemental Fig.1).
In order to gain insight into the immunologic mechanisms employed by the hydrogel adjuvant, cytokine expression levels after PRRSV challenge were compared among vaccinated groups of pigs. We found that the Th1-related cytokine IFN-γ in the sera of pigs vaccinated with MLV+H9e was significantly higher than that of pigs vaccinated with MLV alone after both homologous and heterologous challenges (Fig3a, b). IFN-γ is a key cytokine that is associated with host cell-mediated immunity (CMI) response, which is secreted by natural killer cells and several different T cell subpopulations, and its expression is often decreased by PRRSV infection [21, 22]. These studies suggest that decreased IFN-γ expression allows PRRSV to evade the host immune response and result in chronic PRRS infections. Interestingly, a recent study using two different PRRSV strains reported that systemic enhancement of IFN-γ further activates natural killers and T cell subpopulations creating a positive feedback loop for the rapid clearance of PRRSV [23].
Therefore, the elevated production of IFN-γ observed in the pigs vaccinated with H9e+MLV could explain the increased PRRS viral clearance and improved protective immune response we observed.
The expression of inflammatory cytokine IL-8, but not TNF-α, was increased in pigs vaccinated with H9e-MLV when pigs were challenged with homologous VR-2332 virus (Fig. 3b and d). In previous studies, low serum IL-8 levels are related to persistent PRRSV infection, and elevated IL-8 levels in serum is correlated with the clearance of PRRS virus [24, 25]. Although our results also indicated IL-8 may play a role in vaccination-induced clearance of PRRS virus, further experimentation is required to fully characterize the ability of H9e adjuvant to modulate IL-8 expression levels.
The Th2-related cytokine IL-4 was increased in the sera of pigs vaccinated with MLV+H9e compared with the pigs vaccinated with MLV alone only after homologous VR-2332 challenge. IL-4 expression has been shown to control macrophage inflammatory activities in the pig [26]. While IL-4 expression levels in PRRSV-infected pigs can remain unaltered [27], recent studies suggest that natural PRRSV infection can significantly induce the expression of IL-4 [28], suggesting that PRRSV-mediated IL-4 induction may be strain dependent. In our hands, the increased IL-4 expression after VR-2332 challenge correlated well with enhanced protection of pigs vaccinated with MLV+H9e than that of pigs vaccinated with MLV-alone. This indicates IL-4 may play a positive role in the immune response to PRRSV infection.
PRRSV infection or vaccination has been shown to induce a strong immunosuppressive response characterized by promoting the secretion of IL-10 to antagonize the protective Th1 immune response [29, 30]. In our study, we found that the concentrations of IL-10 in the serum and tissues of pigs vaccinated with MLV alone were consistently higher than that from pigs vaccinated with MLV+H9e (Fig. 3e). IL-10 is mainly produced by a small subpopulation of T lymphocytes termed T-regulatory cells [31]. Consistent with IL-10 levels, the frequency of T regulatory cells in MLV+H9e vaccinated pigs was dramatically reduced in blood, lung MNCs, and TBLNs post infection (Fig. 4c). Therefore, it is likely that the reduced T-regulatory cell population and production of IL-10 may contribute to the enhanced Th1 response and efficient elimination of PRRSV after challenge in the pigs vaccinated with MLV+H9e.
CD4+CD8+ T cells, which include T-helper, cytolytic, and memory properties, are a major type I IFN-γ cytokine secreting population [32]. In our study, pigs vaccinated with MLV+H9e generated significantly higher Th/memory cell populations both before and after challenge compared to the unvaccinated and MLV vaccinated pigs. This result is consistent with the observation that IFN-γ production is elevated in pigs vaccinated with MLV+H9e. The high frequency of functional T memory cells may contribute to rapid recall response for the quick elimination of subsequent PRRS virus exposure [33]. The ability of H9e adjuvant to shift MLV vaccine from mainly humoral, to a response having both humoral and cell-mediated immune responses suggest that CMI may be important for increased vaccine protection potential. Our results support the notion that MLV+H9e may act to enhance IFN-γ and reduce IL-10 production via increasing Th/memory and decreasing Treg lymphocyte populations, thereby causing a shift to a Th1-type immune response to provide a better protection against PRRSV infection.
We have previously shown that flexible polymer adjuvants such Montanide™ Gel01 ST also can enhance the protective effects of modified live PRRSV vaccines: however, their enhanced protective effects are limited to homologous re-infection [11]. Interestingly, it was demonstrated that the addition of Gel01 adjuvant to MLV vaccine could enhance vaccine-induced antibody-mediated immunity but did not promote a stronger cellular-mediated immunity. Furthermore, Gel01 adjuvanted MLV did not show improved efficacy in reducing heterologous challenge-induced viremia as compared with MLV alone. Thus, these results and our previous work suggest that the vaccine’s ability to generate a cellular-mediated immune response may be essential to mediate its cross-protective efficacy against PRRSV infection.
Conclusion
This study shows that H9e hydrogel as an adjuvant for MLV can improve vaccination-induced host protection against RRRSV infection by increased circulation of the vaccine virus in the blood, enhanced antibody production, and increased CMI responses. We believe that the addition of H9e hydrogel adjuvant to existing vaccines is an exciting method to improve PRRS live vaccine efficacy. These results encourage us to explore the ability of H9e to work as a universal adjuvant for a broad range of animal vaccines.
Supplementary Material
Acknowledgements
We thank Dr. Brooke Bloomberg and the rest of the comparative medicine staff at Kansas State University for their veterinarian help. We thank Dr. Kay Faaberg for her critical review of our manuscript. We would also like to thank Nanhua Chen, Theresa Quintana, and Sarah Ebarb for their help in handling our animals. This research was supported in part by KBA-CBRI 611310, NIH R21 AI085416, NIH NCRR P20-RR017686, Kansas State University Research Foundation, and USDA Agricultural Experimental Station at KSU (contribution number 13-227-J).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, et al. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS One. 2007;2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chand RJ, Trible BR, Rowland RR. Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol. :256–263. doi: 10.1016/j.coviro.2012.02.002. 2012/06/20 ed. [DOI] [PubMed] [Google Scholar]
- 3.Rowland RR, Lunney J, Dekkers J. Control of porcine reproductive and respiratory syndrome (PRRS) through genetic improvements in disease resistance and tolerance. Frontiers in genetics. 2012;3:260. doi: 10.3389/fgene.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thanawongnuwech R, Suradhat S. Taming PRRSV: revisiting the control strategies and vaccine design. Virus Res. 154:133–140. doi: 10.1016/j.virusres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Botner A, Strandbygaard B, Sorensen KJ, Have P, Madsen KG, Madsen ES, et al. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet Rec. 1997;141:497–499. doi: 10.1136/vr.141.19.497. [DOI] [PubMed] [Google Scholar]
- 6.Deville S, Arous JB, Ionkoff G, Kukushkin S, Bertranda F, Baybikov T, et al. Load reduction in live PRRS vaccines using oil and polymer adjuvants. Procedia in Vaccinology. 2012;6:134–140. doi: 10.1016/j.provac.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwivedi V, Manickam C, Patterson R, Dodson K, Murtaugh M, Torrelles JB, et al. Cross-protective immunity to porcine reproductive and respiratory syndrome virus by intranasal delivery of a live virus vaccine with a potent adjuvant. Vaccine. 2011;29:4058–4066. doi: 10.1016/j.vaccine.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinkhani H. 3D in vitro technology for drug discovery. Curr Drug Saf. 7:37–43. doi: 10.2174/157488612800492753. [DOI] [PubMed] [Google Scholar]
- 10.Huang HZ, Shi JS, Laskin J, Liu ZY, McVey DS, Sun XZS. Design of a shear-thinning recoverable peptide hydrogel from native sequences and application for influenza H1N1 vaccine adjuvant. Soft Matter. 2011;7:8905–8912. [Google Scholar]
- 11.Li X, Galliher-Beckley A, Nietfeld J, Faaberg K, Shi J. Montanide TM Gel01 ST Adjuvant Enhances PRRS Modified Live Vaccine Efficacy by Regulating Porcine Humoral and Cellular Immune Responses. World Journal of Vaccines. 2013;03:1–9. [Google Scholar]
- 12.Ferrari L, Martelli P, Saleri R, De Angelis E, Cavalli V, Bresaola M, et al. Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection. Vet Immunol Immunopathol. 2013;151:193–206. doi: 10.1016/j.vetimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Zhang C. Porcine reproductive and respiratory syndrome virus vaccines: current status and strategies to a universal vaccine. Transbound Emerg Dis. 2013 doi: 10.1111/tbed.12016. [DOI] [PubMed] [Google Scholar]
- 14.Charerntantanakul W. Adjuvants for porcine reproductive and respiratory syndrome virus vaccines. Vet Immunol Immunopathol. 2009;129:1–13. doi: 10.1016/j.vetimm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi V, Manickam C, Binjawadagi B, Joyappa D, Renukaradhya GJ. Biodegradable nanoparticle-entrapped vaccine induces cross-protective immune response against a virulent heterologous respiratory viral infection in pigs. PLoS One. 2012;7:e51794. doi: 10.1371/journal.pone.0051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H, Herrera AI, Luo Z, Prakash O, Sun XS. Structural transformation and physical properties of a hydrogel-forming peptide studied by NMR, transmission electron microscopy, and dynamic rheometer. Biophys J. 103:979–988. doi: 10.1016/j.bpj.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J, Ma PX. Nano-fibrous tissue engineering scaffolds capable of growth factor delivery. Pharm Res. 2011;28:1273–1281. doi: 10.1007/s11095-011-0367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Kim JS, Chu HS, Kim GW, Won JI, Jang JH. Electrospun nanofibrous scaffolds for controlled release of adeno-associated viral vectors. Acta Biomater. 2011;7:3868–3876. doi: 10.1016/j.actbio.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Webber MJ, Matson JB, Tamboli VK, Stupp SI. Controlled release of dexamethasone from peptide nanofiber gels to modulate inflammatory response. Biomaterials. 2012;33:6823–6832. doi: 10.1016/j.biomaterials.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo B, Lager KM, Henningson JN, Miller LC, Schlink SN, Kappes MA, et al. Experimental infection of United States swine with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. Virology. 2013;435:372–384. doi: 10.1016/j.virol.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Y, Han M, Kim C, Calvert JG, Yoo D. Interplay between interferon-mediated innate immunity and porcine reproductive and respiratory syndrome virus. Viruses. 2012;4:424–446. doi: 10.3390/v4040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Zhou Y, Tong G. Mechanisms of suppression of interferon production by porcine reproductive and respiratory syndrome virus. Acta Virol. 2012;56:3–9. doi: 10.4149/av_2012_01_3. [DOI] [PubMed] [Google Scholar]
- 23.Wesley RD, Lager KM, Kehrli ME., Jr Infection with Porcine reproductive and respiratory syndrome virus stimulates an early gamma interferon response in the serum of pigs. Can J Vet Res. 2006;70:176–182. [PMC free article] [PubMed] [Google Scholar]
- 24.Lunney JK, Fritz ER, Reecy JM, Kuhar D, Prucnal E, Molina R, et al. Interleukin-8, interleukin-1beta, and interferon-gamma levels are linked to PRRS virus clearance. Viral Immunol. 2010;23:127–134. doi: 10.1089/vim.2009.0087. [DOI] [PubMed] [Google Scholar]
- 25.Petry DB, Lunney J, Boyd P, Kuhar D, Blankenship E, Johnson RK. Differential immunity in pigs with high and low responses to porcine reproductive and respiratory syndrome virus infection. J Anim Sci. 2007;85:2075–2092. doi: 10.2527/jas.2006-721. [DOI] [PubMed] [Google Scholar]
- 26.Murtaugh MP, Johnson CR, Xiao Z, Scamurra RW, Zhou Y. Species specialization in cytokine biology: is interleukin-4 central to the T(H)1-T(H)2 paradigm in swine? Dev Comp Immunol. 2009;33:344–352. doi: 10.1016/j.dci.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Diaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259. doi: 10.1016/j.virol.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 28.Dwivedi V, Manickam C, Binjawadagi B, Linhares D, Murtaugh MP, Renukaradhya GJ. Evaluation of immune responses to porcine reproductive and respiratory syndrome virus in pigs during early stage of infection under farm conditions. Virol J. 2012;9:45. doi: 10.1186/1743-422X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song S, Bi J, Wang D, Fang L, Zhang L, Li F, et al. Porcine reproductive and respiratory syndrome virus infection activates IL-10 production through NF-kappaB and p38 MAPK pathways in porcine alveolar macrophages. Dev Comp Immunol. 2013;39:265–272. doi: 10.1016/j.dci.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Suradhat S, Thanawongnuwech R. Upregulation of interleukin-10 gene expression in the leukocytes of pigs infected with porcine reproductive and respiratory syndrome virus. J Gen Virol. 2003;84:2755–2760. doi: 10.1099/vir.0.19230-0. [DOI] [PubMed] [Google Scholar]
- 31.Charerntantanakul W, Platt R, Roth JA. Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol. 2006;19:646–661. doi: 10.1089/vim.2006.19.646. [DOI] [PubMed] [Google Scholar]
- 32.Gerner W, Kaser T, Saalmuller A. Porcine T lymphocytes and NK cells--an update. Dev Comp Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 33.Xiao Z, Batista L, Dee S, Halbur P, Murtaugh MP. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J Virol. 2004;78:5923–5933. doi: 10.1128/JVI.78.11.5923-5933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.